Translate this page into:
Can an inadequate cervical cytology sample in ThinPrep be converted to a satisfactory sample by processing it with a SurePath preparation?
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The Norwegian Cervical Cancer Screening Program recommends screening every 3 years for women between 25 and 69 years of age. There is a large difference in the percentage of unsatisfactory samples between laboratories that use different brands of liquid-based cytology. We wished to examine if inadequate ThinPrep samples could be satisfactory by processing them with the SurePath protocol.
Materials and Methods:
A total of 187 inadequate ThinPrep specimens from the Department of Clinical Pathology at University Hospital of North Norway were sent to Akershus University Hospital for conversion to SurePath medium. Ninety-one (48.7%) were processed through the automated “gynecologic” application for cervix cytology samples, and 96 (51.3%) were processed with the “nongynecological” automatic program.
Results:
Out of 187 samples that had been unsatisfactory by ThinPrep, 93 (49.7%) were satisfactory after being converted to SurePath. The rate of satisfactory cytology was 36.6% and 62.5% for samples run through the “gynecology” program and “nongynecology” program, respectively. Of the 93 samples that became satisfactory after conversion from ThinPrep to SurePath, 80 (86.0%) were screened as normal while 13 samples (14.0%) were given an abnormal diagnosis, which included 5 atypical squamous cells of undetermined significance, 5 low-grade squamous intraepithelial lesion, 2 atypical glandular cells not otherwise specified, and 1 atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion. A total of 2.1% (4/187) of the women got a diagnosis of cervical intraepithelial neoplasia 2 or higher at a later follow-up.
Conclusions:
Converting cytology samples from ThinPrep to SurePath processing can reduce the number of unsatisfactory samples. The samples should be run through the “nongynecology” program to ensure an adequate number of cells.
Keywords
Background
cervical cytology
inadequate
SurePath
ThinPrep
unsatisfactory
INTRODUCTION
The Norwegian Cervical Cancer Screening Program recommends that women between the ages 25 and 69 participate in cervical cytology screening every 3 years (www.kreftregisteret.no). If there are cytologic changes in this sample, the women are triaged and followed up according to specified guidelines. In the case of unsatisfactory/inadequate samples, the recommendation is for renewed cytology test within 3 months. If a woman has several unsatisfactory cytology samples, she will be referred to a gynecologist for further follow-up (www.kreftregisteret.no).
In Norway, there are 17 cytology laboratories covering a population of 5 million people. All the laboratories receive most of their samples from general practitioners in primary screening. There is a large difference in the percentage of unsatisfactory samples between laboratories that use different brands of liquid-based cytology (LBC).[1] In Norway, laboratories that use ThinPrep (Hologic, Bedford, MA, USA) report around 5%–7% inadequate samples while laboratories that use SurePath (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) report a rate of around 0.5%–1.0%.[2] Most of the laboratories in Norway use ThinPrep, but four laboratories have recently started using SurePath. One laboratory reduced their rate from 5.6% in 2015 to 1.4% in 2016 when they changed LBC from ThinPrep to SurePath.
One difference in the two techniques is the inclusion of the sampling brush in the medium of the SurePath container, whereas it is discarded with ThinPrep.[3] As approximately half of the cellular material is attached to the brush, its inclusion with the sample will retain a larger quantity of material for SurePath than for ThinPrep. This could be part of the explanation of why SurePath samples have a higher satisfactory rate than ThinPrep.[4] For optimal results, the collection vial containing the sampling brush is shaken vigorously when it arrives at the laboratory to make a larger number of cells available for processing. Akershus University Hospital (Ahus) uses a “paint shaker,” of the kind used commercially to mix pigment with paint base, for this purpose.
One of the issues with the use of ThinPrep is that blood, mucus, gynecological gel, and inflammatory cells from the sample can clog the filter during the process of preparation.[56] There are protocols for manual “washing” of ThinPrep samples to lyse red blood cells that can improve the adequacy of samples.[5] Different hospital laboratories report varying degrees of success using these protocols.[78] The manual procedures consume extra time in the laboratory and each sample ends up being microscopically evaluated twice.
The Department of Clinical Pathology at the University Hospital of North Norway (UNN) receives all the cervical cytology samples from women in Troms and Finnmark county, annually 25,000–30,000 samples. UNN has used ThinPrep LBC since 2006. Following the switch from conventional smears to ThinPrep, there was an increase in the percentage of unsatisfactory samples. Of all samples, 7.6% (2052/26,886) were inadequate in 2014. In this department, the increase in sample yield after washing was found to be small and was not deemed cost effective.
The preparation of samples in SurePath medium is based on centrifugation as opposed to filters. The blood and gel are automatically removed before preparation, and the number of inflammatory cells is reduced.[9] We wished to examine if the sample adequacy of unsatisfactory ThinPrep samples could be improved by transferring them to the SurePath medium and processing them with the SurePath protocol. The Pathology Department at Ahus uses SurePath in their routine and collaborated with UNN in this project. UNN sent 187 unsatisfactory ThinPrep samples to Ahus for preparation and evaluation.
MATERIALS AND METHODS
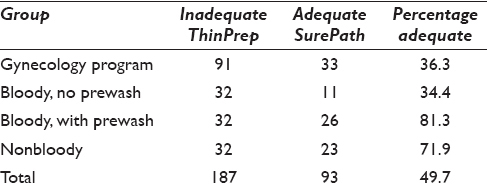
In 2013, the Pathology Department at UNN initially sent a batch of 91 inadequate ThinPrep specimens to Ahus for conversion to SurePath medium. These were processed through the automated “gynecologic” application for cervix cytology samples. In 2014, the second batch was sent, divided into three separate categories: 32 bloody samples to be run without prewashing, 32 bloody samples to be run after prewashing, and 32 nonbloody samples [Table 1]. These samples were processed with the “nongynecological” automatic program, where a larger proportion of cells are extracted for evaluation.
The converted samples were initially evaluated by a cytotechnologist (Mette Kristin Pedersen) for adequacy and then screened by a pathologist (Sveinung Wergeland Sørbye). Criteria for cellularity require the presence of at least 5000 cells in the specimen equivalent to 8–9 cells per ×40 high-powered field across diameter.[1] The evaluation was performed with investigators blinded to earlier results, previous specimens, and clinical information. The results of the screening were registered as an internal quality control and not as part of the patients’ medical records. Screening of subsequent ThinPrep samples from these patients was also performed blinded to previous results in both ThinPrep and converted SurePath samples. In this study, the diagnoses from the converted SurePath samples and human papillomavirus (HPV) messenger RNA analysis (PreTect SEE) of the inadequate ThinPrep sample were compared to the follow-up tests from the patients, in the form of ThinPrep samples, and/or follow-up HPV DNA testing (Cobas 4800) and/or histological biopsies. The women with unsatisfactory ThinPrep sample at UNN in 2013–2014 were followed until December 31 2015.
Converting from ThinPrep sample to SurePath kit
Conversion of the first batch of samples was performed as follows: all remaining material in the ThinPrep container was centrifuged, and the resulting pellet was transferred to a BD SurePath™ collection vial. These samples were then processed as regular gynecological samples with PrepMate BD™ and BD density reagent before another two centrifugation steps to remove unwanted debris (blood, inflammatory cells, mucus, and gynecological gel).
The resulting cell pellet was resuspended in BD Totalys™ Slideprep with 1 ml tris-buffered water. The samples were run through the standardized “gynecological program,” where 200 μl of the cell suspension is used for processing and staining. At Ahus, >99% of routine samples return satisfactory results on this program.
From the second batch of samples, 32 of the bloody ThinPrep specimens were centrifuged for 10 min at 2148 rpm. The precipitate was then washed with a solution of 9:1 Cytolyt® solution (Hologic, Bedford, MA, USA) and concentrated acetic acid. The unwashed bloody samples, the washed samples, and the nonbloody samples were then spun for 10 min at 2148 rpm. The resulting cell pellets were then transferred to BD Surepath™ collection vials and processed like the first batch with PrepMate BD™ and BD density reagent and a two-step centrifugation and resuspension in BD Totalys™ Slideprep with 1 ml tris-buffered water.
The second batch samples were prepared and stained with a “nongynecological” program, which gives the option to choose the amount of cell suspension to use on each sample slide. The volumes used were 400–800 μl of the suspension, where 800 μl is the maximum volume available for selection in this program.
The Regional Committee for Medical and Health Research Ethics, North Norway, approved the study as a quality assurance study in laboratory work fulfilling the requirements for data protection procedures within the department (REK Nord 2014/787). Norwegian regulations exempt quality assurance studies from written informed consent from the patients (https://lovdata.no/dokument/SF/forskrift/2000-12-15-1265).
RESULTS
Out of 187 samples that had been unsatisfactory by ThinPrep, 93 (49.7%) were satisfactory after being converted to SurePath. Out of the 91 unsatisfactory samples from 2013 that were run through the “gynecology” program, 33 samples (36.6%) became satisfactory. One example is displayed in Figure 1. Out of the 96 samples from 2014 that were run through the “nongynecology” program, the percentage of satisfactory samples from the different groups was 34.4% (11/32), 81.3% (26/32), and 71.9% (23/32) for bloody sample with no prewash, bloody samples after prewash, and nonbloody samples, respectively.
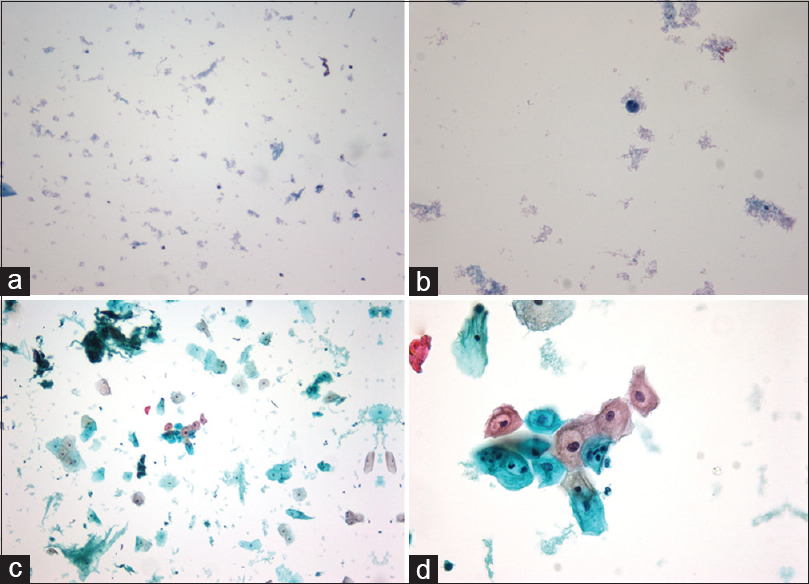
- (a) ThinPrep × 10 before conversion, (b) ThinPrep × 40 before conversion, (c) SurePath × 10 after conversion, (d) SurePath × 40 after conversion
Of the 93 samples that became satisfactory after conversion from ThinPrep to SurePath, 80 (86.0%) were screened as normal while 13 samples (14.0%) were given an abnormal diagnosis, which included 5 atypical squamous cells of undetermined significance (ASC-US), 5 low-grade squamous intraepithelial lesion (LSIL), 2 atypical glandular cells not otherwise specified (AGC-NOS), and 1 atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion [Table 2].
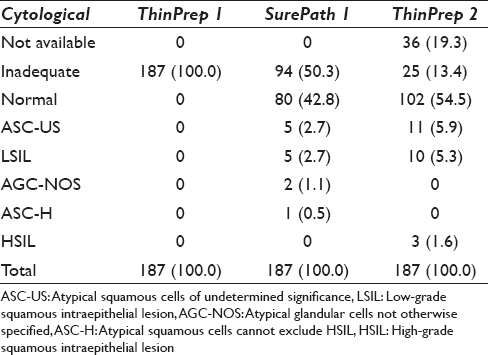
Of the 187 patients, 80.7% (151/187) had follow-up tests sent to the same hospital, whereas 19.3% (36/187) had not submitted to repeat testing at the time of the study. Out of 151 follow-up tests, 83.4% were deemed satisfactory while 16.6% (25/151) were still unsatisfactory by ThinPrep. Of the 25 women with repeated unsatisfactory cytologies, 9 had a follow-up HPV DNA analysis and 6 had cervical biopsies taken (data not shown).
Of the 13 SurePath samples that had cytologic abnormalities on screening, follow-up tests from 5 were normal and 5 had LSIL. One had a second unsatisfactory sample, and two were lost to follow-up. Four of the five women with LSIL had an HPV DNA analysis done, which all came back with positive results. Two women had biopsies performed, which were negative (normal/cervical intraepithelial neoplasia 1 [CIN1]) while two women are still under surveillance. One of the samples diagnosed as AGC-NOS on SurePath was unsatisfactory by ThinPrep in follow-up. The HPV DNA test was positive for HPV type 18, and this woman was referred for colposcopy and biopsy at a gynecologist. The biopsies showed CIN2 and she was treated with conization [Table 3].

Sixty-six out of the 88 women (82.5%) with normal SurePath tests had follow-up ThinPrep tests. 74.2% (49/66) were normal, 12.1% (8/66), abnormal, and 13.6% (9/66) had a second unsatisfactory ThinPrep sample (data not shown). The eight abnormal samples included five ASC-US and three LSIL. Seven of the eight abnormal ThinPrep samples had HPV DNA test run. Three of these were positive and five were negative. All three HPV DNA-positive women are still under surveillance [Table 3]. Biopsies were not performed.
DISCUSSION
Norway has one of the highest rates of unsatisfactory cases in the world. In 2015, the rate was 3.99% (17,516/439,494). There are 6000 general practitioner and gynecologist sampling cervical cytology giving an average of 72 samples annually. When many doctors take <1 sample every week, and some only one sample every month, this could explain why the rate of unsatisfactory is high in Norway. The SurePath method is more robust and less dependent of the sampling technique. The University Hospital of Norway (UNN) had in 2015 a rate of unsatisfactory cases of 8.67% with ThinPrep, which is one of the highest rates in Norway. There is a huge difference within different doctors sending cytology samples to UNN. Some of the gynecologists have a rate of unsatisfactory cases of 1.2% while some of the general practitioners have up to 85% unsatisfactory cases.
The marketing of LBC stressed that the monolayer technology would provide better preservation of cells, better samples, and fewer unsatisfactory tests (http://www.hologic.com). However, all laboratories in Norway that use ThinPrep have noticed a marked increase in the number of unsatisfactory samples.[210] At the Department of Clinical Pathology UNN, we had only a slight increase in inadequate samples initially, due to our pragmatic attitude toward defining a sample as unsatisfactory. In 2012, our percentage of unsatisfactory samples was lower than the national average (2.9% vs. 3.5%).[10] Since 2013, we have followed the guideline criteria for defining a satisfactory sample more stringently, and our levels rose to 7.1% unsatisfactory in 2013 and 7.6% in 2014. The national average for this period was 4.5% and 4.3%, respectively.[2]
In this project, we have shown that approximately half of the unsatisfactory ThinPrep samples can be made adequate by converting them to a SurePath procedure (performed at Ahus). This finding is in accordance with Randolph et al.,[6] thus reducing the rate of unsatisfactory specimens from 7.0% to 3.5%. This reduces our percentage of unsatisfactory samples to below the national average but does not achieve the 0.5%–1.0% rate of laboratories that primarily use the SurePath process.[2] In contrast, Kalinicheva et al.[8] found increased cellularity in 48% of specimens that had been reprocessed after a wash protocol. Of these, 29% were satisfactory and showed good cellularity in 22%, whereas 7% had borderline cellularity.
The unsatisfactory samples received at Ahus had already had cells extracted from the medium at UNN for the first attempt at analysis. This is probably another explanation for the small number of cells received at Ahus and for their need to run the samples through the “nongynecology” program, which uses a larger dose of fluid. Results after running the samples through the “gynecology” program yielded a satisfactory rate of 36.3% (33/91), whereas the “nongynecology” program yielded 62.5% satisfactory samples (60/96). Kalinicheva et al. found lubricant to be the main cause of unsatisfactory specimens (68%) using ThinPrep. They obtained a satisfactory rate of 29% after applying a wash protocol on the unsatisfactory samples.
Some laboratories report increased yields by “washing” blood-tinged ThinPrep samples before processing. The ThinPrep samples that were prewashed before conversion to SurePath had an 81.3% (23/32) satisfactory rate. These are good results, but perhaps more notable was that 71.9% (23/32) of nonbloody unsatisfactory ThinPrep samples became satisfactory after converting to SurePath. Centrifugation to obtain a pellet of cells is a technique that allows to throw the supernatant that includes red blood cells and sometimes gel (lubricant/lube). Therefore, the material put into the SurePath liquid is not exactly the same as the material that was initially in the ThinPrep fixative. Using the SurePath preparation technique, there are less problems with red blood cells and gel cluttering the filter in the ThinPrep machine. This gives better quality of the slide and less inadequate samples.
We have not compared the results of “washing” blood-tinged ThinPrep samples using glacial acetic acid wash (AAG treatment) before ThinPrep processing versus SurePath transfer. We could use “split samples” to do both AAG treatment of ThinPrep to compare the results with SurePath using material from the same sample, but in samples with low cellularity, an additional 50% reduction in cellularity using split samples would probably give a higher rate of inadequate samples using both methods.
We have not tried to transfer to Hologic technique of the unsatisfactory cases obtained with SurePath applying an equivalent method to compare the results, but all laboratories with experience with both methods have less unsatisfactory cases using SurePath than ThinPrep.
Of the 187 women with unsatisfactory ThinPrep samples, 126 (67.4%) were followed up with a satisfactory ThinPrep while 61 (32.6%) had either an unsatisfactory follow-up test or no follow-up test.
A total of 2.1% (4/187) of the women in our material got a diagnosis of CIN2 or higher at a later follow-up. In the Norwegian screening population, this percentage was 0.64% (2427/378,855) in 2014.[2] Thus, the women with unsatisfactory samples in our project had an increased risk of cervical dysplasia compared to a randomly selected woman from the screening population.
CONCLUSIONS
Converting cytology samples from ThinPrep to SurePath processing can reduce the number of unsatisfactory samples. The samples should be run through the “nongynecology” program to ensure an adequate number of cells, and bloody samples should be prewashed before conversion. In general, the use of SurePath decreases the number of inadequate samples due to the sample brush being included with the fluid, thus increasing the number of cells available for analysis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
BE and MKP prepared and processed the SurePath specimens, evaluated the converted samples for adequacy and helped to draft the manuscript. MEJW drafted the manuscript. SWS participated in the design of the study, screened the converted samples, performed follow-up, performed the statistical analysis and drafted the manuscript. TS helped to draft the manuscript. YC participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
The Regional Committee for Medical and Health Research Ethics, North Norway, approved the study as a quality assurance study in laboratory work fulfilling the requirements for data protection procedures within the department (REK Nord 2014/787).
LIST OF ABBREVIATIONS (In alphabetic order)
AGC-NOS- Atypical glandular cells not otherwise specified
AHUS- Akershus University Hospital
ASC-H- Atypical squamous cells cannot exclude HSIL
ASC-US- Atypical squamous cells of undetermined significance
BD- Becton Dickinson and Company
CIN- Cervical intraepithelial neoplasia
HSIL- High-grade squamous intraepithelial lesion
HPV- Human papillomavirus
LBC- liquid-based cytology
LSIL- Low-grade squamous intraepithelial lesion
mRNA- messenger ribonucleic acid
REK- The Regional Committee for Medical and Health Research Ethics
UNN- University Hospital of North Norway
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Unsatisfactory rates vary between cervical cytology samples prepared using ThinPrep and SurePath platforms: A review and meta-analysis. BMJ Open. 2012;2:e000847.
- [Google Scholar]
- The Norwegian Cervical Cancer Screening Programme. Annual Report 2013-2014. The Cancer Registry of Norway 2015
- [Google Scholar]
- Comparing SurePath, ThinPrep, and conventional cytology as primary test method: SurePath is associated with increased CIN II+ detection rates. Cancer Causes Control. 2016;27:15-25.
- [Google Scholar]
- A study of cellular counting to determine minimum thresholds for adequacy for liquid-based cervical cytology using a survey and counting protocol. Health Technol Assess. 2015;19:i.
- [Google Scholar]
- The unsatisfactory ThinPrep Pap Test: Missed opportunity for disease detection? Am J Clin Pathol. 2002;117:457-63.
- [Google Scholar]
- Reprocessing unsatisfactory ThinPrep papanicolaou tests using a modified SurePath preparation technique. Cancer Cytopathol. 2014;122:343-8.
- [Google Scholar]
- The effectiveness of acetic acid wash protocol and the interpretation patterns of blood contaminated cervical cytology ThinPrep(®) specimens. Cytojournal. 2015;12:23.
- [Google Scholar]
- Etiologic factors related to unsatisfactory ThinPrep(®) cervical cytology: Evaluation and potential solutions to improve. Cytojournal. 2015;12:21.
- [Google Scholar]
- Comparison of BD Surepath and ThinPrep Pap systems in the processing of mucus-rich specimens. Cancer Cytopathol. 2010;118:244-9.
- [Google Scholar]
- The Norwegian Cervical Cancer Screening Programme. Annual Report 2012. The Cancer Registry of Norway 2014
- [Google Scholar]








