Translate this page into:
Clear cell neuroendocrine tumor of pancreas: Endoscopic Ultrasound-guided fine needle aspiration diagnosis of an uncommon variant
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The cytomorphologic features of clear cell neuroendocrine tumor of pancreas have been rarely reported in cytology literature. The cytomorphology of this rare variant mimics many primary and metastatic clear cell tumors of the pancreas. However, a precise cytological diagnosis can be rendered by awareness of this entity and judicious use of immunohistochemistry. We report one such case in a young woman diagnosed on endoscopic ultrasound fine needle aspiration. The tumor cells showed positive staining with synaptophysin, chromogranin, and also with inhibin.
Keywords
Clear
endoscopic ultrasound-guided fine needle aspiration
inhibin
neuroendocrine tumor
INTRODUCTION
With the advent of endoscopic ultrasound guided fine needle aspiration (EUS FNA) of pancreatic masses, large numbers of pancreatic tumors are being diagnosed by cytopathologists. FNA smears are often the only material available for preoperative tissue diagnosis.[1] Adenocarcinomas are the most common tumors. Pancreatic endocrine neoplasms (PENs) constitute only 1–2% of all pancreatic tumors.[2] Cytomorphologic features combined with immunohistochemistry on cell blocks or smears are sufficient for diagnosis of PEN in most of the cases.[3] Clear cell variant of PEN is uncommon. It can be morphologically confused with many other primary and metastatic tumors of pancreas.[4]
To the best of our knowledge, only six case reports of FNA diagnosis of this rare variant are available.[5678] We report a case of clear cell variant of PEN, diagnosed on EUS FNA with the aid of immunohistochemistry.
CASE REPORT
A 32-year-old woman was evaluated for abdominal pain in another hospital. A mass was seen between the pancreatic head and duodenal wall on ultrasound examination of abdomen. She was referred to our institution for further evaluation. EUS confirmed a 5.5 cm × 4.5 cm hypoechoic mass between pancreatic head and duodenal wall. The mass appeared to be outside the duodenal wall. No calcifications or vessels were seen inside the mass. Multiple small nodes were seen around the lesion. No vascular involvement was seen. The possibilities considered on EUS were gastrointestinal stromal tumor, lymphoma, and solid and papillary epithelial neoplasm (SPEN) of pancreas. EUS FNA was done. Air dried and alcohol fixed smears were prepared from the FNA material. Material was also procured in formalin for cell block preparation.
Cytological evaluation
May-Grunwald Giemsa and Papanicolaou stained smears were examined. Smears were of good cellularity. The cells were present dispersed singly, in small groups and fragments. In few fragments, cells were seen adhering to fibrovascular cores [Figure 1]. The cells were round in shape and monomorphic. The nuclei had fine nuclear chromatin and small nucleoli. The most striking feature was that the cytoplasm of almost all the cells had multiple fine vacuoles imparting a foamy appearance to the cells. Most of the cells had eccentric nuclei [Figures 2 and 3]. At low power examination, these cells were almost indistinguishable from foamy histiocytes. Occasional bare nuclei were seen in the background. Hematoxylin and eosin stained sections from cell block showed small nests of similar foamy cells entrapped in blood clot. The cells had well defined cytoplasmic membranes [Figure 4]. Keeping in view, the cytologic features of the tumor, location of the tumor, and the young age of the patient possibilities of neuroendocrine tumor (NET) with clear cell change and SPEN with clear cell change were considered. Other differentials considered were primary clear cell adenocarcinoma, metastatic clear cell carcinoma (adrenal, renal, female genital tract origin), or perivascular epithelioid cell tumors (PEComas). Immunohistochemistry done on cell block revealed tumor cells staining positive with cytokeratin, chromogranin [Figure 5], and synaptophysin. The nuclei of tumor cells showed negative staining with beta catenin. Cytological diagnosis of NET with clear cell change was rendered. The Ki-67 proliferation index was 8%. The tumor cells showed strong positive membranous staining with inhibin [Figure 6]. In view of inhibin positivity in clear cell NET, evaluation of the patient for Von Hippel-Lindau (VHL) disease was suggested.
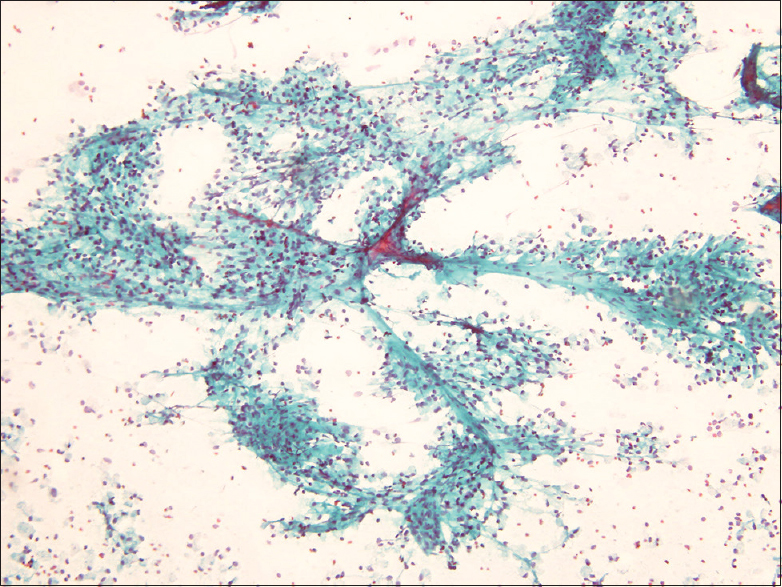
- Tumor cells were seen adherent to fibrovascular cores and scattered singly (Pap, ×200)
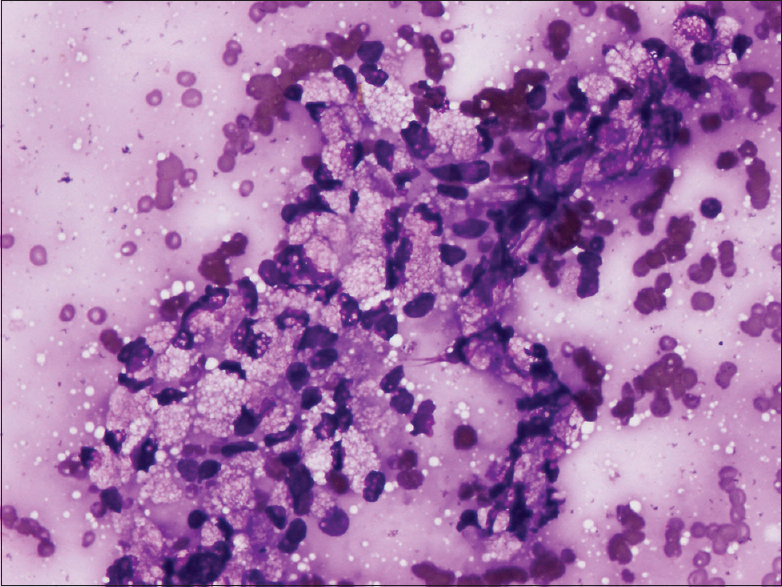
- Most of the cells had multiple fine vacuoles in the cytoplasm (MGG, ×400)
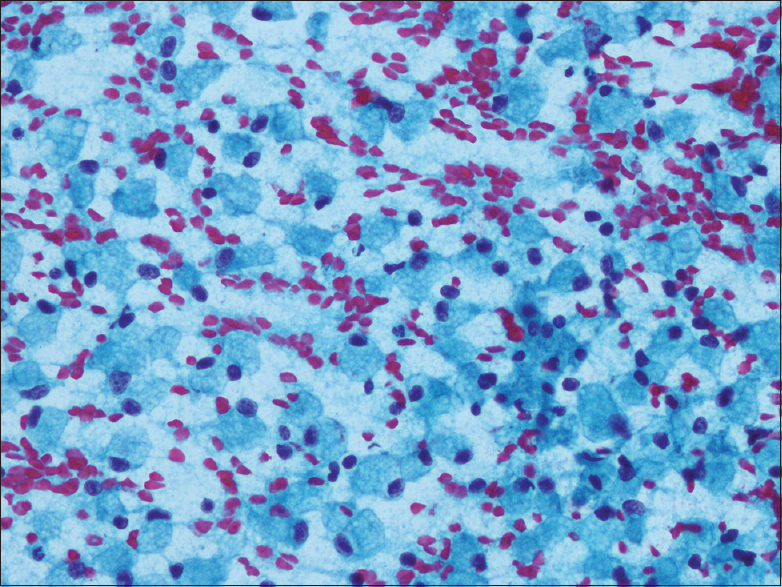
- Many singly scattered foamy tumor cells simulating histiocytes (Pap, ×400)
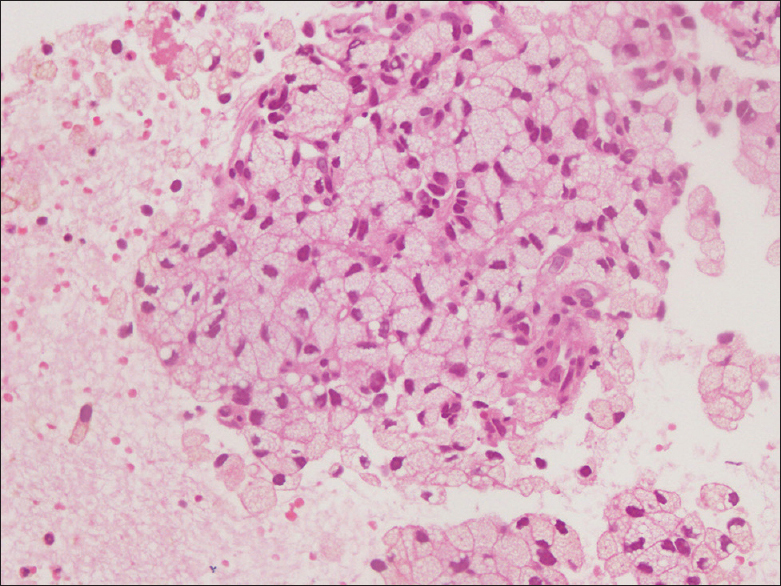
- Section from cell block shows small nests of tumor cells (H and E, ×400)
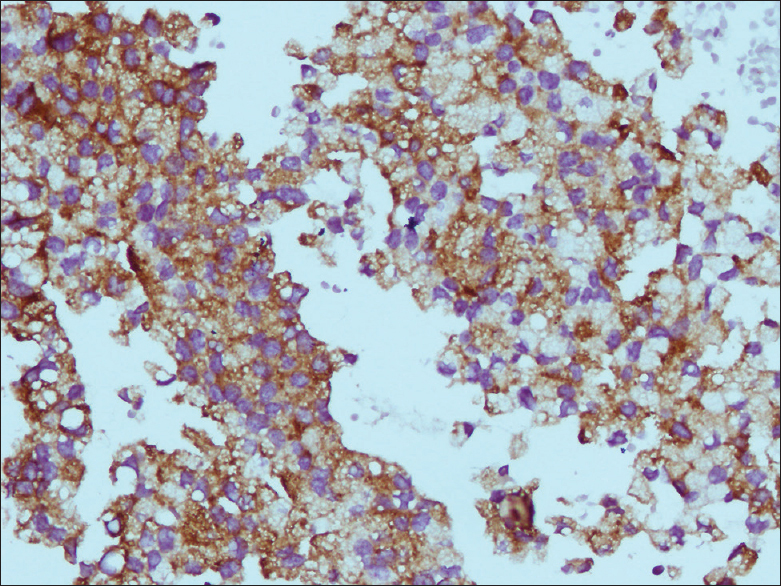
- Chromogranin (×400)
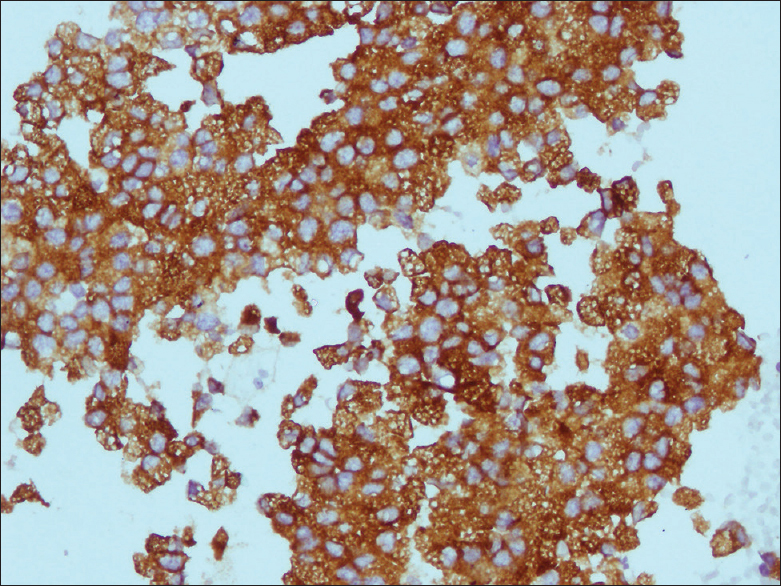
- Inhibin (×400)
Clinical follow-up
The plain and postcontrast computed tomography scans of the patient revealed a 46.9 mm × 66.5 mm × 51.7 mm solid mass adjacent to pancreas. There was loss of fat plains in few regions of pancreas. The mass lesion showed vascularity and changes suggestive of old hemorrhage. Multiple focal lesions were also observed in various segments of liver. Both the adrenal glands and kidneys were normal. The endometrial cavity showed a small collection (11.9 mm) and left ovary had a simple cyst (39.1 mm × 39.5 mm). The patient opted for treatment in another hospital.
DISCUSSION
Clear cell tumors are infrequently encountered in pancreas and include a variety of primary and metastatic tumors. The primary tumors of pancreas with clear cell change include clear cell adenocarcinomas, clear cell variant of NET, clear cell variant of SPEN, serous cystadenomas, and PEComas.[49] Though pancreas is a rare site for metastases, renal cell carcinoma is the most common cancer metastatic to pancreas.[10] In the present case, careful attention to cytological features and immunohistochemistry helped arrive at a diagnosis of NET.
Diagnosis of NET is usually straightforward and can be rendered confidently in the presence of characteristic morphologic features; cell monotony, plasmacytoid cells, and salt and pepper chromatin. This is commonly supplemented with immunohistochemical staining with synaptophysin and/or chromogranin. The morphologic patterns of NET may vary considerably and can pose a diagnostic challenge in some cases. The variants of NET described in pancreas include clear cell, oncocytic, pleomorphic, and rhabdoid type.[11]
Clear cell change in NETs is an uncommon occurrence. The cytoplasmic clearing can be due to the accumulation of glycogen, mucin, or lipids. It can also be produced by the swelling of cytoplasmic organelles. In the earliest reported case of clear cell islet cell tumor, the clear cell morphology was due to the accumulation of lipid and glycogen and cytoplasmic swelling.[12] Later, Ordonez and Silva[13] described an islet cell tumor with “vacuolated lipid-rich” cytoplasm. Histochemical staining did not demonstrate mucin or glycogen in their case. They did not observe swelling of cytoplasmic organelles on ultra-structural examination and clearing was attributed to the accumulation of massive amount of lipids. Singh et al.[14] studied 11 cases of NETs with microvesicular foamy cytoplasm. Electron microscopy was done in three cases and showed lipid vesicles. They preferred the term lipid-rich variant for these cases.
The terms lipid-rich and clear cell variants have been used interchangeably in literature.[1516] These tumors can be sporadic or syndromic (associated with VHL disease or multiple endocrine neoplasia type 1 [MEN I] syndrome).[1415]
NET with clear cell changes is prone to misdiagnosis without the aid of immunocytochemistry. The earliest documented case of clear cell islet cell tumor was initially misinterpreted as an adrenocortical carcinoma. Ultra-structural and immunohistochemical findings proved that it was metastatic islet cell tumor.[12] Four of the 11 cases of lipid rich NETs studied by Singh et al.[14] were initially misinterpreted as adrenocortical tumor, renal cell carcinoma, solid pseudopapillary tumor, and adenocarcinoma each. Immunohistochemistry showed positive staining with chromogranin and synaptophysin in all the cases.
Very few cases of clear cell NET of pancreas have been reported in cytology literature. Absence of architectural features renders the diagnosis more challenging in small biopsies and fine needle aspiration cytology.[8] Safo et al. described EUS FNA features of clear cell PEN in a patient with VHL syndrome.[5] A similar case was described by Chen et al. in a patient without VHL syndrome.[6] Ayub and Dodge also elaborated cytologic features of lipid-rich PEN in a 71-year-old patient.[7] Cytological diagnosis in all these three cases was confirmed by immunostaining with neuroendocrine markers. Levy et al. reported another 3 cases of NET with prominent cytoplasmic vacuolization in most of the cells.[8] A definite cytologic diagnosis could be given in two out of these three cases, after immunohistochemistry with synaptophysin and chromogranin was done on cell block. The third case was diagnosed as “neoplasm” as no ancillary studies could be done.
In the present case, the tumor cells also showed positive staining with inhibin. Inhibin positivity has been reported in clear cell NETs in association with VHL disease and also in a single sporadic case of NET. Inhibin staining was absent in all NETs in a study of 8 tumors in MEN I patients. Whether inhibin positivity in clear cell tumors could suggest the possibility of VHL disease needs to be substantiated by larger case studies.[1517]
CONCLUSION
The above case highlights the cytomorphologic features of an unusual variant of NET and also highlights the role of cell block and immunohistochemistry in arriving at precise cytological diagnoses in pancreatic tumors.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. GK worked up the case along with PB and KV. GK, VS, PB, and KV drafted the manuscript and reviewed the literature.
ETHICS STATEMENT BY ALL AUTHORS
As this is the case report without identifiers, our institution does not require approval from the Institutional Review Board (or its equivalent).
LIST OF ABBREVIATIONS (In alphabetic order)
EUS FNA - Endoscopic Ultrasound Guided Fine Needle Aspiration
NET - Neuroendocrine Tumor
PEComas - Perivascular Epithelioid Cell Tumors
PENs - Pancreatic Endocrine Neoplasms
SPEN - Solid and Papillary Epithelial Neoplasm
VHL - Von Hippel-Lindau
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am. 2012;22:155-67. vii
- [Google Scholar]
- Endoscopic ultrasound-guided fine needle aspiration cytology of pancreatic neuroendocrine tumours: Cytomorphological and immunocytochemical evaluation. Cytopathology. 2006;17:10-7.
- [Google Scholar]
- Clear cell carcinoma of the pancreas: Histopathologic features and a unique biomarker: Hepatocyte nuclear factor-1β. Mod Pathol. 2008;21:1075-83.
- [Google Scholar]
- Endoscopic ultrasound-guided fine-needle aspiration diagnosis of clear-cell pancreatic endocrine neoplasm in a patient with von Hippel-Lindau disease: A case report. Diagn Cytopathol. 2009;37:365-72.
- [Google Scholar]
- Fine needle aspiration cytology of a clear cell (lipid-rich) pancreatic neuroendocrine tumour in a patient without von Hippel-Lindau disease. Cytopathology. 2013;24:197-8.
- [Google Scholar]
- Lipid-rich variant of pancreatic endocrine neoplasms: A case report. Acta Cytol. 2010;54(Suppl):829-34.
- [Google Scholar]
- Cytoplasmic vacuolization: An under-recognized cytomorphologic feature in endocrine tumors of the pancreas. Diagn Cytopathol. 2013;41:623-8.
- [Google Scholar]
- The clear cell variant of solid pseudopapillary tumor of the pancreas: A previously unrecognized pancreatic neoplasm. Am J Surg Pathol. 2006;30:1237-42.
- [Google Scholar]
- Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. 2010;211:749-53.
- [Google Scholar]
- Pathology diagnosis of pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci. 2015;22:586-93.
- [Google Scholar]
- Islet cell tumour with vacuolated lipid-rich cytoplasm: A new histological variant of islet cell tumour. Histopathology. 1997;31:157-60.
- [Google Scholar]
- Lipid-rich variant of pancreatic endocrine neoplasms. Am J Surg Pathol. 2006;30:194-200.
- [Google Scholar]
- Lipid-rich (“clear cell”) neuroendocrine tumors of the pancreas in MEN I patients. Endocr Pathol. 2012;23:243-6.
- [Google Scholar]
- Clear cell carcinoid of the appendix: An uncommon variant of lipid-rich neuroendocrine tumor with a broad differential diagnosis. Endocr Pathol. 2010;21:258-62.
- [Google Scholar]
- Inhibin-expressing clear cell neuroendocrine tumor of the ampulla: An unusual presentation of von Hippel-Lindau disease. Virchows Arch. 2013;463:593-7.
- [Google Scholar]








