Translate this page into:
The effect of the small amount of formaldehyde in the SurePath liquid when establishing protocols for immunocytochemistry
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
SurePath® is an ethanol-based liquid fixative. In addition to ethanol, it also contains a small amount of formaldehyde (<0.2%). The aim of this study was to investigate the immunoreactivity of cells stored for different lengths of time in the SurePath liquid.
Materials and Methods:
Rest material from one malignant and three benign effusions were fixed in SurePath for 1–12 days. Cytospins were incubated with cytokeratin 7 antibody (AB) to evaluate the staining intensity of carcinoma cells and benign, reactive mesothelial cells. Protocols varied as to pretreatment and AB incubation time.
Results:
Reduced immunostaining intensity was seen within 5 days of storage in the SurePath liquid. It was restored when the pretreatment time was prolonged.
Conclusions:
The small amount of formaldehyde in the SurePath liquid seems to affect the immunoreactivity. Local immunocytochemistry protocols in the cytology laboratories should consider this when optimizing their procedures. Postfixation with formalin should be omitted.
Keywords
Formaldehyde effect
immunocytochemistry
SurePath
INTRODUCTION
SurePath® (Becton Dickinson Pty Ltd.) is an ethanol-based liquid fixative, originally developed for liquid-based preparations of cervical cytological specimens and suited for automated screening devices. The morphology using Papanicolaou's staining method is excellent, and mucus, blood, and inflammatory cells are to a large extent removed.[1] Today it is used for all kinds of cytological material, namely, gynecological and nongynecological exfoliative material as well as fine-needle aspirations.
In addition to ethanol, SurePath also contains a small amount of formaldehyde (<0.2%).[23] Formaldehyde is a cross-linking fixative which causes epitope masking. In immunocytochemistry (ICC) and immunohistochemistry (IHC) demasking is essential when formalin is used as primary or as postfixation. Demasking with heat-induced-epitope retrieval (HIER) has been extensively studied in histological material and shown to cause both false negative and false negative immunostaining results.[45]
The effect of formaldehyde in the SurePath liquid is not well known. The aim of this study was to investigate the immunoreactivity of cells stored for a different length of time in the SurePath liquid.
MATERIALS AND METHODS
Effusions were chosen as test material because they come in large quantities and there is usually abundant rest material after standard cytological diagnostics. We obtained anonymized material from four cases [Table 1]. Effusions were received fresh/unfixed and stored at −4°C until preparation. All four were collected during 1 month. The benign cases were chosen among cases that had shown a fair number of mesothelial cells in the routine diagnostic work-up. There were one malignant (M1) and three benign effusions (B1–B3).

Cytokeratin 7 (CK 7) antibody (AB) (confirm anti-CK 7 [SP53], {REF 790-4462}).[6] Ventana Medicals Systems was chosen because it is positive in mesothelial cells and in the vast majority of carcinomas cells diagnosed in effusions. Positive or negative controls were not used in this study, but day 0 represented the known (and expected) positive reaction of carcinoma and mesothelial cells and negativity of other cells.
CC1 was used as buffer. This is a tris buffer with pH 8, 5 that hydrolyze the cross-linking in the protein molecules.[7]
The rest material after routine diagnostics had been suspended in phosphate-buffered saline and a set of cytospins were made as day 0, and corresponding to an ICC protocol without SurePath fixation. The cells from the effusions were then left in the SurePath liquid for 1, 5 and 12 days, respectively, before preparation. The prepared cytospins were kept in the freezer at −86°C. The slides were taken out and thawed 1 day before ICC, and kept in the refrigerator until the immunostaining. The ICC was done on a Ventana Benchmark (Roche Diagnostics Norge AS) using the Ultra view DAB Detection Kit[8] and CK7 AB. The details of the ICC pretreatment, AB incubation time and protocol variants are shown in Tables 2 and 3. AB incubation and HIER times were chosen according to the options given in the protocols variants given in the staining machine.
The staining intensity of carcinoma and mesothelial cells were coarsely evaluated with day 0 as baseline for adequate staining. Staining intensity of cells from day 1, 5, and 12 using SurePath as fixative was then compared with the baseline staining of the four cases and recorded as clearly reduced/not clearly reduced, but without trying to quantify. Percentage of positive cells was not evaluated. Staining was considered optimal when both carcinoma and mesothelial cells had a strong brown cytoplasmic staining. Nonspecific staining of macrophages, granulocytes, and erythrocytes should be minimal or absent.
Staining intensity was documented by images of all results of the protocol variants. Images at ×40 original magnification were used as the basis for evaluation of staining intensity.
Our study represented a bachelor student project with limited time and equipment available. We thus only had two options for pretreatment time, and it was not possible to extend the fixation time for more than 12 days.
RESULTS
The staining results are shown in Figures 1–3. Morphological details were not affected, and cellular details were equal in all protocols.
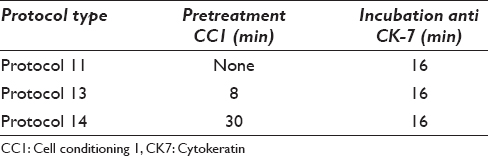
- Day 0 and day 1. (a) Protocol 11 (NBF+) M1 day 0. (b) Protocol 11 (NBF+) M1 day 1. (c) Protocol 13 (NBF−) M1 day 1. (d) Protocol 13 (NBF−) B1 day 1
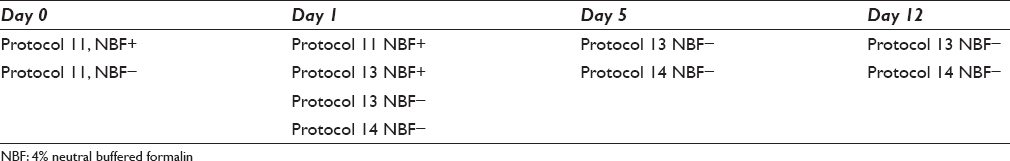
- Day 5. (a) Protocol 13 M1 day 5. (b) Protocol 13 B1 day 5
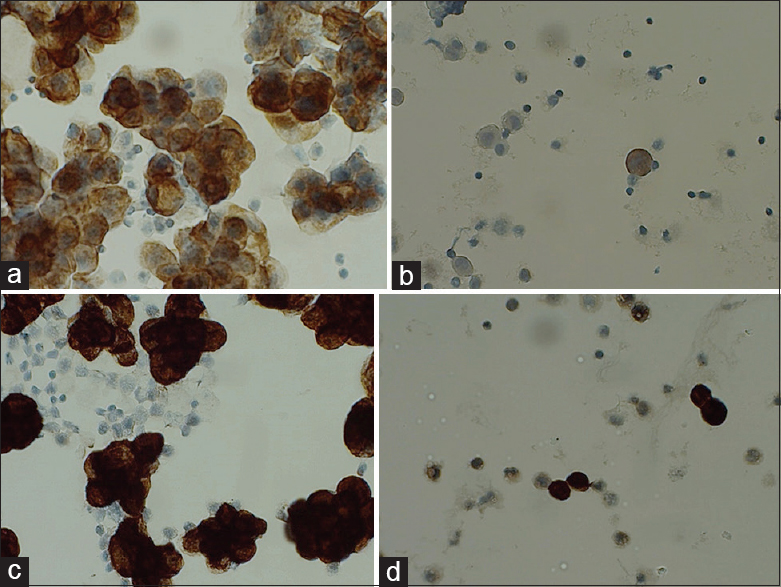
- Day 12. (a) Protocol 13 M1 day 12. (b) Protocol 13 B1 day 12. (c) Protocol 14 M1 day 12. (d) Protocol 14 B1 day 12
Day 0 [Figure 1a]: All cases showed a strong cytoplasmic staining of carcinoma and mesothelial cells (protocol 11, no HIER).
Day 1 [Figure 1b–d]: Protocol 11 that did not include HIER revealed negative or very weak staining. B1 showed some unspecific staining of macrophages. B3 had many neutrophils that stained intense due to the insufficient blocking of endogen peroxidase. The two parallel series of cases from day 0 and day 1 using protocols 11 and 13 revealed a clearly reduced staining intensity when including postfixation in 4% formalin. In contrast, there was no difference when neutral buffered formalin (NBF) was omitted.
Day 5: Protocol 13 [Figure 2] showed a reduced staining compared to day 1. B1 was virtually negative. Protocol 14 [Figure 3c and d] had a longer pretreatment time. These all showed an intense brown cytoplasmic staining, but also some nonspecific staining.
Day 12: The carcinoma cells in M1 were still positive in protocol 13 [Figure 3a and b], but reduced compared to day 1 and both B1 and B3 were negative. In B3, neutrophils were still positive, whereas the mesothelial cells were completely negative. Mesothelial cells were still positive in B2, but with a weak staining intensity. Protocol 14 [Figure 3c and d] gave strong brown cytoplasmic staining of the target cells in all four samples.
DISCUSSION
The main aim of this small study was to see whether cells kept in SurePath liquid fixation would experience reduced immunostaining intensity when stored in the liquid for more than 1 day and thus risk false negative ICC results. It is well known that postfixation with NBF[9] preserve cellular details and thus enhance IHC results. Our results show clearly that when SurePath is used as fixative, postfixation with NBF should be omitted [Figure 1a–c]. Over-fixation with formalin due to extensive cross-linking[1011] and reduced or false negative IHC staining is a well-known phenomenon, which is probably what happened to our cells in protocol 11 NBF+.
In addition, our results indicate that even the small amount of formaldehyde (<0.2%) in the SurePath liquid give cross-linking that might mask our epitopes. This must be taken into account when optimizing local ICC protocols and pretreatment should be adjusted to the number of days the cells might be stored in the SurePath liquid before eventual ICC. Longer pretreatment time restored immunoreactivity, indicating that the reduced staining in protocol 13 was due to extensive cross-linking and not to degeneration of the cells.
Otali et al.[10] investigated the effect of 10% formalin fixation for 5 min and up to 180 h before transferring the cells to 70% ethanol. They showed that the immunoreactivity was reduced after 12 h and this is comparable to our findings. Another study[11] investigated the effect of different fixatives on immunoreactivity. They found no difference in cells stored in up to 40 days. However, they did not investigate SurePath, and they used cell blocks for their immunostaining. Their results cannot be compared directly with ours.
The manufacturer (Becton, Dickinson and Company) recommends a maximum storing time in the SurePath liquid of 4 weeks in room temperature and 6 months in a refrigerator. This, however, refers to HPV DNA testing in cervical samples where morphology is not relevant.[12] Extensive pretreatment might damage the morphology and could also enhance the risk of false positive immune reactions.
When storing the cells for several weeks, there is probably a high risk of false negative ICC results. Our study was a bachelor student project, and can be considered a small pilot that needs to be followed up in a larger study with more cases and variations in protocols. An open system as, for example, DAKO Autostainer, would enable an even broader testing of protocols.[13] Even so, SurePath fixation liquid should perhaps not be used for long time storage of cells in projects where cells are collected during weeks or months and the actual analyzes as ICC are done much later.
ICC is a valuable tool in routine diagnostics for classification of malignant cells and to assess predictive and prognostic markers (f.ex. ER, HER-2 in breast carcinomas) in primary tumors or in recurrences and metastases as well as in scientific projects. Protocols should always be optimized and with appropriate controls. Use of histological protocols is not optimal. Laboratories using SurePath, are encouraged to optimize their protocols with respect to storage time and necessary demasking of epitopes due to the formaldehyde content in the fluid. Different antibodies might require different handling and all routinely used AB's should be tested.
CONCLUSION
The small amount of formaldehyde in the SurePath liquid seems to affect the immunoreactivity and local ICC protocols in the cytology laboratories should consider this when optimizing their procedures.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
None of the authors have any competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors confirm authorship.
ETHICS STATEMENT BY ALL AUTHORS
All authors confirm that there are no ethical concerns. The study has been approved by the institutional review board.
LIST OF ABBREVIATIONS (In alphabetic order)
AB - Antibody
CC1 - cell conditioning buffer 1
CK - Cytokeratin
ER - Estrogen receptor
HER-2 - Epidermal growth factor receptor 2
HIER - Heat epitope retrieval
HPV DNA - Human papilloma virus DNA (desoxyribonucleic acid)
ICC - Immunocytochemistry
IHC - Immunohistochemistry
NBF - Neutral buffered formalin.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Comparison of BD Surepath and ThinPrep Pap systems in the processing of mucus-rich specimens. Cancer Cytopathol. 2010;118:244-9.
- [Google Scholar]
- 2014. Væskebasert celleprøvetaking fordeler og utfordringer. In: Department of HealthN, editor. Available form: https://www.kreftregisteret.no/contentassets/b59a5d4ab11349b492a1ba762325423d/11vaskebasert-cytologi--fordeler-og-utfordringer-mleide
- PrepStain° System Product Insert. Available form: https://www.bd.com/tripath/downloads/msds_pi/prepstain/prepstain_pi_precoat
- Antigen retrieval immunohistochemistry: Review and future prospects in research and diagnosis over two decades. J Histochem Cytochem. 2011;59:13-32.
- [Google Scholar]
- Antigen retrieval: Its significance and drawbacks in immunohistochemistry. Kaibogaku Zasshi. 1996;71:615-28.
- [Google Scholar]
- 2009. Ventana. Confirm Anti-Cytokeratin 7 (SP52) Rabbit Monoclonal Primary Antibody. Available from: http://www.productlibrary.ventana.com/ventana_portal/executeSearch.do
- Cell Conditioning Solution (CC1). Available form: http://www.ventana.com/documents/msds/05279801001US
- Ultraview Universal DAB Detection Kit. Available form: http://www.ventana.com/documents/msds/05269806001US
- 2008. Anvendt Immunhistokemi. (Immunohistochemistry in practice). (7th ed). København, Udg: Odontologisk Boghandel; Available form: https://nota.dk/bibliotek/bog/anvendt-immunhistokemi
- The effect of antigen retrieval on cells fixed in 10% neutral buffered formalin followed by transfer to 70% ethanol. PLoS One. 2013;8:e82405.
- [Google Scholar]
- Fixation effect of SurePath preservative fluids using epidermal growth factor receptor mutation-specific antibodies for immunocytochemistry. Cancer Cytopathol. 2014;122:145-52.
- [Google Scholar]
- 2011. Becton Dickinson Pty Ltd. SurePathTM Collection Product Insert. Available from: http://www.bd.com/tripath/downloads/surepath_ctgc/779-07084-00_Rev_B_PI_SP.pdf
- EnVision™ FLEX, High pH, (Dako Autostainer/Autostai ner Plus). Available from: http://www.dako.com/no/download.pdf?objectid=119237003








