Translate this page into:
Analysis of clinicopathological characteristics and prognostic factors in 54 metaplastic breast carcinoma patients from northwest China

*Corresponding author: Xulong Zhu, Department of Surgical Oncology and Pathology, Shaanxi Provincial People’s Hospital, Xian, China. zhuxulongmd@163.com
-
Received: ,
Accepted: ,
How to cite this article: Du J, Wu S, Liu J, Guo B, Li J, Li W, et al. Analysis of clinicopathological characteristics and prognostic factors in 54 metaplastic breast carcinoma patients from northwest China. CytoJournal. 2024;21:31. doi: 10.25259/Cytojournal_15_2024
Abstract
Objective:
Metaplastic breast carcinoma (MBC) is a special type of morphologically heterogeneous and aggressively invasive breast cancer. MBC is characterized by the transformation of tumor epithelium into squamous epithelium and/or mesenchymal components, including differentiation into spindle cells, chondrocytes, and osteocytes. Due to its rarity and invasiveness, there is a paucity of research on MBC prognosis. Furthermore, there are currently no treatment guidelines for MBC. This study analyzed the clinicopathological characteristics, immunophenotype, and prognostic features of MBC. Our aim was to better characterize MBC, thereby identifying potential prognostic factors and new treatment methods. Moreover, we also describe an MBC case treated experimentally with anti-vascular targeted therapy.
Material and Methods:
We retrospectively analyzed clinical pathological data on 54 female patients with MBC from Shaanxi Provincial People’s Hospital and the XiJing Hospital of Air Force Medical University. These cases were diagnosed with MBC between January 1st, 2013, and October 1st, 2018. All patients were from the northwest region of China. The gross morphological, histological, and immunohistochemical features of MBC were analyzed. Kaplan–Meier analysis was used to calculate the survival rate, and univariate analysis was performed to identify significant prognostic factors. In addition, the treatment of an MBC patient with anti-angiogenic therapy was described, and a relevant literature review was conducted.
Results:
MBC was diagnosed in 32 left breasts and 22 right breasts from 54 women aged 21–76 years (median age of 57 years). The maximum tumor diameter ranged from 0.6 to 14 cm (average of 4.1 cm). Of the 54 patients, 47 underwent surgical treatment, with lymph node metastasis found in 17.0% (8/47). According to the World Health Organization classification criteria for breast tumors, the study cohort consisted of 15 cases of squamous cell carcinoma, ten cases of spindle cell carcinoma, nine cases of carcinoma with associated stromal differentiation, 18 cases of mixed carcinoma, and two cases of adenocarcinoma with squamous differentiation. Based on the American Joint Committee on Cancer clinical staging criteria, the patients were classified as Stage I (10 cases, 18.5%), Stage II (26 cases, 48.1%), Stage III (11 cases, 20.4%), and Stage IV (7 cases, 13.0%). Immunohistochemical analysis revealed that 94.4% of patients had triple-negative breast cancer (TNBC), 47 cases showed mutant tumor protein 53 (TP53) expression, 29 cases showed positive epidermal growth factor receptor (EGFR) expression, 43 cases showed positive E-cadherin expression, and 37 cases showed positive Cluster of Differentiation 24 expression. The Ki-67 index ranged from 20% to 90%. Univariate analysis showed that the Ki-67 index was not significantly associated with either progression-free survival (PFS) or overall survival (OS) in MBC patients. Patients with negative axillary lymph nodes had significantly better PFS and OS than those with positive nodes (P < 0.05), and patients with clinical stage I-II disease had better PFS and OS than those with stage III-IV disease (P < 0.05). Patients treated with anthracycline-containing chemotherapy had significantly better PFS than those who did not receive chemotherapy. Univariate analysis revealed that the high expression of EGFR correlated with worse PFS (P < 0.05). The type of surgical approach employed did not affect the prognosis of MBC patients. Following the application of anti-angiogenic therapy, a rapid partial response was observed in an MBC patient with carcinoma and associated stromal differentiation. This patient subsequently underwent surgery and radiation therapy and has now achieved over 6 years of PFS.
Conclusion:
MBC is a heterogeneous group of tumors with high malignancy and poor prognosis. The large majority is TNBC and exhibits unique immune phenotypes. The poor PFS of MBC patients may be related to EGFR expression, which could become a potential therapeutic target in these patients. Surgery remains the primary treatment method for MBC. The present study found that sentinel lymph node biopsy was feasible in appropriate patients, and that chemotherapy regimens incorporating anthracycline-class drugs did not appear to improve OS. Anti-angiogenic therapy holds promise as a potentially effective treatment approach for MBC, and the optimization of systemic treatment strategies should be a priority in the management of these patients.
Keywords
Metaplastic breast carcinoma
prognostic factors
anti-angiogenic targeted therapy
epidermal growth factor receptor
progression-free survival
INTRODUCTION
Metaplastic breast carcinoma (MBC) is a rare and highly invasive subtype of infiltrating breast cancer that accounts for <1% of all breast cancers.[1] There is relatively limited literature on metaplastic breast carcinoma compared to non-special type invasive breast cancer. MBCs are known to encompass a range of different histological types. The 2019 edition of the WHO classification of breast cancer identified the following MBC subtypes: spindle cell metaplastic carcinoma, squamous cell metaplastic carcinoma, metaplastic carcinoma with heterologous differentiation, mixed metaplastic carcinoma, low-grade adenosquamous carcinoma, and fibromatosis-like metaplastic carcinoma.[2] MBC usually exhibits aggressive characteristics such as larger tumor size, higher stage and grade, and a poor 5-year survival rate.[1] Due to its low incidence, there is still only a limited understanding of MBC. Most studies to date indicate that MBC is negative for estrogen receptor (ER), partial response (PR), and human epidermal growth factor receptor 2 (HER-2), thus resembling the characteristics of triple-negative breast cancer (TNBC).
The pathogenesis of MBC is believed to involve the overexpression of epithelial-mesenchymal transition (EMT) markers and cancer stem cell (CSC)-related markers.[3-5] EMT is a process by which epithelial cells acquire a mesenchymal phenotype, thus enabling them to migrate and invade. Loss of E-cadherin expression and the upregulation of stromal proteins such as vimentin and smooth muscle actin are key steps in EMT. Epidermal growth factor receptor (EGFR) is closely associated with cell proliferation, differentiation, and growth and, therefore, serves as a target molecule in various tumor types. The tumor protein 53 (TP53) encoded by the TP53 gene plays a crucial role in many cancer types. Loss of this gene can result in the dysregulation of cell proliferation, apoptosis, and DNA repair. Moreover, mutant TP53 can act as an oncogene and causes abnormal cell proliferation, cellular transformation, and ultimately the formation of malignant tumors.[6] The Ki-67 protein is a short-lived and nonhistone nuclear protein and is a commonly used marker for assessing tumor cell proliferation in clinical practice. The expression of Ki-67 is closely associated with the development, prognosis, and lymph node metastasis of various cancer types. ER, PR, and HER-2 are important prognostic indicators for breast cancer. However, due to the complexity of malignant breast tumors and especially metaplastic cancer, these markers are often negative. Hence, the use of traditional indicators may not effectively reflect the clinical characteristics and prognosis of individual patients. Moreover, the immunophenotype of MBC is not consistent, possibly reflecting the heterogeneity of its morphology and molecular characteristics. This warrants further investigation of the diversity of MBC immunophenotypes and their prognostic impact. In the present work, we investigated the clinical and pathological characteristics of MBC by analyzing the tumor stem cell marker cluster of differentiation 24 (CD24), the epithelialmesenchymal stem cell marker E-cadherin, the cell proliferation marker Ki-67, as well as EGFR and TP53. In addition, we evaluated the prognostic significance of each of these markers in MBC.
The rarity of MBC has meant that no specific guidelines are available for its treatment. The management of MBC is similar to that of non-special type infiltrating breast cancer, with the main treatment options being surgery and chemotherapy. An analysis of MBC patients in the SEER database found that those who underwent surgery had significantly better progression-free survival (PFS) and overall survival (OS) than those who did not undergo surgery.[7] Ong et al. reported that MBC patients who underwent chemotherapy showed improved survival (hazard ratio [HR] 0.69, 95% confidence interval [CI] 0.53–0.89, P = 0.004).[8] Improved survival rates were also reported in MBC patients who received anthracycline-based chemotherapy after surgery.[9] The present study also compared the effect of different treatment strategies on the prognosis and survival of MBC patients, including different surgical approaches and treatment with anthracycline-containing chemotherapy regimens. In a preliminary study by our group, we used anti-angiogenic therapy in a patient with locally advanced MBC and achieved a favorable outcome, thus providing direction for future treatment choices. The present study also analyzed the pathological characteristics and prognostic features of MBC patients from northwest China, some of which may serve as potential therapeutic targets.
MATERIAL AND METHODS
Patients
Collecting female patients who were pathologically diagnosed with MBC from two centers, Shaanxi Provincial People’s Hospital and Xijing Hospital of Air Force Medical University from January 1, 2013, to October 1, 2018. The patients’ permanent residence addresses were limited to the northwest region of China. The main clinical pathological data collected include: Age, tumor size, location, menopausal status, tumor type, axillary lymph node metastasis, staging, operation choosing, treatment modalities, histopathological diagnosis, immunohistochemical results (immunohistochemical markers performed or feasible for this study), traceable follow-up records, and the absence of any other malignant tumor history. A total of 54 patients with MBC who met the inclusion criteria were included in the study.
Criteria for axillary lymph node metastasis
The post-operative routine histopathological evaluation involved cutting the lymph nodes into several tissue slices at 2 mm intervals, each of which was embedded in paraffin to create tissue blocks. Each tissue block was stained with hematoxylin and eosin (H&E). In cases where the tissue blocks were not fully sectioned or when tumor lesions within the lymph nodes were in an indeterminate state between isolated tumor cells and micrometastasis or between micrometastasis and macrometastasis, additional consecutive sections were obtained. Immunohistochemical staining was used as an additional diagnostic aid for cases with difficult H&E staining diagnoses (e.g., lobular carcinoma-like metastasis and lymph nodes after neoadjuvant therapy). Isolated tumor cells were defined as tumor foci with a diameter ≤0.2 mm and <200 tumor cells on a single section, designated as pN0(i+). Micrometastasis was defined as tumor foci with a maximum diameter >0.2 mm but not exceeding 2 mm, designated as pN1mi. Macrometastasis was defined as tumor foci with a maximum diameter >2 mm. Both macrometastasis and micrometastasis were considered positive for axillary lymph node metastasis. Cases with pN0(i+) after neoadjuvant chemotherapy were considered positive for axillary lymph node metastasis, while cases with pN0(i+) without neoadjuvant chemotherapy were considered negative for axillary lymph node metastasis.[10,11]
Immunohistochemistry and interpretation criteria
All specimens were fixed in 10% formalin (F111936, Shanghai Aladdin Biochemical Technology Co., Ltd. Shanghai, China.), routinely embedded in paraffin, and cut into consecutive sections at a thickness of 4 mm. Immunohistochemical staining was performed using the Elivision two-step method. The expression of proteins was considered positive when brownish-yellow reactions were observed in the corresponding cellular compartment, such as cytoplasm, cell membrane, and/or nucleus. The interpretation of results was independently performed by two pathologists who assessed the staining intensity and the ratio of positive cells under a microscope. Five high-power fields with relatively uniform expression were selected from each slide, and the percentage of positive cells was calculated after counting a total of 500 cancer cells.
Staining for Ki-67 and TP53 was considered positive when brownish-yellow particles were observed in the nucleus. The Ki-67 index was categorized according to the proportion of positive cells as follows: Tumors with ≤5% positive cells were considered negative (−), 6–30% positive cells were considered weakly positive (+), 31–50% positive cells were considered positive (++), and >50% positive cells were considered strongly positive (+++). The expression pattern for mutant TP53 is considered to be all-or-none. If >90% of cancer cells had brown-yellow particles in their nuclei, the tumor was judged to have TP53 mutation. If there are no brown-yellow particles in the nuclei under the microscope, the tumor is also judged to have TP53 mutation. Expression of wild-type TP53 takes the form of brown-yellow particles in the nucleus that are unevenly distributed or have varying intensity. EGFR staining occurred in the cytoplasm and cell membrane of tumor cells, and tumor cells were counted under high-power magnification. Tumors with greater than or equal to 10% positive cells for EGFR were considered positive (+), while those with <10% positive cells were considered negative (−). E-cadherin was primarily expressed in the cell membrane, with a small amount in the cytoplasm. Positive staining for E-cadherin appeared as brownish-yellow or reddish-brown particles, and a semi-quantitative assessment was performed based on the percentage of positive cells. Tumors were considered positive for E-cadherin expression when the proportion of positive cancer cells was greater than or equal to 50%, and negative or low if the proportion was <50%. CD24 was mainly expressed in the cell membrane and/or cytoplasm. Tumors with >1% of cancer cells staining were considered positive for CD24, while the others were considered negative.
Treatment
Among 54 patients with MBC, surgical treatment was performed in 47 cases. Among them, 46 patients underwent mastectomy, and one underwent breast-conserving surgery. Axillary lymph node dissection was performed in 35 patients, and sentinel lymph node biopsy was performed in 12 patients. Thirty-eight patients received chemotherapy regimens containing anthracyclines, including epirubicin/adriamycin, cyclophosphamide, and fluorouracil, epirubicin/adriamycin, cyclophosphamide, sequential paclitaxel, paclitaxel, epirubicin/adriamycin, and paclitaxel, epirubicin/adriamycin, and cyclophosphamide (TAC). Two patients received anti-angiogenic therapy, with one patient taking apatinib mesylate and another patient taking anlotinib hydrochloride. Twenty-one patients received local radiotherapy. Only one patient with PR-positive status received endocrine therapy among the 54 patients.
Follow-up
Fifty-four patients were followed up through medical record review, outpatient visits or telephone calls, and the follow-up ended on 2023-10-01. Three patients were lost to follow-up (5.6%): one patient was calculated based on the date of the last treatment discharge, while the other two patients were calculated based on the date of the last follow-up. The follow-up period ranged from 1 to 110 months, and the endpoint event was death or the last follow-up for any reason. The median follow-up duration was 38.8 months, with a minimum follow-up of 5 years and four cases with a follow-up duration of at least 10 years.
Statistical analysis
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences 22.0 (developed by International Business Machines, Chicago, United States). Kaplan–Meier survival analysis was carried out to determine the rates of OS and PFS, with the log-rank test used to evaluate differences between groups. The chi-square test was used to evaluate the influence of each factor on prognosis. Univariate analysis was also performed as indicated. Multivariate analyses were conducted using Cox proportional hazard regression models. Survival rates are presented with their 95% CIs. P < 0.05 was considered as statistically significant.
RESULTS
Clinical and pathological characteristics
All 54 patients with MBC were female from the northwest region of China and aged from 21 to 76 years (mean of 55.76 years, and median of 57 years). Six cases were aged ≤39 years, 28 were aged 40–59 years, and 20 were aged ≥60 years. Furthermore, 35 (64.8%) women were postmenopausal and 19 (35.2%) were premenopausal. The primary tumor originated in the left breast in 32 cases (59.3%) and in the right breast in 22 cases (40.7%). The maximum tumor diameter ranged from 0.6 cm to 14 cm, with an average diameter of 4.1 cm. Among the 47 patients who underwent surgical treatment, 8 (17.0%) had positive axillary lymph node metastasis and 39 (83.0%) had negative lymph nodes. According to the 2019 WHO criteria for the classification of breast tumors, this study cohort was comprised 15 cases of metaplastic squamous cell carcinoma, ten cases of spindle cell metaplastic carcinoma, nine cases of metaplastic carcinoma with heterologous stromal differentiation, 18 cases of mixed metaplastic carcinoma, and two cases of low-grade adenosquamous carcinoma subtype [Figure 1]. In the 18 cases of mixed metaplastic carcinoma, the squamous cell carcinoma component accounted for 10–90% of cancer cells. These included 11 cases of non-special type carcinoma and four cases of special type breast carcinoma. The 15 cases of metaplastic squamous cell carcinoma included nine cases of highly differentiated epithelial metaplastic squamous carcinoma showing obvious keratin pearls and/or intercellular bridges, and six cases of poorly differentiated squamous cell carcinoma. The ten cases of spindle cell carcinoma subtype were characterized by bundles or fascicles of morphologically mild spindle cells, with nuclear grading showing mainly mild to moderate atypia, and positive expression of epithelial markers on immunohistochemistry. Among the nine cases of metaplastic carcinoma with heterologous stromal differentiation, five cases had chondrosarcoma metaplasia in the stromal component, three cases had osteosarcoma metaplasia, and one case had rhabdomyosarcoma metaplasia. According to the American Joint Committee on Cancer (AJCC) clinical staging criteria,[12] 10 cases (18.5%) were stage I, 26 cases (48.1%) were stage II, 11 cases (20.4%) were stage III, and 7 cases (13.0%) were stage IV. Immunohistochemical staining showed that 51 cases (94.4%) were TNBC, two cases were HER-2 positive, and one case showed positive expression of both PR and HER-2. Six cases (11.1%) showed positive expression of Androgen receptor (AR), and 47 cases (87.0%) were positive for TP53 protein expression. The Ki-67 index ranged from 20% to 90%, with 11 cases (20.4%) having a Ki-67 index ≤30%, 12 cases (22.2%) having a Ki-67 index between 30% and 50%, and 31 cases (57.4%) having a Ki-67 index >50%. Positive expression for EGFR was observed in 29 cases (53.7%), positive expression for E-cadherin in 43 cases (79.6%), and positive expression of CD24 in 37 cases (68.5%). Figure 2 shows the positive expression of E-cadherin, CD24, EGFR, and P53 in metaplastic carcinoma.
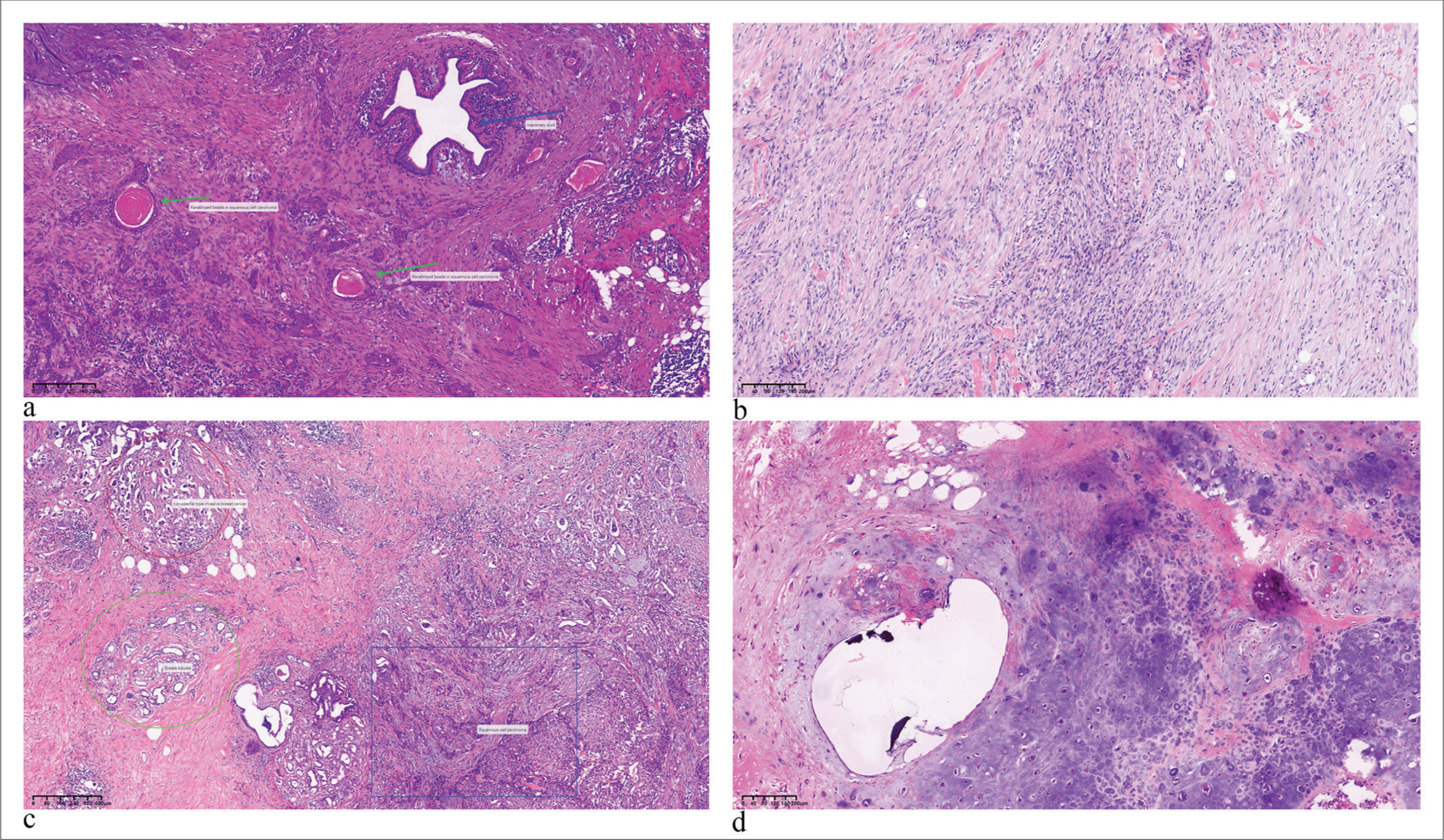
- HE pictures of different pathological types of metaplastic carcinoma. (a) Metaplastic squamous carcinoma (Tumor cells exhibit a nest-like arrangement, breast ducts visible in the picture, Blue arrows represent breast ducts, and green arrows represent metaplastic squamous carcinoma (×40). (b) Spindle cell metaplastic carcinoma (Tumor cells are arranged in fine stripes with little cell atypia, (×100). (c) Mixed metaplastic carcinoma (Blue rectangle, red circle, and green oval respectively indicate squamous cell carcinoma, non-specific infiltrating breast carcinoma, and normal breast lobules respectively, (×40). (d) Metaplastic carcinoma with chondrogenic differentiation (×100) (HE: Hematoxylin and Eosin).
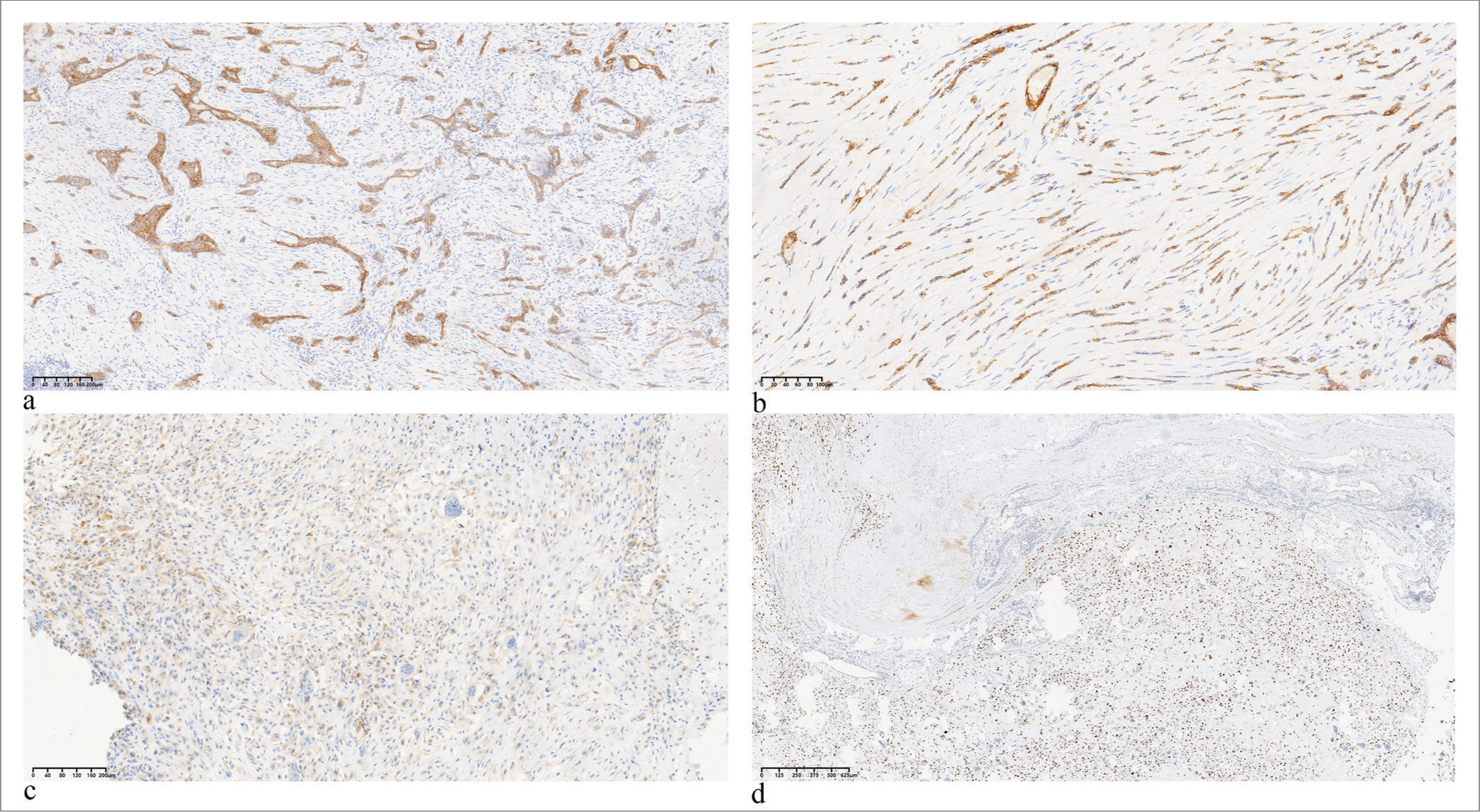
- Four positive expression markers in metaplastic carcinoma. (a) E-cadherin (×40), (b) cluster of differentiation CD24 (×40), (c) epidermal growth factor receptor (×40), and (d) P53 (×100). (EGFR: Epidermal growth factor receptor)
Among the 47 patients who underwent surgical treatment, 46 underwent mastectomy and one underwent breast-conserving surgery. Axillary lymph node dissection was performed in 35 patients (74.5%), and sentinel lymph node biopsy in 12 patients (25.5%). Of the total cohort, 38 cases (80.9%) received chemotherapy regimens containing anthracycline drugs, and 9 cases (19.1%) received other treatments or did not receive post-operative treatment [Table 1].
| Variable | n | % |
|---|---|---|
| Age (year) | ||
| ≤39 | 6 | 11.1 |
| 40–59 | 28 | 51.9 |
| ≥60 | 20 | 37.0 |
| Menopausal status | ||
| Yes | 35 | 64.8 |
| No | 19 | 35.2 |
| Tumor size (cm) | ||
| ≥4 | 22 | 40.7 |
| ≥4 | 32 | 59.3 |
| Location | ||
| Left sided | 32 | 59.3 |
| Right sided | 22 | 40.7 |
| Axillary lymph node status | ||
| Positive | 8 | 17.0 |
| Negative | 39 | 83.0 |
| Histological pattern | ||
| Metaplastic squamous carcinoma | 15 | 27.8 |
| Spindle cell metaplastic carcinoma | 10 | 18.5 |
| Metaplastic carcinoma with heterologous mesenchymal differentiation | 9 | 16.7 |
| Mixed metaplastic carcinoma | 18 | 33.3 |
| Adenocarcinoma with squamous differentiation | 2 | 3.7 |
| AJCC staging | ||
| Stage I/II | 36 | 66.7 |
| Stage III/IV | 18 | 33.3 |
| AR | ||
| Positive | 6 | 11.1 |
| Negative | 48 | 88.9 |
| TP53 | ||
| Mt | 47 | 87.0 |
| Wt | 7 | 13.0 |
| EGFR | ||
| Positive | 29 | 53.7 |
| Negative | 25 | 46.3 |
| E-cadherin | ||
| Positive | 43 | 79.6 |
| Negative | 11 | 20.4 |
| CD24 | ||
| Positive | 37 | 68.5 |
| Negative | 17 | 31.5 |
| Ki-67 | ||
| ≤30% | 11 | 20.4 |
| 30–50% | 12 | 22.2 |
| >50% | 31 | 57.4 |
| Surgical method | ||
| Sentinel lymph node dissection | 12 | 25.5 |
| Axillary lymph node dissection | 35 | 74.5 |
| Chemotherapy | ||
| Yes | 38 | 80.9 |
| No | 9 | 19.1 |
AJCC: American joint committee on cancer, AR: androgen receptor, EGFR: Epidermal growth factor receptor, CD24: Cluster of differentiation 24, MBC: Metaplastic breast carcinoma, n: Number of cases, Stage I-IV: AJCC staging of the carcinoma, Mt: Mutant, Wt: Wild type
Survival and recurrence/metastasis status
The 1-, 3-, and 5-year OS rate for the 54 MBC patients was 100.0%, 74.1%, and 68.5%, respectively, while the corresponding PFS rate was 94.4%, 68.5%, and 55.6%. Nine patients experienced recurrence or metastasis after treatment, with four deaths. These included three cases of local recurrence, two cases of liver metastasis, two cases of lung metastasis, one case of bone metastasis, and one case of multiple pulmonary and cerebral metastases. Of the four deceased patients, one had multiple pulmonary and cerebral metastases, two had liver metastasis, and one had lung metastasis. The pathological subtypes of recurrence/metastasis included four cases of mixed adenocarcinoma, the cases of adenocarcinoma with heterologous stromal differentiation, and two cases of squamous cell carcinoma. The probability of recurrence or distant metastasis was 5.6% (3/54) for pure epithelial type and 11.1% (6/54) for epithelial/mesenchymal hybrid type, with no statistically significant difference between the two [Table 2].
| 1-year | 3-year | 5-year | |
|---|---|---|---|
| OS rates | 100.0% | 74.1% | 68.5% |
| PFS rates | 94.4%, | 68.5% | 55.6% |
OS: Overall survival, PFS: Progression-free survival, MBC: Metaplastic breast carcinoma
Analysis of prognostic factors
Univariate analysis and the log-rank test were used to evaluate the impact of various factors on PFS and OS, with the results shown in Table 3. These included patient age, menopausal status, tumor size and location, pathological type, axillary lymph node metastasis, AJCC staging, surgical approach, treatment with anthracycline-containing chemotherapy, and the expression of AR, Ki-67, EGFR, TP53, E-cadherin, and CD24. Kaplan–Meier survival analysis was also conducted for each of these factors. The results showed that patients with AJCC staging I-II tumors had significantly better PFS and OS than those with stage III-IV tumors (P = 0.016 and P = 0.009, respectively). The presence of axillary lymph node metastasis was associated with significantly worse PFS and OS (P = 0.004 and P = 0.003, respectively). Patients with negative EGFR expression had better PFS than those with positive expression (c2 = 5.266, P = 0.031), but there was no significant difference in OS between the two groups. Patients who received anthracycline-containing chemotherapy had better PFS than those who did not (c2 = 6.566, P = 0.025), although no significant difference was observed for OS [Table 3]. These findings suggest that early tumor stage, absence of lymph node metastasis, treatment with anthracycline-containing chemotherapy, and low EGFR expression may be favorable prognostic factors for metaplastic carcinoma. Moreover, the results showed that patient age, menopause, tumor size, tumor location, tumor pathological type, surgical method, and the expression of AR, Ki-67, TP53, E-cadherin, and CD24 were not significantly correlated with patient survival.
| Variable | PFS | OS | ||
|---|---|---|---|---|
| χ2 | P | χ2 | P | |
| Age (year) | 0.307 | 0.980 | 1.805 | 0.479 |
| ≤39 | ||||
| 40–59 | ||||
| ≥60 | ||||
| Menopausal status | 3.279 | 0.108 | 1.273 | 0.191 |
| Yes | ||||
| No | ||||
| Tumor size (cm) | 1.283 | 0.585 | 3.345 | 0.322 |
| ≥4 | ||||
| ≥4 | ||||
| Location | 0.679 | 0.612 | 0.358 | 0.749 |
| Left sided | ||||
| Right sided | ||||
| Axillary lymph node status | 27.988 | 0.004 | 34.623 | 0.003 |
| Yes | ||||
| No | ||||
| Histological pattern | 1.361 | 0.329 | 0.958 | 0.693 |
| Metaplastic squamous carcinoma | ||||
| Spindle cell metaplastic carcinoma | ||||
| Metaplastic carcinoma with heterologous mesenchymal differentiation | ||||
| Mixed metaplastic carcinoma | ||||
| Adenocarcinoma with squamous differentiation | ||||
| AJCC clinical staging | 6.051 | 0.016 | 8.623 | 0.009 |
| Stage I/II | ||||
| Stage III/IV | ||||
| AR | 0.725 | 0.469 | 0.436 | 0.879 |
| Positive | ||||
| Negative | ||||
| TP53 | 0.271 | 0.603 | 0.153 | 0.696 |
| Mt | ||||
| Wt | ||||
| EGFR | 5.266 | 0.031 | 0.983 | 0.152 |
| Positive | ||||
| Negative | ||||
| E-cadherin | 0.862 | 0.353 | 0.391 | 0.532 |
| Positive | ||||
| Negative | ||||
| CD24 | 3.518 | 0.060 | 6.455 | 0.115 |
| Positive | ||||
| Negative | ||||
| Ki-67 | 2.324 | 0.381 | 2.902 | 0.223 |
| ≤30% | ||||
| 30–50% | ||||
| >50% | ||||
| Surgical method | 1.025 | 0.062 | 2.032 | 0.072 |
| Axillary lymph node dissection | ||||
| Sentinel lymph node dissection | ||||
| Chemotherapy | 6.566 | 0.025 | 2.024 | 0.085 |
| Yes | ||||
| No | ||||
OS: Overall survival, PFS: Progression-free survival, AJCC: American joint committee on Cancer, EGFR: Epidermal growth factor receptor, CD24: Cluster of differentiation 24, MBC: Metaplastic breast carcinoma, Mt: Mutant, Wt: Wild type, Stage I-IV: AJCC staging of the carcinoma
Based on the results of univariate analysis, Cox multivariate regression analysis was then performed for axillary lymph node metastasis, AJCC stage, EGFR expression, and treatment with anthracycline-containing chemotherapy [Table 4]. Survival analysis curves were generated [Figure 3] and demonstrated the prognostic correlations of axillary lymph node metastasis and EGFR expression with PFS in metaplastic carcinoma. The results showed that axillary lymph node metastasis (HR = 0.321, 95% CI: 0.151–1.176, P = 0.049) and high EGFR expression (HR = 0.288, 95% CI: 0.094–0.888, P = 0.030) were independent predictors of PFS. AJCC stage was not an independent risk factor for PFS (HR = 0.918, 95% CI: 0.530-9.592, P = 0.761), and treatment with anthracycline-containing chemotherapy did not affect PFS (95% CI: 0.433–6.325, P = 0.396).
| P-value | Exp (B) | 95.0% Exp (B) CI | ||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Axillary lymph node status | 0.049 | 0.321 | 0.151 | 1.176 |
| EGFR | 0.030 | 0.288 | 0.094 | 0.888 |
| AJCC staging | 0.761 | 0.918 | 0.530 | 9.592 |
| Chemotherapy | 0.396 | 0.792 | 0.433 | 6.325 |
PFS: Progression-free survival, EGFR: Epidermal growth factor receptor, CI: Confidence interval, AJCC: American Joint Committee on Cancer, Exp(B): Hazard ratio (HR)
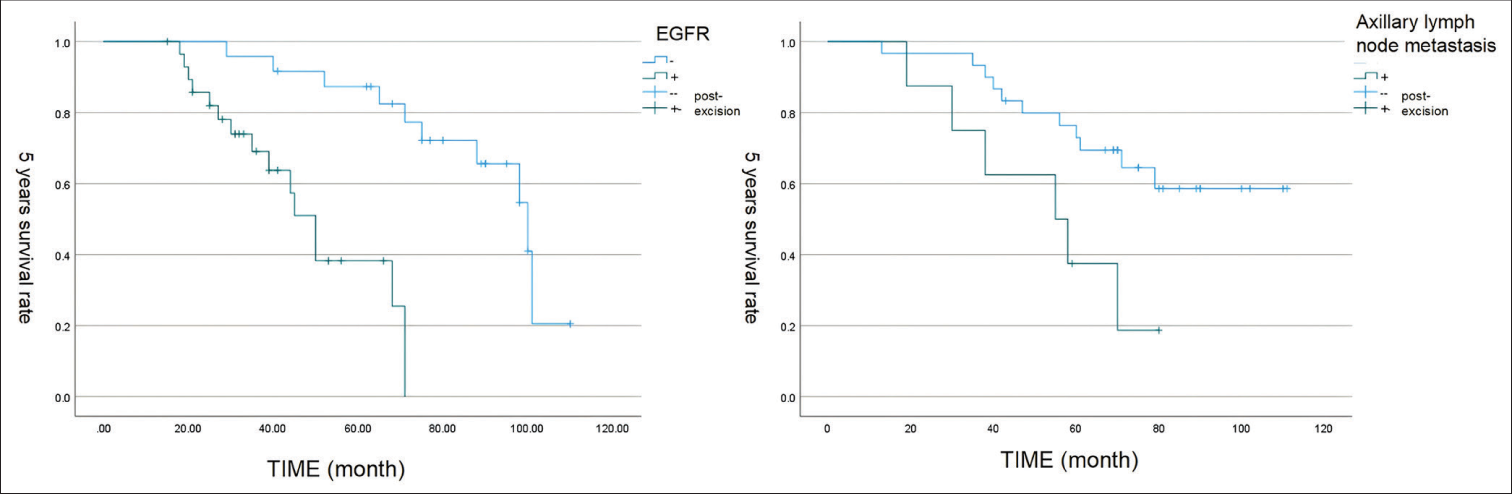
- The survival curve of epidermal growth factor receptor and axillary lymph node metastasis. (EGFR: Epidermal growth factor receptor)
Clinical information of the patient
In June 2017, a premenopausal woman aged 44 years discovered a mass in the upper outer quadrant of her left breast. The mass rapidly increased in size within 3 months, accompanied by redness, swelling, and pain on the surface. The patient experienced an elevated body temperature and promptly sought medical attention at a local hospital. Ultrasound revealed a breast mass measuring 4.5 cm × 3.5 cm × 3.0 cm with the formation of an abscess. The initial diagnosis at the local hospital was breast inflammation, and the patient underwent drainage surgery. However, the mass continued to grow, and hence, the patient was subsequently transferred to the People’s Hospital of Shaanxi Province for diagnosis and treatment. By this time, the tumor size had grown to approximately 15 cm × 12 cm × 10 cm and occupied a significant portion of the left breast, with noticeable changes in the nipple position. The skin surface of the tumor showed signs of inflammation, with an outwardly protruding wound at the center resembling a fish mouth that continuously produced a large amount of exudate. A palpable enlarged lymph node measuring 2 cm in its longest diameter was detected in the left axilla. Systemic examination indicated no distant metastasis of the tumor. A core needle biopsy of the tumor was performed at our hospital. This revealed a clustered and sheet-like distribution of tumor cells under the microscope, a significant increase in the nuclear-cytoplasmic ratio, large and deeply stained cell nuclei, and no apparent pathological mitotic figures. The histopathological diagnosis was reported as “Invasive Breast Cancer (Grade 3).” Immunohistochemistry results showed GATA-3 (+), ER (−), PR (−), HER-2 (−), CK5/6 (+), and a Ki-67 index of 80%. Fine-needle aspiration cytology of the left axillary lymph node suggested metastasis. The patient had no family history of breast or ovarian cancer, and genetic testing revealed no mutations in the BRCA1/2 genes.
In accordance with the treatment protocol for TNBC, the patient underwent neoadjuvant chemotherapy with the TEC regimen (docetaxel/paclitaxel, epirubicin, cyclophosphamide). After two cycles, imaging examinations revealed an enlargement of the breast tumor. Subsequently, the chemotherapy regimen was adjusted to the TCb regimen (docetaxel/paclitaxel, carboplatin). After two cycles, the treatment response was assessed as disease progression (PD). Following this, a multidisciplinary team discussion was held. Based on the clinical presentation and imaging characteristics of the tumor, the possibility of MBC was considered. Literature reports indicate that MBC may not be sensitive to chemotherapy. Therefore, a combination of apatinib mesylate (500 mg, qd, days 1–21) and capecitabine (1.5 g, bid, days 1–14, every 21 days) was given as oral therapy. After two cycles of treatment, the breast tumor decreased in size from 15.0 cm × 13.0cm × 6.5 cm to 8.5 cm × 6.3 cm × 4.3 cm. As the tumor shrank, the exudation from the tumor bed reduced and gradually formed scabs. The treatment response was assessed as partial response (PR), with grade 3 gastrointestinal reactions and grade 1 hand-foot syndrome. From Figure 4, it is evident that the normal shape of the breast gradually becomes visible during the tumor regression process [Figure 4a-d].
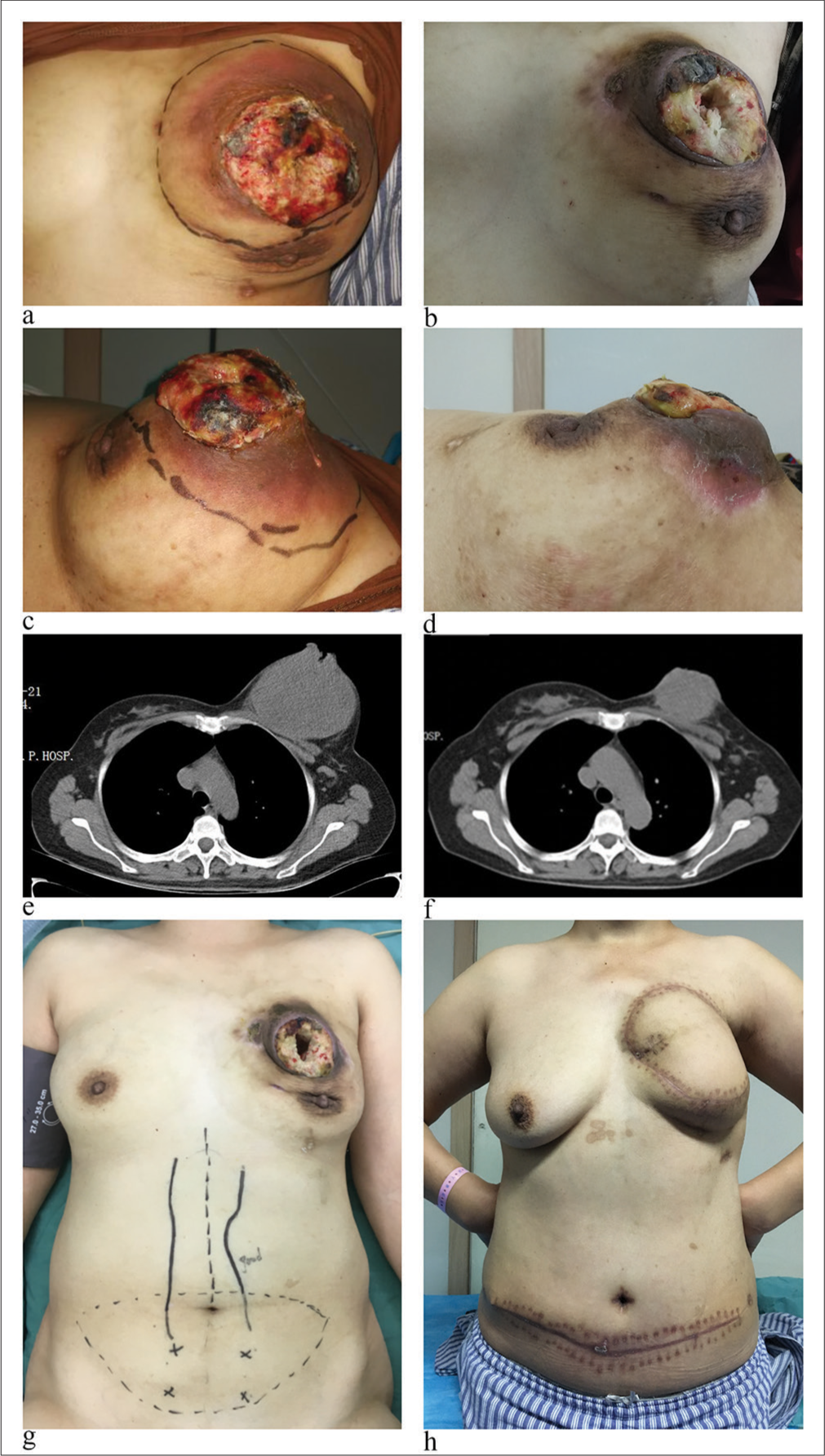
- The comparison of effects before and after anti-angiogenesis treatment: (a and b) Before anti-angiogenesis treatment. (c and d) Effects after 4 cycles of anti-angiogenesis treatment. (e and f) Computed tomography scan images (g and h) comparison before and after surgical treatment.
CT scan images show a centripetal reduction of the tumor, with the tumor base gradually separating from the pectoralis major muscle, revealing a discernible tissue gap [Figure 4e and f]. In January 2018, the patient underwent modified radical mastectomy for breast cancer and pedicled transverse rectus abdominis myocutaneous flap breast reconstruction [Figure 4g and h]. Post-operative pathology confirmed the diagnosis as MBC with chondrosarcomatous differentiation. Microscopically, spindle cells distributed in sheets were seen in some areas, and chondrosarcoma components with mucus production were seen in other areas. The tumor was observed to infiltrate the skin, but there was no involvement of left axillary lymph nodes (0/29). The immunohistochemistry results were as follows: ER (−), PR (−), HER-2 (0), AR (−), Ki-67 (60%), vimentin (partially +), CK5/6 (partially +), P63 (+), E-cadherin (+), TP53 (mutant), CKpan (+), S-100 (+), EGFR (+), and CD24 (−) [Figure 5]. Postoperatively, the tumor achieved a PR, and axillary lymph nodes achieved a complete response (CR). The patient continued to receive four cycles of the original regimen (apatinib mesylate combined with capecitabine) and then continued apatinib until 1 year. The patient also underwent local radiotherapy during this period. As of the latest follow-up, the patient had achieved over 6 years of PFS with a good quality of life.
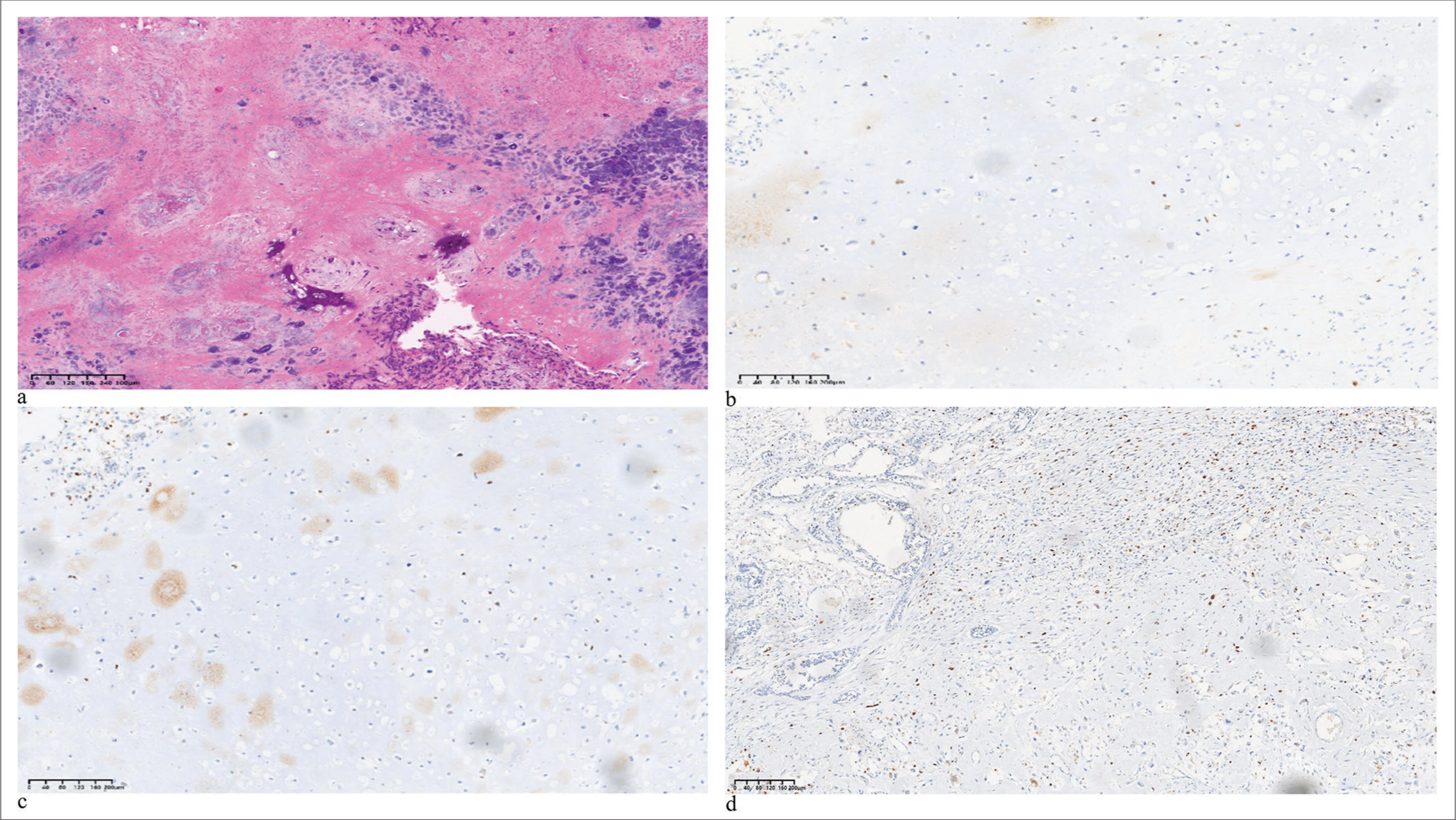
- The (a) hematoxylin and eosin and (b-d) immunohistochemistry (IHC) images of the case patients (IHC markers are as follows: P63, EGFR, Ki-67). (HE: Hematoxylin and eosin, EGFR: Epidermal growth factor receptor)
DISCUSSION
MBC is a highly heterogeneous disease with a low incidence rate. The research to date on MBC has mostly involved small sample sizes, leading to inconsistent findings regarding its clinical and pathological characteristics, treatment approaches, and prognostic factors. The present study focused on MBC patients from the northwest region of China, specifically the Shaanxi, Gansu, Qinghai, and Xinjiang provinces. The patients ranged in age from 21 to 76 years (median age of 57 years), which is slightly higher than the median age of breast cancer patients diagnosed in the general female population in China (45– 55 years),[13] but lower than that of American women (64 years).[14] The spindle cell subtype is generally considered to be the most common of the six MBC subtypes.[15] However, some studies have reported that metaplastic carcinoma with heterologous stromal differentiation is the most common subtype, followed by spindle cell carcinoma and squamous cell carcinoma.[16] In the present study, the most common subtype was mixed metaplastic carcinoma, followed by squamous cell carcinoma, which together accounted for 61.1% of cases. Differences in reported subtype frequencies may be attributed to the relatively small number of cases included in MBC studies, changes in tumor classification schemes over time, interobserver differences, and variations between different patient populations worldwide. These differences may account for some of the discrepancies reported in the literature. Therefore, efforts should be made to conduct similar studies in multiple centers to expand the literature on this rare disease. Our study also found that MBC patients with different histological types had no significant difference in prognosis, in contrast to a previous report.[17] We speculate that this difference may be related to the lower prevalence of spindle cell carcinoma and metaplastic carcinoma with heterologous stromal differentiation in our region compared to study populations from other areas. For non-specific invasive breast cancer, the occurrence and progression of tumors primarily depend on their clinical pathological risk factors. Patients with larger primary tumor size, a greater number of lymph node metastases, or distant metastasis generally have a worse prognosis. We staged and compared patients according to the AJCC clinical staging criteria and found that patients in stages I-II had significantly better PFS and OS compared to patients in stages III-IV. This finding also indicates that the prognosis of MBC follows the general pattern observed in non-specific invasive breast cancer. However, there may be potential confounding factors, such as lymph node metastasis and distant metastasis, which could play a dominant role in poorer prognosis. However, among the 54 patients, only nine experienced distant metastasis, and the sites of metastasis were scattered, making it difficult to perform an equal comparison of case numbers or compare different metastatic organs. We plan to address this limitation in future studies by expanding the sample size. In response to these issues, we further conducted a separate analysis on the impact of lymph node metastasis on prognosis, and these findings will be reflected in the results and discussion sections of our research.
Early-stage, non-special type invasive breast cancer can spread through the lymphatic system, whereas several studies have shown that MBC has a tendency to spread through hematogenous dissemination, leading to organ metastasis such as the lungs and brain.[18,19] The reported incidence of axillary lymph node involvement in MBC ranges from 8% to 40%,[15,20] with 17% of patients in the present study found to have axillary lymph node metastasis. Although this rate of metastasis is not high, survival analysis showed that such patients have relatively poor PFS and OS, indicating that axillary lymph node metastasis is an adverse prognostic factor for MBC. Due to the rarity of this disease, there is currently no standardized treatment regimen. Treatment approaches for MBC mainly reference those used for non-special type TNBC. A previous study showed that curative surgery can significantly improve the prognosis of MBC patients.[17] Due to the low rate of lymph node metastasis in MBC, there is no compelling evidence regarding the necessity for axillary lymph node dissection. Indeed, the present study found that patients who underwent axillary lymph node dissection had a similar prognosis to those who underwent sentinel lymph node biopsy. Therefore, sentinel lymph node biopsy may be considered safe for MBC patients when the surgical indications are well established. With regard to systemic treatment, previous studies have suggested that anthracycline-based chemotherapy drugs have some efficacy against MBC. Anthracycline drugs are alkylating agents and have long been considered cornerstone chemotherapy agents for breast cancer. In recent years, there have been developments and changes in chemotherapy regimens, but anthracyclines are almost the only drugs present in both old and new mainstream chemotherapy regimens for breast cancer. In a study by Han et al.,[21] the TAC neoadjuvant chemotherapy regimen achieved a 17% pathologic CR rate in metastatic breast cancer (MBC) patients. In addition, Aydiner et al.[22] conducted an observational study to assess the survival and treatment response of 54 MBC patients. The study found that MBC patients had a 12.5% response rate to neoadjuvant chemotherapy (anthracycline and taxane-based therapy). Despite the overall lower sensitivity of MBC to chemotherapy, anthracycline chemotherapy drugs still demonstrate certain effectiveness, which may be related to their efficacy in the treatment of soft tissue sarcomas.[21-23] Our study found that MBC patients who received anthracycline-containing chemotherapy regimens showed better prognosis, thus providing some insight for the design of future large-scale clinical studies on MBC treatment regimens. According to the literature, metaplastic breast carcinoma (MBC) is typically a TNBC, meaning the tumor lacks the expression of ER, progesterone receptor (PR), and HER-2.[24] In our study, we observed that 51 cases (94.4% of all patients) had TNBC, so subgroup analysis based on molecular subtypes was not performed.
In cancer cells, wild-type P53 can block cell proliferation in the event of DNA damage, nutrient deficiency, or hypoxia, thereby promoting apoptosis and preventing the development of tumors.[25] However, in the event of mutation, the TP53 gene loses its protective role in cell regulation, leading to abnormal cell growth, cellular transformation, and carcinogenesis.[6] Approximately 80% of patients with TNBC have TP53 mutations, and patients with elevated TP53 expression often have poor prognosis, as consistently shown by molecular testing and immunohistochemistry.[26-28] In the present study, immunohistochemical staining revealed that TP53 was expressed in 87.0% of MBC, which was higher than in non-specific TNBC. However, univariate analysis indicated that TP53 expression did not significantly alter the prognosis of MBC patients. This is inconsistent with its role in malignant transformation, and may be related to the small number of patients in the TP53 negative group. E-cadherin is a calcium-dependent transmembrane glycoprotein that plays a key role in cell-cell adhesion. Disruption of adhesion is an important event in tumor development and metastasis.[29] Decreased expression of E-cadherin is closely related to EMT in various cancer types including breast cancer and is considered be a marker of poor prognosis.[30-32] The occurrence of MBC is thought to be closely related to the EMT process. In the present study of 54 MBC cases, 79.6% showed positive expression for E-cadherin, which was higher than a previous report.[33] We believe that the continuous expression of E-cadherin cell adhesion molecule in metaplastic carcinoma may not only inhibit tumor progression and metastasis but also be associated with the transformation of epithelial cells to mesenchymal cells and angiogenesis in this non-special type of breast cancer. The interference of E-cadherin with other metastasizing factors such as EGFR or the Akt/signal transducer and activator of tranions-mediated pathway has been reported as the main cause of inducing the EMT in triple-negative cancers. However, these observations are highly correlated with the histological types of the included MBC cases and require further validation in larger cohorts.[34,35]
CD24 is a small, highly glycosylated glycosylphosphatidylinositol-anchored protein involved in cancer cell proliferation, metastasis, immune suppression, and escape.[36,37] CD24-positive cell populations found in breast, ovarian, colorectal, and other cancer types have been identified as CSCs.[38-41] Jing X et al.[42] reported that high CD24 expression was positively correlated with poor prognosis and high histological grade in HER2-positive breast cancer and TNBC. In addition, CD24 protein expressed on TNBC cells can interact with Sliglec-10 protein expressed on immune cells. This inhibits phagocytosis of the tumor cells by immune cells and reduces apoptosis, suggesting that CD24 may be an important target for therapy in breast cancer.[42,43] Moreover, more than half of MBC clinical specimens show high expression of CD24. However, the results of univariate analysis in the present study showed that CD24 expression was not significantly correlated with PFS or OS in MBC patients. Ki-67 expression is an important reference index for assessing the proliferative activity of tumor cells. Elevated Ki-67 levels reflect rapid tumor cell proliferation, which, in turn, correlates with distant metastasis and invasion. In the present study, MBC showed high levels of Ki-67 expression, with the index ranging from 20% to 90%. Over 30% of MBC cases had a high Ki-67 index, which was more than that observed in non-specific TNBC.[44] However, a high Ki-67 index in MBC patients were not significantly correlated with PFS or OS. This lack of prognostic significance may again be due to the relatively small number of patients with a low Ki-67 index.
The EGFR family plays an important role in the proliferation, metastasis, cell apoptosis, and drug resistance of various cancer types.[45] EGFR is highly expressed in breast cancer, especially TNBC, and plays a critical role in regulating and maintaining tumor biological characteristics such as stemness, proliferation, invasion, and metastasis.[46-49] Recent studies have shown that EGFR can serve as a molecular marker for breast cancers with a basal-like phenotype, with high EGFR expression being associated with lymph node metastasis and worse PFS.[50,51] In the present study, high EGFR expression was observed in 53.7% of MBC samples, which is similar to the frequency reported in non-specific TNBC.[52] Univariate analysis showed a significant correlation between EGFR expression and both the PFS and OS of MBC patients. In addition, multivariate analysis indicated that high EGFR expression was an independent risk factor for poor prognosis. Since increased EGFR signaling is usually associated with increased EGFR turnover,[52] we plan to use more accurate methods to detect EGFR expression in future studies. This may provide stronger evidence for the potential use of EGFR as a therapeutic target in MBC.
Immune checkpoint inhibitors and other immunotherapy modalities have shown promising results in the treatment of TNBC. The molecular signature of MBCs has similarities to the claudin-low and mesenchymal subtypes of TNBC. However, it was not until 2022 that Professor Adams S. et al.[53] led the SWOG S1609 study, which aimed to represent the first prospective trial of immunotherapy in MBC. Among 17 patients treated with nivolumab and ipilimumab, only three patients achieved objective responses, objective response rate (ORR) was 18%. Median PFS and OS were 2 and 12 months, respectively. Altogether, 65% patients experienced adverse events, 47% patients developed an immune-related adverse event (AE). In recently published studies on the treatment of MBC using anti-PD-1 therapy, the PFS ranged from 5.3 to 8 months, and these studies were conducted on small sample sizes. However, the addition of anti-cytotoxic T lymphocyte-associated antigen-4 to anti-PD-1/PD-L1 regimens have been associated with greater toxicity and higher mortality rates. These smaller studies shed light on the potential of utilizing immunotherapy as treatment options for MBC. However, the optimal drug combinations and potential effective pathological subtypes of anti-PD-1 therapy may require further research to uncover.[53-55] To investigate the impact of tumor mutational burden (TMB), microsatellite instability (MSI) in MBC patients, Adams S et al.[53] analyzed 19 MBC patients’ tumor next generation sequencing data, which showed low TMB and absence of MSI. This is consistent with Tray et al.[56] previously published large dataset of 192 MBC demonstrating a low TMB across these tumors (median 2.7 mutations/Mb) along with a low microsatellite stability (0/192 MpBC was MSI high).[53,56] Mesenchymal TNBC is one subgroup labeled by various investigators as “claudin-low,” “mesenchymal,” or “mesenchymal stem-like” and some of them are actually metaplastic breast cancer. Mesenchymal TNBCs are enriched in EMT and CSC features, raising the possibility of targeting this axis for treatment. Mesenchymal stem-like tumors are also enriched in genes involved in angiogenesis including vascular endothelial growth factor (VEGF), endothelial PAS domain-containing protein 1, and TEK tyrosine kinase. There have been a few completed and ongoing clinical trials specifically on therapeutics against tumor microenvironment factors. For example, Basho et al.[57] conduct a phase 1 clinical trial to assess the safety and efficacy of mammalian target of rapamycin inhibition with temsirolimus or everolimus in combination with VEGF inhibitor bevacizumab and liposomal doxorubicin in 52 patients with advanced MBC. The ORR was 21%.[57-60]
TNBC is significantly associated with marked overexpression and more frequent gene amplification of VEGF. There is also some evidence suggesting that TNBC patients may benefit from anti-angiogenic therapy.[61] Apatinib is a tyrosine kinase inhibitor that targets VEGF receptor-2 (VEGFR-2). Pharmacological studies indicate that apatinib effectively inhibits tumor angiogenesis by blocking the signaling cascade following VEGF-VEGFR-2 binding. The efficacy of this drug in the treatment of TNBC has been demonstrated.[44] Furthermore, preclinical research and clinical trials have shown that apatinib has effective anti-angiogenic and antitumor activities in advanced sarcoma.[62] Based on these earlier findings, we administered apatinib mesylate in combination with chemotherapy to a patient with MBC exhibiting chondrosarcomatous differentiation. Encouragingly, the tumor consistently decreased in size during treatment, and partial clinical relief was achieved preoperatively. Maintenance treatment was continued postoperatively with chemotherapy and anti-angiogenic therapy. This result is consistent with the treatment outcome for a late-stage MBC patient reported by Zou et al.[63] Their patient was diagnosed with metaplastic carcinoma with heterologous mesenchymal differentiation. She underwent treatment with anlotinib (12 mg/day, 2 weeks on, 1 week off) and achieved a durable PR of more than 25 months.[52] Given the outcome of this case, we believe that MBC patients may benefit from anti-angiogenic therapy, thus offering a potential treatment option for MBC with heterologous mesenchymal differentiation.
SUMMARY
This study included 54 cases of MBC from two influential medical centers in Northwest China. The study analyzed the age of onset, tumor size, pathological subtypes, AJCC staging, operation choosing, and systemic treatment in this relatively homogeneous population. It also conducted a pioneering analysis of the correlation between molecular biomarkers associated with the pathogenesis of MBC, such as CD24 (a tumor stem cell marker), E-cadherin (an EMT marker), EGFR, P53, and the cell proliferation index Ki-67. The study aimed to clarify their expression in MBC and their correlation with prognosis. The results of univariate analysis demonstrated that axillary lymph node metastasis, AJCC staging, EGFR expression, and receiving anthracycline-containing chemotherapy regimens were correlated with the prognosis of MBC. The results of multivariate analysis indicated that axillary lymph node involvement and EGFR expression were independent prognostic factors for MBC. Patients with axillary lymph node metastasis and high expression of EGFR had a relatively poorer prognosis. Due to the rarity and heterogeneity of this disease, although there have been several single-center studies analyzing its diagnosis, classification, and prognosis, there is limited research on treatment options. The study shared a successful case of using anti-angiogenic therapy in an MBC patient, with the hope of providing a reference for new treatment approaches in MBC.
However, this study still has some limitations. Despite including cases of metaplastic breast carcinoma from two medical centers in Northwest China over the past 5 years, the sample size still needs to be increased. In addition, the Northwest region of China comprises multiple ethnic groups, including Han, Hui, Uyghur, and Mongolian. In this study, 52 patients were Han Chinese, and only two patients were from ethnic minorities. Therefore, we did not include the ethnic composition of patients in the study. Other potential confounding variables, such as patients’ smoking and drinking history, comorbidities, socioeconomic status, psychological status, and family genetic factors, were not considered, which may introduce errors and biases in the research results. In the future, we will continue to expand the sample size, collect more detailed patient information, conduct stratified analysis, and perform longer follow-ups.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available on request from the corresponding author, [XZ], upon reasonable request.
ABBREVIATIONS
CD24–Cluster of differentiation 24
CSCs–Cancer stem cells
EGFR–Epidermal growth factor receptor
EMT–Epithelial-mesenchymal transition
ER–Estrogen receptor
GPI–Glycosylphosphatidylinositol
H&E–Hematoxylin and eosin
HER-2–Human epidermal growth factor receptor 2
MBC–Metaplastic breast carcinoma
MDT–Multidisciplinary team
MSI–Microsatellite instability
ORR–Objective response rate
OS–Overall survival
PCR–Pathologic complete response
PFS–Progression-free survival
PR–Partial response
TMB–Tumor mutational burden
TNBC–Triple-negative breast cancers
TRAM–Transverse rectus abdominis myocutaneous
VEGF–Vascular endothelial growth factor
WHO–World Health Organization
AUTHOR CONTRIBUTIONS
XZ and JHL: Conceived and designed the study; JD, SW and WL: Performed analysis and interpretation of the data and wrote the manuscript; JYL, BG, HS and YZ: Contributed to MBC database administration; WS and ZL: Prepared the article images. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This retrospective study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital (2021071609). The informed and written consent was obtained from the patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
The research was supported by funding from the National Key Research and Development Program of China (2022YFE0104200), Shaanxi Breast Disease Clinical Medical Research Center (grant no. S2021-0-ZC-LCZX-0002); Basic Research Program of Natural Science in Shaanxi Province (2023JC-YB-345) and Shaanxi Provincial People’s Hospital Elite Talent Project (2022JY-46).
References
- Metaplastic breast cancer: Prognostic and the rapeutic considerations. J Surg Oncol. 2021;123:6-70.
- [CrossRef] [Google Scholar]
- The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77:181-5.
- [CrossRef] [Google Scholar]
- Expression of markers of stem cell characteristics, epithelial-mesenchymal transition, basal-like phenotype, proliferation, and androgen receptor in metaplastic breast cancer and their prognostic impact. Acta Oncol. 2021;60:1233-9.
- [CrossRef] [Google Scholar]
- Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: Identification of genes potentially related to epithelial-mesenchymal transition. Oneogene. 2007;26:7859-71.
- [CrossRef] [Google Scholar]
- Metaplastie breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transidon. Mod Pathol. 2012;25:178-84.
- [CrossRef] [Google Scholar]
- Regulation of PRMT5-MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. Proc Natl Acad Sci USA. 2019;116:17990-8000.
- [CrossRef] [Google Scholar]
- Nomogram prediction model for prognosis of triple negative metaplastic breast cancer based on SEER database China: Nanchang University; 2022.
- [Google Scholar]
- Metaplastic breast cancer treatment and outcomes in 2500 patients: A retrospective analysis of a national oncology database. Ann Surg Oncol. 2018;25:2249-60.
- [CrossRef] [Google Scholar]
- Metaplastic breast carcinoma: Case series and review of the literature. Asian Pac J Cancer Prev. 2012;13:4645-9.
- [CrossRef] [Google Scholar]
- Axillary nodal evaluation in breast cancer: State of the art. Radiology. 2020;295:500-15.
- [CrossRef] [Google Scholar]
- Predicting the risk of axillary lymph node metastasis in early breast cancer patients based on ultrasonographic-clinicopathologic features and the use of nomograms: A prospective single-center observational study. Eur Radiol. 2022;32:8200-12.
- [CrossRef] [Google Scholar]
- Eighth Edition of the AJCC cancer staging manual: Breast cancer. Ann Surg Oncol. 2018;25:1783-5.
- [CrossRef] [Google Scholar]
- A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011;11:364.
- [CrossRef] [Google Scholar]
- Review of metaplastic carcinoma of the breast: Imaging findings and pathologic features. J Clin Imaging Sci. 2012;2:21.
- [CrossRef] [Google Scholar]
- Metaplastic carcinoma of the breast is more aggressive than triple-negative breast cancer: A study from a single institution and review of literature. Clin Breast Cancer. 2017;17:382-91.
- [CrossRef] [Google Scholar]
- Survival outcomes for metaplastic breast cancer differ by histologic subtype. Ann Surg Oncol. 2021;28:4245-53.
- [CrossRef] [Google Scholar]
- Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J Surg Oncol. 2013;11:129-38.
- [CrossRef] [Google Scholar]
- Clincopathologic features and outcomes of metaplastic breast carcinoma: Comparison with invasive ductal carcinoma of the breast. Y Onsei Med J. 2010;51:864-9.
- [CrossRef] [Google Scholar]
- Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349-53.
- [CrossRef] [Google Scholar]
- Metaplastic breast carcinoma: A clinical-pathologic study of 97 cases with subset analysis of response to neoadjuvant chemotherapy. Mod Pathol. 2019;32:807-16.
- [CrossRef] [Google Scholar]
- Metaplastic breast carcinoma versus triplenegative breast cancer: Survival and response to treatment. Medicine (Baltimore). 2015;94:e2341.
- [CrossRef] [Google Scholar]
- Doxorubicin versus CY-VADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: A randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537-45.
- [CrossRef] [Google Scholar]
- Metaplastic breast carcinoma: More than a special type. Nat Rev Cancer. 2014;14:147-8.
- [CrossRef] [Google Scholar]
- Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node-negative breast cancer patients. J Natl Cancer Inst. 1992;84:1109-14.
- [CrossRef] [Google Scholar]
- p53 protein expression in breast cancer as related to histopathological characteristics and prognosis. Int J Cancer. 1993;55:51-6.
- [CrossRef] [Google Scholar]
- A common gain of function of p53 cancer mutants in inducing genetic instability. Oncogene. 2010;29:949-56.
- [CrossRef] [Google Scholar]
- The role of E-Cadherin expression in primary site of breast cancer. Arch Gynecol Obstet. 2022;305:913-20.
- [CrossRef] [Google Scholar]
- Evaluation of the value of GATA3 combined with E-cadherin in the diagnosis of breast cancer. J BUON. 2019;24:1038-44.
- [Google Scholar]
- Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6-11.
- [CrossRef] [Google Scholar]
- Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Mod Pathol. 2012;25:178-84.
- [CrossRef] [Google Scholar]
- The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol. 2012;29:526-33.
- [CrossRef] [Google Scholar]
- CAPE-pNO2 inhibited the growth and metastasis of triplenegative breast cancer via the EGFR/STAT3/Akt/E-cadherin signaling pathway. Front Oncol. 2019;9:461.
- [CrossRef] [Google Scholar]
- Differential expression of E-cadherin and P-cadherin in breast cancer molecular subtypes. Anticancer Res. 2020;40:5557-66.
- [CrossRef] [Google Scholar]
- Glycosylation modulates plasma membrane trafficking of CD24 in breast cancer cells. Int J Mol Sci. 2021;22:8165.
- [CrossRef] [Google Scholar]
- Novel insights into the function of CD24: A driving force in cancer. Int J Cancer. 2021;148:546-59.
- [CrossRef] [Google Scholar]
- Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci USA. 2010;107:3722-7.
- [CrossRef] [Google Scholar]
- CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672-80.
- [CrossRef] [Google Scholar]
- Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937-44.
- [CrossRef] [Google Scholar]
- Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010;288:99-106.
- [CrossRef] [Google Scholar]
- CD24 is a potential biomarker for prognosis in human breast carcinoma. Cell Physiol Biochem. 2018;48:111-9.
- [CrossRef] [Google Scholar]
- Nuclear CD24 drives tumor growth and is predictive of poor patient prognosis. Cancer Res. 2017;77:4858-67.
- [CrossRef] [Google Scholar]
- The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Sci Rep. 2020;10:225.
- [CrossRef] [Google Scholar]
- A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat Cell Biol. 2007;9:961-9.
- [CrossRef] [Google Scholar]
- SGCE promotes breast cancer stem cells by stabilizing EGFR. Adv Sci (Weinh). 2020;7:1903700.
- [CrossRef] [Google Scholar]
- Faciogenital dysplasia 5 supports cancer stem cell traits in basal-likebreast cancer by enhancing EGFR stability. Sci Transl Med. 2021;13:eabb2914.
- [CrossRef] [Google Scholar]
- EGFR Promotes the development of triple negative breast cancer through JAK/STAT3 signaling. Cancer Manag Res. 2020;12:703-17.
- [CrossRef] [Google Scholar]
- TGF-β transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Mol Oncol. 2018;12:305-21.
- [CrossRef] [Google Scholar]
- Prognostic evaluation of epidermal growth factor receptor (EGFR) genotype and phenotype parameters in triple-negative breast cancers. Cancer Genomics Proteomics. 2017;14:181-95.
- [CrossRef] [Google Scholar]
- EGFR/HER2 in breast cancer: A biological approach for molecular diagnosis and therapy. Expert Rev Mol Diagn. 2008;8:417-34.
- [CrossRef] [Google Scholar]
- Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429-34.
- [CrossRef] [Google Scholar]
- A multicenter phase II trial of ipilimumab and nivolumab in unresectable or metastatic metaplastic breast cancer: Cohort 36 of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART, SWOG S1609) Clin Cancer Res. 2022;28:271-8.
- [CrossRef] [Google Scholar]
- A case series of metastatic metaplastic breast carcinoma treated with anti-PD-1 therapy. Front Oncol. 2021;11:635237.
- [CrossRef] [Google Scholar]
- Immune-checkpoint inhibition for metastatic triple-negative breast cancer: Safety first? Nat Rev Clin Oncol. 2019;16:399-400.
- [CrossRef] [Google Scholar]
- Metaplastic breast cancers: Genomic profiling, mutational burden and tumor-infiltrating lymphocytes. Breast. 2019;44:29-32.
- [CrossRef] [Google Scholar]
- Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: Evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol. 2017;3:509-15.
- [CrossRef] [Google Scholar]
- Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116-24.
- [CrossRef] [Google Scholar]
- Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68.
- [CrossRef] [Google Scholar]
- Immunohistochemical features of claudinlow intrinsic subtype in metaplastic breast carcinomas. Breast. 2012;21:354-60.
- [CrossRef] [Google Scholar]
- Multicenter phaseII study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961-9.
- [CrossRef] [Google Scholar]
- Apatinib as targeted therapy for advanced bone and soft tissue sarcoma: A dilemma of reversing multidrug resistance while suffering drug resistance itself. Angiogenesis. 2020;23:279-98.
- [CrossRef] [Google Scholar]
- A case report of targeted therapy with anlotinib in a patient with advanced breast metaplastic carcinoma. Onco Targets Ther. 2021;14:4599-607.
- [CrossRef] [Google Scholar]








