Translate this page into:
A preliminary study of sirtuin-1 on angiotensin II-induced senescence and inflammation in abdominal aortic aneurysms

*Corresponding author: Xiangyu Zhang, Department of General Surgery, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China. 3314023@zju.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Zhang X, Chen H, Pang T, Liang K, Mei J, Zhu Y, et al. A preliminary study of sirtuin-1 on angiotensin II-induced senescence and inflammation in abdominal aortic aneurysms. CytoJournal. 2024;21:32. doi: 10.25259/Cytojournal_80_2024
Abstract
Objective:
Recent evidence suggests the involvement of senescence and inflammation in abdominal aortic aneurysm (AAA). Considering the role of sirtuin-1 (SIRT1) in delaying senescence, we aimed to preliminarily investigate the potential mechanism underlying the effects of SIRT1 in senescence and inflammation during AAA.
Material and Methods:
A cell AAA model was established using angiotensin II (Ang II) as the inducer, which was applied to treat human aortic vascular smooth muscle cells (HASMCs). The senescence and cell cycle of treated HASMCs were evaluated based on senescence-associated (SA)-b-galactosidase (b-gal) assay and flow cytometry, respectively. The levels of inflammatory cytokines and proteins related to senescence-associated secretory phenotype (SASP), along with nuclear factor-kappa B (NF-kB) and mitogen-activated protein kinases (MAPK) pathways, as well as SIRT1, were gauged. The correlation between SIRT1 and NF-kB and MAPK pathway-related proteins was further estimated.
Results:
In Ang II-treated HASMCs, reduced SIRT1 and B-cell lymphoma-2 levels yet increased levels of SASP-related proteins P16 and P21, inflammatory cytokines, as well as Bax and caspases were all visible. In the meantime, Ang II exposure enhanced the number of b-gal-positive HASMCs and promoted cell cycle arrest. SIRT1 was also repressed following Ang II treatment and negatively correlated with NF-kB and MAPK pathway-related proteins (P < 0.05). Furthermore, the overexpression of SIRT1 diminished the levels of SASP-related proteins and reduced the phosphorylation of extracellular regulated kinase 1/2 and P65 in Ang II-treated HASMCs (P < 0.05).
Conclusion:
Taken together, our results indicate that SIRT1 overexpression attenuates the inflammatory and senescent responses of HASMCs in the Ang II-induced AAA cell model. This finding suggests that SIRT1 can be a highly promising target for clinical treatment of AAA.
Keywords
Sirtuin-1
Cellular senescence
Aging
Angiotensin II
Abdominal aortic aneurysm
INTRODUCTION
Abdominal aortic aneurysm (AAA) refers to a pathologic event characterized by progressive abdominal aortic dilatation of 30 mm or more predisposing the abdominal aorta to rupture, making it a life-threating cardiovascular disease (CVD) conferring significant mortality.[1] At present, AAA can only be treated by surgery, and effective medical therapy remains urgently missing.[2] However, patients who fail to meet the surgical indications require long-term follow-up, thus bringing heavy burden to patients both economically and psychologically.[3] Therefore, identifying and recognizing possible therapeutic targets and drugs to retard aneurysm progression or to prevent rupture in AAA can be of great significance in clinical practice and ultimately help extend human life expectancy.[4]
While the pathophysiology of AAA formation, expansion, and rupture has not been fully understood, existing studies have indicated the substantial roles of local inflammation, smooth muscle cell death, and cellular senescence in the mechanisms of the occurrence and development of AAA.[4-6] “Cellular senescence” is defined as a process resulting from various stresses, thereby causing an irreversible growth arrest state, which accompanies stable exit from the cell cycle.[7-10] When it comes to AAA, only some preliminary data have supported the steep increase on the incidence of AAA in patients over 65 years old, thereby indicating the role of aging as a major risk factor of AAA.[11] Moreover, AAA is a degenerative heart condition that is partially distinguished by weakened abdominal aorta. Therefore, it serves as a potential indicator of alterations in the internal microenvironment of local cellular and extracellular matrix components, which is a result of vascular restructuring. Throughout the vascular restructuring process, a key aspect is the decline in vascular smooth muscle cells and the penetration of immune inflammation, aligning closely with the defining traits of senescent cells, that is, diminished proliferation capability and senescence-associated secretory phototype (SASP).[4] This phenomenon implies that cellular senescence could potentially be a significant factor in the development of AAA. Thus, given the inadequate understanding on the specific mechanisms underlying senescence in AAA, it has become the objective of our current study to bridge this research gap by conducting a preliminary investigation.
Next, we shift our focus on relevant biomarkers for the management of senescence. In this regard, the sirtuin family catches our attention. Sirtuins are class III nicotinamide adenine dinucleotide-dependent histone deacetylases that regulate a variety of physiological functions.[12,13] Among these, sirtuin 1 (SIRT1) has been revealed to be implicated in regulating cellular senescence and aging.[13] Thus, studies have investigated increased SIRT1 signaling as a potential strategy for different aging-related diseases.[14] In fact, the involvement of SIRT1 in the aging of brain, placenta, and eye has already been investigated.[15-17] Further, SIRT1 has been proven to play a protective effect in cardiovascular diseases (CVDs), as supported by the discoveries highlighting the involvement of SIRT1 in some relevant CVD models, such as atherosclerosis, myocardial infraction, and more importantly, AAA.[18-20] Thus, we will continue to investigate the relevant molecular mechanisms concerning the involvement of SIRT1 in AAA, with particular emphasis on the potential downstream pathways implicated.
MATERIAL AND METHODS
Cell culture and intervention
Human aortic smooth muscle cells (HASMCs) were ordered from ScienCell Research Laboratories (#6110, Carlsbad, California, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (11966025, Thermo Scientific, Waltham, Massachusetts, USA) supplemented with 10% fetal bovine serum (16000044, Hyclone, Thermo scientific, USA) and 1% penicillin-streptomycin (10378016, Hyclone, Thermo Scientific, USA) in an incubator at 37 ℃ with 5% carbon dioxide. Cells were identified through short tandem repeat (STR) profiling analysis, thereby yielding negative mycoplasma detection results.
Lentivirus encoding SIRT1 was generated and ordered from Genomeditech Company (Shanghai, China). In accordance with the manufacturer’s guidelines, lentiviral vectors overexpressing SIRT1 were used to transfect HASMCs. Stable cell expression was then ensured through selection using 50 μg/mL puromycin (A1113803, Thermo Scientific, USA). Following the construction, these cells infected with lentivirus overexpressing SIRT1 were starved for 24 h in the culture medium containing 0.4% serum and 1% penicillin-streptomycin, followed by the intervention of 100 nM angiotensin II (Ang II, HY-13948, MedChemExpress, Monmouth Junction, New Jersey, USA) for 24 h for the AAA modeling in vitro.[21] In addition, cells using an equal volume of saline were also treated for 24 h and applied as the control (Con).
Assessment of senescence
Senescent HASMCs were identified by determining the activity of b-galactosidase (b-gal) at pH 6.0 using a senescence-associated b-gal assay kit (C0602, Beyotime, China).[22] In detail, cultured cells were rinsed with phosphate-buffered saline (PBS) and fixed at room temperature for 15 minutes. After two additional washings in PBS, HASMCs were incubated with b-gal staining solution (1 mg/mL, pH 6.0) at 37 ℃ for 12 h. The blue stained cells were identified, and at least, three representative fields of view were visualized in BioTek Cytation 5 Cell Imaging Multimode Reader (Agilent, Santa Clara, California, USA). The b-gal density was calculated based on the number of b-gal-positive cells from a minimum of 200 cells.
Cell cycle analysis
The HASMCs were harvested following trypsin (V5280, Worthington, Columbus, OH, USA) digestion and rinsed twice in PBS followed by centrifugation for 10 min. Then, the cells were resuspended in PBS and then fixed in pre-cooled 70% ethanol (-20 ℃, A507050, Sangon, Shanghai, China) at 4 ℃ overnight. The next day, after discarding the excessive ethanol, cells were rinsed in PBS twice and a staining solution comprising ribonuclease A (150 μL, A003431, Sangon, China) and propidium iodide (150 μL, A601112, Sangon, China) was added for 4 h in the dark. The proportion of cells in each cell cycle was then analyzed through CytoFlex flow cytometer (Beckman Coulter, Indianapolis, Indiana, USA) and the affiliated software CytExpert 2.3 (Beckman Coulter, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
A commercial total RNA isolation reagent (TQ102-01, Xinbei, Shanghai, China) was applied to extract the total RNA from the HASMCs. Hereafter, the concentration and purity of extracted RNA were determined through spectrophotometry with a NanoDrop 2000 (Thermo Scientific, USA). NovoScript Plus All-in-one 1st Strand complementary DNA (cDNA) Synthesis SuperMix kit (E047, Novoprotein, Suzhou, China) was then applied for the synthesis of cDNA through reverse transcription. The synthesized cDNA was amplified through the NovoStart® SYBR qPCR SuperMix Plus reagent (No. E096; Novoprotein, Suzhou, China), after which the qRT-PCR was initiated in CFX96 RT-PCR instrument (Bio-Rad, Hercules, California, USA) under the recommended conditions of 95℃ for 1 min and 40 cycles of 95℃ for 20 s, 60℃ for 20 s, and 72℃ for 30 s. The comparative threshold cycle (2-ΔΔCt) method was applied to determine the relative gene expression using b-actin as the housekeeping control.[23] The sequences of primers for this assay are listed in Table 1.
Immunoblotting
Radioimmunoprecipitation assay lysis buffer (P0013B, Beyotime, Beijing, China) containing 1 mM phenylmethanesulfonyl fluoride (ST505, Beyotime, Beijing, China) was adopted to extract the total protein from the HASMCs. Following the quantification on the concentration using a relevant bicinchoninic acid protein assay kit (ZJ102, Epizyme, Shanghai, China), the protein sample was detached in 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis separation gel and then transferred to polyvinylidene fluoride membranes. The membranes were then blocked in 5% defatted milk and incubated with the primary antibodies against SIRT1 (#9475, 1:1000, Cell Signaling Technology, Danvers, Massachusetts, USA), p16 (#18769, 1:1000, Cell Signaling Technology, USA), p21 (ab109520, 1:1000, Abcam, Cambridge, UK), extracellular signal-regulated kinase 1/2 (ERK1/2, ab184699, 1:10000, Abcam, UK), p-ERK1/2: phosphorylated-ERK1/2 (p-ERK1/2, #9101, 1:1000, Cell Signaling Technology, USA), phosphorylated-P65 (p-P65, #3033, 1:1000, Cell Signaling Technology, USA), p65 (#8242, 1:1000, Cell Signaling Technology, USA), and housekeeping control GAPDH (#5174, 1:1000, Cell Signaling Technology, USA) at 4 ℃ overnight. Further, horseradish peroxide-conjugated goat anti-rabbit immunoglobulin G secondary antibody (#35401, 1:2000, Cell Signaling Technology, USA) was applied to incubate with the membrane at ambient temperature for 1 h. After washing in Tris-buffered saline with Tween-20 (TBST), the membrane was visualized in an electrochemiluminescence chemiluminescent assay kit (BL520A, Biosharp, Beijing, China), and the gray scale quantification was implemented in ImageJ 1.42 (IBM Corporation, Armonk, New York, USA).
| Gene | Primers (5’-3’) | |
|---|---|---|
| Forward | Reverse | |
| SIRT1 | TAGACACGCTGGAACAGGTTGC | CTCCTCGTACAGCTTCACAGTC |
| IL1B | CCACAGACCTTCCAGGAGAATG | GTGCAGTTCAGTGATCGTACAGG |
| TNFA | CTCTTCTGCCTGCTGCACTTTG | ATGGGCTACAGGCTTGTCACTC |
| IL6 | AGACAGCCACTCACCTCTTCAG | TTCTGCCAGTGCCTCTTTGCTG |
| IL18 | GATAGCCAGCCTAGAGGTATGG | CCTTGATGTTATCAGGAGGATTCA |
| RELA | TGAACCGAAACTCTGGCAGCTG | CATCAGCTTGCGAAAAGGAGCC |
| RELB | TGTGGTGAGGATCTGCTTCCAG | TCGGCAAATCCGCAGCTCTGAT |
| MAPK1 | ACACCAACCTCTCGTACATCGG | TGGCAGTAGGTCTGGTGCTCAA |
| MAPK8 | ACACCAACCTCTCGTACATCGG | TGGCAGTAGGTCTGGTGCTCAA |
| BAX | TCAGGATGCGTCCACCAAGAAG | TGTGTCCACGGCGGCAATCATC |
| BCL2 | ATCGCCCTGTGGATGACTGAGT | GCCAGGAGAAATCAAACAGAGGC |
| CASP1 | GCTGAGGTTGACATCACAGGCA | TGCTGTCAGAGGTCTTGTGCTC |
| CASP3 | GGAAGCGAATCAATGGACTCTGG | GCATCGACATCTGTACCAGACC |
| ACTB | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
SIRT1: Sirtuin 1, IL1B: Interleukin 1 beta, TNFA: Tumor necrosis factor-alpha, IL6: Interleukin 6, IL18: Interleukin 18, RELA: RELA proto-oncogene, NF-kB subunit, RELB: RELB proto-oncogene, NF-kB subunit, MAPK1: mitogen-activated protein kinase 1, MAPK8: mitogen-activated protein kinase 8, BAX: BCL2 associated X, BCL2: BCL2 apoptosis regulator, CASP1: Caspase 1, CASP3: Caspase 3, ACTB: β-actin
Statistics
All experimental data were derived from over three independent trials and expressed as mean ± standard deviation. Independent samples t-test was employed to compare the data of two groups. Pearson’s correlation test was applied in the correlation analysis. The statistical analysis was performed in GraphPad Prism 7 (GraphPad Software Inc., La Jolla, California, USA). The data were considered statistically significant when the calculated P-value was lower than 0.05.
RESULTS
Lower SIRT1 expression and higher senescence-related protein expression in Ang II-induced HASMCs
With the purpose of exploring involvement in AAA, a cellular Ang II-induced HASMCs model was established to mimic AAA in vitro. Accordingly, the expression level of SIRT1 was quantified through qRT-PCR and immunoblotting. Relevant results proved that the messenger RNA (mRNA) expression of SIRT1 was lower in AAA-modeled HASMCs (P < 0.0001) [Figure 1a]. Similar results regarding the protein expression of SIRT1 (P < 0.01) were obtained [Figure 1b and c].
Next, we determined the involvement of senescence in AAA. After gauging the levels of senescence-relevant proteins P16 and P21, the results revealed that the expression levels of both proteins were all evidently elevated in the Ang II-induced HASMCs compared to those treated only with saline (P < 0.01) [Figure 2a-d].
Higher inflammatory cytokine levels in Ang II-induced HASMCs and the negative correlation between SIRT1 and inflammatory cytokines
Given that senescence and inflammation are interconnected mediators of aging or aging-related disease, we calculated the levels of inflammation-related cytokines in saline- or Ang II-treated HASMCs accordingly. The relevant results demonstrated that the levels of all cytokines, such as Interleukin 1 beta (IL1B, P < 0.05), tumor necrosis factor-alpha (TNFA, P < 0.001), Interleukin 6 (IL6, P < 0.05), and Interleukin 18 (IL18, P < 0.05), were all visibly increased following the intervention of Ang II [Figure 3a-d].
Then, with the aim of preliminarily investigating the involvement of SIRT1 in regulating these cytokines in Ang II-treated HASMCs, the correlation between SIRT1 and the inflammatory cytokines was determined. Based on the Pearson’s correlation test, the results showed that SIRT1 was negatively correlated with IL1B (P = 0.012) [Figure 3e], TNFA (P = 0.01) [Figure 3f], IL6 (P = 0.026) [Figure 3g], and IL18 (P = 0.032) [Figure 3h].
Higher mRNA expression levels of nuclear factor-kappaB (NF-Κb)- and mitogen-activated protein kinases (MAPK)-related proteins in Ang II-induced HASMCs and the negative correlations between SIRT1 and these proteins
In this work, we also aimed to explore the possible downstream pathways underlying the effects of SIRT1 in Ang
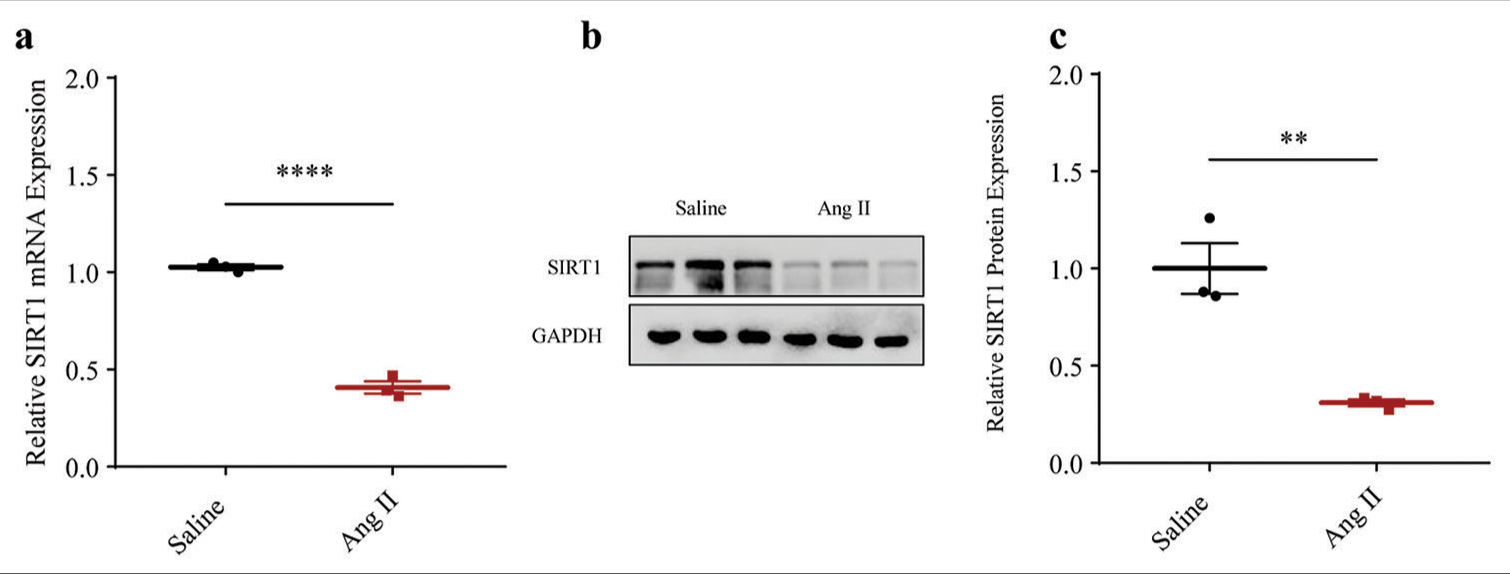
- Lower SIRT1 expression in Ang II-induced HASMCs. (a) Quantified SIRT1 expression in Ang II-induced modeled or saline-treated control HASMCs via qRT-PCR. (b and c) Relative SIRT1 protein expression in Ang II-induced modeled or saline-treated HASMCs based on immunoblotting. All experimental data were derived from over three independent tests and expressed as mean ± standard deviation. (**P < 0.01, ****P < 0.0001. SIRT1: Sirtuin 1, Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells, qRT-PCR: Quantitative real-time polymerase chain reaction, SIRT1: Sirtuin 1.)
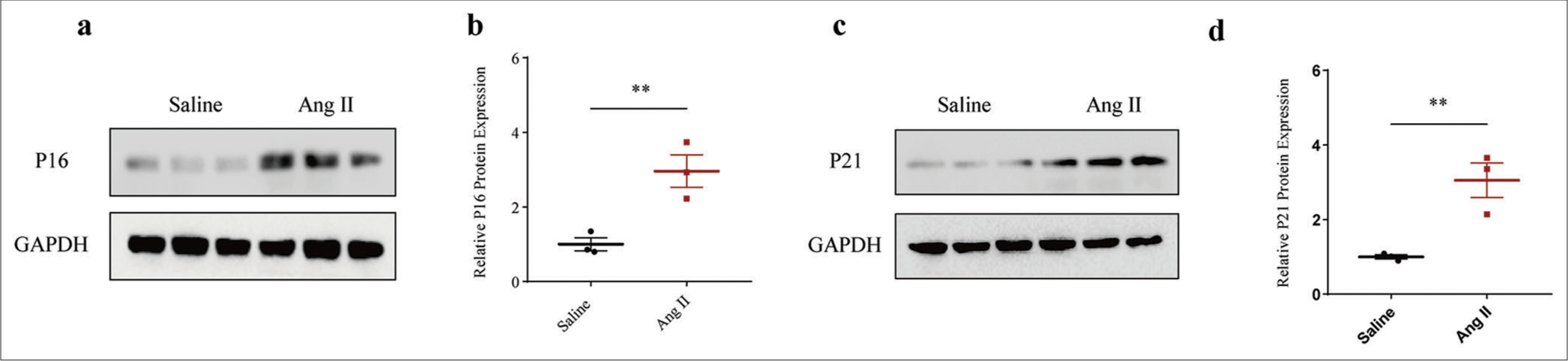
- Higher senescence-related protein expression in Ang II-induced HASMCs. (a and b) Relative P16 protein expression in Ang II-induced modeled or saline-treated control HASMCs based on immunoblotting. (c and d) Relative P21 protein expression in Ang II-induced modeled or saline-treated HASMCs based on immunoblotting. All experimental data were derived from over three independent tests and expressed as mean ± standard deviation. (**P < 0.01. Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells.)
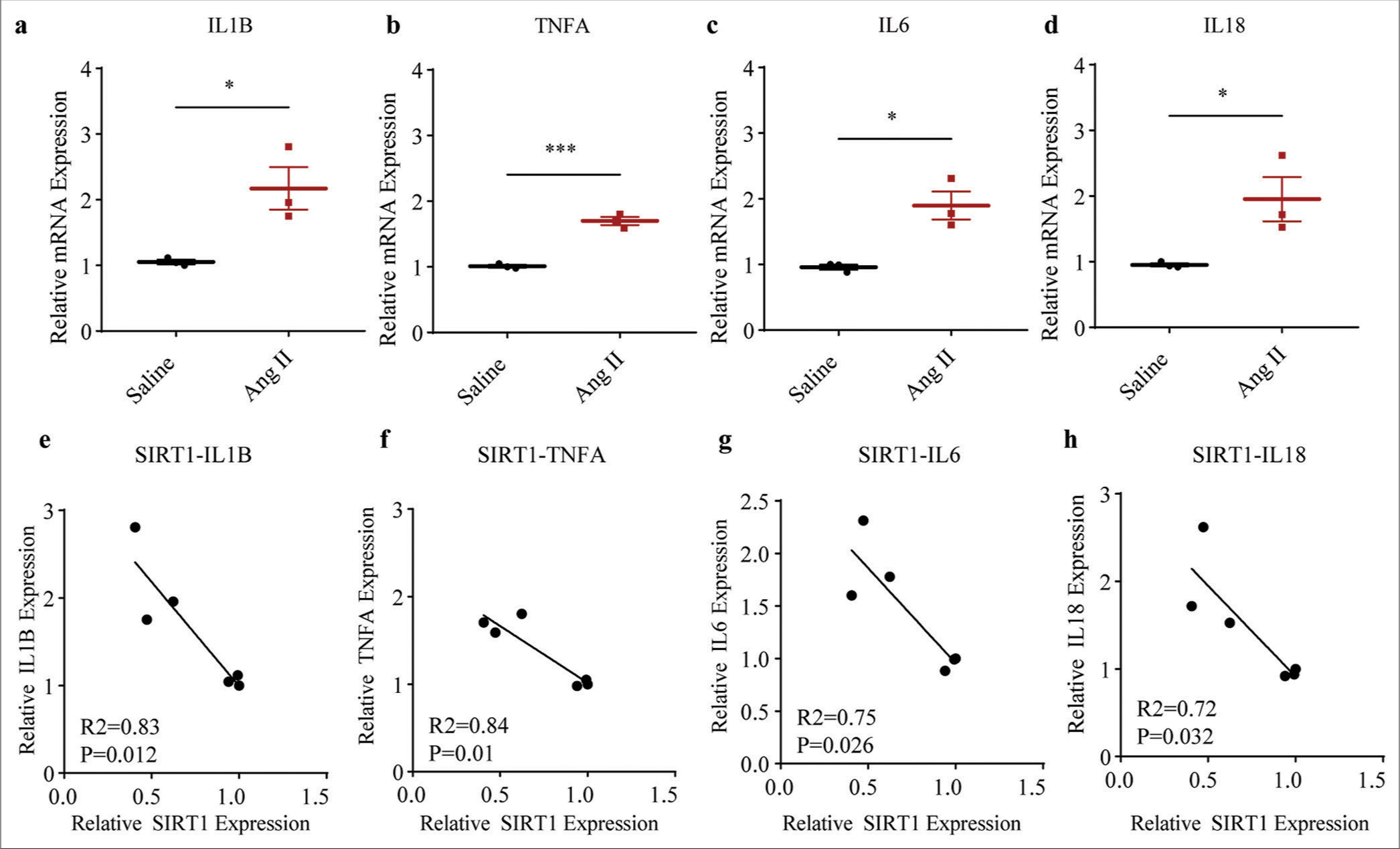
- Higher inflammatory cytokine levels in Ang II-induced HASMCs and the negative correlations between SIRT1 and inflammatory cytokines. (a-d) qRT-PCR assay was applied to determine the difference in the mRNA expression levels of inflammatory cytokines, including IL1B (a), TNFA (b), IL6 (c), and IL18 (d) following Ang II and saline induction in HASMCs. (e-h) Correlations between SIRT1 and pro-inflammatory cytokines in Ang II-induced modeled or saline-treated HASMCs, as revealed by Pearson’s correlation test. All experimental data were derived from over three independent tests and expressed as mean ± standard deviation. (*P < 0.05, ***P < 0.001. SIRT1: Sirtuin 1, Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells, IL1B: Interleukin 1 beta, TNFA: Tumor necrosis factor alpha, IL6: Interleukin 6, IL18: Interleukin 18, mRNA: Messenger RNA, qRT-PCR: Quantitative real-time polymerase chain reaction.)
II-treated HASMCs. Two classic pathways, including NF-kB and MAPK, attracted our attention. Evidently, the mRNA expression levels of proteins related to both NF-kB (including RELA, P < 0.01 and RELB, P < 0.001) and MAPK (including MAPK1 and MAPK8, both P < 0.01) were all increased in HASMCs following Ang II modeling [Figure 4a-d]. In the meantime, negative correlations were also observed in the SIRT1 and the mRNA expression levels of these proteins, including RELA (P = 0.006), [Figure 4e], RELB (P = 0.001), [Figure 4f], MAPK1 (P = 0.005), [Figure 4g], and MAPK8 (P = 0.0001), [Figure 4h], suggesting the negative modulation of SIRT1 on these pathways in Ang II-treated HASMCs.
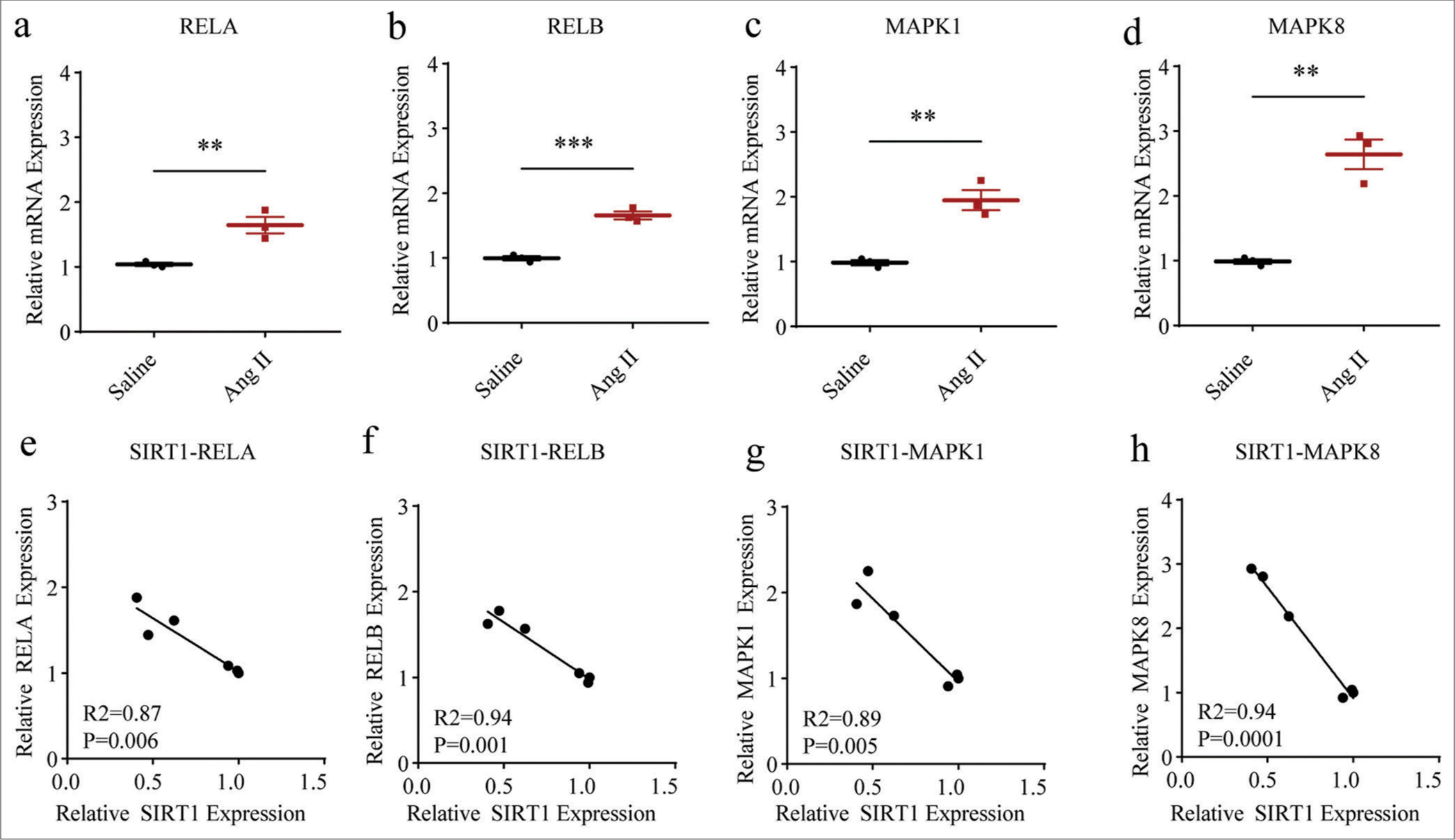
- Higher NF-kB- and MAPK-related proteins levels in Ang II-induced HASMCs and the negative correlations between SIRT1 and these proteins. (a-d) qRT-PCR was adopted to assess the difference in the mRNA expression levels of NF-kB- and MAPK-related proteins, including RELA (a), RELB (b), MAPK1 (c), and MAPK8 (d) in HASMCs after Ang II and saline induction. (e-h) Correlations between SIRT1 and NF-kB- and MAPK-related proteins in Ang II-induced modeled or saline-treated HASMCs, as revealed by Pearson’s correlation test. All experimental data were derived from over three independent tests and expressed as mean ± standard deviation. (**P < 0.01, ***P < 0.001. SIRT1: Sirtuin 1, Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells, MAPK: Mitogen-activated protein kinase, NF-kB: Nuclear factor-kappa B, mRNA: Messenger RNA, qRT-PCR: Quantitative real-time polymerase chain reaction.)
Determination of the SASP, cell cycle, and cell apoptosis in Ang II-induced HASMCs
The SASP in Ang II-induced HASMCs were determined based on the visualization on the senescent HASMCs and the calculation on b-gal density. The results showed that Ang II exposure led to increased senescent HASMCs, as indicated by the increase on the blue stained cells and the b-gal density (P < 0.01) [Figure 5a and b]. The determination of the cell cycle and apoptosis of Ang II-induced HASMCs was then carried out based on flow cytometry test and qRTPCR test (which was employed to quantify the levels of relevant proteins). The results showed that Ang II exposure led to the decreased percentage of cells in S phase (P < 0.01) [Figure 5c and d]. Further, quantification results revealed that Ang II intervention in HASMCs upregulated the level of pro-apoptotic proteins BAX (P < 0.05) [Figure 5e] and downregulated the level of apoptosis suppressor BCL2 (P < 0.01) [Figure 5f]. In addition, increased CASP1 (P < 0.01) and CASP3 (P < 0.001) levels were confirmed in HASMCs following Ang II intervention [Figure 5g and h].
Effects of SIRT1 overexpression on Ang II-induced senescence and inflammation in HASMCs
We sought to determine the effects of SIRT1 on senescence in Ang II-induced HASMCs. Thus, lentivirus overexpression SIRT1 was constructed and applied to HASMCs followed by Ang II modeling. SIRT1 was successfully overexpressed by lentivirus transfection using qRT-PCR (P < 0.01), [Figure 6a]. The levels of senescence-related proteins were then calculated, and the low levels of proteins P16 (P < 0.01) [Figure 6b and c] and P21 (P < 0.01) [Figure 6d and e] in SIRT1-overexpressed Ang II-induced HASMCs were observed.
We also calculated the expression levels of proteins related to both NF-kB and MAPK pathways in Ang II-induced HASMCs following SIRT1 overexpression. The results clearly showed that the phosphorylation of both ERK1/2 (P < 0.05) [Figure 7a and b] and P65 (P < 0.0001), [Figure 7a and c] in Ang II-induced HASMCs was sharply diminished after the overexpression of SIRT1.
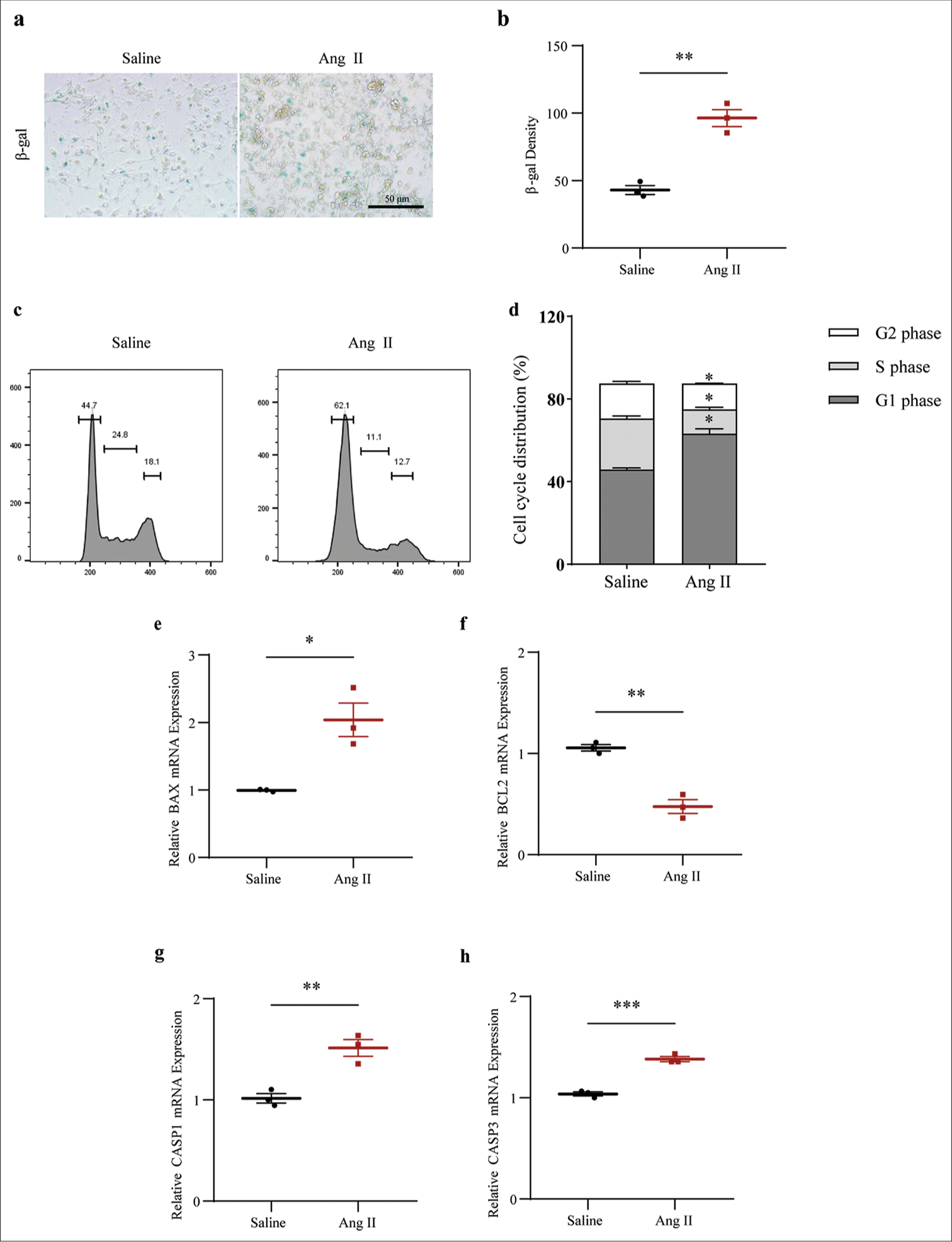
- Determination of the senescence-associated secretory phototype, cell cycle, and cell apoptosis in Ang II-induced HASMCs. (a and b) Visualized senescent HASMCs in different groups and the quantified b-gal density based on SA b-gal staining test (scale bar: 50 μm). (c and d) Relevant results regarding cell cycles in the HASMCs of different groups based on flow cytometry. (e-h) Based on qRT-PCR, the differences in mRNA expression levels of apoptosis-related proteins BAX (e), BCL2 (f), CASP1 (g), and CASP3 (h) in HASMCs of Ang II and Saline groups were assessed. All experimental data were derived from over three independent tests and expressed as mean ± standard deviation. (*P < 0.05, **P < 0.01, ***P < 0.001. SIRT1: Sirtuin 1, Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells, SA b-gal: Senescence-associated b-gal, BAX: BCL2 associated X, BCL2: BCL2 apoptosis regulator, CASP1: Caspase 1, CASP3: Caspase 3, mRNA: Messenger RNA, qRT-PCR: Quantitative real-time polymerase chain reaction.)
Finally, our results revealed that after the overexpression of SIRT1, the blue-stained cell and b-gal densities were significantly lower than those of the control group (P < 0.01), implying that SIRT1 overexpression significantly suppressed Ang II-induced senescence levels in HASMCs [Figure 8a and b]. We also observed that the mRNA expression level of apoptosis-related gene BAX was reduced following SIRT1 overexpression, while that of BCL2 was increased (P < 0.0001) [Figure 8c and d]. In the meantime, the levels of CASP1 and CASP3 were also significantly downregulated after SIRT1 overexpression relative to controls (P < 0.0001) [Figure 8e and f]. Overall, these results further support that SIRT1 has anti-aging, apoptotic, and inflammatory effects on Ang II-induced HASMCs, thereby confirming the potential protective role of SIRT1 in regulating AAA.
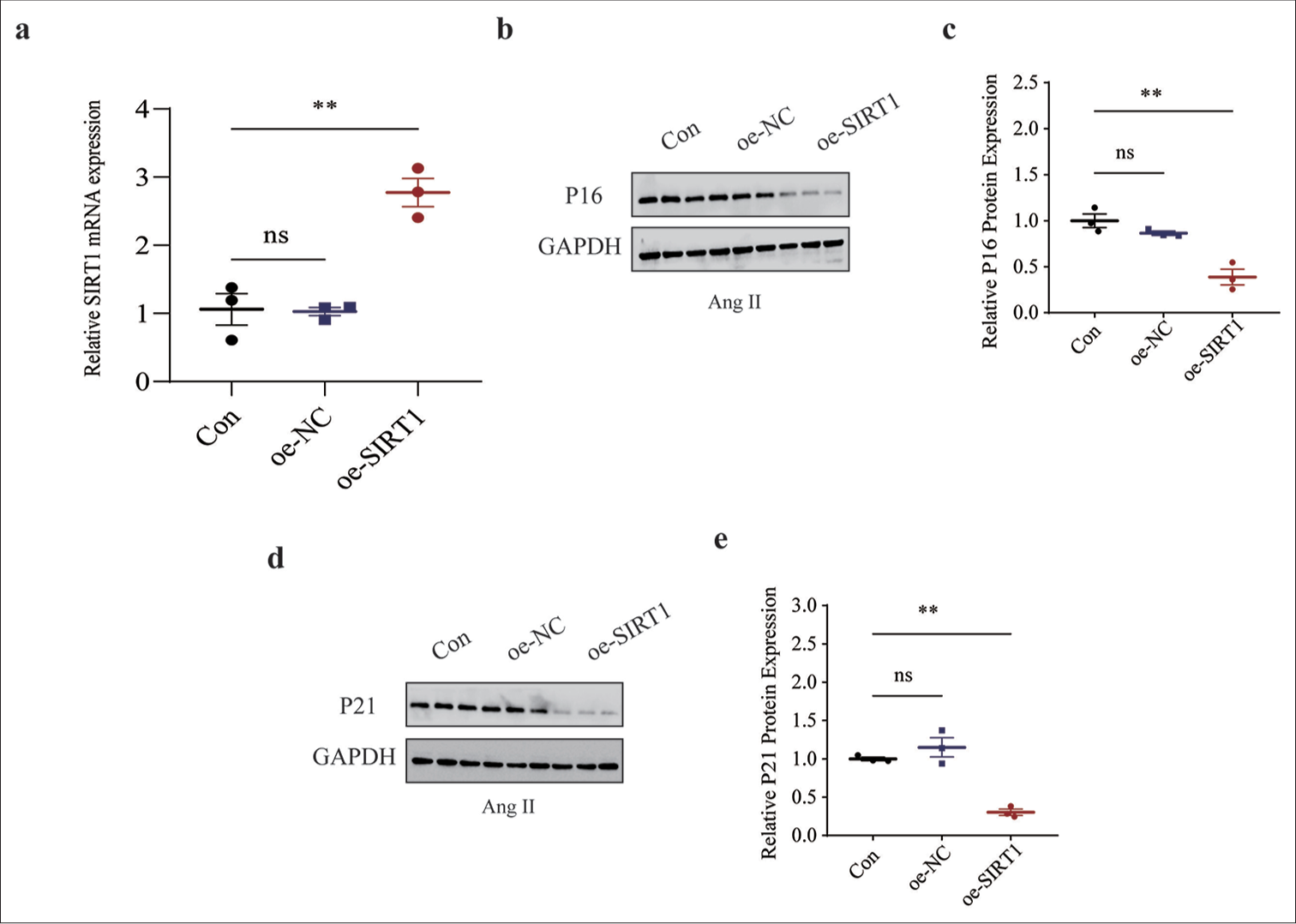
- Effects of overexpressed SIRT1 on senescence-related protein expressions in Ang II-induced HASMCs. (a) qRT-PCR was used to validate the transfection efficiency of lentivirus overexpressing SIRT1. (b and c) Relative P16 protein expression in Ang II-induced HASMCs following SIRT1 overexpression based on immunoblotting. (d and e) Relative P21 protein expression in Ang II-induced HASMCs following SIRT1 overexpression based on immunoblotting. All experimental data were derived from over three independent tests and expressed as mean ± standard deviation. (ns: P > 0.05, **P < 0.01. SIRT1: Sirtuin 1, Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells, Con: Control, oe-NC: Negative control of overexpression plasmid, oe-SIRT1: SIRT1 overexpression plasmid, qRT-PCR: Quantitative real-time polymerase chain reaction, ns: no significance, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.)
DISCUSSION
Ang II is typically considered an endogenous vasoconstrictor and a hormone implicated in the renin-angiotensin system that regulates blood pressure; however, increasing discoveries have now underlined its involvement beyond hemodynamic regulation and implication in the inflammatory process and the pathobiology of vascular diseases.[24,25] Furthermore, increasing evidence has shown the effects of Ang II as an inducer in AAA. For instance, triggering receptor expressed on myeloid cells 1 (TREM-1) can orchestrate Ang II-induced monocyte trafficking and promote experimental AAA in mice.[26] Another study on eosinophils demonstrated their protective effects against Ang II-induced AAA.[27] Further, adipocyte-derived serum Amyloid A has been found to aggravate Ang II-induced AAA in obese C57BL/6J mice.[28] In the present study, an in vitro AAA model using HASMCs was replicated and applied to evaluate the involvement of SIRT1 and investigate the relevant mechanisms. Relevant results have proven that SIRT1 may contribute to the repression on Ang II-induced senescence and inflammation in HASMCs and to the modulation of the levels of proteins related to MAPK and NF-kB pathways, thus hinting at the potential involvement of these two pathways in the repressive effects of SIRT1.
Evidence has also been presented regarding the crucial roles of SIRT family members in health and disease conditions. Of note, this protein family has been found to play various roles in cellular biology, such as inflammation, metabolism, oxidative stress, and apoptosis, among others, which can also delay cellular senescence and extend the organismal lifespan.[29,30] The inflammatory process has been suggested to play a crucial role in AAA and substantially affect the determinants of aortic wall remodeling.[31] Some inflammatory cytokines, including IL1B, TNFA, IL6, and IL18, may also be involved in AAA. For example, IL1B is a pro-inflammatory cytokine that is produced and secreted by monocytes and macrophages in response to inflammatory stimulation, which, together with TNFA, has been related to the progressive inflammatory process promoting aneurysm progression.[32,33] IL6, a central coordinator of inflammatory responses, can stimulate the production of monocyte chemoattractant protein-1 to induce the accumulation and maturation of monocytes/macrophages, a cell population involved in producing proteases that drive matrix degradation and aortic dilation.[34-36] IL18 is a member of the IL1 cytokine family, which stimulates the innate immune and acquired immune responses.[37] Furthermore, IL18 reduction seems to be associated with the reduced AAA formation.[38] Extensive investigations have proven the specific effects of SIRT1 on specific pro-inflammatory cytokines in diverse diseases. For example, activated SIRT1 can attenuate the inflammation of articular chondrocytes and reduce IL1B and IL6 levels.[39] Despite the inadequate discussion on the directive effects of SIRT1 on TNFA and IL18, a study that used SRT1720 (a SIRT1 activator) has confirmed the diminished level of TNFA in a nitroglycerin-induced mouse model of chronic migraine.[40] In the meantime, SRT1720 has also been found to reverse the effects of ferric ammonium citrate on increasing IL18 levels in THP-1 macrophages.[41] In the present study, the levels of IL1B, TNFA, IL6, and IL18 were all proven to be elevated in HASMCs following Ang II exposure. Furthermore, negative correlations were observed in SIRT1 and these pro-inflammatory cytokines. However, whether SIRT1 could also negatively modulate these cytokines in vivo or in vitro has not been detailed, making it a limitation of this study.
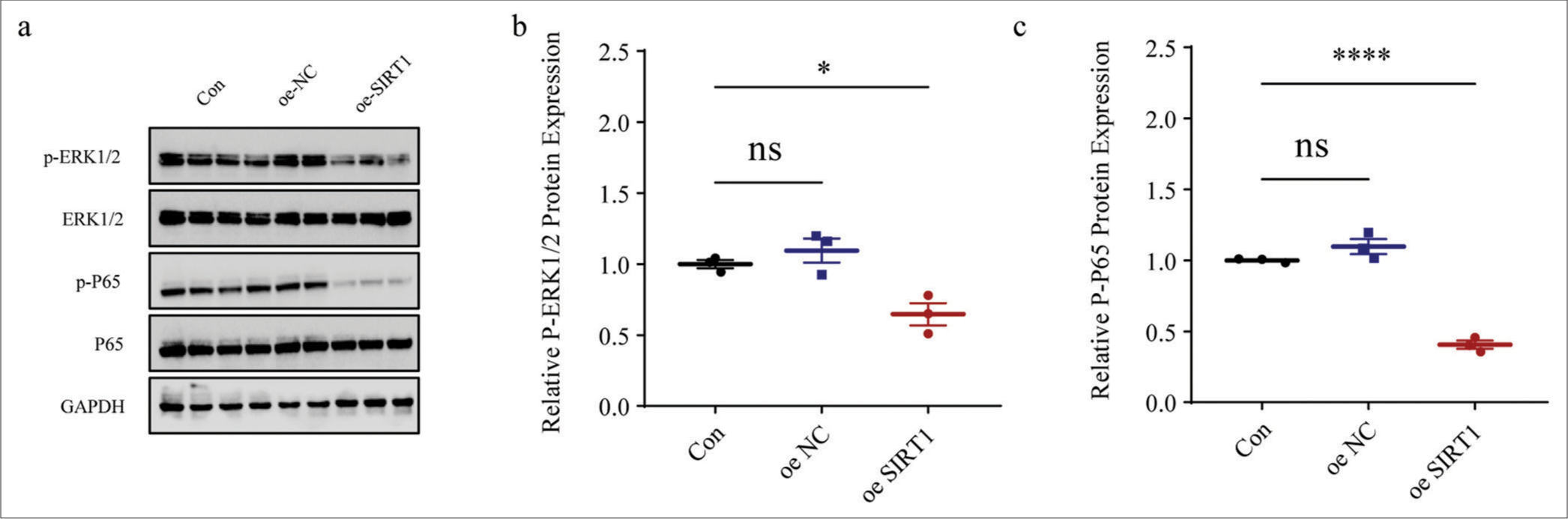
- Effects of SIRT1 overexpression on NF-kB- and MAPK-related proteins levels in Ang II-induced HASMCs. (a-c) Quantified phosphorylation levels of ERK1/2 and P65 in Ang II-induced HASMCs following SIRT1 overexpression based on immunoblotting. All experimental data were derived from over three independent tests and expressed as mean ± standard deviation. (ns: P > 0.05, *P < 0.05, ****P < 0.0001. SIRT1: Sirtuin 1, Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells, Con: control, oe-NC: Negative control of overexpression plasmid, oe-SIRT1: SIRT1 overexpression plasmid, p-ERK1/2: Phosphorylated-ERK1/2, ERK1/2: Extracellular signal-regulated kinase 1/2, p-P65: Phosphorylated-P65, NF-kB: Nuclear factor-kappa B, MAPK: Mitogen-activated protein kinases, ns: no significance, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.)
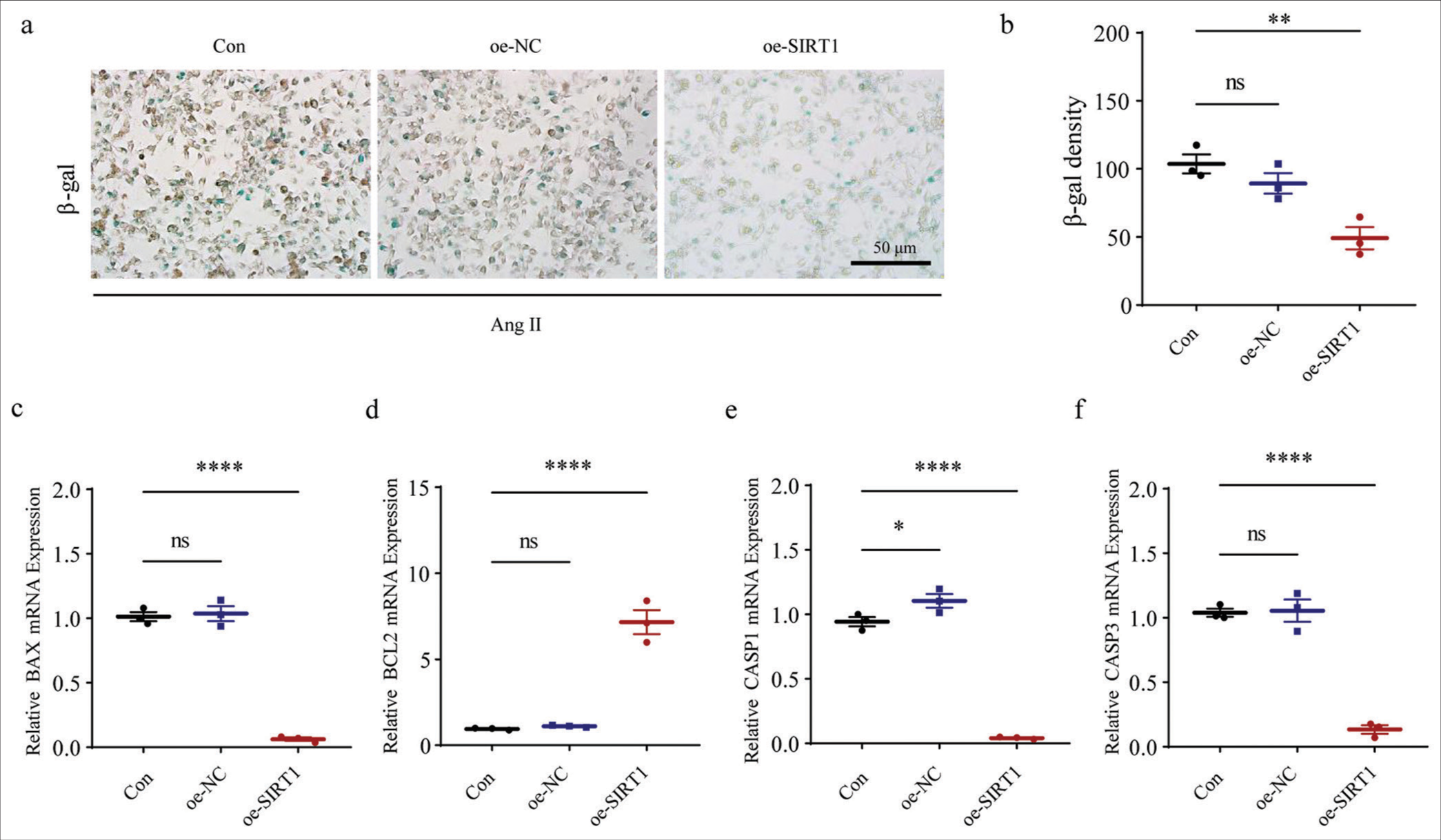
- Effects of SIRT1 overexpression on Ang II-induced senescence and apoptosis in HASMCs. (a and b) SA b-gal staining tests were performed to visualize the results of staining and b-gal density quantification of HASMCs after SIRT1 overexpression. (c-f) Effects of SIRT1 overexpression on the expression levels of apoptosis-related gene BAX (c), BCL2 (d), CASP1 (e), and CASP3 (f) mRNA in HASMCs. (ns: P > 0.05, *P < 0.05, **P < 0.01, ****P < 0.0001. SIRT1: Sirtuin 1, Ang II: Angiotensin II, HASMCs: Human aortic smooth muscle cells, Con: Control, oe-NC: Negative control of overexpression plasmid, oe-SIRT1: SIRT1 overexpression plasmid, SA b-gal: Senescence-associated b-gal, BAX: BCL2 associated X, BCL2: BCL2 apoptosis regulator, CASP1: Caspase 1, CASP3: Caspase 3, mRNA: Messenger RNA, ns: no significance, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.)
Additional studies have also proven the involvement of cellular senescence in the pathogenesis of AAA.[4] For instance, aging could aggravate the aortic aneurysm and dissection through microRNA (miRNA)-1204, an miRNA that leads to the acquisition of SASP by vascular smooth muscle cells.[42] Cellular senescence involves cell cycle arrest and the release of inflammatory cytokines, and several relevant biomarkers of cellular senescence have already been identified, including SA-b-gal, p16, and p21, with the latter two involved in the direct modulation of SASP.[10,43-45] Likewise, studies on the senescence in AAA have suggested the increased accumulation of senescence markers P16 and P21 along with the aggravation of AAA.[46,47] Notably, SIRT6, another sirtuin family member, has been found to attenuate the senescence in vascular smooth muscle cells and reduce the levels of P16 and P21 on the development of AAA based on an Ang II infusion model.[44] While linking SIRT1 with the senescence and the corresponding markers, an existing study on preeclampsia has suggested that the SIRT1 plasmid could decrease the SA-b-gal-positive staining and the protein level of p21.[48] Relevant results have supported the finding that SIRT1, which is lower-expressed in Ang II-induced HASMCs, could neutralize the effects of Ang II on promoting the senescence and cell cycle arrest of HASMCs, along with the repression of P16 and P21 levels.
We also aimed to investigate the possible downstream pathways accounting for the effects of SIRT1 in AAA model cells. As far as we know, SIRT1 can modulate MAPK pathways in ischemic-reperfused cardiomyocytes and deacetylate NF-kB p65 to attenuate hepatic inflammation and fibrosis in macrophages.[49,50] Additional studies have emphasized the involvement of both MAPK and NF-kB pathways in senescence and AAA.[51-53] In the present study, we found that proteins related to MAPK and NF-kB pathways (including RELA, RELB, MAPK1, and MAPK8) were expressed higher in Ang II-treated HASMCs and were negatively correlated with SIRT1. Further immunoblotting results indicated that SIRT1 overexpression could diminish the phosphorylation of P65 and ERK1/2. Nonetheless, whether these pathways were indeed involved in the anti-aging effects of SIRT1 on AAA require further examination, which, we believe, will be covered in our future research. Nonetheless, it should be noted that all these results were solely concluded from relevant cell assays in vitro. In the future, an equivalent animal model in vivo is required to consolidate the conclusion that we have drawn in this work. Nevertheless, our results further proved the anti-aging effect of SIRT1 and suggested the potential efficacy of SIRT1 in AAA or other aging-related diseases.
SUMMARY
Taken together, our study preliminarily investigates the involvement of SIRT1 and provides mechanistic evidence on the role of SIRT1 as a regulator of HASMC senescence in an Ang II-mimicked AAA model. The findings revealed that SIRT1 overexpression attenuated Ang II-induced inflammation and senescence in HASMCs and that both NF-kB and MAPK pathways may help explain the underlying effects of SIRT1. This finding suggests that SIRT1 may have a potential therapeutic role in the management of AAA, as well as provide new insights and perspectives on the clinical management of AAA patients.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
ABBREVIATIONS
AAA: Abdominal aortic aneurysm
CVDs: Cardiovascular diseases
SASP: Senescence-associated secretory phototype
NAD: Nicotinamide adenine dinucleotide
SIRT1: Sirtuin 1
HASMCs: Human aortic smooth muscle cells
DMEM: Dulbecco’s modified Eagle’s medium
STR: Short tandem repeat
Ang II: Angiotensin II
Con: Control
PBS: Phosphate buffered saline
β-gal: β-galactosidase
qRT-PCR: Quantitative real-time PCR
ACTB: β-actin
RIPA: Radioimmunoprecipitation assay
BCA: Bicinchoninic acid
SDS-PAGE: Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
PVDF: Polyvinylidene fluoride
ERK1/2: Extracellular signal-regulated kinase ½
p-ERK1/2: Phosphorylated-ERK1/2
p-P65: Phosphorylated-P65
ECL: Electrochemiluminescence
IL1B: Interleukin 1 beta
TNFA: Tumor necrosis factor-alpha
IL6: Interleukin 6
IL18: Interleukin 18
NF-kB: Nuclear factor-kappa B
MAPK: Mitogen-activated protein kinase
BAX: BCL2-associated X
BCL2: BCL2 apoptosis regulator
CASP1: Caspase 1
CASP3: Caspase 3
RAS: Renin-angiotensin system
MCP-1: Monocyte chemoattractant protein-1
FAC: Ferric ammonium citrate
miRNA: MicroRNA
AUTHOR CONTRIBUTIONS
XYZ and HHC: Designed the study; TSP and KL: Acquired the data; JHM, YFZ and JY: Improved the figure quality; XYZ, HHC and JY: Drafted the manuscript; TSP, KL, and XYZ: Revised the manuscript. All authors contributed to this present work. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study did not involve direct studies of human or animal samples.
CONFLICT OF INTEREST
The authors declare no conflict of interest
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
This study was supported by Zhejiang Provincial Natural Science Foundation (LQ18H020001).
References
- The pathophysiologic basis of abdominal aortic aneurysm progression: A critical appraisal. Expert Rev Cardiovasc Ther. 2015;13:839-51.
- [CrossRef] [Google Scholar]
- Abdominal aortic aneurysms part two: Surgical management, postoperative complications and surveillance. J Perioper Pract. 2021;31:319-25.
- [CrossRef] [Google Scholar]
- Cellular senescence and abdominal aortic aneurysm: From pathogenesis to therapeutics. Front Cardiovasc Med. 2022;9:999465.
- [CrossRef] [Google Scholar]
- Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015;13:975-87.
- [CrossRef] [Google Scholar]
- Novel mechanisms of abdominal aortic aneurysms. Curr Atheroscler Rep. 2012;14:402-12.
- [CrossRef] [Google Scholar]
- Cellular senescence and its effector programs. Genes Dev. 2014;28:99-114.
- [CrossRef] [Google Scholar]
- The RhoA nuclear localization changes in replicative senescence: New evidence from in vitro human mesenchymal stem cells studies. Biocell. 2022;46:2053-8.
- [CrossRef] [Google Scholar]
- Amonafide induces HUVEC senescence by inhibiting autophagy. Discov Med. 2023;35:264-74.
- [CrossRef] [Google Scholar]
- Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92-102.
- [CrossRef] [Google Scholar]
- Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol Ther. 2018;188:140-54.
- [CrossRef] [Google Scholar]
- Endothelial SIRT1 as a target for the prevention of arterial aging: Promises and challenges. J Cardiovasc Pharmacol. 2021;78:S63-77.
- [CrossRef] [Google Scholar]
- Effects of resveratrol and other polyphenols on sirt1: Relevance to brain function during aging. Curr Neuropharmacol. 2018;16:126-36.
- [CrossRef] [Google Scholar]
- SIRT1 regulates trophoblast senescence in premature placental aging in preeclampsia. Placenta. 2022;122:56-65.
- [CrossRef] [Google Scholar]
- SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28:711-32.
- [CrossRef] [Google Scholar]
- Impairment of sirtuin 1-mediated DNA repair is involved in bisphenol A-induced aggravation of macrophage inflammation and atherosclerosis. Chemosphere. 2021;265:128997.
- [CrossRef] [Google Scholar]
- Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. J Cell Mol Med. 2021;25:2944-55.
- [CrossRef] [Google Scholar]
- Activating transcription factor 4 aggravates angiotensin II-induced cell dysfunction in human vascular aortic smooth muscle cells via transcriptionally activating fibroblast growth factor 21. Korean J Physiol Pharmacol. 2022;26:347-55.
- [CrossRef] [Google Scholar]
- The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med. 2017;21:621-7.
- [CrossRef] [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8.
- [CrossRef] [Google Scholar]
- Association of the serum angiotensin II level with disease severity in severe fever with thrombocytopenia syndrome patients. Intern Med. 2016;55:895-900.
- [CrossRef] [Google Scholar]
- Transcriptomics of angiotensin II-induced long noncoding and coding RNAs in endothelial cells. J Hypertens. 2022;40:1303-13.
- [CrossRef] [Google Scholar]
- TREM-1 orchestrates angiotensin II-induced monocyte trafficking and promotes experimental abdominal aortic aneurysm. J Clin Invest. 2021;131:142468.
- [CrossRef] [Google Scholar]
- Eosinophils protect mice from angiotensin-II perfusion-induced abdominal aortic aneurysm. Circ Res. 2021;128:188-202.
- [CrossRef] [Google Scholar]
- Adipocyte-derived serum amyloid a promotes angiotensin II-induced abdominal aortic aneurysms in obese C57BL/6J mice. Arterioscler Thromb Vasc Biol. 2022;42:632-43.
- [CrossRef] [Google Scholar]
- The sirtuin family in health and disease. Signal Transduct Target Ther. 2022;7:402.
- [CrossRef] [Google Scholar]
- Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019;52:24-34.
- [CrossRef] [Google Scholar]
- Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol. 2017;14:457-71.
- [CrossRef] [Google Scholar]
- Transgenic mouse model for imaging of interleukin-1b-related inflammation in vivo. Sci Rep. 2015;5:17205.
- [CrossRef] [Google Scholar]
- IL-1b (Interleukin-1b) and TNF-a (Tumor Necrosis Factor-a) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler Thromb Vasc Biol. 2018;38:457-63.
- [CrossRef] [Google Scholar]
- Interleukin-6 receptor signaling and abdominal aortic aneurysm growth rates. Circ Genom Precis Med. 2019;12:e002413.
- [CrossRef] [Google Scholar]
- Elevated wall tension initiates interleukin-6 expression and abdominal aortic dilation. Ann Vasc Surg. 2018;46:193-204.
- [CrossRef] [Google Scholar]
- Exploration of combinational therapeutic strategies for HCC based on TCGA HCC database. Oncologie. 2022;24:101-11.
- [CrossRef] [Google Scholar]
- Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front Immunol. 2022;13:919973.
- [CrossRef] [Google Scholar]
- Pharmacological inhibition of gasdermin D suppresses angiotensin II-induced experimental abdominal aortic aneurysms. Biomolecules. 2023;13:899.
- [CrossRef] [Google Scholar]
- SIRT1: A potential therapeutic target in autoimmune diseases. Front Immunol. 2021;12:779177.
- [CrossRef] [Google Scholar]
- MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J Neuroinflammation. 2021;18:287.
- [CrossRef] [Google Scholar]
- SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1B and IL-18. Biochem Biophys Res Commun. 2021;561:33-9.
- [CrossRef] [Google Scholar]
- Aging aggravates aortic aneurysm and dissection via miR-1204-MYLK signaling axis in mice. Nat Commun. 2024;15:5985.
- [CrossRef] [Google Scholar]
- Cellular senescence: The good, the bad and the unknown. Nat Rev Nephrol. 2022;18:611-27.
- [CrossRef] [Google Scholar]
- Bhlhe40 protects cochlear hair cell-like HEI-OC1 cells against HOtriggered oxidative injury. Biocell. 2024;48:991-9.
- [CrossRef] [Google Scholar]
- The senescence-associated secretory phenotype and its physiological and pathological implications. Nat Rev Mol Cell Biol 2024 Online ahead of print
- [CrossRef] [Google Scholar]
- Trimethylamine N-oxide promotes abdominal aortic aneurysm formation by aggravating aortic smooth muscle cell senescence in mice. J Cardiovasc Transl Res. 2022;15:1064-74.
- [CrossRef] [Google Scholar]
- PM2.5 promotes abdominal aortic aneurysm formation in angiotensin II-infused apoe-/-mice. Biomed Pharmacother. 2018;104:550-7.
- [CrossRef] [Google Scholar]
- MiRNA-494 induces trophoblast senescence by targeting SIRT1. Hypertens Pregnancy. 2023;42:2219774.
- [CrossRef] [Google Scholar]
- SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci. 2012;69:2245-60.
- [CrossRef] [Google Scholar]
- Activating SIRT1 deacetylates NF-kB p65 to alleviate liver inflammation and fibrosis via inhibiting NLRP3 pathway in macrophages. Int J Med Sci. 2023;20:505-19.
- [CrossRef] [Google Scholar]
- Selenomethionine mitigate PM2.5-induced cellular senescence in the lung via attenuating inflammatory response mediated by cGAS/STING/NF-kB pathway. Ecotoxicol Environ Saf. 2022;247:114266.
- [CrossRef] [Google Scholar]
- Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600-4.
- [CrossRef] [Google Scholar]
- Cellular signaling in abdominal aortic aneurysm. Cell Signal. 2020;70:109575.
- [CrossRef] [Google Scholar]








