Translate this page into:
Midkine promotes thyroid cancer cell migration and invasion by activating the phosphatidylinositol 3 kinase/protein kinase B/mammalian target of rapamycin pathway

*Corresponding author: Yongfeng Zhao, Department of Ultrasound, The Third Xiangya Hospital of Central South University, Changsha, China. redscv@csu.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Yuan L, Zhou P, Liu W, Jiang L, Xia M, Zhao Y. Midkine promotes thyroid cancer cell migration and invasion by activating the phosphatidylinositol 3 kinase/protein kinase B/mammalian target of rapamycin pathway. CytoJournal. 2024;21:41. doi: 10.25259/Cytojournal_47_2024.
Abstract
Objective:
Thyroid cancer (TC) therapy, which is routinely used at present, can improve patients’ survival rates. However, lymph node metastasis results in a higher degree of TC malignancy in patients who experience recurrence and/or death. The elucidation of new mechanisms of TC metastasis can help identify new therapeutic targets. Midkine (MDK) is expressed aberrantly in various cancers. However, the regulatory mechanisms of MDK in TC remain largely unknown. Hence, this study mainly explores the effect and molecular function of MDK in TC.
Material and Methods:
MDK gene expression and protein levels were analyzed using the Gene Expression Profiling Interactive Analysis and the Human Protein Atlas online databases. MDK messenger RNA (mRNA) in TC was analyzed by quantitative real-time polymerase chain reaction. MDK, phosphatidylinositol 3 kinase (PI3K), phosphorylated AKT (p-AKT), and phosphorylated mammalian target of rapamycin (p-mTOR) protein in TC were analyzed by Western blotting. Transwell and wound healing assays were performed to evaluate TC cell metastasis.
Results:
MDK mRNA was significantly highly expressed in most patients with TC (P < 0.05). Moreover, MDK gene expression levels correlated with different TC stages. MDK protein was negative in normal tissues and positive in TC tissues. MDK mRNA and protein were significantly highly expressed in TC cells (P < 0.01). Compared with metastasis in the control group, that in the MDK group is significantly suppressed by MDK knockdown (P < 0.001). MDK knockdown also significantly inhibited PI3K, p-AKT, and p-mTOR protein expression in TPC-1 and K1 cells (P < 0.001). The activation of PAmT-P significantly enhanced the PI3K, p-AKT, and p-mTOR protein expression in TPC-1 and K1 cells (P < 0.001) and promoted metastasis (P < 0.001), thereby disrupting the inhibitory effect of the MDK knockdown.
Conclusion:
Our findings confirmed that MDK promotes TC migration and invasion by activating PAmT-P. MDK is a novel molecular target for the treatment of patients with metastatic TC.
Keywords
Thyroid cancer
Neoplasm metastasis
Neoplasm invasiveness
Midkine
INTRODUCTION
Thyroid cancer (TC) is a common malignant endocrine cancer that poses a considerable threat to human health and development.[1] Furthermore, TC-related morbidity has steadily increased in recent years.[1] TC encompasses various malignant subtypes derived from different cell types in the thyroid gland. The degree of cell differentiation strongly affects disease progression, treatment, and overall survival.[2] Radioactive iodine-131 therapy, which is routinely used at present for the treatment and management of TC, can improve the survival rates of most patients with TC.[3] However, lymph node metastasis may cause a high degree of TC malignancy in patients with recurrent and/or distant metastases.[4] Furthermore, early lymph node metastasis in TC is generally more common than in other types of cancer. However, due to the lack of distinct early symptoms in TC, lymph node metastasis usually found before TC diagnosis, which was a major cause for poor prognosis of TC.[5] Distant metastasis to multiple organs in TC leads to a decrease in the disease-specific survival rate from 77.6% to 5.3%.[6] The molecular mechanisms that control TC proliferation and metastasis are complex and involve the overexpression or restraint of genes through epigenetic regulation.[7] Moreover, the molecular pathogenesis of TC proliferation and metastasis remains mostly unknown. Thus, the identification of potential molecular markers with therapeutic targets is critical for improving the prognosis of patients with TC.
Midkine (MDK) has dynamic biological functions in many biological processes such as embryo process, cardiovascular disease, inflammation, and immune disease.[8] Aberrantly high MDK expression levels in most human malignant tumors seem to promote tumor progression by actively regulating cell transformation, metastasis, angiogenesis, and antitumor immunity.[9] MDK is remarkably overexpressed in cancer tissues and the serum of patients with TC; it also has potential as a diagnostic marker for TC.[10,11] Furthermore, clinical analysis has revealed a strong correlation between elevated MDK expression and lymph node metastases in papillary TC.[12] However, the role of MDK in the migration and invasion of TC cells and related mechanisms remains unclear.
MDK regulates multiple downstream pathways to exert its oncogenic effects. MDK knockdown reduces the proliferation and migration of cancer stem cells by silencing the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) pathway in prostate cancer.[13] The activation of the phosphoinositol-dependent protein kinase-1/AKT signaling pathway by MDK enhances the survival of hepatocellular carcinoma (HCC).[14] MDK enhances the proliferation and migration of glioblastoma and breast cancer by activating the PI3K/Akt and nuclear factor-kappa-B signaling pathways, respectively.[15,16] Moreover, MDK is a PI3K pathway activator that enhances tumor survival and metastasis.[17-19] The PI3K/Akt/mammalian target of rapamycin (mTOR) pathway (PAmT-P) was the central hub regulating cell proliferation, metastasis, and angiogenesis in TC.[20] Silencing PAmT-P is a target for treating TC.[21,22] However, whether MDK regulates that PAmT-P expression in TC remains unclear. In this study, the correlation between MDK expression and TC clinical stage was assessed using online bioinformatics, and the biological functions of MDK in TC were assessed. Finally, we investigated the regulatory mechanisms underlying the role of MDK in PAmT-P signaling in TC.
MATERIAL AND METHODS
Bioinformatics analyses
The gene expression profiling interactive analysis (GEPIA) 2.0 database (http://gepia2.cancer-pku.cn/#index) was employed to assess the MDK gene expression profiles in multiple tumors and normal tissues, including TC, and the MDK expression levels at different pathological stages of TC. The MDK protein in TC and normal thyroid glands was analyzed through immunohistochemical staining using the Human Protein Atlas database (https://www.proteinatlas.org/).
Cell incubation and transfection
Five TC cell lines CAL-62 (CL-0618), TPC-1 (CL-0643), K1 (CL-0062), TTA1 (CL-0673), and HTh7 (CL-0647), and the normal thyroid cell line Nthy-ori3-1 (CL-0817) were obtained from Cellcook (Guangzhou Cellcook Biotech, Guangzhou, China) and cultivated in Roswell Park Memorial Institute-1640 medium (SH30809.01B, Hyclone, South Logan, UT, USA) adding 10% fetal bovine serum (FBS) (SH30087.01, Hyclone) at 37°C with 5% carbon dioxide. The cell lines were authenticated by the genomics unit at CLMA using ShortTandem Repeat profiling (AmpFLSTR Identifler Plus polymerase chain reaction [PCR] Amplification Kit). Mycoplasma test was measured in all cell lines every other week using the MycoAlert Mycoplasma Detection Kit (Lonza). Small interfering RNA negative control (si-NC, 5'- TTCTCCGAACGTGTCACGTTT -3') with three small interfering RNA MDK (si-MDK) (siMDK-1: 5'- CTGCAAGTACAAGTTTGAGAACT -3', siMDK-2: 5'- GGGTTTCCGCGAGGGCACCTGCG -3', and si-MDK-3: 5'- CGCCAAAAAGAAAGATAAGGTGA -3'), short hairpin (shRNA) against MDK (shMDK, 5'-CGCCAAAAAGAAAGATA AGGTGACTCGAGTCA CCTTATCTTTCTTTTTGGCG -3'), and an shRNA NC (shNC, 5'- TTCTCCGAACGTGTCACGTTTCTC GAGAAACGTGAC ACGTTCGGAGAA -3') were obtained from GenePharma (Shanghai, China) and transfected into TC cell lines using lipofectamine 2000 (11668019, Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells using TRIzol reagent (15596026, Invitrogen, Carlsbad, CA, USA). Total RNA was reverse-transcribed into complementary DNA using an RT Reagent Kit (Takara). PCR was performed using an ABI PCR system (Applied Biosystems, Foster City, CA, USA) and Synergetic Binding Reagent Green (RR820A, Takara, Dalian, China). The primer sequences are MDK forward: 5'-CGCGGTCGCCAAAAAGAAAG-3', reverse: 5'-ACTTGCAGTCGGCTCCAAAC-3'; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward: 5'-GAAGGTGAAGGTCGGAGTC-3', reverse: 5'-GAAGATGGTGATGGGATTTC-3'. The MDK messenger RNA (mRNA) relative expression levels were calculated using the 2−ΔΔCT method, and GAPDH was used as an internal parameter for MDK.
Transwell migration and invasion assay
The upper chambers of the Transwell chambers (Corning, Steuben County, NY, USA) were either precoated with Becton, Dickinson and Company (BD) Matrigel (for invasion) or not precoated with BD Matrigel (for migration). After transfection, 1 × 105 TPC-1 and K1 cells were added to the upper chambers containing the FBS-free medium (FBS free). The lower Transwell chambers were supplemented with 10% FBS. After 48 h of cultivation, the cells were shaped with methanol, and the cells that did not migrate or invade the upper chamber were removed. Then, the surface cells at the bottom of the membrane in the lower chamber were stained with crystal violet for 10 min. Finally, the cells in five randomly selected areas were imaged and counted under a microscope (U-CTR30-2, OLYMPUS CKX41, Tokyo, Japan).
Wound healing assays
After transfection, 1 × 106 TPC-1 and K1 cells were added to a six-well plate containing culture medium and cultured for 24 h under conventional conditions. Then, a scratch wound was created by applying sterile plastic and washing thrice with Phosphate buffer saline. Afterward, TPC-1 and K1 cells were incubated in the culture medium for another 48 h. Five random fields of each wound were measured and photographed (U-CTR30-2, OLYMPUS CKX41).
Cell proliferation assay
The proliferation of TPC-1 and K1 was assessed using the Cell Counting Kit-8 (CCK-8) assay (C0038, CCK-8, Beyotime, Shanghai, China). In brief, TPC-1 and K1 were incubated for 48 h, and 10 µL CCK-8 was added for further incubation at 2 h at 37°C. Then, OD450 nm was measured using a multiscan MK3 microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Western blotting
Radio-immunoprecipitation assay lysis buffer containing phenylmethanesulfonyl fluoride (Beyotime) was used to extract the total protein from TC cells. The protein was quantified using the bicinchoninic acid protein quantification kit (BCA1, Sigma, St. Louis, MO, USA). The 30 µg protein was separated through sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). After being stabilized, the membranes were immersed with the listed diluted primary antibodies at 4°C throughout 12 h: Anti-MDK (1:1000, ab52637, Abcam, Cambridge, MA, USA), anti-PI3K (1:1500, ab302958, Abcam), anti-phosphorylated (p-AKT) (1:1000, ab38449, Abcam), anti-AKT (1:5000, ab8805, Abcam), anti-phosphorylated mTOR (p-mTOR) (1:1000, ab314037, Abcam), anti-mTOR (1:1500, ab134903, Abcam), or anti-GAPDH (1:5000, ab8245, Abcam) antibodies. After being washed, the membrane was incubated with a horseradish peroxidase-labeled secondary antibody (1:5000, Abcam) for 2 h at 25°C. Then, additional washing followed. Afterward, protein expression was assessed by applying an enhanced chemiluminescence detection kit (P0018S, Beyotime, Shanghai, China) with the ChemiDoc XRS system (Bio-Rad). Grayscale values are analyzed using ImageJ 1.44p (National Institutes of Health, USA).
Statistical analysis
The statistical analyses of data were carried out by the Statistical Package for the Social Sciences version 22.0 (IBM Corp., Armonk, NY, USA) and expressed as means ± standard deviation. A t-test was used to assess discrepancies between the cancer and non-cancer groups or one-way analysis of variance was used to assess discrepancies between more than two groups. The significance level was P < 0.05.
RESULTS
MDK expression was dramatically enhanced in TC tissues and cells
The MDK gene expression profile in multiple cancers was first analyzed to observe and study the role of MDK in TC. Gene expression profiling interactive analysis showed that MDK was dramatically highly expressed in most human cancers [Figure 1a]. The GEPIA database also revealed that MDK gene levels were dramatically elevated in TC tissues (P < 0.05); [Figure 1b]. In addition, the MDK expression correlated with different TC stages (P < 0.001); [Figure 1c]. Then, the MDK protein was negative expression in normal tissues and positive expression in TC tissues [Figure 2]. This finding suggests that MDK protein expression was enhanced in TC tissues. The MDK mRNA levels were assessed in TC and normal thyroid cells. Compared with the MDK mRNA and protein levels in Nthy-ori3-1, those in all five TC cell lines were elevated, particularly in the TPC-1 and K1 cell lines, (all P < 0.001); [Figure 3]. Therefore, TPC-1 and K1 were chosen for further study.
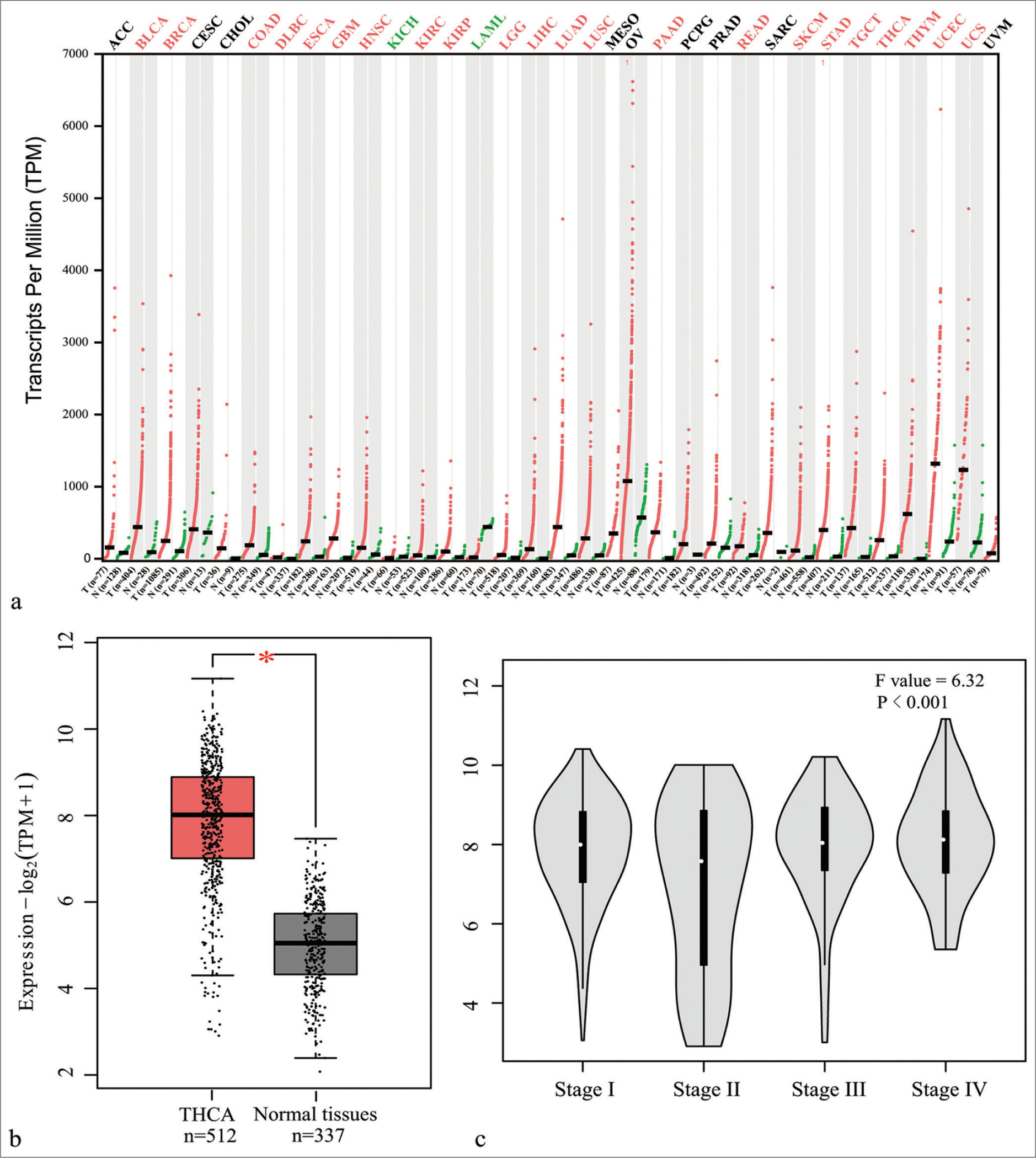
- MDK gene level was evaluated using the GEPIA online database. (a) MDK gene expression profile in different cancer tissues; red and green indicate an increase and decrease, respectively, in MDK expression in tumors. (b) MDK gene expression in thyroid carcinoma tissues and normal tissues; (c) MDK gene level at different TC stages (*P < 0.05). (MDK: Midkine, GEPIA: Gene expression profiling interactive analysis, THCA: Thyroid carcinoma, n: Number, TPM: Transcripts per million, TC: Thyroid cancer. ACC: Adrenocortical carcinoma; BLCA: Bladder Urothelial Carcinoma; BRCA: Breast invasive carcinoma; CESC: Cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL: Cholangio carcinoma; COAD: Colon adenocarcinoma; DLBC: Lymphoid Neoplasm Diffuse Large B-cell Lymphoma; ESCA: Esophageal carcinoma; GBM: Glioblastoma multiforme; HNSC: Head and Neck squamous cell carcinoma; KICH: Kidney Chromophobe; KIRC: Kidney renal clear cell carcinoma; KIRP: Kidney renal papillary cell carcinoma; LAML: Acute Myeloid Leukemia; LGG: Brain Lower Grade Glioma; LIHC: Liver hepatocellular carcinoma; LUAD: Lung adenocarcinoma; LUSC: Lung squamous cell carcinoma; MESO: Mesothelioma; OV: Ovarian serous cystadenocarcinoma; PAAD: Pancreatic adenocarcinoma; PCPG: Pheochromocytoma and Paraganglioma; PRAD: Prostate adenocarcinoma; READ: Rectum adenocarcinoma; SARC: Sarcoma; SKCM: Skin Cutaneous Melanoma; STAD: Stomach adenocarcinoma; TGCT: Testicular Germ Cell Tumors; THCA: Thyroid carcinoma; THYM: Thymoma; UCEC: Uterine Corpus Endometrial Carcinoma; UCS: Uterine Carcinosarcoma; UVM: Uveal Melanoma).
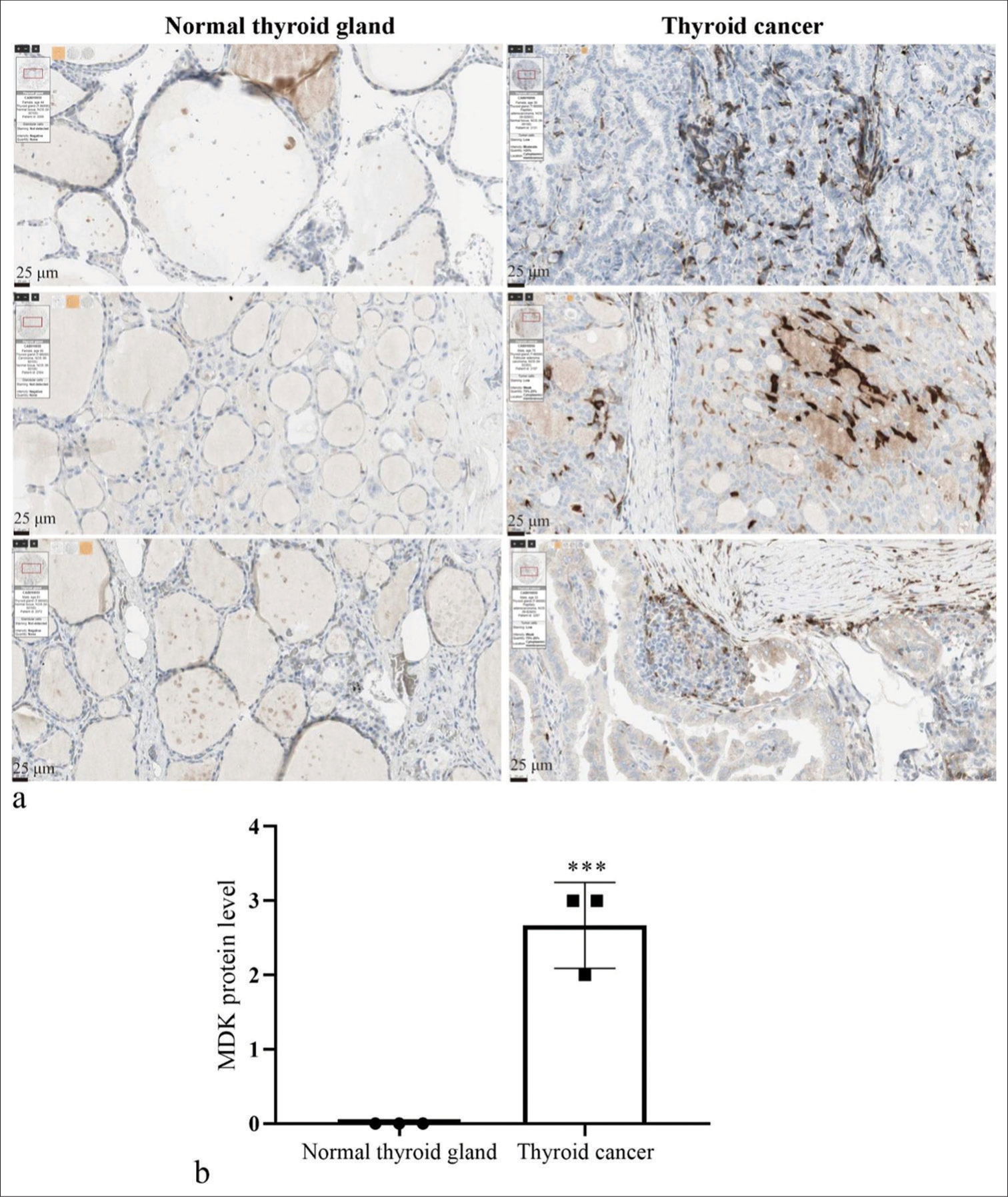
- MDK expression was enhanced in TC tissues. (a) MDK protein levels were measured by immunohistochemistry staining in TC with normal thyroid gland tissues using the Human Protein Atlas database. (b) Histogram represents the MDK expression. *** P < 0.001, n = 3. (MDK: Midkine, TC: Thyroid cancer.).
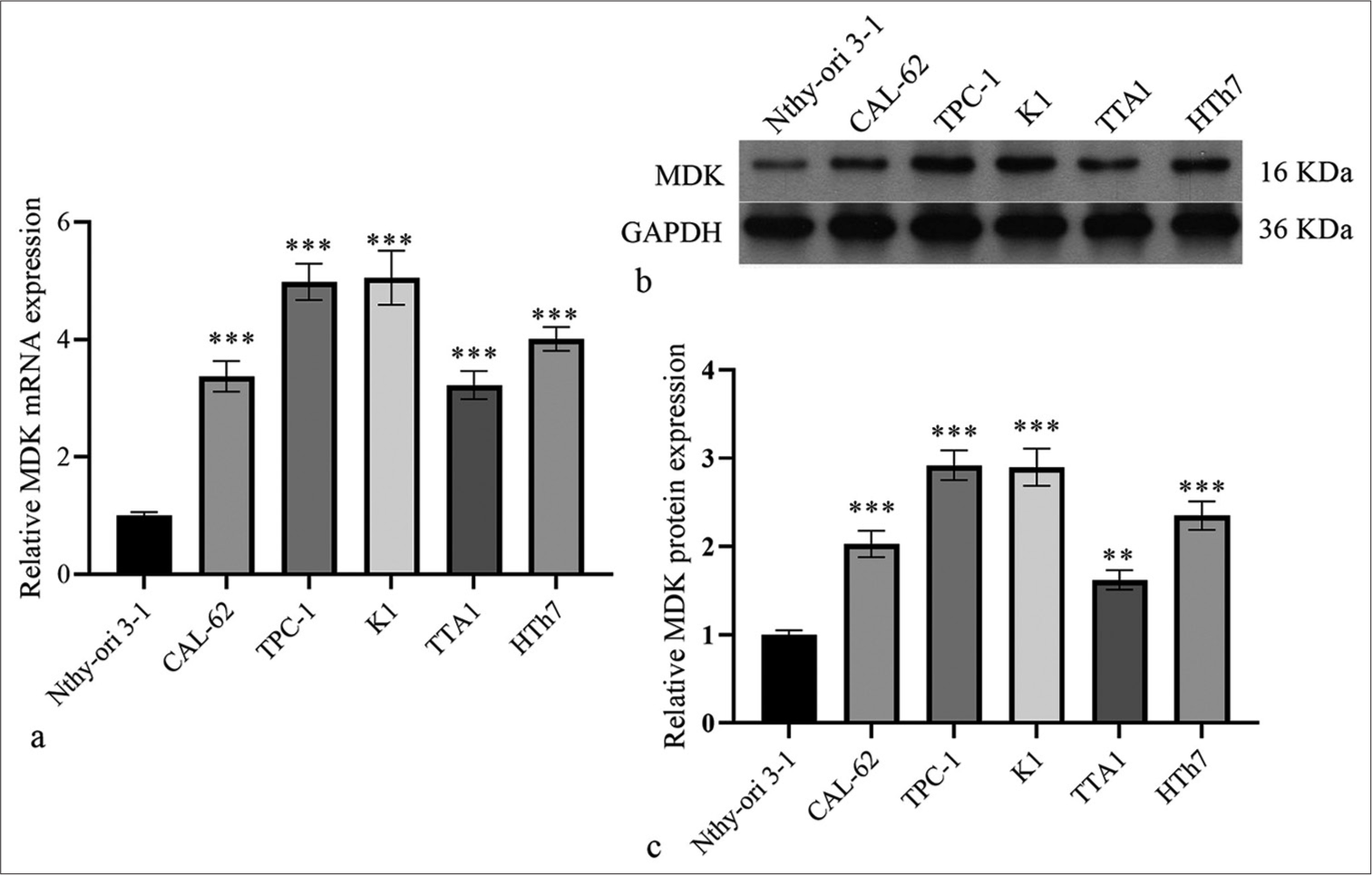
- MDK messenger RNA and protein expression levels were dramatically elevated in TC cells. (a-c) MDK mRNA (a) and protein (b and c) in Nthy-ori3-1 cells and five TC cell lines were evaluated by qRT-PCR and Western blotting, respectively. (**P < 0.01 and ***P < 0.001 compared with Nthy-ori3-1 cells). n = 3. (MDK: Midkine, TC: Thyroid cancer, qRT-PCR: Quantitative real-time polymerase chain reaction.).
Knocking down MDK suppressed TC cell migration and invasion
si-MDK-1/2/3 was transfected in TPC-1 and K1 cells to knock down the MDK expression and study the possible biological functions of MDK in TC. qRT-PCR analysis showed that the MDK mRNA level was dramatically decreased in TPC-1, particularly in si-MDK-2 group (all P < 0.001); [Figure 4a]. Therefore, we used si-MDK-2 sequences, which had been introduced into an interfering plasmid, to generate the shMDK plasmid for further study. Compared with MDK mRNA and protein in the shNC group, those in the shMDK group were strongly blocked (all P < 0.001); [Figure 4b and c]. Functionally, Transwell assays demonstrated that MDK knockdown strongly suppressed TPC-1 and K1 cell metastasis compared to shNC group (all P < 0.001), [Figure 5a and b]. MDK knockdown dramatically attenuated the migration of TPC-1 and K1 cells (all P < 0.001); [Figure 5c]. In addition, MDK knockdown attenuated the proliferation of these cells (all P < 0.001); [Supplementary Figure S1a].
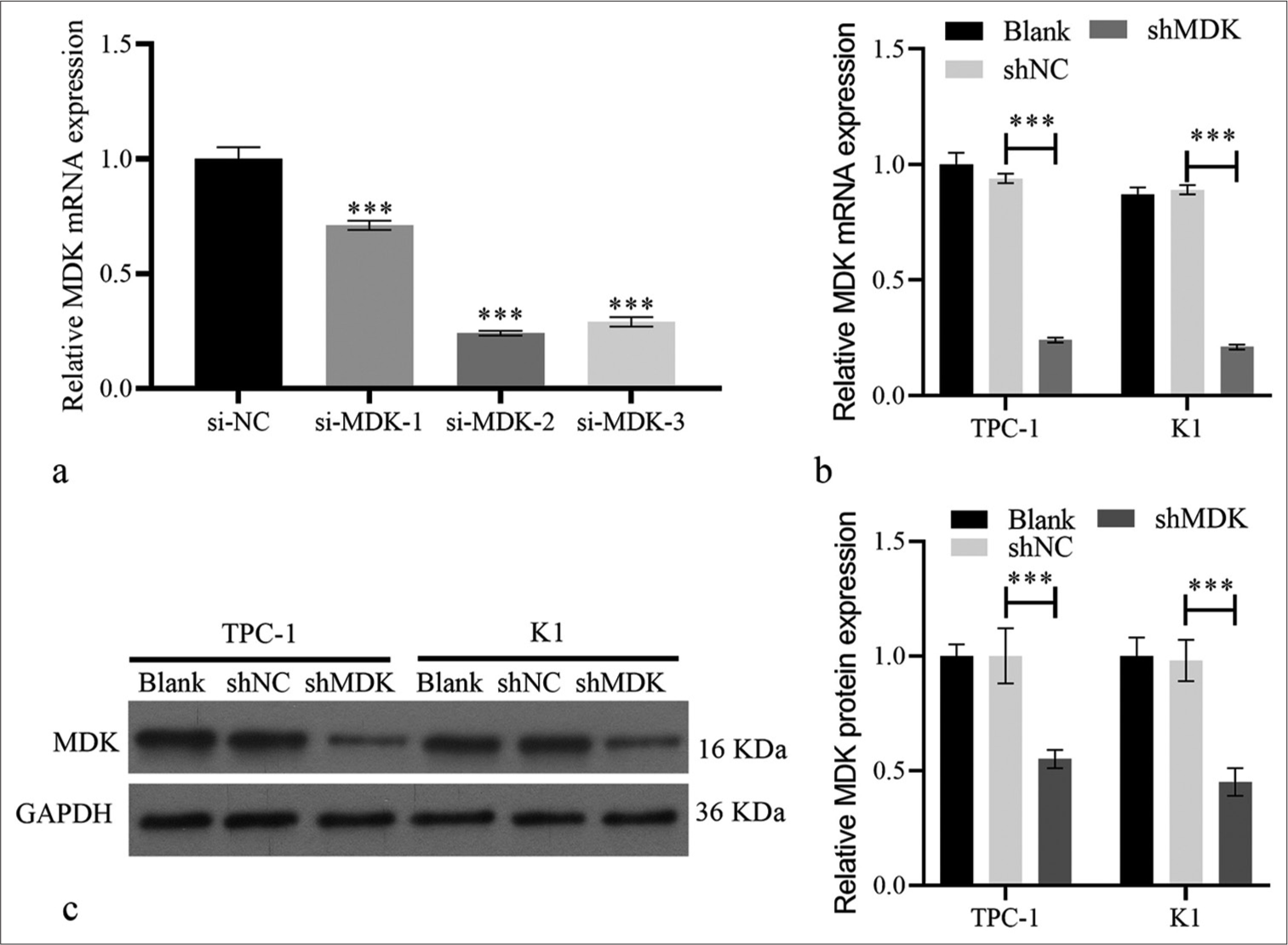
- MDK expression was silenced in TC cells. (a) MDK messenger RNA expression was quantified using qRT-PCR transfected with si-MDK-1, si-MDK-2, si-MDK-3, or si-NC in TPC-1 cells. (b and c) MDK mRNA (b) and protein (c) in TPC-1 and K1 were measured by qRT-PCR and Western blotting, respectively, after being transfected with shMDK and shNC at 48 h. (***P < 0.001). n = 3. (MDK: Midkine, si-NC: Negative control of small interfering RNA, si-MDK: Small interfering RNA MDK, shNC: Negative control of short hairpin RNA, shMDK: Short hairpin RNA of MDK, qRT-PCR: Quantitative real-time polymerase chain reaction.).
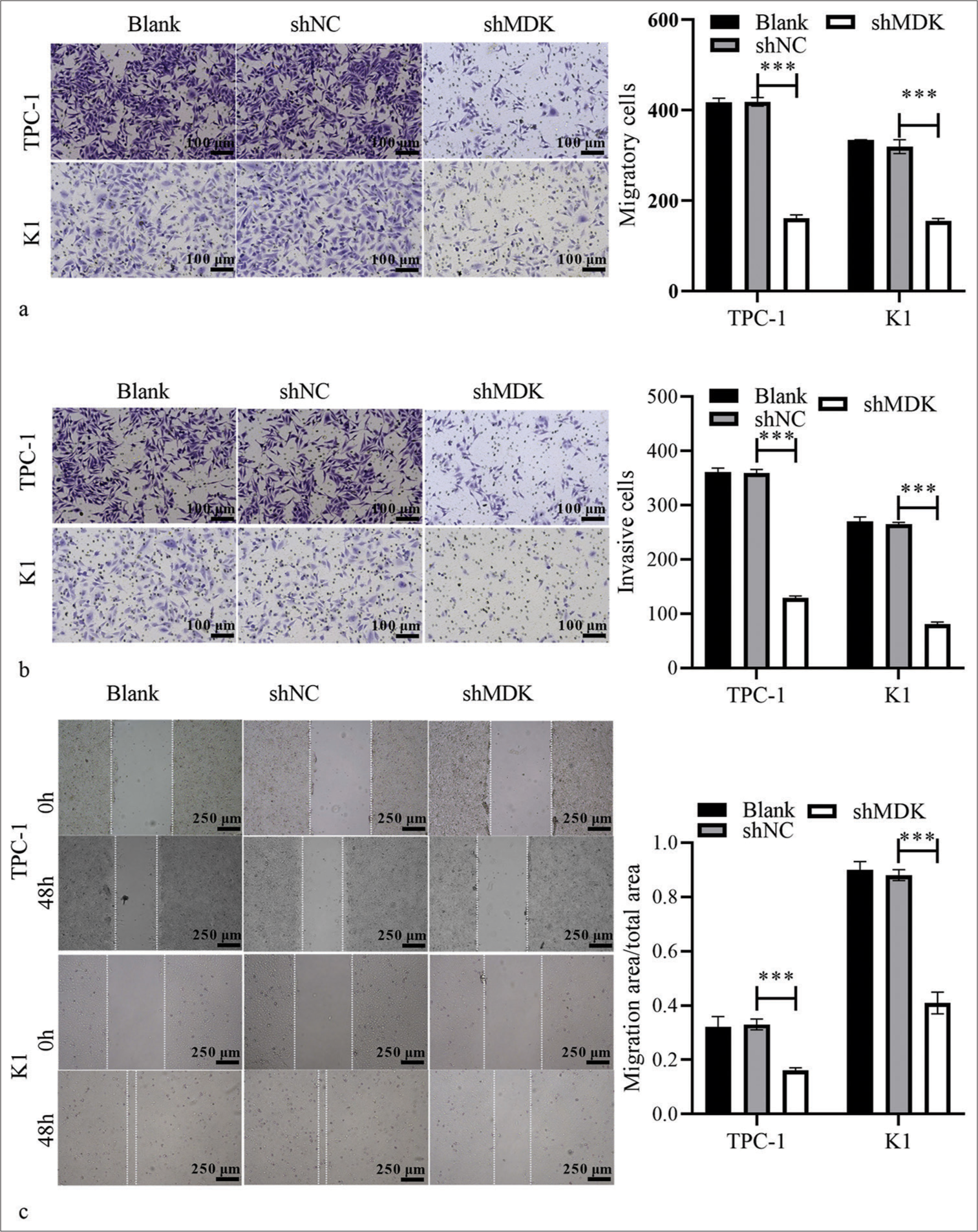
- MDK knockdown suppressed TC cell metastasis. (a and b) The migratory (a) and invasive (b) cells after transformation with shMDK or shNC were assessed using Transwell assays. (c) The migration of TPC-1 and K1 cells after transformation with shMDK or shNC was assessed using wound-healing assays (***P < 0.001). n = 3. (MDK: Midkine, shNC: Negative control of short hairpin RNA, shMDK: Short hairpin RNA of MDK, TC: Thyroid cancer.).
MDK knockdown suppressed PAmT-P in TC cells
We also explored whether PAmT-P was controlled by MDK. As expected, Western blotting indicated that MDK knockdown remarkably reduced the PI3K level, p-AKT/AKT, and p-mTOR/mTOR in TPC-1 and K1 cell compared with those in the shNC-transfected cells (All P < 0.001); [Figure 6].
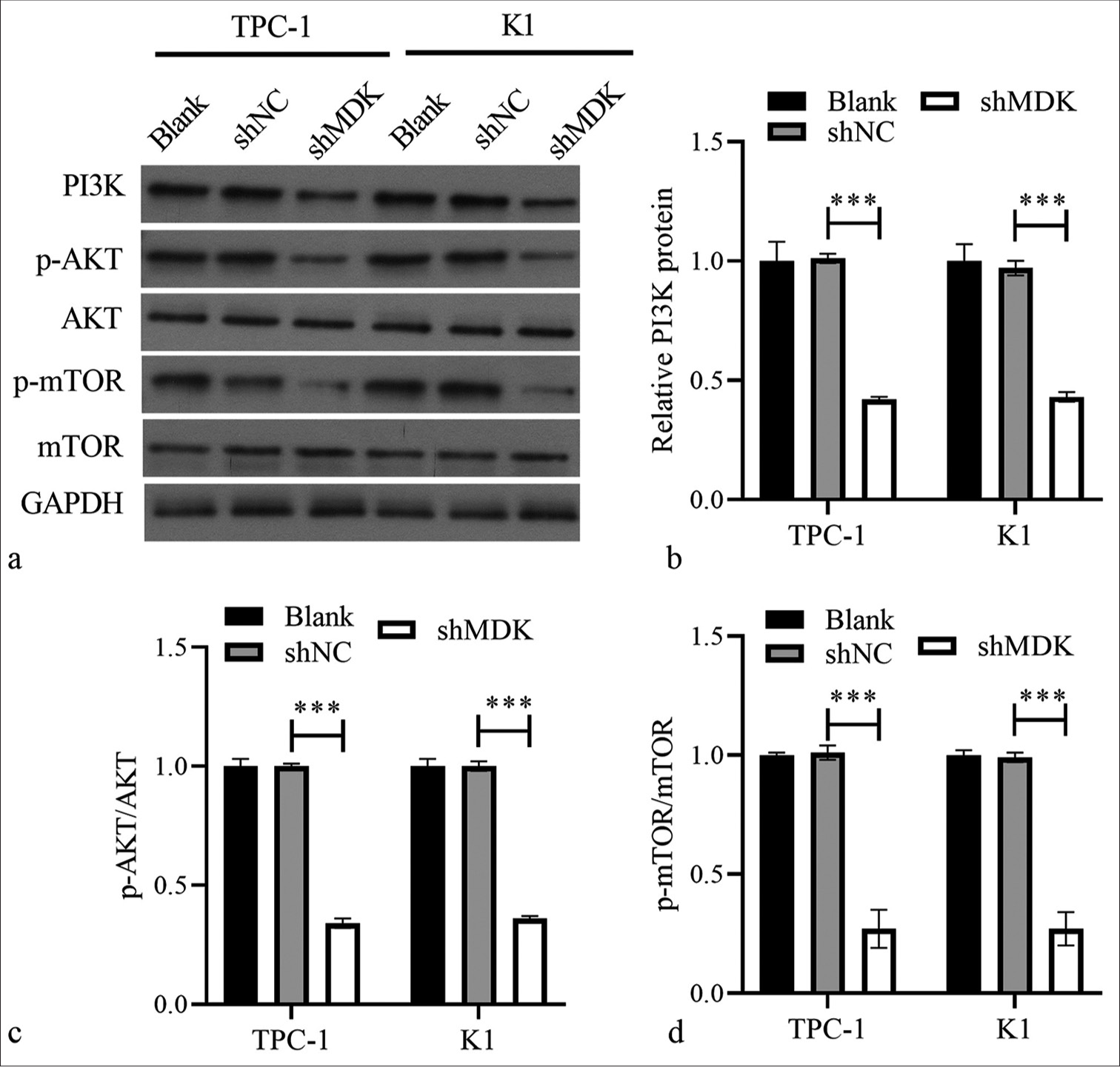
- MDK knockdown suppressed PAmT-P-related protein expression in TPC-1 and K1 cells. (a) The protein levels of PI3K, p-AKT/AKT, and p-mTOR/mTOR were measured using Western blotting. (b-d) Histogram represents the PI3K protein level, p-AKT/AKT, and p-mTOR/mTOR after MDK knockdown (*** P < 0.001). (MDK: Midkine, shNC: Negative control of short hairpin RNA, shMDK: Short hairpin RNA of MDK, PI3K: Phosphatidylinositol 3 kinase, AKT: Protein kinase B, mTOR: Mammalian target of rapamycin, p-AKT: Phosphorylated AKT, p-mTOR: Phosphorylated mTOR, PAmT-P: PI3K/Akt/mammalian target of rapamycin pathway.).
Activating PAmT-P reverted the inhibitory effect of MDK knockdown on TC cells
MDK-knockdown TC cells were treated with a PAmT-P activator (insulin-like growth factor-1 [IGF-1], 100 ng/mL; Sigma) to confirm that MDK knockdown suppressed TC through PAmT-P. PI3K, p-AKT, and p-mTOR protein levels were markedly elevated in TC cells following the MDK knockdown (all P < 0.001); [Figure 7]. We further investigated whether the activation of PAmT-P in TC cells with MDK knockdown could reverse the effect of MDK knockdown in TC cells. Transwell and wound healing assays revealed that activating PAmT-P sharply enhanced the metastatic ability of MDK-knockdown TC cells (all P < 0.001); [Figure 8]. In addition, the activation of PAmT-P sharply enhanced the proliferation of MDK-knockdown TC cells (all P < 0.001); [Supplementary Figure S1b].
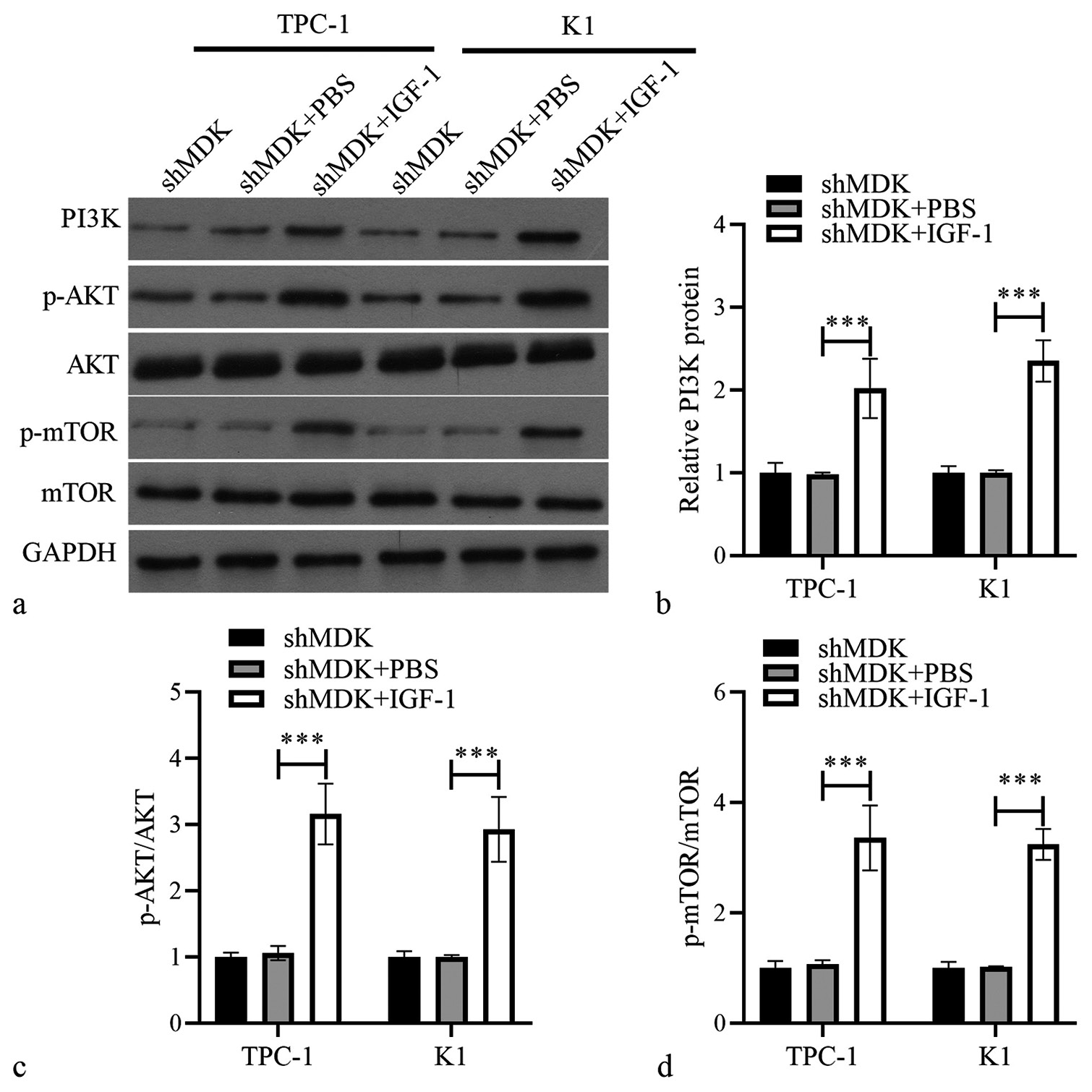
- Activating PAmT-P elevated PAmT-P-related protein in TPC-1 and K1 cells transformed with shMDK. (a) The protein levels of PI3K, PI3K, p-AKT/AKT, and p-mTOR/mTOR were measured using Western blotting. (b-d) Histogram represents the PI3K protein level, p-AKT/AKT, and p-mTOR/mTOR after IGF-1 treatment (***P < 0.001). n = 3. (MDK: Midkine, shMDK: Short hairpin RNA of MDK, PI3K: Phosphatidylinositol 3 kinase, AKT: Protein kinase B, mTOR: Mammalian target of rapamycin, p-AKT: Phosphorylated AKT, p-mTOR: Phosphorylated mTOR, PBS: Phosphate buffer saline, IGF-1: Insulin-like growth factor 1, PAmT-P: PI3K/Akt/mammalian target of rapamycin pathway.).
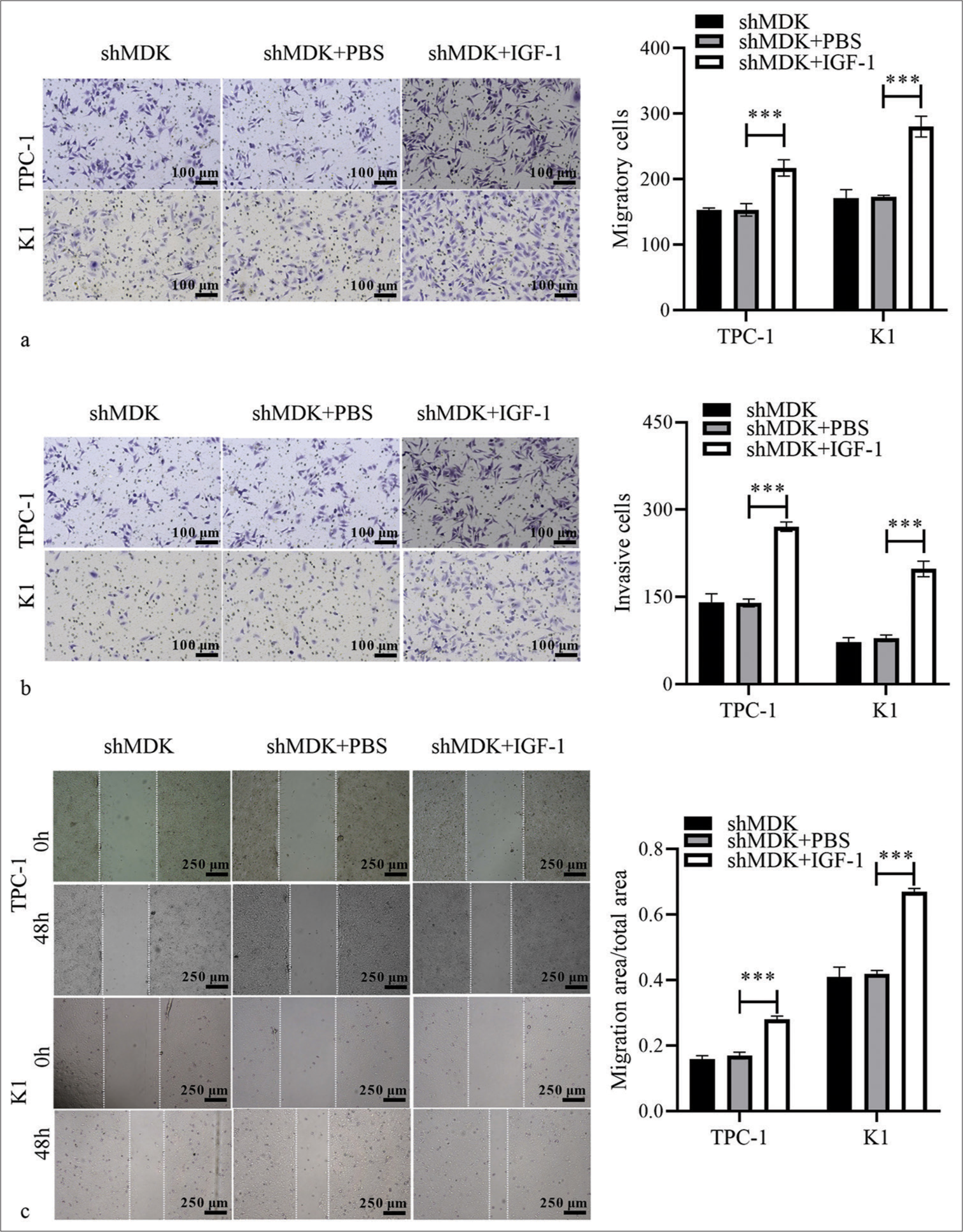
- Activation of PAmT-P can reverse the repressive effect of MDK knockdown on TC cells. (a and b) The migratory and invasive abilities of MDK-knockdown TPC-1 and K1 cells after IGF-1 treatment were evaluated using Transwell assays. (c) The migratory abilities of MDK knockdown TPC-1 or K1 cells after IGF-1 treatment were analyzed using wound-healing assays. (***P < 0.001). n = 3. (MDK: Midkine, shMDK: Short hairpin RNA of MDK, PBS: Phosphate buffer saline, IGF-1: Insulin-like growth factor 1, TC: Thyroid cancer, PAmT-P: PI3K/Akt/mammalian target of rapamycin pathway.)
DISCUSSION
The mortality rate of TC is lower than that of other malignancies, and most patients with TC have a good prognosis. However, metastatic TC remains a clinical challenge. Despite considerable advances in surgical resection, radiotherapy, and levothyroxine multimodal therapy, TC prognosis remains unsatisfactory.[23,24] Therefore, the identification of novel therapeutic targets for metastatic TC is imperative. Here, MDK was highly expressed in TC, and its expression correlated with different TC stages. MDK knockdown strongly suppressed metastasis and impeded PAmT-P has been defined in the Introduction section (PAmT-P) expression in TC. However, PAmT-P activation disrupted the inhibitory function of MDK knockdown on TC metastasis. Therefore, MDK exhibited enhanced properties in TC, indicating that it may be a promising biomarker for TC treatment.
MDK is aberrantly expressed in at least 20 cancers. It is a critical mediator of cancer development and progression of cancers.[25,26] For example, MDK facilitates the migration of breast cancer cells by increasing NR3C1 expression.[16] MDK knockdown therapy reduces endothelial cell angiogenesis and weakens non small-cell lung cancer metastasis.[27] circularMDK is the circularRNA (circRNA) of the MDK gene and is significantly upregulated in HCC, leading to the activation of the PAmT-P signaling pathway; it also acts as a novel oncogenic circRNA.[28] However, its role played by MDK in TC remains unclear. Here, MDK expression in TC cells was consistent with previous findings and was highly elevated. We also found that its expression was correlated with different TC stages, suggesting that this protein may play a different role at each TC stage. Meng et al. showed that serum MDK can serve as a biomarker for differentiated TC cells and predict the presence or absence of metastasis before the first ablative therapy; this finding indicates that MDK may be related to TC metastasis.[29] MDK knockdown strongly inhibited TC cell migration and invasion, suggesting that MDK plays a key role in TC metastasis.
The abnormal activation of PAmT-P is found in TC and contributes to TC cell proliferation and metastasis.[21,30] Chen et al. demonstrated that S100 protein superfamily A6 modulates TC cell proliferation and metastasis through PAmT-P.[22] Furthermore, PAmT-P activation partially eliminates the inhibitory effect of the TANK-binding kinase 1 knockdown on TC.[31] IGF-1 activates PAmT-P in TC and in other conditions.[32-34] Here, we showed that MDK knockdown blocked PAmT-P in TC and that activating PAmT-P by IGF-1 reversed the inhibitory effect of MDK knockdown on TC metastasis. Consistently, MDK knockdown reduced cancer stem cell migration by silencing PI3K/AKT in prostate cancer, HCC, and glioblastoma.[13-15] These data indicated that MDK knockdown restrained the metastatic behavior of TC by inhibiting PAmT-P. However, the molecular mechanisms underlying PAmT-P regulation by MDK in metastatic TC require further investigation. In addition, MDK may play a therapeutic role in other cancers with abnormal activation of other PI3K pathways.
However, this study has two limitations. First, whether MDK has an oncogene effect on TC in animals needs to be studied further. Second, the molecular mechanisms underlying MDK regulation in the upstream and downstream regions require further verification.
SUMMARY
MDK expression was highly increased in TC. It also promoted TC cell metastasis by activating PAmT-P. Therefore, MDK can be a useful molecular target to weaken metastatic TC.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated during and/or analyzed during the present study will be available from the corresponding author on reasonable request.
ABBREVIATIONS
mRNA: messenger RNA
TC: Thyroid cancer
MDK: Midkine
shNC: Negative control of short hairpin RNA
shMDK: Short hairpin RNA of MDK
PI3K: Phosphatidylinositol 3 kinase
AKT: Protein kinase B
mTOR: Mammalian target of rapamycin
p-AKT: Phosphorylated AKT
p-mTOR: Phosphorylated mTOR
PAmT-P: PI3K/Akt/mammalian target of rapamycin pathway
IGF-1: Insulin-like growth factor 1
AUTHOR CONTRIBUTIONS
LY: Drafted the study protocol, performed the experiments, and wrote the first draft of the manuscript; PZ: Conceptualized and designed the trial and supervised its implementation; WL, LJ, and MX: Performed the experiments and interpretation of results; YZ: Drafted the study protocol, supervised trial implementation, and has critically revised the manuscript. All authors have read and approved the final version of the study protocol and agreed with the order of authorship.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Because this article did not contain any human or animal experiments, ethics approval and informed patient consent was not required.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
Supplementary material associated with this article can be found:
References
- Notch signaling in thyroid cancer. Adv Exp Med Biol. 2021;1287:155-68.
- [CrossRef] [PubMed] [Google Scholar]
- Non-coding RNA in thyroid cancer-Functions and mechanisms. Cancer Lett. 2021;496:117-26.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of papillary thyroid carcinoma: An overview. Updates Surg. 2017;69:145-50.
- [CrossRef] [PubMed] [Google Scholar]
- Patient and tumor factors contributing to distant metastasis in well-differentiated thyroid cancer: A retrospective cohort study. J Otolaryngol Head Neck Surg. 2020;49:78.
- [CrossRef] [PubMed] [Google Scholar]
- Incidental positive lymph nodes in patients with papillary thyroid cancer is independently associated with recurrent disease. J Surg Oncol. 2017;116:275-80.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-organ distant metastases confer worse disease-specific survival in differentiated thyroid cancer. Thyroid. 2014;24:1594-9.
- [CrossRef] [PubMed] [Google Scholar]
- New therapies for advanced thyroid cancer. Front Endocrinol (Lausanne). 2020;11:82.
- [CrossRef] [PubMed] [Google Scholar]
- Shedding more light on the role of Midkine in hepatocellular carcinoma: New perspectives on diagnosis and therapy. IUBMB Life. 2021;73:659-69.
- [CrossRef] [PubMed] [Google Scholar]
- Midkine (MDK) growth factor: A key player in cancer progression and a promising therapeutic target. Oncogene. 2020;39:2040-54.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic performance of Midkine ratios in fine-needle aspirates for evaluation of Cytologically indeterminate thyroid nodules. Diagn Pathol. 2021;16:92.
- [CrossRef] [PubMed] [Google Scholar]
- Serum midkine as a surrogate biomarker for metastatic prediction in differentiated thyroid cancer patients with positive thyroglobulin antibody. Sci Rep. 2017;7:43516.
- [CrossRef] [PubMed] [Google Scholar]
- Strong immunoexpression of midkine is associated with multiple lymph node metastases in BRAFV600E papillary thyroid carcinoma. Hum Pathol. 2015;46:1557-65.
- [CrossRef] [PubMed] [Google Scholar]
- Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed Pharmacother. 2018;107:793-805.
- [CrossRef] [PubMed] [Google Scholar]
- Long non-coding RNA maternally expressed gene 3 affects cell proliferation, apoptosis and migration by targeting the microRNA-9-5p/midkine axis and activating the phosphoinositide-dependent kinase/AKT pathway in hepatocellular carcinoma. Oncol Lett. 2021;21:345.
- [CrossRef] [PubMed] [Google Scholar]
- Midkine promotes glioblastoma progression via PI3K-Akt signaling. Cancer Cell Int. 2021;21:509.
- [CrossRef] [PubMed] [Google Scholar]
- Midkine promotes breast cancer cell proliferation and migration by upregulating nr3c1 expression and activating the nf-kb pathway. Mol Biol Rep. 2022;49:2953-61.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of the growth factor MDK/midkine by a novel small molecule compound to treat non-small cell lung cancer. PLoS One. 2013;8:e71093.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of Midkine suppresses prostate cancer CD133(+) stem cell growth and migration. Am J Med Sci. 2017;354:299-309.
- [CrossRef] [PubMed] [Google Scholar]
- The possible interactions and therapeutic roles of lithium chloride and Midkine on cancer treatment. Crit Rev Oncog. 2019;24:35-45.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic alterations in MAPK and PI3K/Akt signaling pathways and the generation, progression, diagnosis and therapy of thyroid cancer. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012;29:1221-5.
- [Google Scholar]
- Circ_100395 impedes malignancy and glycolysis in papillary thyroid cancer: Involvement of PI3K/AKT/mTOR signaling pathway. Immunol Lett. 2022;246:10-7.
- [CrossRef] [PubMed] [Google Scholar]
- S100A6 promotes the development of thyroid cancer and inhibits apoptosis of thyroid cancer cells through the PI3K/AKT/mTOR pathway. Pathol Res Pract. 2023;242:154325.
- [CrossRef] [PubMed] [Google Scholar]
- PCNP is a novel regulator of proliferation, migration, and invasion in human thyroid cancer. Int J Biol Sci. 2022;18:3605-20.
- [CrossRef] [PubMed] [Google Scholar]
- Ccl18 knockdown suppresses cell growth and migration in thyroid cancer. J Healthc Eng. 2022;2022:1548155.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Midkine in cancer drug resistance: Regulators of its expression and its molecular targeting. Int J Mol Sci. 2023;24:8739.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring midkine: The utility of midkine as a biomarker in cancer and other diseases. Br J Pharmacol. 2014;171:2925-39.
- [CrossRef] [PubMed] [Google Scholar]
- Midkine is a potential therapeutic target of tumorigenesis, angiogenesis, and metastasis in non-small cell lung cancer. Cancers (Basel). 2020;12:2402.
- [CrossRef] [PubMed] [Google Scholar]
- M6A-mediated upregulation of circMDK promotes tumorigenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. Mol Cancer. 2022;21:109.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of serum midkine as a biomarker in differentiated thyroid cancer. Life Sci. 2015;130:18-24.
- [CrossRef] [PubMed] [Google Scholar]
- GOLM1 promotes prostate cancer progression through activating PI3K-AKT-mTOR signaling. Prostate. 2018;78:166-77.
- [CrossRef] [PubMed] [Google Scholar]
- TBK1 promotes thyroid cancer progress by activating the PI3K/Akt/mTOR signaling pathway. Immun Inflamm Dis. 2023;11:e796.
- [CrossRef] [PubMed] [Google Scholar]
- M2like tumourassociated macrophagesecreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol Med Rep. 2021;24:604.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of lidocaine on polycystic ovary syndrome through modulating ovarian granulosa cell physiology via PI3K/AKT/mTOR pathway. Cytotechnology. 2022;74:283-92.
- [CrossRef] [PubMed] [Google Scholar]
- Death receptor 3 is involved in preeclampsia through regulating placental trophoblast cell physiology by inactivating the PI3K/AKT pathway. Immun Inflamm Dis. 2023;11:e995.
- [CrossRef] [PubMed] [Google Scholar]








