Translate this page into:
Receptor interacting serine/threonine kinase 2 promotes rheumatoid arthritis progression and partially regulates nuclear factor kappa B pathway

*Corresponding author: Deheng Liu, Department of Hand and Foot Surgery, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Qingdao, China. liudeheng_ql@163.com
-
Received: ,
Accepted: ,
How to cite this article: Wang Y, Xu M, Liu X, Liu D. Receptor interacting serine/threonine kinase 2 promotes rheumatoid arthritis progression and partially regulates nuclear factor kappa B pathway. CytoJournal. 2024;21:56. doi: 10.25259/Cytojournal_63_2024
Abstract
Objective:
Rheumatoid arthritis (RA) is a disabling systemic autoimmune disease worldwide; however, its molecular pathway remains largely unknown. Thus, this study aimed to explore the effects of receptor-interacting serine/threonine kinase 2 (RIPK2) on RA progression and its underlying mechanism.
Material and Methods:
RIPK2 expression was analyzed using real-time quantitative polymerase chain reaction, immunohistochemical staining, and Western blot (WB) analysis in RA synovial tissues or cells. Cell viability or proliferation was determined using the cell counting kit-8 and 5-ethynyl-2'-deoxyuridine. Cell metastasis was analyzed using the transwell assay and wound healing assay. Flow cytometry was adopted to measure cell apoptosis. The level of inflammation-related factors was measured using the enzyme-linked immunosorbent assay. WB analysis was used to determine the expression level of nuclear factor kappa B (NF-κB) pathway-related genes.
Results:
RIPK2 was highly expressed in RA synovial tissues and cells. Transfection with RIPK2 short hairpin RNA plasmids reduced the gene expression level of RIPK2 in RA fibroblast-like synoviocytes (FLS) cells. Notably, RIPK2 silencing hindered the proliferation, invasion, and migration of tumor cells as well as accelerated the apoptosis of RA-FLS cells. Furthermore, RIPK2 silencing suppressed the RA-FLS cell inflammatory response and NF-κB pathway.
Conclusion:
RIPK2 silencing could retrain the malignant behavior and inflammatory response of RA-FLSs and partially modulate the NF-κB pathway.
Keywords
Rheumatoid arthritis
Fibroblast-like synoviocytes
Receptor interacting serine/threonine kinase 2
Nuclear factor kappa B pathway
INTRODUCTION
Rheumatoid arthritis (RA) is a common disabling systemic inflammatory autoimmune disease, and it primarily causes infection, respiratory diseases, osteoporosis, cardiovascular diseases, and cancer.[1] With poor prognosis and high risk of death, RA affects 0.5–1% of the global population and seriously impairs the quality of life of patients.[2] RA fibroblast-like synoviocytes (FLS) participate in the chronic inflammation of synovial joints, and FLS can induce cartilage and bone damage, thereby playing a crucial role in RA.[3,4] Through the pathogenesis of RA, activated FLS exhibits similar proliferation and metastatic characteristics to tumor cells, which are the main triggering factors for abnormal proliferation and joint destruction.[4] The current treatment options for RA include surgery, disease-modifying anti-rheumatic drugs, corticosteroids, and non-steroidal anti-inflammatory drugs, which may be limited because of their side effects and contraindications.[5] Therefore, understanding the pathogenesis of RA is the key to finding specific treatment methods.
Receptor interacting serine/threonine kinase 2 (RIPK2), also known as nuclear factor kappa B (NF-κB) activator, has a carboxy-terminal caspase activation and recruitment domain.[6] The activation of RIPK2 may activate IκB kinases (IKKs), causing NF-κB dimers to translocate into the nucleus and transcribe target genes.[7] RIPK2 plays a vital role in the occurrence, development, and prognosis of tumors.[8,9] Knocking down RIPK2 could inhibit subcutaneous tumor growth, autophagy, and liver metastasis of pancreatic cancer (PC), and RIPK2 may be an immunotherapy target in PC.[10] RIPK2 could promote the development of colon cancer by regulating the NF-κB pathway.[11] In addition, RIPK2 functions as an oncogene in the regulation of gastric cancer progression.[12] RIPK2 encodes a downstream adaptor protein of the nucleotide oligomerization domain 1/2 (NOD1/2), which plays an essential role in inflammation and innate immunity.[13,14] Jurynec et al. found a link between RIPK2 and osteoarthritis (OA) while analyzing a family with dominant inheritance of early-onset OA.[15] However, few studies were performed on the correlation between RIPK2 and RA pathogenesis. Moreover, other mechanisms by which RIPK2 short hairpin RNA (shRNA) inhibits tumor growth have not been elucidated, and its role in cell apoptosis remains unknown.
In this study, the effects of RIPK2 silencing on the malignant behavior and inflammatory response to RA were explored by transfecting RIPK2 shRNA into RA-FLS cells to provide insights into RIPK2 as a potential target for RA therapy.
MATERIAL AND METHODS
Tissue samples
Synovial tissues were obtained from 30 patients with RA. In addition, RA was diagnosed in accordance with the 2010 American College of Rheumatology/European League Against Rheumatism criteria for classification.[16] Healthy synovial tissues from 30 patients receiving emergent traumatic amputation were collected. This research was approved by the Medical Ethics Committee of Yantaishan Hospital (No.: 2024064), and patients signed an informed consent for therapies. All experiments were performed in accordance with the Declaration of Helsinki.
Cell lines and plasmid transfection
Human normal FLS (#9315) and RA-FLS (#8889) were provided by Jennio Biotech Co., Ltd. (Guangzhou, China). The cells were identified using short tandem repeat and tested for mycoplasma. All cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, LM87076C, LMAI Bio, Shanghai, China) with 10% fetal bovine serum (CG1110A, CELLiGENT, Beijing, China) at 37°C with 5% CO2. Timely cell exchange and passage were performed to ensure a good growth state of cells. Negative control plasmid (shNC) and RIPK2 shRNA (sh-RIPK2) were purchased from Genepharma (Shanghai, China). The sh-NC and shRIPK2 were transfected into RA-FLS using Lipofectamine 2000 (11668019, Thermo, Waltham, MA, USA). The aforementioned sequences were as follows: sh-RIPK2-1:5'-GGACATCGACCTGTTATTAAT-3' and sh-RIPK2-2:5'-CACCAATCCTTTGCAGATAAT-3'. A scramble sequence was used as control.
Cell counting kit-8 (CCK-8)
RA-FLS were seeded in 96-well plates containing 200 μL of DMEM for 24 h, 48 h, and 72 h. The DMEM supplemented with 10 μL of CCK-8 reagent (20 μL/well, BB-4202-250T, BestBio, Shanghai, China) was added to each experimental well. The optical density was measured at 450 nm on a microplate reader (iMARK, Bio-Rad, California, USA).
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (B610409, Sangon Biotech, Shanghai, China) and then reverse transcribed into complementary DNA under the following conditions: 37°C for 15 min and 98°C for 5 min. RT-qPCR was performed using the miScript Synergetic Binding Reagent ( SYBR) Green polymerase chain reaction (PCR) kit (18064-014, Invitrogen, Carlsbad, CA, USA) on the ABI 7500 Fast real-time PCR system (Life Technologies, Carlsbad, CA, USA). The RIPK2 mRNA expression was calculated using the 2−∆∆Ct method by normalizing to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer sequences for RT-qPCR were as follows: GAPDH forward, 5'-GAATCATGTGGATCCTCTCAGC-3'; reverse, 5'-GTGGGTGTCGCTGTTGAAGTC-3' and RIPK2 forward, 5'-GAATCATGTGGATCCTCTCAGC-3', reverse, 5'-TGATTTCCAGGACAGTGATGC-3'.
5-Ethynyl-2'-deoxyuridine (EdU) assay
A total of 200 μL (50 μmol/L) of the EdU medium (61135-33-9, Absin, Shanghai, China) were added after 48 h transfection. Then, the cells were fixed in 4% paraformaldehyde (691, Fuchen, Tianjin, China), treated with 4',6-diamidino-2-phenylindole (DAPI) solution (C0065, Solarbio, Beijing, China) for 5 min, and observed under a fluorescence microscope (FSX100, Olympus, Tokyo, Japan). Positive rate = EdU-labeled cell number/DAPI-labeled cell number × 100%.
Transwell assay
The invasion ability was determined using transwell chambers (3413, Corning, Corning, NY, USA) coated with Matrigel (119876-18-6, Yeasen, Shanghai, China). Then, RA-FLS (1 × 105 cells) transfected with sh-RIPK2 were added to the apical chamber, and a DMEM medium was added into the lower chamber. Then, the cells on the apical surface of the chambers were removed, and those in the lower chamber were stained by crystal violet (C0121, Beyotime, Shanghai, China) for 15 min. Finally, the cells were observed and recorded.
Wound healing assay
When cell confluence reached 80%, the transfected cells were replaced with a fresh serum-free medium and cultured for 12 h. A 200 μL spearhead was used to line perpendicular to the culture plate, and cell debris was washed off with phosphate-buffered saline (PBS). A DMEM medium was added to each well and then incubated in 37°C and 5% CO2 for 24 h. The scratch width was photographed at 0 and 24 h on a microplate reader (iMARK, Bio-Rad, California, USA).
Flow cytometry
RA-FLS (2 × 105 cells/well) with sh-RIPK2 transfection was inoculated into 6-well plates. The cells were washed with precooled PBS and resuspended in 500 μL of the Annexin V-FITC/PI (#45115, CST, Massachusetts, USA) mixture buffer to avoid the light. Then, apoptosis was analyzed using flow cytometry.
Western blot (WB)
Total proteins were extracted using a radio-immunoprecipitation assay lysate (P0013B, Beyotime, Shanghai, China). Protein concentration was analyzed using a Bicinchoninic acid (BCA) kit (ST2222-1g, Beyotime, Shanghai, China). Then, the proteins were transferred to Polyvinylidene fluoride (PVDF) membranes (Millipore) after being separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After sealing at room temperature for 2 h, RIPK2, IKKβ, p-IKKβ, IκBα, p-IκBα, and GAPDH (Abcam, UK) antibodies were added at 4°C overnight. Next, the membrane was incubated with a secondary antibody (1:2000, 7074, CST, Danvers, MA, USA) for 1 h. Then, the proteins were developed using an electrochemiluminescence reagent (3622ES76, Yeasen, Shanghai, China), and the gray value of the strip was measured by Image J (1.8, NIH, Rockville, MD, USA).
The primary antibodies used are as follows: anti-RIPK2 (1:1000, DF6967, Affinity, Beijing, China), anti-p-IkBα (phosphorylated inhibitor of kappa B alpha, 1:1000, bs-5515R, Bioss, Shanghai, China), anti-IkBα (1:1000, HZ-R1287, Shanghai Huzhen, Shanghai, China), anti-p-IKKβ (phosphorylation of IkappaB kinase beta, 1:1000, BS4236, Bioworld, Shanghai, China), anti-IKKβ (1:1000, 7557R, YaJi, Shanghai, China), and anti-GAPDH (1:10000, AF0006, Beyotime, Beijing, China).
Immunohistochemical (IHC) staining
RA tissues or healthy synovial tissues were fixed in 10% neutral formalin and embedded in paraffin by conventional dehydration. Then, the dehydrated tissues were continuously sectioned with a thickness of 3–4 μm. IHC experiments were analyzed by RIPK2 polyclonal antibody (1:1000, 4142, CST, Boston, USA) at 4°C overnight. Subsequently, secondary antibodies (A-11011, 1:1000, Invitrogen, Los Angeles, CA, USA) were added. Finally, 3,3'-diaminobenzidine (D8001, Sigma, Saint Louis, MO, USA) color rendering, transparency, and sealing were performed. The IHC results were observed under a microscope (CCD TP510, OPTEC, Chongqing, China). The positive reaction was yellowish brown in the tumor cells.
Enzyme-linked immunosorbent assay (ELISA)
Cell supernatants were harvested at 48 h post-transfection. The contents of interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, and IL-1β were determined by ELISA kit. The ELISA kits for IL-1β (70-EK201), IL-8 (70-EK108), TNF-α (WM-YX10110), and IL-6 (CSB-E04638h) were obtained from Wuhan Huamei Biotech Co. (Wuhan, China). The detection reagent was added to the samples. The absorbance value was detected at 450 nm.
Statistical analysis
All experiments were performed in triplicate. GraphPad Prism 8.0.2 was used to analyze the statistical data. All data were represented as mean ± standard deviation. The differences between two groups were assessed using Student’s t-tests and the Chi-squared test. For multiple groups, data were analyzed using one-way analysis of variance. P < 0.05 indicates significant difference.
RESULTS
RIPK2 is highly expressed in RA synovial tissues and RA-FLS cells
To demonstrate whether RIPK2 was expressed in RA, the expression level of RIPK2 in RA tissues and healthy synovial tissues was determined. RIPK2 has a higher expression in RA tissues than in normal synovial tissues [Figure 1a], (P < 0.001). Consistent with patient samples, the expression of RIPK2 in RA-FLS cells is higher than that in FLS cells [Figure 1b], (P < 0.001). To further visualize RIPK2 expression in RA, IHC and WB were performed in 30 RA tissues or 30 healthy synovial tissues. The data displayed that RIPK2 was highly expressed in RA tissues ([Figure 1c-f], (P < 0.0001, P < 0.001).
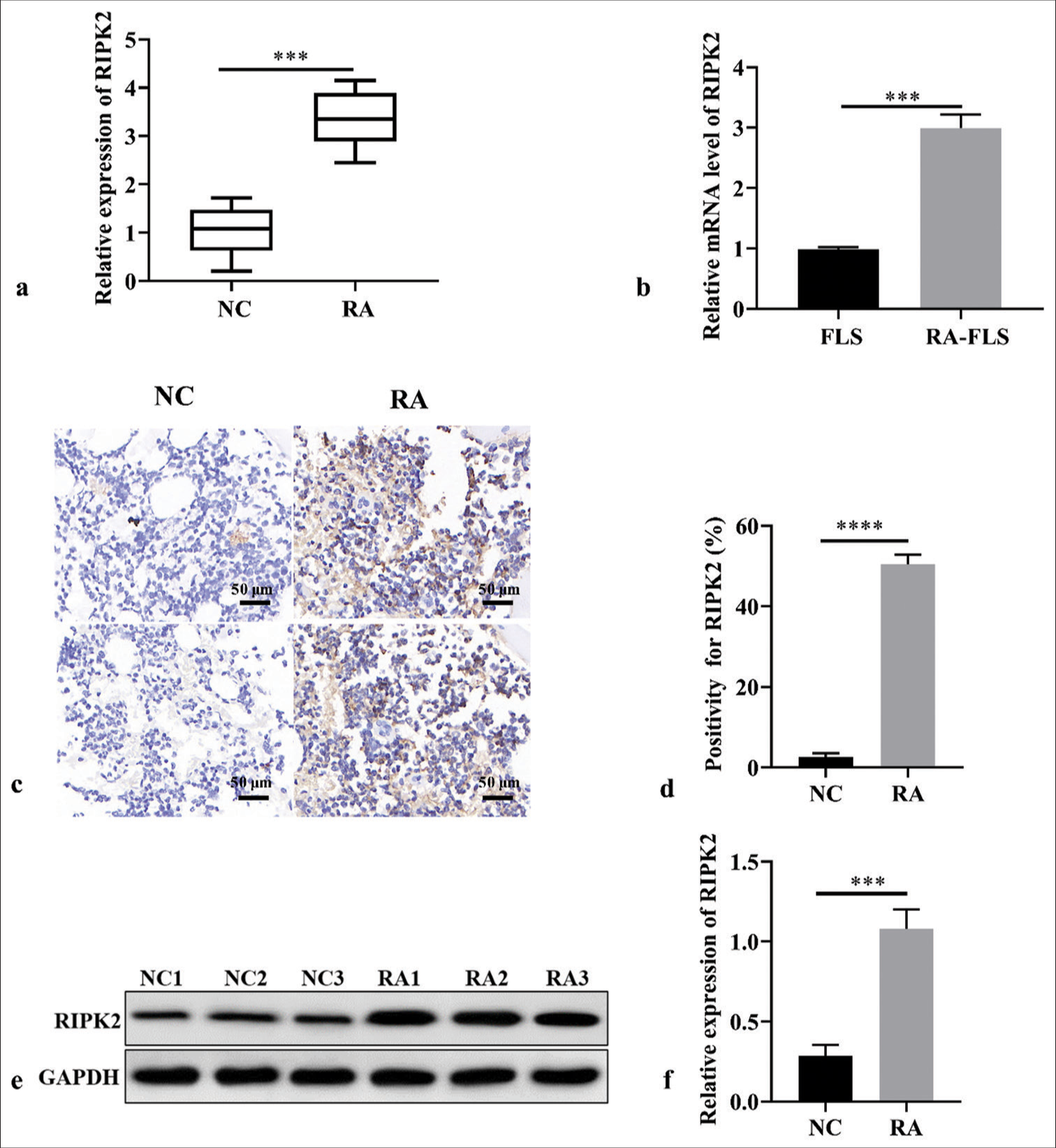
- RIPK2 expression in RA tissues and RA-FLS cells. (a) RIPK2 expression in RA and healthy synovial tissues (n = 3). (b) RIPK2 expression in RA-FLS cells using RT-qPCR (n = 3). (c and d) The ICH results indicated that RIPK2 was highly expressed in RA (n = 3). (e and f) The WB results indicated that RIPK2 was highly expressed in RA (n = 3). (***P < 0.001, ****P < 0.0001. RIPK2: Receptor interacting serine/threonine kinase 2, NC: Negative control, RA: Rheumatoid arthritis, FLS: Fibroblast-like synoviocytes, GADPH: Glyceraldehyde-3-phosphate dehydrogenase, RT-qPCR: Real-time quantitative polymerase chain reaction, IHC: Immunohistochemical, WB: Western blot).
RIPK2 silencing suppresses the viability and proliferation of RA-FLS
To determine the mechanism by which RIPK2 affects RA cells, related biological experiments were performed in vitro. First, the transfected efficiency of sh-RIPK2 was evaluated using RT-qPCR and WB in RA-FLS, and the result indicated that RIPK2 expression was significantly decreased under shRIPK2 treatment [Figure 2a-c], (P < 0.01). RIPK2 shRNAs have similar transfected efficiency in RA-FLS, so RIPK2 shRNA1 was utilized for the follow-up experiments. The CCK-8 and EdU assay data shown in Figure 2d-f demonstrate that the cell viability and proliferation of RA-FLS were reduced upon RIPK2 silencing (P < 0.01). Collectively, RIPK2 silencing can suppress the proliferation and viability of RA-FLS.
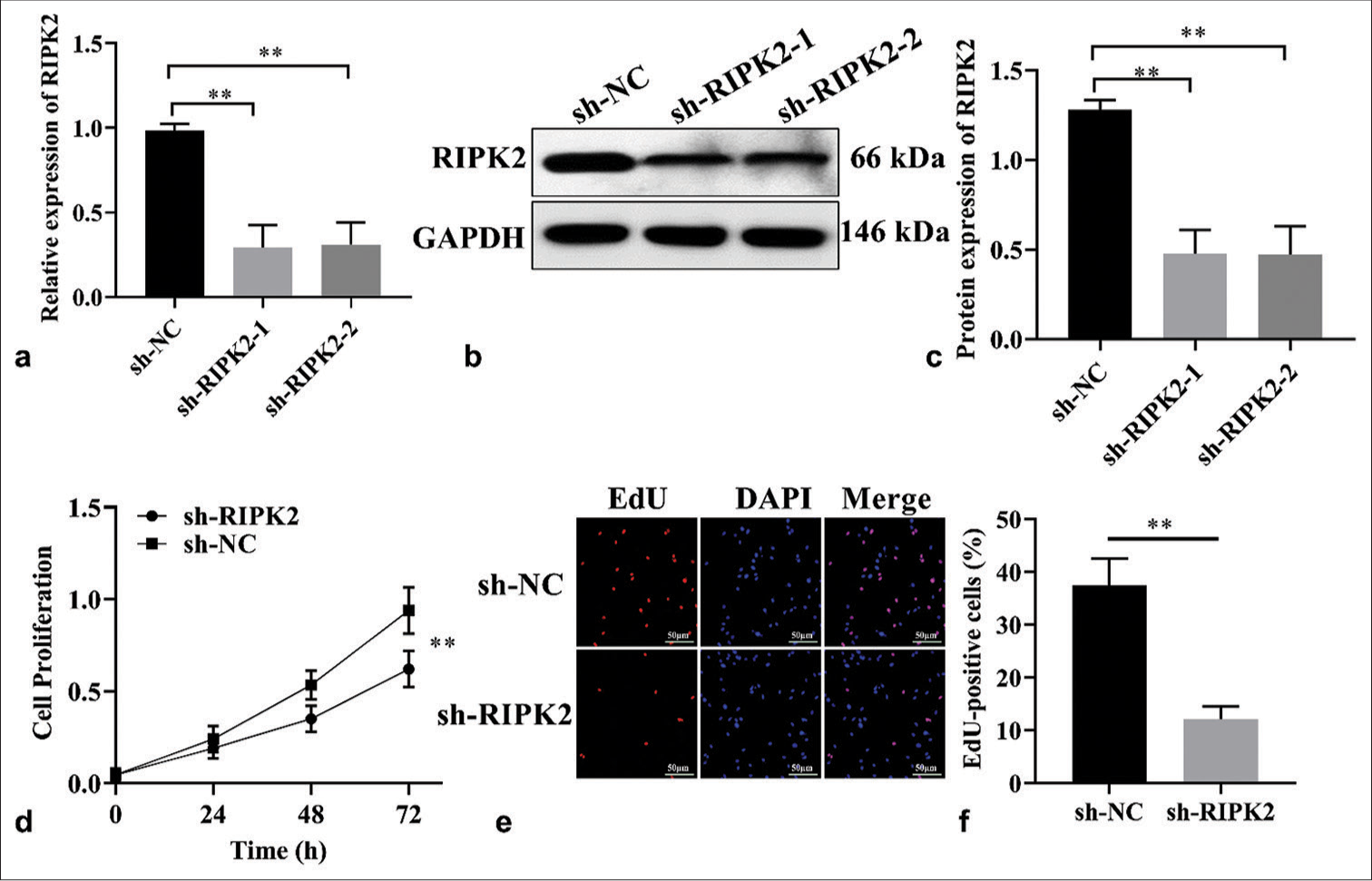
- RIPK2 knockdown suppresses cell proliferation. (a-c) Analysis of RIPK2 expression in shRIPK2 RA-FLS by RT-qPCR and WB (n = 3). (d) The growth of sh-RIPK2 RA-FLS was suppressed (n = 3). (e and f) EdU assay showed that cell proliferation was reduced upon RIPK2 silencing in RAFLS cells (n = 3). (**P < 0.01. EdU: 5-ethynyl-2'-deoxyuridine, GAPDH: Glyceraldehyde 3-phosphate dehydrogenase, DAPI: 4', 6-diamidino-2-phenylindole, RIPK2: Receptor interacting serine/threonine kinase 2, NC: Negative control, RA-FLS: Rheumatoid arthritis-fibroblast-like synoviocytes, RT-qPCR: Real-time quantitative polymerase chain reaction, WB: Western blot.).
RIPK2 silencing suppresses the migration and invasion of RA-FLS cells
The migration activity of RA-FLS in the sh-RIPK2 group was decreased compared with the sh-NC group [Figure 3a and b], (P < 0.001). Considering that invasion can lead to bone and cartilage destruction in RA, the Matrigel-coated transwell membrane was used to further visualize the regulatory effect of RIPK2 knockdown on cell invasion. The cell invasion ability of RA-FLS was reduced upon RIPK2 silencing [Figure 3c and d], (P < 0.05). Collectively, RIPK2 silencing can suppress the migration and invasion of RA-FLS cells.
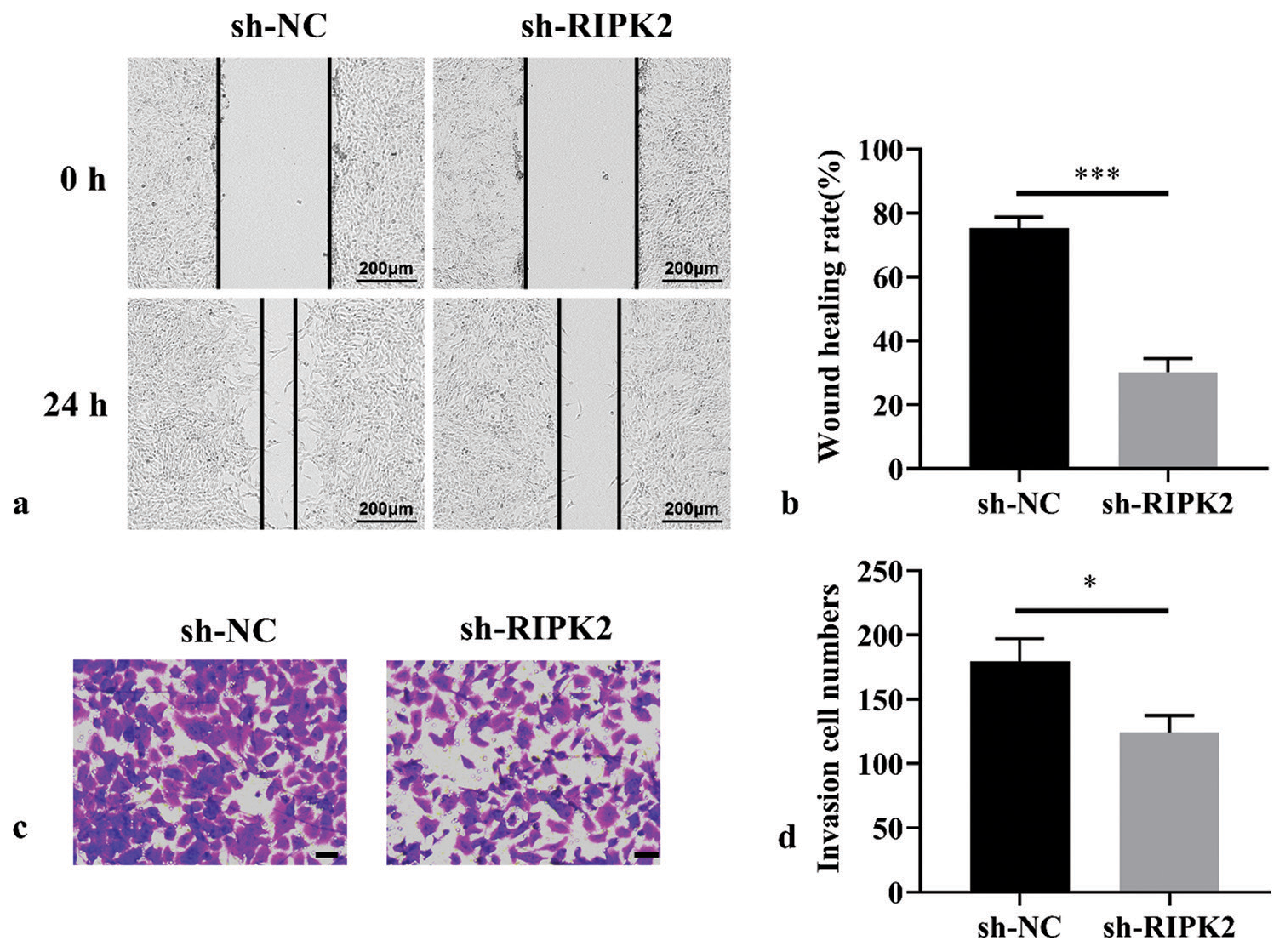
- RIPK2 knockdown suppresses the migration and invasion of RA-FLS. (a and b) Cell migration ability of RA-FLS suppressed by RIPK2 knockdown as analyzed by wound healing assay (n = 3). (c and d) Cell invasion ability of RA-FLS suppressed by RIPK2 as analyzed by transwell assay (n = 3, scale bar: 50 μm). (*P < 0.05, ***P < 0.001. RIPK2: Receptor interacting serine/threonine kinase 2, NC: Negative control, RA-FLS: Rheumatoid arthritis-fibroblast-like synoviocytes.).
RIPK2 silencing promotes the apoptosis and suppresses the inflammatory response
The inhibition of RIPK2 silencing on RA-FLS proliferation prompted us to explore its impact on apoptosis. RIPK2 silencing promotes apoptosis in RA-FLS cells compared with the sh-NC group [Figure 4a and b], (P < 0.001). Moreover, we analyzed the effect of RIPK2 on inflammatory response. Thus, we used ELISA to estimate the level of inflammatory cytokines. The results indicated that RIPK2 silencing in RAFLS cells reduced the levels of IL-1β, IL-8, TNF-α, and IL-6 [Figure 4c-f], (P < 0.05, P < 0.01, P < 0.001).
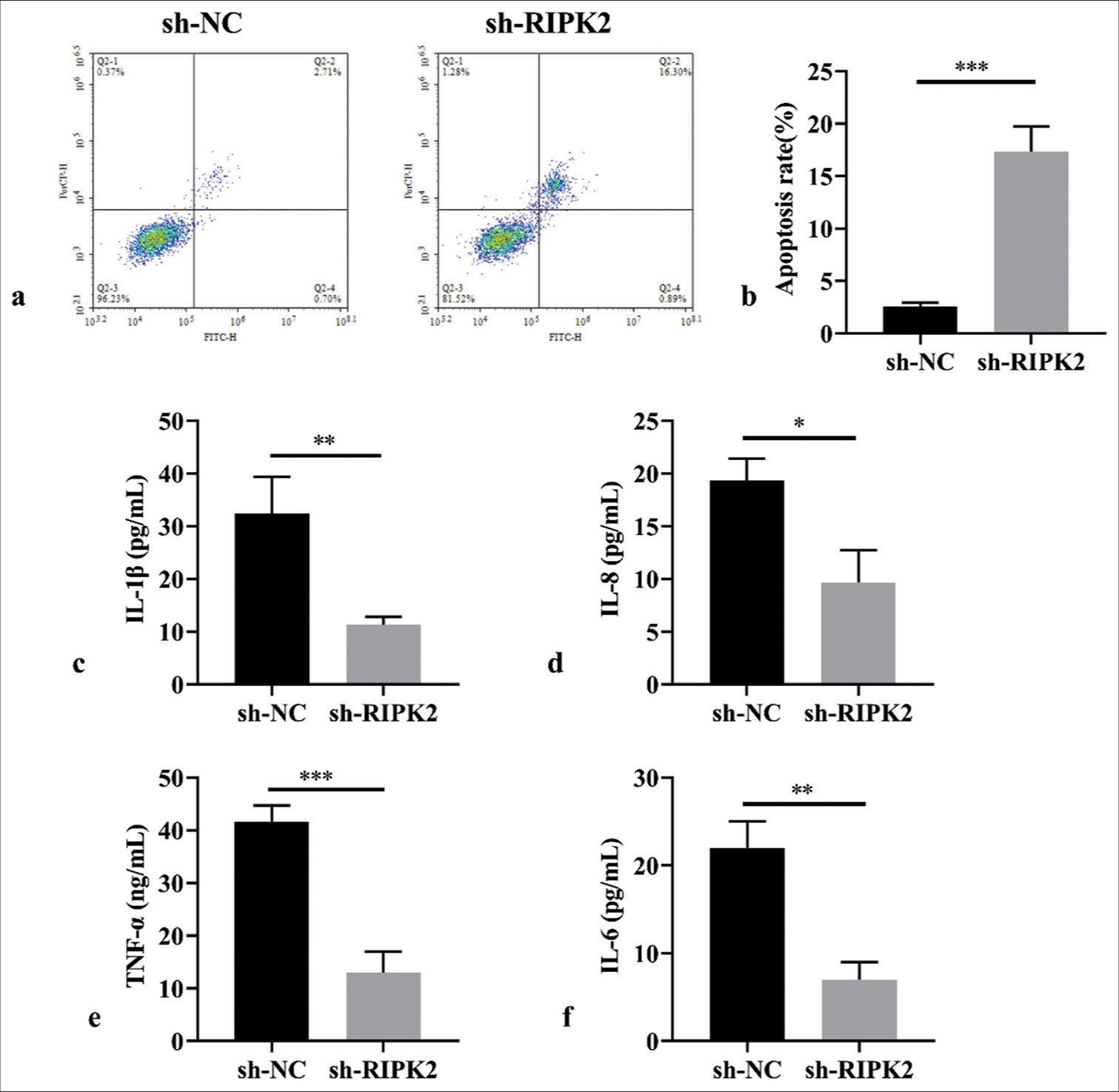
- RIPK2 silencing promotes apoptosis and suppresses the inflammatory response of RA-FLS. (a and b) Effects of RIPK2 on apoptosis analyzed by flow cytometry (n = 3). (c-f) ELISA analysis of inflammatory factors following RIPK2 suppression (n = 3). (*P < 0.05, **P < 0.01, ***P < 0.001. IL-6: Interleukin-6, TNF-α: Tumor necrosis factor-α, RIPK2: Receptor interacting serine/threonine kinase 2, NC: Negative control, RA-FLS: Rheumatoid arthritis-fibroblast-like synoviocytes, ELISA: Enzyme-linked immunosorbent assay.).
RIPK2 silencing partially regulates the NF-κB pathway of RA-FLS cells
WB assay showed a marked decrease in NF-κB phosphorylation in the presence of sh-RIPK2 compared with sh-NC treated RA-FLS [Figure 5], (P < 0.01, P < 0.001). Collectively, RIPK2 affects the progression of RA-FLS cells by activating the NF-κB pathway.
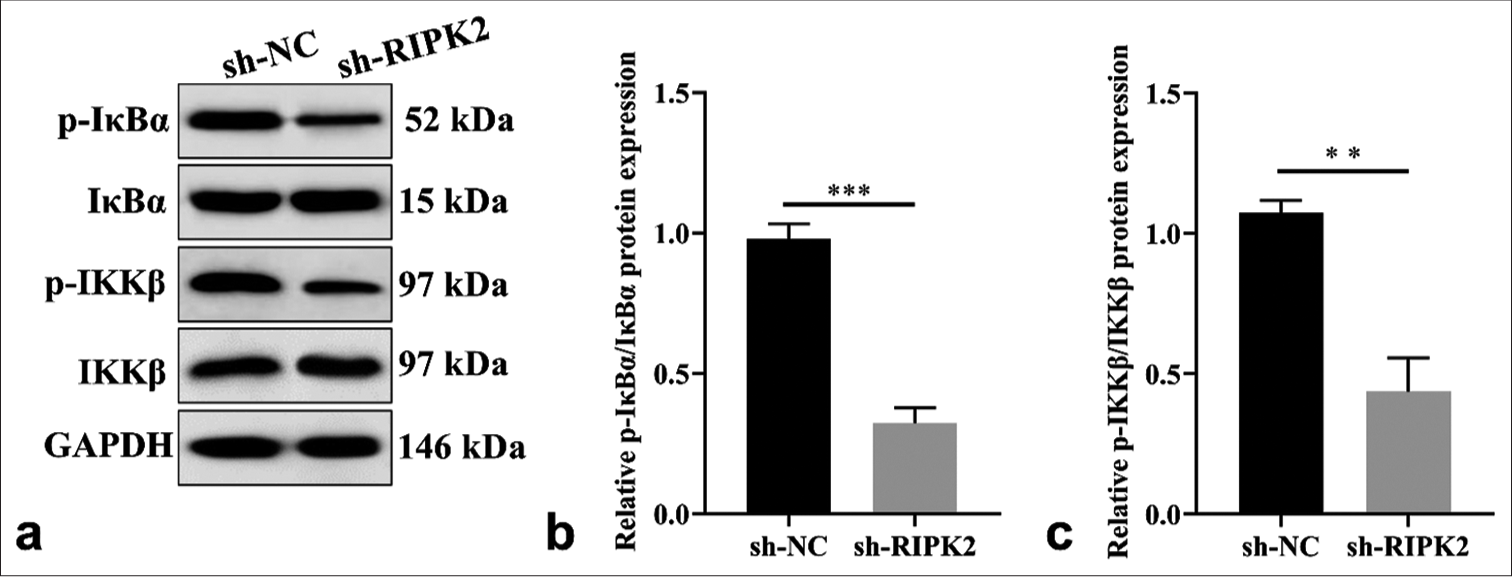
- RIPK2 silencing regulates the NF-κB pathway in RA-FLS cells. (a-c) The level of p-IκBα/IκBα and p-IKKβ/IKKβ was detected by WB (n = 3). (**P < 0.01; ***P < 0.001. p-IκBα: Phosphorylated inhibitor of kappa B alpha, p-IKKβ: Phosphorylation of IkappaB kinase beta, NF-κB: Nuclear factor kappa B, RIPK2: Receptor interacting serine/threonine kinase 2, NC: Negative control, RA-FLS: Rheumatoid arthritis-fibroblast-like synoviocytes, WB: Western blot).
DISCUSSION
RA is characterized by synovial tissue proliferation and unrestricted chronic inflammation, ultimately resulting in cartilage and bone destruction, joint swelling, or joint function loss.[17] The unclear pathogenesis of RA poses a challenge for its treatment. In general, individual immune imbalance, genetic susceptibility, and environmental factors are implicated in the initiation of RA.[18] In this study, RIPK2 was highly expressed, and RIPK2 silencing remarkably hindered the proliferation and migration of RA in vitro. Mechanistically, RIPK2 silencing restrained the invasion of RA in vitro. RIPK2 silencing promotes apoptosis, suppresses inflammatory response, and inhibits RA development. Mechanistic studies have shown that RIPK2 knockdown repressed the NF-κB pathway. The abovementioned findings indicate that RIPK2 might serve as a novel diagnostic biomarker in RA.
RIPK2 has been proven as a key molecule that regulates cell death pathways and inflammatory signaling.[19] Evidence shows that RIPK2 may be associated with chronic inflammation of inflammatory bowel disease,[20,21] and RIPK2 expression was positively associated with the malignant progression of colorectal cancer.[22] According to Yan et al., RIPK2 can regulate the disease progression and poor prognosis of PC, and RIPK2 inhibition can effectively impair PC metastatic outgrowth.[23] The effect of the NOD2/RIPK2 pathway on RA has been investigated, and high levels of NOD2/RIPK2 were found in synovial fluid T cells, FLS, or peripheral blood mononuclear cells.[24,25] NOD2/RIPK2 can disturb inflammatory responses by influencing TNF-α and IL-1β levels. The direct regulatory effect of RIPK2 on immune cell function in RA will be the focus of our further research. However, little research has reported on the direct role of RIPK2 in RA cell proliferation and metastasis. This study was the first to show that RIPK2 knockdown suppressed the proliferation, invasion, and migration of RA-FLS. Moreover, RIPK2 silencing promotes apoptosis, suppresses inflammatory response, and inhibits RA development.
During the progression of chronic inflammation, the NF-κB pathway has been considered an atypical pro-inflammatory signaling pathway for a long time, which is hyperactivated at high frequencies in tumors and chronic inflammatory diseases.[26] RIPK2 is a core molecule involved in the NF-kB activation and cytokine production mediated by NOD activation.[27] Existing evidence shows that the activation of RIPK2 may activate IKKs, causing NF-κB dimers to translocate into the nucleus and transcribe target genes.[7] The effect of RIPK2 on NF-κB signaling is evident.[28,29] Therefore, it is not difficult to infer that one of the main regulatory factors of NF-κB pathway, RIPK2, is also highly expressed and unfriendly to anti-tumor therapy.[30] Yang et al. revealed that RIPK2 plays an important role in modulating gastric cancer malignant behavior through the NF-kB pathway.[12] In human astrocytomas, RIPK2 promotes glioma cell growth by inhibiting tumor necrosis factor receptor-associated factor protein 3 (TRAF3) and activating p38 and NF-κB signaling.[31] Here, the function of RIPK2 on the NF-kB pathway was explored. WB analysis displayed a marked decrease in NF-κB phosphorylation in the presence of shRIPK2 compared with sh-NC-treated RA-FLS, which is consistent with previous literature reports.
Given the certain limitations of our study, the relationship between RIPK2 and the NF-κB pathway was not further explored. We only observed the expression of RIPK2 in synovial tissue in little RA patients. In addition, this study must conduct experiments to verify the mechanism of RIPK2 in vivo, which will be the focus of our future study.
SUMMARY
RIPK2 was highly expressed in RA, and RIPK2 silencing regulated the malignant behavior and restrained inflammatory responses in RA-FLS. Therefore, RIPK2 is a novel oncogene in RA, which may serve as a potentially valuable prognostic biomarker and may provide a novel approach for the clinical treatment of RA.
AVAILABILITY OF DATA AND MATERIALS
The dataset that supported the results and findings of this research is available from the corresponding author on reasonable request.
ABBREVIATIONS
CCK-8 – Cell counting kit-8
DMEM – Dulbecco’s Modified Eagle’s Medium
EdU assay – 5-Ethynyl-2'-deoxyuridine
ELISA – Enzyme-linked immunosorbent assay
GAPDH – Glyceraldehyde-3-phosphate dehydrogenase
IHC staining – Immunohistochemical
NF-κB – Nuclear factor kappa B
NOD1/2 – Nucleotide oligomerization domain 1/2
OA – Osteoarthritis
PC – Pancreatic cancer
RA – Rheumatoid arthritis
RIPK2 – Receptor interacting serine/threonine kinase 2
RT-qPCR – Real-time quantitative polymerase chain reaction
AUTHOR CONTRIBUTIONS
YZW: Designed the research study; MYX: Performed the research; XXL: Collected and analyzed the data; DHL: Has been involved in drafting the manuscript and all authors have been involved in revising it critically for important intellectual content. All authors give final approval of the version to be published.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research was approved by the Medical Ethics Committee of Yantaishan Hospital (No: 2024064) dated 2024-04-19, and all patients signed informed consent for the therapies. All experiments were performed in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Cardiovascular risk assessment and therapeutic implications in rheumatoid arthritis. J Cardiovasc Transl Res. 2020;13:878-90.
- [CrossRef] [PubMed] [Google Scholar]
- Autophagy inhibitor regulates apoptosis and proliferation of synovial fibroblasts through the inhibition of PI3K/AKT pathway in collagen-induced arthritis rat model. Am J Transl Res. 2017;9:2065-76.
- [CrossRef] [PubMed] [Google Scholar]
- Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16:316-33.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9:880.
- [CrossRef] [PubMed] [Google Scholar]
- RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380-6.
- [CrossRef] [PubMed] [Google Scholar]
- The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic NOD2 promotes hepatocarcinogenesis via a RIP2-mediated proinflammatory response and a novel nuclear autophagy-mediated DNA damage mechanism. J Hematol Oncol. 2021;14:9.
- [CrossRef] [PubMed] [Google Scholar]
- RIPK2 is an unfavorable prognosis marker and a potential therapeutic target in human kidney renal clear cell carcinoma. Aging (Albany NY). 2021;13:10450-67.
- [CrossRef] [PubMed] [Google Scholar]
- Paired protein kinases PRKCI-RIPK2 promote pancreatic cancer growth and metastasis via enhancing NF-κB/JNK/ERK phosphorylation. Mol Med. 2023;29:47.
- [CrossRef] [PubMed] [Google Scholar]
- RIPK2 promotes the progression of colon cancer by regulating BIRC3-mediated ubiquitination of IKBKG. Exp Cell Res. 2023;429:113644.
- [CrossRef] [PubMed] [Google Scholar]
- Knockdown of RIPK2 inhibits proliferation and migration, and induces apoptosis via the NF-κB signaling pathway in gastric cancer. Front Genet. 2021;12:627464.
- [CrossRef] [PubMed] [Google Scholar]
- Immune modulating effects of receptor interacting protein 2 (RIP2) in autoinflammation and immunity. Clin Immunol. 2021;223:108648.
- [CrossRef] [PubMed] [Google Scholar]
- RIPK2 as a promising druggable target for autoimmune diseases. Int Immunopharmacol. 2023;118:110128.
- [CrossRef] [PubMed] [Google Scholar]
- A hyperactivating proinflammatory RIPK2 allele associated with early-onset osteoarthritis. Hum Mol Genet. 2018;27:2383-91.
- [CrossRef] [PubMed] [Google Scholar]
- 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580-8.
- [CrossRef] [PubMed] [Google Scholar]
- HDAC2 exacerbates rheumatoid arthritis progression via the IL-17-CCL7 signaling pathway. Environ Toxicol. 2023;38:1743-55.
- [CrossRef] [PubMed] [Google Scholar]
- RIP kinases as modulators of inflammation and immunity. Nat Immunol. 2018;19:912-22.
- [CrossRef] [PubMed] [Google Scholar]
- Dysregulation of intestinal epithelial cell RIPK pathways promotes chronic inflammation in the IBD Gut. Front Immunol. 2019;10:1094.
- [CrossRef] [PubMed] [Google Scholar]
- RIPK2 NODs to XIAP and IBD. Semin Cell Dev Biol. 2021;109:144-50.
- [CrossRef] [PubMed] [Google Scholar]
- Receptor-interacting serine/threonine-protein kinase-2 as a potential prognostic factor in colorectal cancer. Medicina (Kaunas). 2021;57:709.
- [CrossRef] [PubMed] [Google Scholar]
- Receptor-interacting protein kinase 2 (RIPK2) stabilizes c-Myc and is a therapeutic target in prostate cancer metastasis. Nat Commun. 2022;13:669.
- [CrossRef] [PubMed] [Google Scholar]
- Differential expressions of NOD-like receptors and their associations with inflammatory responses in rheumatoid arthritis. Clin Exp Rheumatol. 2017;35:630-7.
- [Google Scholar]
- Identification of novel protein kinase receptor type 2 inhibitors using pharmacophore and structure-based virtual screening. Molecules. 2018;23:453.
- [CrossRef] [PubMed] [Google Scholar]
- Negative regulatory NLRs mitigate inflammation via NF-κB pathway signaling in inflammatory bowel disease. Biomed J. 2023;46:100616.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of RIPK2-mediated NOD1 signaling promotes proliferation and invasion of ovarian cancer cells via NF-κB pathway. Histochem Cell Biol. 2022;157:173-82.
- [CrossRef] [PubMed] [Google Scholar]
- RIP2 enhances cell survival by activation of NF-κB in triple negative breast cancer cells. Biochem Biophys Res Commun. 2018;497:115-21.
- [CrossRef] [PubMed] [Google Scholar]
- Knockdown of receptor-interacting serine/threonine protein kinase-2 (RIPK2) affects EMT-associated gene expression in human hepatoma cells. Anticancer Res. 2012;32:3775-83.
- [Google Scholar]
- RIPK2: A promising target for cancer treatment. Front Pharmacol. 2023;14:1192970.
- [CrossRef] [PubMed] [Google Scholar]
- RIP2 promotes glioma cell growth by regulating TRAF3 and activating the NFκB and p38 signaling pathways. Oncol Rep. 2018;39:2915-23.
- [CrossRef] [PubMed] [Google Scholar]








