Translate this page into:
Prenyl diphosphate synthase subunit 2 is downregulated in abdominal aortic aneurysm and retards the progression of abdominal aortic aneurysm

*Corresponding author: Xiujie Fan, Medical Laboratory Diagnosis Center, Jinan Central Hospital, Jinan, China. fanxiujiejn@163.com
-
Received: ,
Accepted: ,
How to cite this article: Yong J, Tang S, Yu L, Li M, Zhang F, Fan X. Prenyl diphosphate synthase subunit 2 is downregulated in abdominal aortic aneurysm and retards the progression of abdominal aortic aneurysm. CytoJournal. 2024;21:63. doi: 10.25259/Cytojournal_70_2024
Abstract
Objective:
Abdominal aortic aneurysm (AAA) is a complex and fatal vascular disease for which specific treatments are still lacking. This study explored the effect and possible mechanisms of prenyl diphosphate synthase subunit 2 (PDSS2) on angiotensin II (Ang II)-induced AAA in human vascular smooth muscle cells (VSMCs).
Material and Methods:
The AAA cell model was established by treating VSMCs with 1 μM Ang II for 24 h. The effect of Ang II on VSMC viability was detected by cell counting kit-8 assay. The role of PDSS2 on VSMC proliferation was examined using the 5-ethynyl-2'-deoxyuridine method. The influence of Ang II and PDSS2 on VSMC apoptosis was analyzed by flow cytometry. The expression changes of PDSS2, apoptosis-related proteins, and phosphatidylinositol 3 kinase/protein kinase B/mechanistic target of rapamycin (PI3K/AKT/mTOR) pathway-related proteins were detected by Western blot analysis.
Results:
After treatment with Ang II, the VSMCs showed decreased viability and increased apoptosis (P < 0.01). PDSS2 expression was low in the AAA tissues and Ang II-treated VSMCs (P < 0.01). PDSS2 promoted the proliferation and blocked the apoptosis of Ang II-treated VSMCs, and si-PDSS2 showed the opposite effect (P < 0.01). PDSS2 also decreased the levels of p-mTOR, p-AKT, and p-PI3K, which, in turn, were increased by si-PDSS2 (P < 0.01).
Conclusion:
PDSS2 was downregulated in AAA and retarded the progression of VSMCs partially through the PI3K/AKT/mTOR pathway. This work explored the molecular mechanism of PDSS2 in the prevention, diagnosis, and treatment of AAA.
Keywords
Abdominal aortic aneurysm
Prenyl diphosphate synthase subunit 2
Phosphatidylinositol 3 kinase/protein kinase B/mechanistic target of rapamycin
Angiotensin II
INTRODUCTION
Abdominal aortic aneurysm (AAA) is a disease in which the affected vessels form permanent abnormal dilation, causing local weakness and hypotonia of the vessel wall.[1] The abdominal aorta is usually swollen and enlarged in diameter by more than 50% that of normal lumen.[2]
The incidence of AAA in elderly men over 65 years old is as high as 6%.[3] Most patients have no evident clinical symptoms in the early stage, and severe abdominal pain usually indicates tumor rupture.[4] AAA tumor rupture is the most common cause of death, with a mortality rate of up to 80%. According to statistics, approximately 200,000 people worldwide die every year due to AAA rupture.[5] The popularization of imaging technology has improved the early diagnosis rate of AAA, providing additional time for drug treatment and avoiding delayed surgery.[6] To date, no effective drugs are available to inhibit AAA expansion. Therefore, studying and understanding the pathogenesis of AAA are the key to finding specific treatment methods.
Prenyl diphosphate synthase subunit 2 (PDSS2) is located in the 16.3–21 region of the long arm of human chromosome 6 (6Q16.3–21) and contains eight exons and seven introns.[7] PDSS2 is a key enzyme involved in the biosynthesis of coenzyme q10 (CoQ10, which participates in mitochondrial oxidative phosphorylation and ATP production and plays an important role in energy conversion. In addition to its involvement in cellular energy metabolism, CoQ10 acts as a fat-soluble antioxidant in cell biofilm, scavenging oxygen free radicals and regulating cell apoptosis. CoQ10 also inhibits the proliferation of cancer cells,[8] alleviates inflammation in patients with cancer,[9] and slows down the progression of cardiovascular disease.[10,11] Clinical studies have shown that oral CoQ10 administration is effective in treating neurodegenerative diseases related to mitochondrial dysfunction and aging, such as Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease. As confirmed in hepatocellular carcinoma and kidney diseases, PDSS2 can cause illnesses by affecting CoQ10.[12,13] PDSS2 is used as a novel tumor inhibitor for a variety of solid tumors. Kandz et al.[14] detected a reduced level of PDSS2 mRNA in hepatocellular carcinoma tissues and speculated that PDSS2 is a tumor suppressor factor for this disease. In lung cancer, PDSS2 is underexpressed and inhibits the growth and movement of lung cancer cells.[15] In cardiovascular diseases, PDSS2 knockdown destroys the myofibrillar and cytoskeletal structures, resulting in the functional defects of congenital heart disease and heart failure.[16] PDSS2 also exerts a cardioprotective role by promoting the activation of Nrf2 pathway and inhibiting ferroptosis in atherosclerosis cells.[7]
Here, we explored whether PDSS2 is involved in AAA progression. We detected the expression level of PDSS2 in AAA and studied its molecular mechanism to further investigate whether PDSS2 can be used as an effective and new target for AAA.
MATERIAL AND METHODS
Collection of clinical samples
The AAA tissues removed from patients undergoing AAA surgery were collected, and normal abdominal aortic segments were selected as the control group. Before surgery, none of the patients with AAA had clinically unstable states, such as ruptured aneurysms. The mean age of the control group was 67.68 ± 11.01 years, and the percentage of male patients was 84%. The mean age of the AAA group was 68.96 ± 8.89 years, and the percentage of male patients was 88%. This study was authorized and supervised by the Ethics Committee of our hospital (Yantai Yuhuangding Hospital, 2024-130) and was conducted in accordance with the principles of the Helsinki Declaration. All the patients provided written consent before the study.
Cell culture
Human vascular smooth muscle cells (VSMCs) were obtained from Jennio (JNO-H0515, Guangzhou, China) and cultured in smooth muscle cell medium (SMCM, ZQ-1325, ZQXZBIO, Shanghai, China) containing 10% fetal bovine serum (C2027050, Gibco, California, USA) at 37°C and 5% carbon dioxide (CO2). When the cell fusion reached approximately 80%, angiotensin II (Ang II, MP5422, Shanghai Maokang Biotechnology Co., Ltd., Shanghai, China) was added for follow-up experiments. The VSMCs used in the study were identified by short tandem repeat and tested negative for mycoplasma.
Cell transfection
Logarithmic growth VSMCs (6 × 105 cells/well) were collected and inoculated onto a 6-well plate. PDSS2 overexpression (pcDNA3.1-PDSS2), negative control (NC, pcDNA3.1), siNC (sc-37007, Santa Cruz Biotechnology, Inc., Texas, USA), and si-PDSS2 (sc-76100, Santa Cruz Biotechnology, Inc., Texas, USA) were transfected according to the Lipofectamine 3000 kit (L3000015, Invitrogen, Carlsbad, California, USA). After 48 h of transfection, the transfection efficiency was verified by Western blot experiment.
Real-time quantitative polymerase chain reaction (RTqPCR)
The total RNA of VSMCs in each group was extracted in accordance with the instructions of RNAiso Plus kit (9109, Takara, Tokyo, Japan). According to the reverse transcription kit (QP056, iGeneBio, Guangzhou, China), the total RNA was reverse transcribed into cDNA, followed by RT-qPCR. The expression of PDSS2 was measured by the 2−ΔΔCt method. The primer sequences for RT-qPCR are PDSS2 F: 5'-TCTAGCAAATGCCTGCAATG-3', PDSS2 R: 5'-TCTGCTCCTTCCAAGTCGAT-3', Tubulin F: 5'-TCCATGAAGGAGGTCGATGA-3', Tubulin R: 5'-CAGACGGCTGTCTTGACATT-3'.
Cell counting kit-8 (CCK-8) assay
The VSMCs from each group were added to 96-well plates and incubated in a 5% CO2 incubator at 37°C. After 24 h intervention with Ang II culture solution, 10 μL CCK-8 reagent (HZ0037, Huzhen Biology, Shanghai, China) was added to the cells at 37°C for 2 h. At 450 nm wavelength, the absorbance value of each well was detected with an enzyme labeler (DR-200B, DeLang, Nanjing, China).
Western blot assay
The total protein of each group was extracted using a protein lysate at 4°C, and the protein concentration was assessed using the bicinchoninic acid protein assay kit (B00101, Donghuan, Shanghai, China). After gel electrophoresis, the protein samples were transferred to the polyvinylidene fluoride membrane (IPVH00010, Millipore, Boston, Massachusetts, USA) and sealed with 5% skim milk. The primary antibodies (anti-tubulin: ab6046, 1:500, Abcam, Cambridge, UK; anti-cleaved caspase-3: ab32042, 1:500, Abcam, Cambridge, UK; anti-Bcl-2: ab182858, 1:2000, Abcam, Cambridge, UK; anti-Bax: XGK96889, 1:500, Sig Biotechnology, Shanghai, China; anti-PDSS2: XGK0946, 1:500, Sig Biotechnology, Shanghai, China; anti- phosphatidylinositol 3 kinase (PI3K): 60225, 1:5000, Proteintech, Wuhan, China; anti-p-PI3K: ab191606, 1:1000, Abcam, Cambridge, UK; anti- protein kinase B (AKT): A18675, 1:1000, ABclonal, Wuhan, China; anti-p-AKT: AP1208, 1:1000, ABclonal, Wuhan, China; anti- mechanistic target of rapamycin (mTOR): A11345, 1:1000, ABclonal, Wuhan, China; anti-p-mTOR: AP0115, 1:1000, ABclonal, Wuhan, China; and anti-GAPDH: XGK1981, 1:500, Sig Biotechnology, Shanghai, China) were added, and the samples were incubated at 4°C for 24 h. The corresponding horseradish peroxidase-labeled IgG diluent (ab205718, Abcam, Cambridge, UK) was added and the samples were incubated for 1 h. The dilution ratio was 1:2000. After the enhanced chemiluminescence luminescent solution (HY-K1005, MedChemExpress, Shanghai, China) was added, exposure and photography were performed on an automatic gel imager Bio-Rad XR protein gel imager (Bio Rad, CA, USA). Image J software (National Institutes of Health, Maryland, USA) was used for the quantitative analysis of gray values.
5-Ethynyl-2'-deoxyuridine (EdU) staining
In brief, 100 μL of cell suspension was added to a 96-well plate and cultured in an incubator for 48 h (5% CO2, 37°C). Each well was then added with 200 μL of EdU reagent (D-AKE2031, Biogradetech, California, USA) and incubated at 37°C for 2 h. After cleaning, 300 μL of cell fixative (4% paraformaldehyde, PN4204, G-Clone, Beijing, China) was added to each well, followed by incubation for 30 min at room temperature. Each well was then added with 300 μL of penetrant 0.5% TritonX-100, CS9013, G-Clone, Beijing, China) and incubated for 10 min to decolorize. Volume 200 μL of ×1 Apollo staining reaction solution was added to each well to avoid light for 30 min, followed by 200 μL of Hoechst 33342 reaction solution. The samples were incubated for 30 min. The experimental results were observed and recorded under an inverted microscope (DSY2000X, UOP, Chongqing, China) immediately after staining.
Flow cytometry
The VSMCs of each group were prepared into cell suspension. In brief, 200 μL of cell suspension was added into a new centrifuge tube. The cells were washed with PBS and stained with FITC-Annexin V and PI (E-CK-A211, Elabscience, Wuhan, China) under dark light. The apoptosis of the cells in each group was observed and recorded by a Flow cytometer (NovoCyte Quanteon, Agilent, Santa Clara, California, USA).
Statistical analysis
Data were statistically analyzed by GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, California, USA) and displayed as mean ± standard deviation. Differences between two groups were analyzed by t-test. When P < 0.05, the difference was considered statistically significant.
RESULTS
Ang II induces the apoptosis of VSMCs
As a mature modeling method, Ang II-induced VSMCs have been widely used to study the pathogenesis and mechanism of AAA. Here, CCK-8 experiment revealed that the cell viability significantly decreased under 0.5 and 1 μM Ang II treatment (P < 0.01)[Figure 1a]. In our experiment, the VSMCs were treated with 1 μM Ang II for 24 h. EdU test results displayed that the percentage of EdU-positive cells was notably reduced after 1 μM Ang II treatment for 24 h (P < 0.01) [Figure 1b and c]. In addition, Ang II accelerated the apoptosis rate of the VSMCs (P < 0.01) [Figure 1d and e]. We also observed decreased Bcl-2 activity and increased Bax and caspase-3 activity in the Ang II-induced VSMCs (P < 0.01) [Figure 1f and g]. These findings suggested that Ang II treatment impaired cell proliferation and triggered apoptosis, leading to VSMC injury.
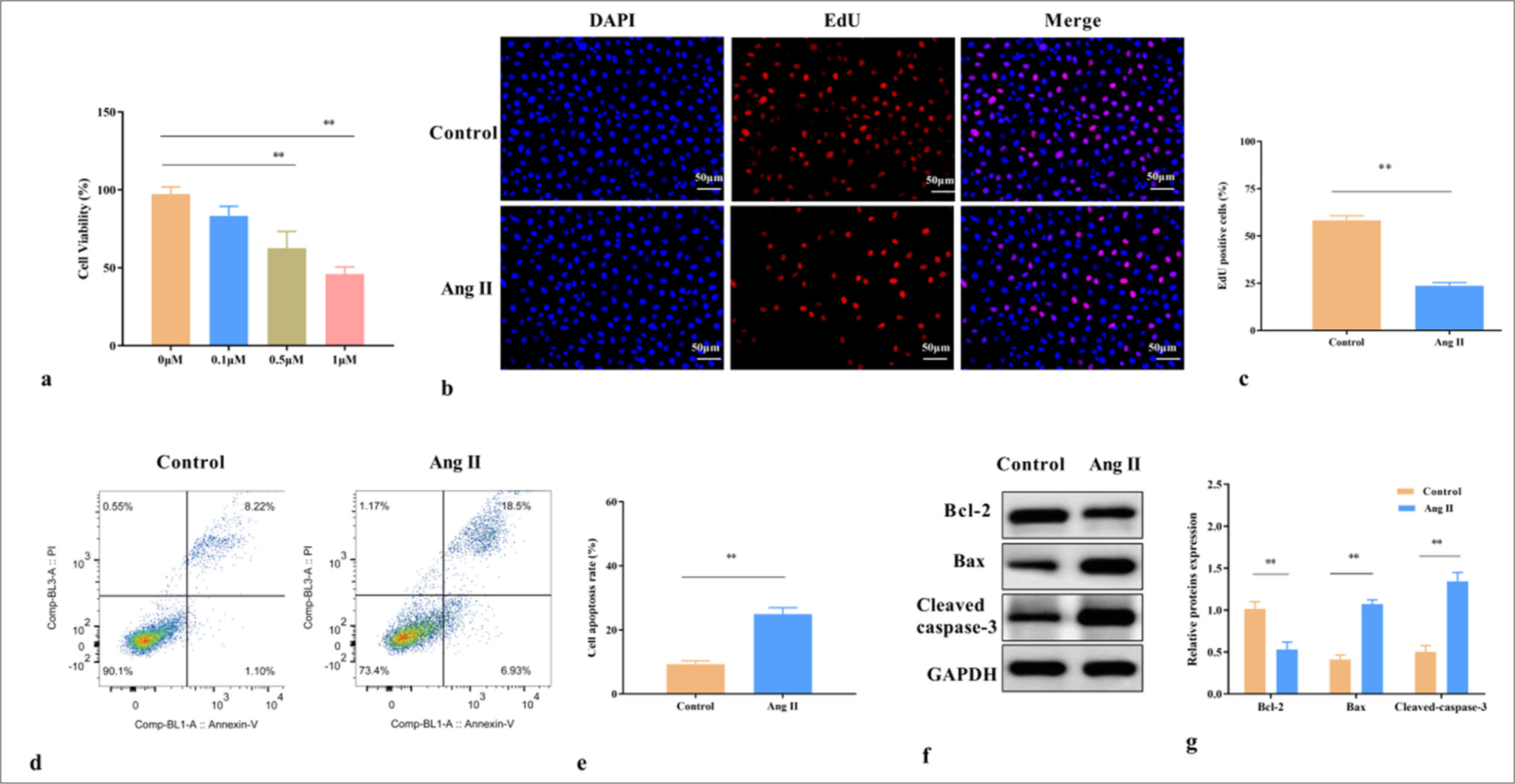
- Ang II induces the apoptosis of VSMCs. (a) Effects of different concentrations of Ang II (0, 0.1, 0.5, and 1 μM) on VSMC viability. (b) EdU positive rate of VSMCs treated with 1 μM Ang II. (c) Statistical analysis of EdU positive cell rate. (d) Cell apoptosis of VSMCs treated with 1 μM Ang II. (e) Statistical analysis of cell apoptosis. (f) Expression of apoptosis-related proteins in VSMCs treated with 1 μM Ang II. (g) Statistical analysis of protein expression. ✶✶P < 0.01, n = 3. Ang II: Angiotensin II, VSMCs: Vascular smooth muscle cells, EdU: 5-ethynyl-2'-deoxyuridine, DAPI: 4’,6-diamidino-2-phenylindole, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, Bcl-2: B-cell lymphoma 2, Bax: BCL2-associated X protein.
PDSS2 is downregulated in Ang II-treated VSMCs
Compared with those in normal arterial tissues, the mRNA and protein expression levels of PDSS2 were lower in the aortic tissues of patients with AAA (P < 0.01) [Figure 2a-c]. As expected, PDSS2 was downregulated in the Ang II-treated VSMCs (P < 0.01) [Figure 2d and e]. PDSS2 was overexpressed and knocked down by transfecting PDSS2 vector and siRNAPDSS2, respectively (P < 0.01) [Figure 2f and g]. Therefore, we confirmed that PDSS2 was underexpressed in AAA and speculated that abnormal PDSS2 expression is closely related to AAA progression.
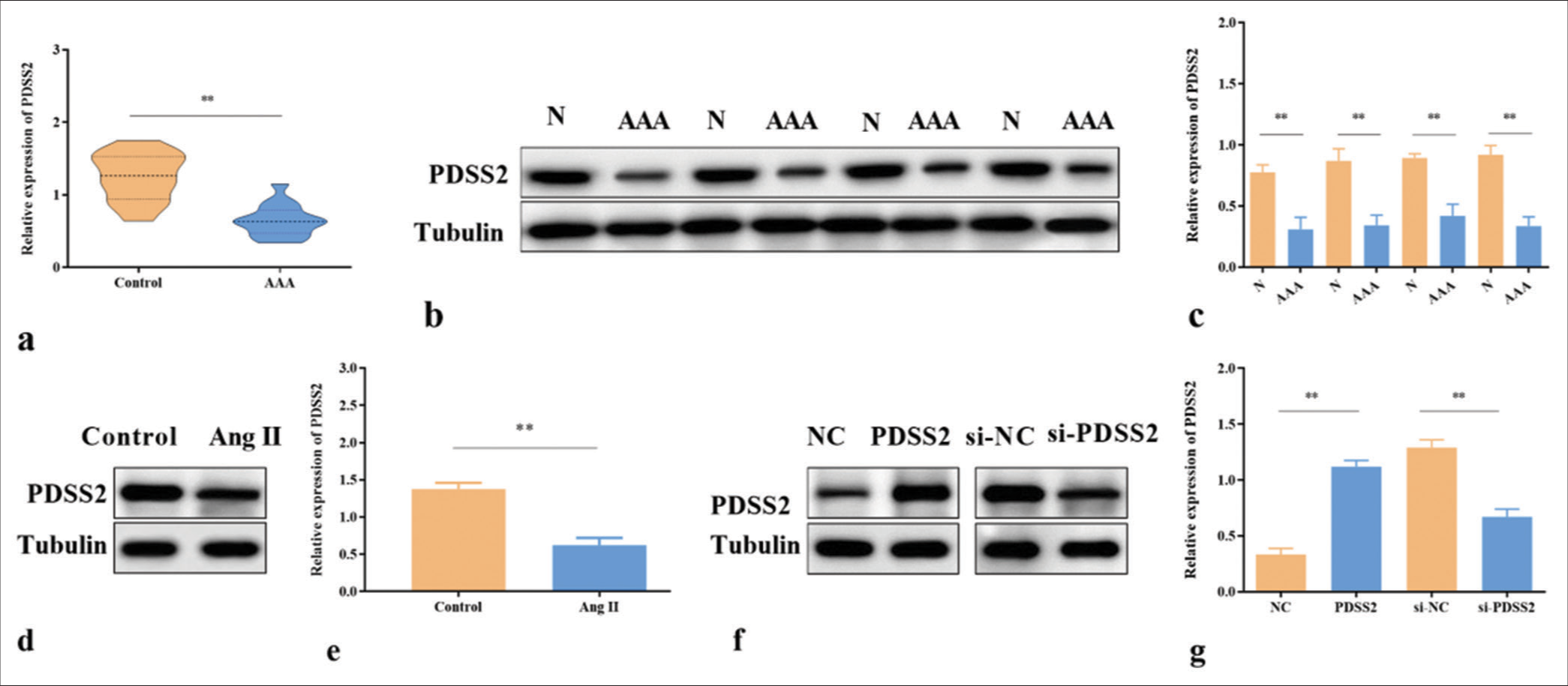
- PDSS2 is downregulated in Ang II-treated VSMCs. (a) PDSS2 mRNA expression in AAA, n = 15. (b) PDSS2 protein expression in AAA, n = 4. (c) Statistical analysis of protein expressions. (d) PDSS2 protein expression in Ang II-treated VSMCs, n = 3. (e) Statistical analysis of protein expression. (f) PDSS2 expression in Ang II-treated VSMCs. (g) Statistical analysis of protein expression. ✶✶P < 0.01. PDSS2: Prenyl diphosphate synthase subunit 2, VSMCs: Vascular smooth muscle cells, Ang II: Angiotensin II, AAA: Abdominal aortic aneurysm, si-NC: Small interfering RNA negative control. N: Normal.
PDSS2 accelerates the proliferation and attenuates the apoptosis of VSMCs treated by Ang II
In the Ang II-treated VSMCs, the EdU positive rate was increased by PDSS2 (P < 0.01) [Figure 3a and b] but decreased by si-PDSS2 (P < 0.01) [Figure 3c and d]. In addition, the apoptosis of Ang II-treated VSMCs was inhibited by PDSS2 (P < 0.01) [Figure 3e and f] and accelerated by si-PDSS2 (P < 0.01) [Figure 3g and h]. PDSS2 upregulated Bcl-2 and downregulated Bax and cleaved caspase-3 (P < 0.01) [Figure 3i and j], and si-PDSS2 had the opposite effect (P < 0.01) [Figure 3k and l]. These data indicated that PDSS2 accelerated the proliferation and attenuated the apoptosis of VSMCs treated by Ang II.
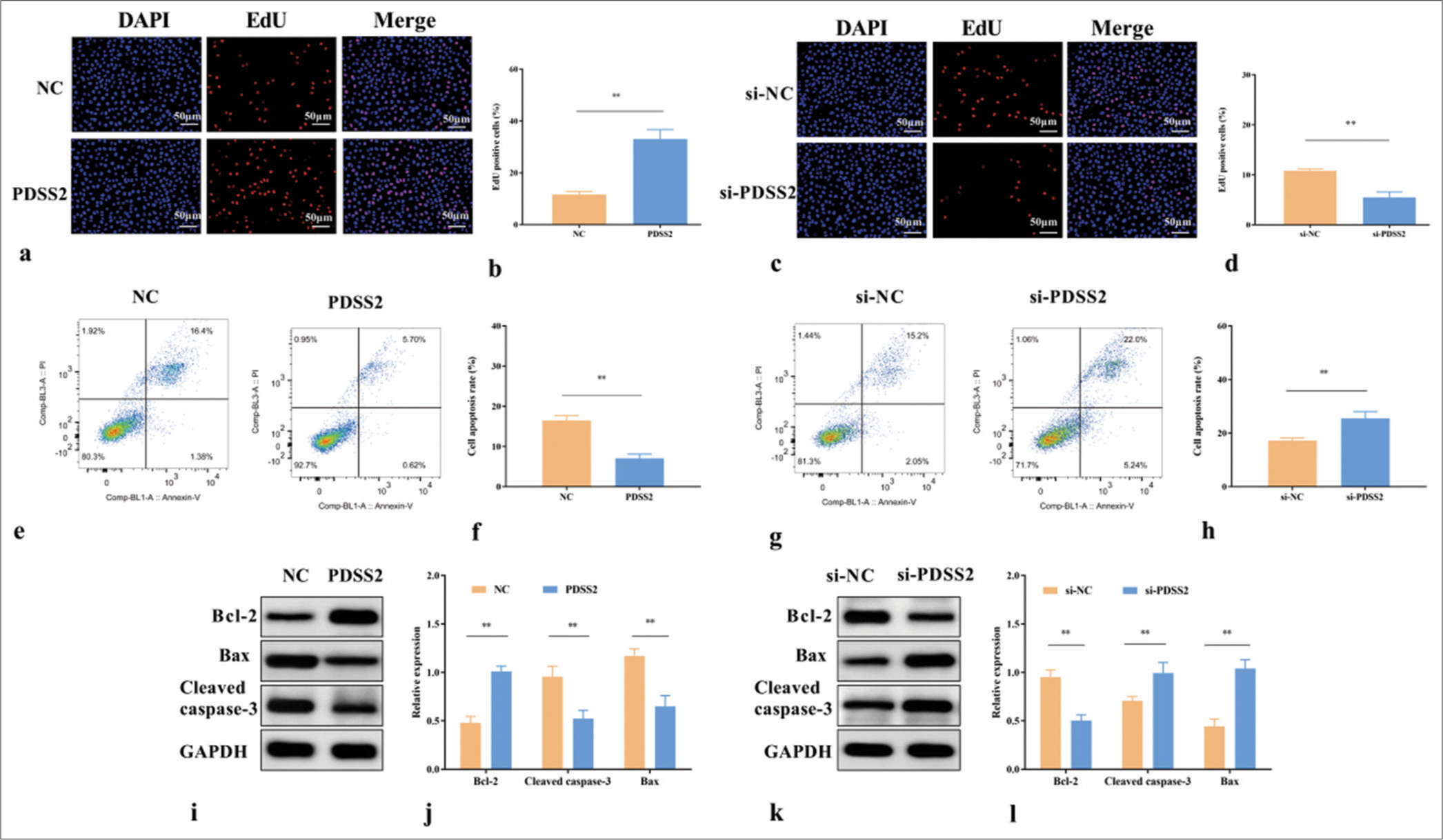
- PDSS2 attenuates the apoptosis of Ang II-treated VSMCs. (a) Effects of PDSS2 on the EdU positive rate of Ang II-treated VSMCs. (b) Statistical analysis of EdU positive cell rate. (c) Effects of si-PDSS2 on the EdU positive rate of Ang II-treated VSMCs. (d) Statistical analysis of EdU positive cell rate. (e) Effects of PDSS2 on the apoptosis of Ang II-treated VSMCs. (f) Statistical analysis of cell apoptosis. (g) Effects of si-PDSS2 on the apoptosis of Ang II-treated VSMCs. (h) Statistical analysis of cell apoptosis. (i) Effects of PDSS2 on the levels of Bcl-2, cleaved caspase-3, and Bax in Ang II-treated VSMCs. (j) Statistical analysis of protein expression. (k) Effects of si-PDSS2 on the levels of Bcl-2, cleaved caspase-3, and Bax in Ang II-treated VSMCs. (l) Statistical analysis of protein expression. ✶✶P < 0.01, n = 3. PDSS2: Prenyl diphosphate synthase subunit 2, VSMCs: Vascular smooth muscle cells, Ang II: Angiotensin II, EdU: 5-ethynyl-2'-deoxyuridine, DAPI: 4’,6-diamidino-2-phenylindole, si-NC: Small interfering RNA negative control, NC: Negative control, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, Bcl-2: B-cell lymphoma 2, Bax: BCL2-associated X protein.
PDSS2 partially weakens the Ang II-induced apoptosis of VSMCs by regulating the PI3K/AKT/mTOR pathway
Western blot assay was performed to determine whether PDSS2 acts on the PI3K/AKT/mTOR pathway. PDSS2 overexpression reduced the phosphorylation levels of mTOR, AKT, and PI3K (P < 0.01) [Figure 4a and b]. By contrast, siPDSS2 increased the phosphorylation levels of mTOR, AKT, and PI3K (P < 0.01) [Figure 4c and d]. Therefore, PDSS2 partially alleviated the Ang II-induced apoptosis of VSMCs by regulating the PI3K/AKT/mTOR pathway.
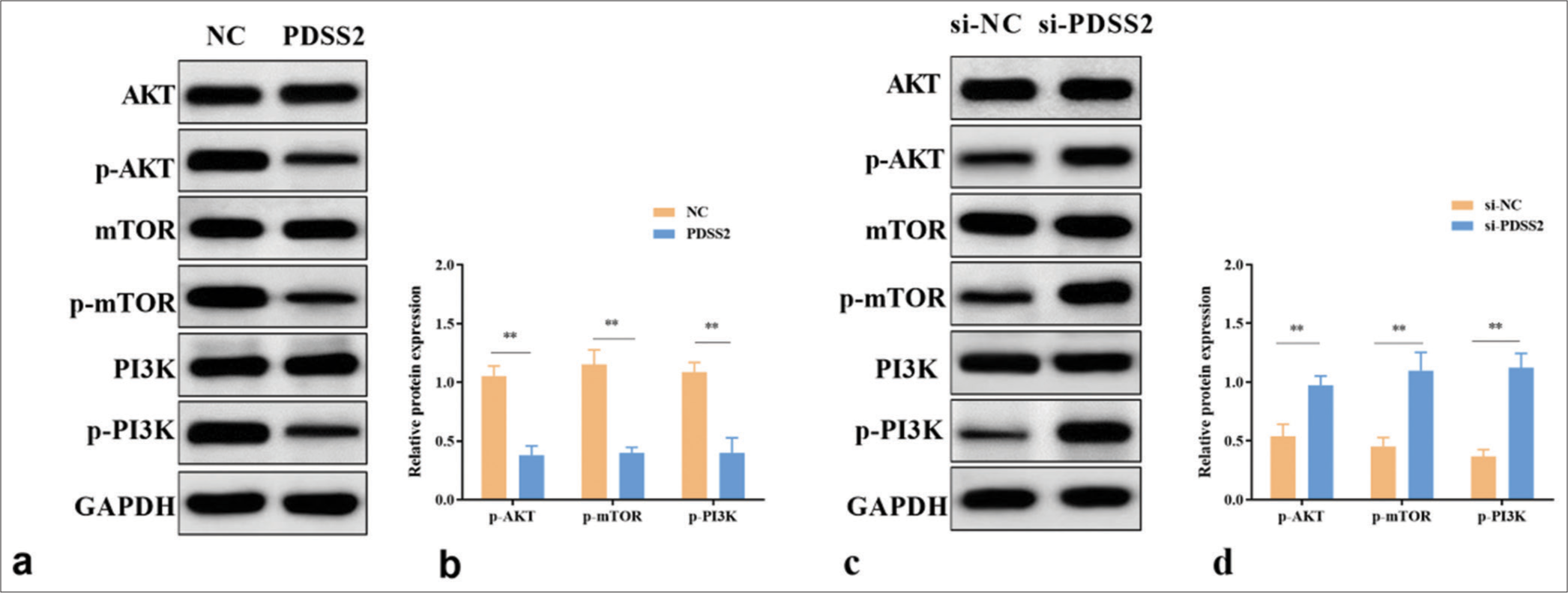
- PDSS2 partially attenuates the Ang II-induced apoptosis of VSMCs by regulating the PI3K/AKT/mTOR pathway. (a) PDSS2 decreased the expression levels of p-mTOR, p-AKT, and p-PI3K. (b) Statistical analysis of protein expression. (c) PDSS2 silencing increased the expression levels of p-mTOR, p-AKT, and p-PI3K. (d) Statistical analysis of protein expression. ✶✶P < 0.01, n = 3. PDSS2: Prenyl diphosphate synthase subunit 2, VSMCs: Vascular smooth muscle cells, PI3K: Phosphatidylinositol 3 kinase, AKT: Protein kinase B, mTOR: Mechanistic target of rapamycin, NC: Negative control, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
AAA is a life-threatening condition in which the dilated aorta can spontaneously rupture. Patients generally have no evident clinical symptoms in the early stage, and the mortality rate can reach to 80% once the AAA ruptures.[17] At present, no effective drug is available to prevent or treat this disease. Therefore, early diagnosis and treatment before AAA rupture have become an important and challenging task. In this study, we focused on the effect of PDSS2 on Ang II-treated VSMCs to explore its mechanism in AAA.
VSMCs are the main constituent cells of the abdominal aorta and play a key role in maintaining the structure, strength, compliance, and function of the aortic wall.[18] The loss of VSMCs can cause serious damage to the structure of the abdominal aorta wall, affecting the tension and strength of the artery wall. The long-term effects of blood pressure may lead to compliant dilation of the artery wall and the formation of aneurysms. From the perspective of histology and anatomy, the apoptosis and extracellular matrix degradation of VSMCs in the abdominal aortic wall are the main histological changes in the occurrence and development of AAA. It has been reported that Ang II treated VSMCs to construct AAA cell models.[19] We discovered that the proliferation of VSMCs decreased and their apoptosis increased after Ang II treatment.
PDSS2 is widely expressed in various tissues at different developmental stages of human body and is involved in the regulation of various cellular life processes and signaling pathways. During cerebellar development, PDSS2 knockdown can lead to cerebellar hypoplasia by reducing cell migration and accelerating ectopic apoptosis.[20] PDSS2 is involved in a variety of cancer processes by controlling tumor cell viability, apoptosis, and metastasis. It has shown potential as a tumor suppressor gene in lung cancer,[21] gastric cancer,[22] and hepatocellular carcinoma.[23] Importantly, Yang et al. found that PDSS2 may play a cardioprotective role in Atherosclerosis, because overexpression of PDSS2 promotes the proliferation and inhibits reactive oxygen species release, iron content, and iron ptosis of human coronary endothelial cells.[7] Consistent with previous evidence, we found that PDSS2 was lowly expressed in AAA. Functional experiments confirmed that PDSS2 overexpression ameliorated the retarded proliferation and reduced apoptosis rate of VSMCs induced by Ang II. Here, we speculated that PDSS2 might inhibit AAA progression by suppressing the apoptosis and promoting the proliferation of VSMCs.
The formation of aneurysm is closely related to the apoptosis, proliferation, and inflammation of vascular endothelial cells. The PI3/AKT pathway affects AAA progression by regulating the survival, proliferation, and extracellular matrix degradation of vascular endothelial cells.[24,25] It also promotes the phenotypic transformation of VSMCs, leading to vascular injury and ultimately aggravating the development of AAA.[26] CoQ10 participates in various diseases by regulating the PI3/AKT pathway and protects against scope-induced Alzheimer’s disease by regulating the PI3K/Akt/glycogen synthase kinase-3 (GSK-3)/cAMP response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B (TrKB) pathway.[27] By blocking the PI3K/AKT signaling pathway, CoQ10 improved heart coefficient and inhibited cardiomyocyte apoptosis in rats with heart failure.[28] Here, we investigated whether PDSS2, a key gene affecting CoQ10 synthesis, has an effect on the PI3/AKT/mTOR pathway. Results showed that PDSS2 decreased the levels of p-mTOR, p-AKT, and p-PI3K. Therefore, we speculated that PDSS2 might slow down AAA progression by blocking the PI3K/AKT/mTOR pathway.
This study also has many shortcomings. Animal models of Ang II-induced AAA were not used to verify the association between PDSS2 and AAA. In addition, the specific mechanism of PDSS2 affecting the PI3K/AKT/mTOR pathway was not verified, which will be the direction of our subsequent experiments.
SUMMARY
This study revealed the role of PDSS2 in AAA. Results showed that PDSS2 reduced the Ang II-induced apoptosis of VSMCs and delayed AAA progression by inhibiting the PI3K/AKT/mTOR pathway. This work provided a theoretical basis and a new research direction for exploring new therapeutic targets of AAA.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author on reasonable request.
ABBREVIATIONS
AAA – Abdominal aortic aneurysm
PDSS2 – Prenyl diphosphate synthase subunit 2
Ang II – Angiotensin II
VSMCs – Vascular smooth muscle cells
PI3K/AKT/mTOR – Phosphatidylinositol 3 kinase/protein kinase B/mechanistic target of rapamycin
RT-qPCR – Real-time quantitative polymerase chain reaction
CCK-8 – Cell counting kit-8
EdU – 5-ethynyl-2'-deoxyuridine
FCM – Flow cytometry
AUTHOR CONTRIBUTIONS
JY: Designed the study, performed trial and data collection, and wrote the original draft; SST and LY: Performed trial and supervised the study; ML and FZ: Performed trial and data collection; XJF: Performed project administration. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was conducted in accordance with the principles of the Declaration of Helsinki, and authorized and supervised by the Ethics Committee of Yantai Yuhuangding Hospital (2024-130), dated 2024.1.8. All the patients signed informed consent forms.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- GP VI-Mediated platelet activation and procoagulant activity aggravate inflammation and aortic wall remodeling in abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2024;44:2294-317.
- [CrossRef] [PubMed] [Google Scholar]
- Infrarenal abdominal aortic aneurysm. Surg Clin North Am. 2023;103:595-614.
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national burden of aortic aneurysm, 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Eur J Prev Cardiol. 2022;29:1220-32.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological therapy of abdominal aortic aneurysm: An update. Curr Vasc Pharmacol. 2018;16:114-24.
- [CrossRef] [PubMed] [Google Scholar]
- PDSS2 inhibits the ferroptosis of vascular endothelial cells in atherosclerosis by activating Nrf2. J Cardiovasc Pharmacol. 2021;77:767-76.
- [CrossRef] [PubMed] [Google Scholar]
- A Review of the potential role of CoQ10 in the treatment of hepatocellular carcinoma. Biochem Genet. 2024;62:575-93.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of coenzyme Q10 Supplementation on inflammation, angiogenesis, and oxidative stress in breast cancer patients: A systematic review and meta-analysis of randomized controlled-trials. Inflammopharmacology. 2021;29:579-93.
- [CrossRef] [PubMed] [Google Scholar]
- Coenzyme Q10 to manage chronic heart failure with a reduced ejection fraction: A systematic review and economic evaluation. Health Technol Assess. 2022;26:1-128.
- [CrossRef] [PubMed] [Google Scholar]
- Coenzyme Q-10 in the treatment of patients with chronic heart failure and reduced left ventricular ejection fraction: Systematic review and meta-analysis. Kardiologiia. 2022;62:3-14.
- [CrossRef] [PubMed] [Google Scholar]
- PDSS2 Deficiency induces hepatocarcinogenesis by decreasing mitochondrial respiration and reprogramming glucose metabolism. Cancer Res. 2018;78:4471-81.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting a Braf/Mapk pathway rescues podocyte lipid peroxidation in CoQ-deficiency kidney disease. J Clin Invest. 2021;131:141380.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical utility of PDSS2 expression to stratify patients at risk for recurrence of hepatocellular carcinoma. Int J Oncol. 2014;45:2005-12.
- [CrossRef] [PubMed] [Google Scholar]
- SKA2-mediated transcriptional downregulation of the key enzyme of CoQ(10) biosynthesis PDSS2 in lung cancer cells. J Cancer. 2023;14:379-92.
- [CrossRef] [PubMed] [Google Scholar]
- Model system identification of novel congenital heart disease gene candidates: Focus on RPL13. Hum Mol Genet. 2019;28:3954-69.
- [CrossRef] [PubMed] [Google Scholar]
- Revealing PPP1R12B and COL1A1 as piRNA pathway genes contributing to abdominal aortic aneurysm through integrated analysis and experimental validation. Gene. 2024;897:148068.
- [CrossRef] [PubMed] [Google Scholar]
- Smooth muscle heterogeneity and plasticity in health and aortic aneurysmal disease. Int J Mol Sci. 2023;24:11701.
- [CrossRef] [PubMed] [Google Scholar]
- RelB represses miR-193a-5p expression to promote the phenotypic transformation of vascular smooth muscle cells in aortic aneurysm. Biochim Biophys Acta Gene Regul Mech. 2023;1866:194926.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebellar defects in Pdss2 conditional knockout mice during embryonic development and in adulthood. Neurobiol Dis. 2012;45:219-33.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-101 inhibits cell migration and invasion in bladder cancer via targeting FZD4. Exp Ther Med. 2019;17:1476-85.
- [CrossRef] [Google Scholar]
- PDSS2 mRNA expression in HCC and role of PDSS2 in growth and invasion of HCC cells. J Trop Med. 2017;17:6.
- [Google Scholar]
- Decreased expression of prenyl diphosphate synthase subunit 2 correlates with reduced survival of patients with gastric cancer. J Exp Clin Cancer Res. 2014;33:88.
- [CrossRef] [PubMed] [Google Scholar]
- Curcumin nicotinate suppresses abdominal aortic aneurysm pyroptosis via lncRNA PVT1/miR-26a/KLF4 axis through regulating the PI3K/AKT signaling pathway. Toxicol Res (Camb). 2021;10:651-61.
- [CrossRef] [PubMed] [Google Scholar]
- FGF21 promotes angiotensin II-induced abdominal aortic aneurysm via PI3K/AKT/mTOR pathway. Vascular. 2024;32(6):1369-1377. 17085381231192688
- [CrossRef] [PubMed] [Google Scholar]
- The role of phosphoinositide 3-kinases in immune-inflammatory responses: Potential therapeutic targets for abdominal aortic aneurysm. Cell Cycle. 2022;21:2339-64.
- [CrossRef] [PubMed] [Google Scholar]
- Spotlight on Coenzyme Q10 in scopolamine-induced Alzheimer's disease: Oxidative stress/PI3K/AKT/GSK 3ß/CREB/BDNF/TrKB. J Pharm Pharmacol. 2023;75:1119-29.
- [CrossRef] [PubMed] [Google Scholar]
- Investigating the mechanism of action of Schisandra Chinensis combined with coenzyme Q10 in the treatment of heart failure based on PI3K-AKT pathway. Drug Des Devel Ther. 2023;17:939-57.
- [CrossRef] [PubMed] [Google Scholar]








