Translate this page into:
Pulmonary extranodal NK/T-cell lymphoma: A clinicopathological analysis of five patients

*Corresponding author: Di Wu, Department of Pathology, Xuzhou Central Hospital, Xuzhou, Jiangsu, China; Xuzhou Clinical School of Xuzhou Medical University, Jiangsu, China. m13852148570@163.com
-
Received: ,
Accepted: ,
How to cite this article: Li Q, Zhang YX, Sun H, Wang X, Wu D. Pulmonary extranodal NK/T-cell lymphoma: A clinicopathological analysis of five patients. CytoJournal. 2025;22:14. doi: 10.25259/Cytojournal_177_2024
Abstract
Objective:
Our goal was to investigate the clinicopathological features of extranodal natural killer (NK)/T-cell lymphoma (ENKTL).
Material and Methods:
A total of five newly identified (5 biopsy samples) untreated cases of pulmonary ENKTL were collected between January 2016 and January 2024. The clinical characteristic pathology features on hematoxylin-eosin-staining sections, immunohistochemistry stating, treatment responses, and prognoses were retrospectively analyzed.
Results:
Among the five patients, four were male and one was female, and their ages varied between 48 and 63 years. All five patients were initially diagnosed with stage IV disease. Histological examination revealed either scattered or localized clusters of highly pleomorphic tumor lymphocytes associated with necrosis and a significant presence of inflammatory cells. Most tumor cells expressed cluster of differentiation (CD)3, T-cell intracellular antigen-1, and granzyme B, whereas there was an absence of CD20, CD79a, or CD5 expression. The expression of CD56 was detected in four out of the five patients. Only two patients were tested for programmed cell death ligand 1, with one out of two patients exhibiting positivity (Tumor Proportion Score (TPS) 80%). The Ki-67 proliferation index varied from 40% to 90%. All patients tested positive for Epstein– Barr virus-encoded ribonucleic acid (RNA) (EBER) through fluorescence in situ hybridization (FISH). Five of the patients died during follow-up. Four of these patients underwent standard chemotherapy, with survival durations ranging from 3 to 24 months. One patient received only supportive treatment, resulting in a survival time of 1 month.
Conclusion:
Pulmonary ENKTL is an uncommon, aggressive cancer associated with a bleak prognosis. The likelihood of misdiagnosis is high because of the presence of necrotic lesions and various cell types. Accurate diagnosis relies heavily on immunohistochemistry and EBER FISH. The aim of our study was to facilitate improved diagnosis of pulmonary ENKTL and to identify treatment strategies for affected individuals.
Keywords
Diagnosis
Epstein–Barr virus-encoded RNA
Extranodal NK/T-cell lymphoma
Prognosis
Pulmonary
INTRODUCTION
Extranodal NK/T-cell lymphoma (ENKTL) is a particularly aggressive form of non-Hodgkin lymphoma characterized by diverse morphology. Epstein–Barr virus (EBV) is present in the majority of these tumors. ENKTL predominantly affects males, with a median age of 50 years, although cases have been reported in individuals aged between 20 and 80 years.[1-3] ENKTL typically affects the nasal cavity and upper respiratory system. Nonetheless, it may also develop in various other locations, such as the skin, digestive tract, testes, salivary glands, pancreas, soft tissues, and bone marrow.[4]
Although the lung is frequently affected by systemic lymphoma, primary pulmonary lymphoma accounts for <1% of all extranodal lymphomas.[5] Pulmonary involvement by ENKTL, whether primary or secondary, is rare and infrequently documented in the literature because of its unclear clinical characteristics and incidence rates.[6] The classification and diagnosis of pulmonary ENKTL require the identification of specific immunological markers, such as cluster of differentiation (CD)3 and CD56 positivity, along with the expression of cytotoxic molecules such as perforin and granzyme B. In addition, the detection of EBV expression through fluorescence in situ hybridization (FISH) is crucial for accurate diagnosis.[7]
Pulmonary ENKTL is typically diagnosed at advanced clinical stages and is characterized by a lack of specific imaging manifestations and difficulty in pathological diagnosis, making biopsy diagnosis particularly challenging. Therefore, we sought to deepen the current understanding of this disease by analyzing the clinicopathological characteristics of 5 patients with pulmonary ENKTL.
MATERIAL AND METHODS
Patient selection
The study was approved by the Xuzhou Central Hospital Biomedical Research Ethics Review Committee and complied with the Helsinki Declaration. We collected samples from patients admitted to the Xuzhou Central Hospital and the Ruijin Hospital from January 2016 to January 2024 with biopsy-confirmed NK/T-cell lymphoma (NKTL). Patients with a prior history of NKTL and those with other malignant tumors were excluded. Ultimately, a total of 5 patients were included in the study. Two experienced pathologists who specialize in hematopoietic and lymphoid tissues reviewed all the samples through routine hematoxylin-eosin staining, immunohistochemistry, and FISH for EBV-encoded RNA (EBER). The diagnosis of pulmonary ENKTL was made on the basis of the World Health Organization classification of tumors of hematopoietic and lymphoid tissues from 2022.[1] The diagnostic criteria include the following: (1) Obvious ulcers and tissue necrosis; (2) angiocentric and vascular destructive growth patterns; (3) tumor cell diversity, including large, medium, and small cells, often intermixed with apoptotic bodies and inflammatory cells; (4) cytotoxic molecules positivity, with negative staining for the surface markers CD3 and CD5 and positive staining for EBER; (5) in the CD56-negative subtype, the majority are EBER- and CD3ε-positive; and (6) associated with EBV. Clinical information and pathological data were collected from medical records and pathology reports submitted through the case management system. This information included the patient’s age, sex, primary symptoms, prior medical history, findings from positron emission tomography/computed tomography (PET/CT), type of treatment administered, and results of laboratory tests. Follow-up data were gathered through direct telephone interviews with patients or their family members.
Immunohistochemical and special staining
Immunohistochemical analysis was conducted on pulmonary ENKTL tissue sections that had been fixed in formalin and embedded in paraffin. This study utilized a variety of monoclonal antibodies at specified dilutions, including CD3 (8920263, DAKO, Glostrup, Denmark), CD56 (ZM-0057, Beijing Zhongshan Golden Bridge Biotechnology Co, Ltd, Beijing, China), Granzyme B (MAB1865, NeoMarkers, Freemont, CA, USA), T-cell intracellular antigen-1 (TIA-1) (ZM-0457, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China), CD20 (Kit-0001, Maixin Biotechnology Co., Ltd., FuZhou, China), CD30 (GA60261-2CN, DAKO; Glostrup, Denmark), Ki-67 (MAB-0672, Maixin Biotechnology Co., Ltd., FuZhou, China), and programmed death ligand 1 (PD-L1) (SP-9001, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). For the immunohistochemistry (IHC) staining procedures, the sections were placed in an oven at 65°C for 30 min in 0.01 M citrate buffer (pH 6.0) autoclaved (95°C, 2 min) to retrieve antigens, washed with phosphate-buffered saline (PBS) 3 times for 5 min each, and then incubated with primary antibodies overnight at 4°C. After the samples were washed with PBS 3 times for 5 min each, they were incubated with an anti-IHC secondary antibody at 37°C for 30 min. The sections were stained with 3,3’-diaminobenzidine (DAB), counterstained with hematoxylin, dehydrated, and sealed with a neutral gel. The assessment of EBV infection was performed through FISH with EBER PNA probes (G111921, DAKO, Glostrup, Denmark), following the manufacturer’s guidelines. Sections (4 μm) were subjected to deparaffinization and then predigested with pepsin diluents (30 min at 37°C) before hybridization with the EBER oligonucleotide probe (8–16 h at 37°C). For detection, horseradish peroxidase-conjugated streptavidin was utilized (30 min at 37°C). The DAB substrate served as the chromogen (1–5 min at room temperature). Each section was counterstained with hematoxylin. Positive hybridization signals were indicated by brown nuclear staining. Nasopharyngeal cancer was employed as a positive control, whereas hybridization procedures without a probe were performed on each sample to serve as negative controls, which were assessed in the same manner. Total DNA was extracted from the samples that had been embedded in paraffin. To verify the precision of the staining techniques, both positive and negative control sections were included.
RESULTS
Clinical features
Among the five patients analyzed, four were males, and one was female. The ages of the patients varied between 48 and 63 years, with a mean age of 55 years and a median age of 52 years. The tumors were found to involve the bilateral lung lobes in three patients and the lower right lobe in two patients. The main clinical symptoms reported included fever (4/5, 80%), which was often accompanied by cough and hemoptysis (3/5, 60%). Chest CT scans and the original CT images were available for all five patients. The majority of patients presented with multiple nodules (4/5) [Figure 1a and b], with four out of five demonstrating bilateral lung nodules; one had lesions confined to only the right lung. The borders of the lesions were typically ill-defined. In addition, three out of five cases involved the nasal cavity at initial diagnosis, one involved the skin of the chest wall, and one involved the brain and ileum. The clinical data for these five patients are summarized in Table 1.
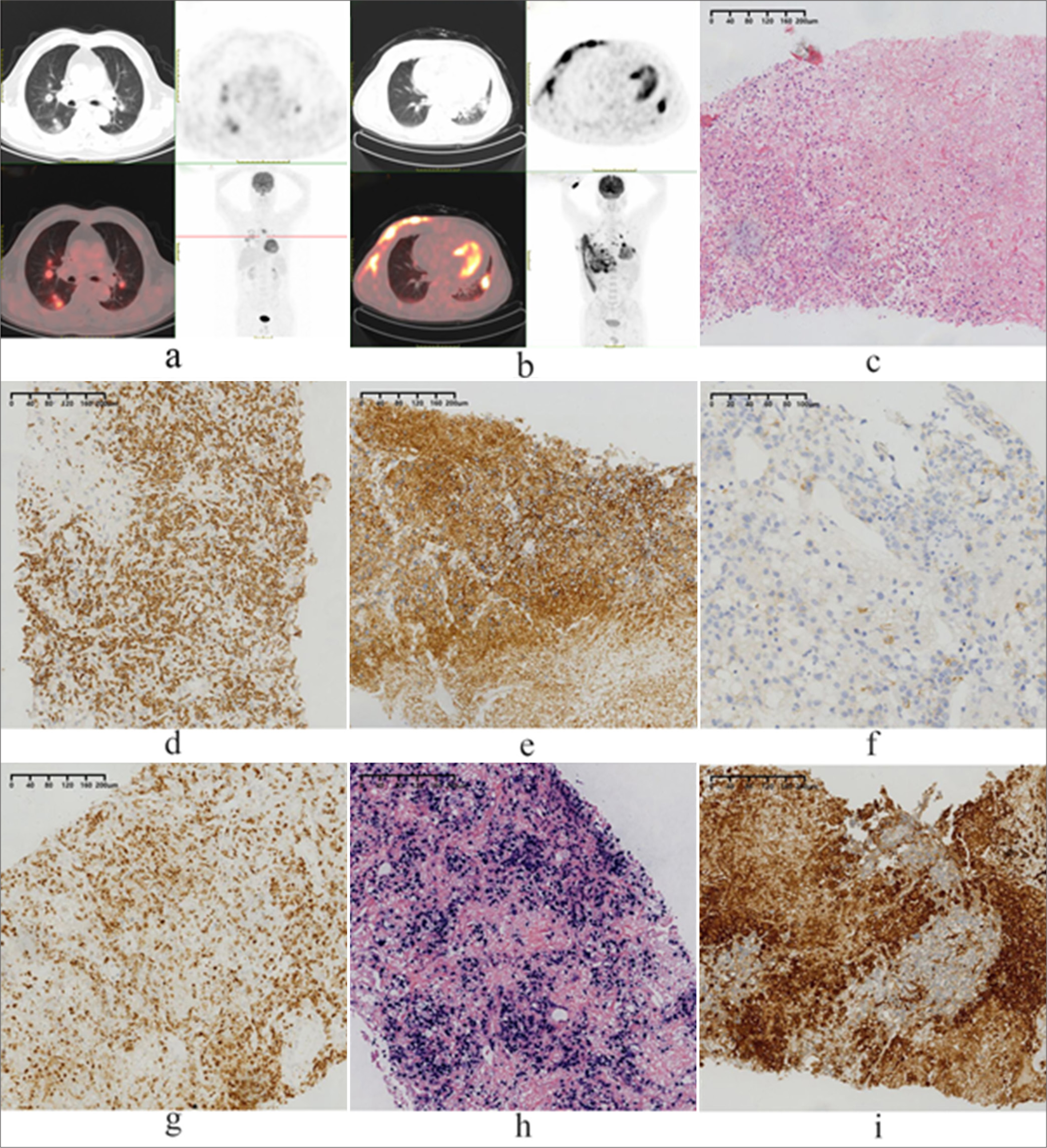
- (a) Case 1 positron emission tomography/computed tomography (PET/CT) scan showing multiple occupying lesions located in the bilateral lung lobes, with involvement of the bronchus and blood vessels. (b) Case 2 PET/CT scan showing pulmonary consolidation with involvement of the chest wall and multiple lymph nodes. (c) Small- to medium-sized tumor cells infiltrate and destroy alveolar walls (hematoxylin and eosin, magnification, ×100); (d) Immunohistochemical staining was positive for CD3ε (magnification, ×100); (e) Immunohistochemical staining was positive for CD56 (magnification, ×100); (f) Immunohistochemical staining was negative for CD56 (magnification, ×200); (g) Immunohistochemical staining was positive for perforin (magnification, ×100); (h) fluorescence in situ hybridization for Epstein–Barr virus-encoded ribonucleic acid (RNA) was positive (magnification, ×100); (i) Immunohistochemical staining was positive for PD-L1 (magnification, ×100).
| Case | Sex | Age (years) | Presentation | Radiographic findings | Accumulation of extrapulmonary organs | Diagnostic intervention | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 63 | Cough | MOL in bilateral lobes | Nasal cavity | PLP | P-GEMOX✶6 | 12 |
| 2 | Male | 52 | Fever | MOL in bilateral lobes | Chest wall | PLP | CHOP✶2 | 3 |
| 3 | Male | 48 | Cough, fever, chest tightness, hemoptysis | Solid nodules of the right lung | Nasal cavity | PLP | ESA✶4, PD-1+Gemox✶6, EBV-CTL✶1 | 24 |
| 4 | Male | 59 | Cough, fever, shiver | MOL in bilateral lobes | Brain, jejunum | PLP | Supporting therapy | 1 |
| 5 | Female | 52 | Fever | MOL in bilateral lobes | Nasal cavity, cheek | PLP | P-GEMOX✶4 | 8 |
MOL: Multiple occupancy lesions, PLP: Percutaneous lung puncture, P-GEMOX: Pegaspargase, gemcitabine, L-asparaginase, and oxaliplatin, CHOP: Cyclophosphamide, doxorubicin, vincristine, prednisone, ESA: Etoposide, steroids, pegaspargase, EBV-CTL: Epstein–Barr virus-specific cytotoxic T lymphocyte, ENKTL: Extranodal natural killer (NK )/T-cell lymphoma, PD-L1: Programmed death ligand-1
Pathology findings
Cytologically, the neoplastic cells varied in size and appearance, including small- to medium-sized cells in one patient, medium-sized cells in three patients, and large pleomorphic cells with clear to pale cytoplasm in one patient. Irregular nuclear folds and distorted nuclei were observed, with medium cytoplasmic content and visible or prominent nucleoli. Mitotic figures were notably abundant. Histomorphologically, alveolar infiltration and destruction were present in all the patients [Figure 1c]. Tumor cells were detected within alveoli in three patients, whereas in one patient, tumor cells surrounded blood vessels, infiltrated vessel walls, and led to map-like necrosis due to vessel destruction. The neoplastic infiltrate included inflammatory non-neoplastic cells such as neutrophilic granulocytes, lymphocytes, and plasma cells, with eosinophils being rare. All five patients had neoplastic cells that were CD3+ and CD2+ [Figure 1d]. Four of the five patients had neoplastic cells that were CD56+ [Figure 1e], and patient 2 was CD56 negative [Figure 1f]. All five patients expressed cytotoxic granules (TIA-1+, granzyme B+, perforin+) [Figure 1g], and EBER was all positive [Figure 1h]. but T-cell surface markers such as CD5, CD4, and CD8 were not detected. None of the patients tested positive for B-cell markers (such as CD20 and CD79a). Two patients were tested for PD-L1, and one of these demonstrated positive staining (TPS 80%) [Figure 1i]. The Ki-67 index ranged from 40% to 90% in five patients, and EBER was detected in all patients through FISH. The histopathological information and immunophenotypes are listed in Table 2.
| Case | Cell size | T-lymphocyte marker | B-lymphocyte marker CD56 | Cytotoxic granules | EBER | PD-L1 | Ki67 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD3 | CD4 | CD5 | CD20 | CD79α | TIA-1 | granzyme B | perforin | ||||||
| 1 | M | + | - | - | - | - | + | + | + | + | + | / | 40 |
| 2 | M | + | - | - | - | - | - | + | + | + | + | / | 60 |
| 3 | M | + | - | - | - | - | + | + | + | + | + | 80% | 80 |
| 4 | S-M | + | - | - | - | - | + | + | + | + | + | 0% | 90 |
| 5 | L | + | - | - | - | - | + | + | + | + | + | / | 70 |
L: Large size, M: Medium size, S: Small size, EBER: EBV-encoded ribonucleic acid (RNA ), PD-L1: Programmed death ligand-1,+: Positive, –: Negative,/: No, ENKTL: Extranodal natural killer (NK )/T-cell lymphoma, TIA-1: T-cell intracellular antigen-1, CD: Cluster of differentiation
Treatment and follow-up
Survival time was calculated from the date of pulmonary ENKTL diagnosis. Patient 1 underwent six courses of pegaspargase, gemcitabine, L-asparaginase, and oxaliplatin (P-GEMOX) chemotherapy, resulting in a survival time of 12 months. Patient 2 received two courses of cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy, with a survival time of 3 months. Patient 3 received four courses of etoposide, steroids, and pegaspargase chemotherapy, followed by PD-L1 monoclonal antibodies combined with the Gemox regimen after disease progression. Despite six courses of treatment, the disease remained uncontrolled. Subsequent reinfusion of EBV-specific cytotoxic T lymphocytes (EBV-CTLs) led to further disease progression and eventual death, with a survival time of 24 months. Patient 4 received only adjuvant supportive treatment and survived for 1 month. Patient 5 underwent four courses of P-GEMOX chemotherapy, resulting in a survival time of 8 months. Among the patients, three out of five underwent bone marrow biopsy, which revealed no neoplastic cell infiltration. The treatment and follow-up information are summarized in Table 1.
DISCUSSION
Pulmonary ENKTL represents a rare subset of lymphoma. ENKTL represents 0.5–1.0% of mature T-cell/NK-cell (T/NK) lymphomas[8] and has a relatively high prevalence in Southeast Asia. ENKTL primarily affects the nasal cavity and is frequently diagnosed in the early stages; however, it tends to infiltrate bone tissue. Extranasal manifestations commonly involve the skin, gastrointestinal tract, testicles, and soft tissues, with pulmonary involvement being exceptionally uncommon. To date, pulmonary ENKTL cases are limited to a few reported instances, primarily as case studies. Large-scale investigations and prognosis studies are lacking.
Activated mature NK cells and cytotoxic T lymphocytes are believed to be the sources of lung ENKTL cells. CD56 is a sensitive marker of NK cells. In our study, CD56 expression was negative in 1 out of 5 patients, suggesting a potential diagnosis of NK cell lymphoma with loss of CD56 expression or cytotoxic T cell markers expression. The exact cause of pulmonary ENKTL is unknown, but all cases are associated with EBV infection, resulting in a type II latent infection pattern with a high frequency of 30 base pair deletions in the latent membrane protein-1 gene. Elevated plasma EBV DNA levels are linked to poor treatment response and prognosis.
The gene expression profile of lung ENKTL resembles that of ENKTL in other locations. The activation of the Janus kinase/ signal transducers and activators of transcription (JAK/STAT) signaling pathway is important in the pathogenesis of ENKTL, with JAK3 phosphorylation at Tyr980 detected in 31–87% of cases and STAT3 phosphorylation at Tyr705 detected in 90% of cases. In addition, the vascular endothelial growth factor receptor, platelet-derived growth factor receptor, Notch, and Aurora kinase A signaling pathways may also play a role in pathogenesis. Unfortunately, due to the retrospective nature of our analysis, we were unable to detect gene expression profile abnormalities specific to lung ENKTL for further investigation.
CT scans usually display ambiguous results, including various patchy lesions, mass-like formations, or several nodules, which can lead to misdiagnosis, especially when considering inflammatory conditions such as acute pneumonia.[9-11] The presence of the halo sign and floating vessel sign on enhanced CT scans can be helpful in making a correct diagnosis. PET/CT plays a crucial role in the diagnostic process of pulmonary ENKTL, particularly in identifying involved lymph nodes and extranodal involvement, including microscopic lesions. All five patients with pulmonary ENKTL in our study underwent PET/CT examinations either at the initial diagnosis or during follow-up visits. Early detection, diagnosis, and treatment are vital for improving patient outcomes.
The typical pathological changes in pulmonary ENKTL include a scattered or focal distribution of significantly atypical tumor cells within various inflammatory cells and extensive necrosis. These tumor cells may exhibit morphological characteristics of large, medium, or small lymphocytes, with a predominance of medium-sized cells. The infiltration of both large and small tumor cells is common and is often accompanied by prominent mitotic figures and nucleoli. Angiocentric growth and destructive vascular infiltration may also be observed in some cases.
Tumor cells generally express T-cell markers (CD3/CD43) and NK-cell markers (CD56), along with cytotoxic granules (TIA-1+, Granzyme B+, perforin+); however, tumor cells do not express B-cell or tissue cell markers. In addition, EBER positivity is essential for diagnosis. For CD56-negative patients, EBER must be included in the diagnostic panel. Only when EBER is positive, and T-cell markers and cytotoxic T-cell markers are simultaneously expressed can the possibility of ENKTL be considered in conjunction with morphological features. For patients who are positive for CD56 and negative for EBER, the diagnosis of ENKTL must be approached with caution. The PD-L1 positivity rate in ENKTL tumor cells ranges from 39% to 100%. The expression of PD-1/PD-L1 is increased through various mechanisms, including EBV infection, gene mutation, and cytokine stimulation.[12] Positive PD-L1 expression appears to be a potential predictor of treatment benefits.[13] In this study, two cases were evaluated for PD-L1 expression, one of which tested positive, resulting in a positivity rate of 50%. The disease progression observed in PD-L1-positive patients following the administration of immune checkpoint inhibitors may be influenced by the limited sample size. The Ki-67 proliferation index exhibits a wide range (40–90%). A previous study indicated that a high Ki-67 index (cutoff value of 60%) is correlated with a poor prognosis.[14] Conversely, another interesting study revealed that a Ki-67 index exceeding 70% does not correlate with ENKTL.[15] In our study, the Ki-67 index for patient 1 was 40% (<60%), and the survival time was longer than that of the other patients. However, owing to the limited number of cases, further analysis and research are needed to determine its relationship with prognosis.
The differential diagnosis of pulmonary ENKTL can be challenging due to the rarity of the disease and the limited amount of tissue available from needle biopsies, particularly in the presence of non-specific inflammation during the early stages or advanced disease resembling aggressive NK cell leukemia. Accurate diagnosis requires a comprehensive approach, including histopathological examination, immunohistochemical staining, and FISH for EBER.
Compared with PTCL, follicular helper T-cell lymphoma (TFH lymphoma), and anaplastic large-cell lymphoma (ALCL), ENKTL is nearly universally EBER positive. In contrast, TFH lymphomas exhibit positive staining for markers associated with lymph node TFH follicular helper T lymphocytes, such as CD10, BCL-6, and chemokine (C-X-C motif) ligand 13. ALCL is characterized by CD30 positivity, whereas T-cell markers may be absent to varying degrees.
Small-cell or mixed pulmonary ENKTL can be easily mistaken for non-specific inflammatory lesions. Both conditions typically present with fever, cough, and dyspnea initially, with no specific imaging findings. On histopathological examination, normal small lymphocytes in the lung may exhibit mild atypia and slight enlargement, leading to potential misdiagnosis. However, features such as infiltrative growth of tumor cells, destruction of alveolar walls, vascular invasion, a significant population of atypical cells, positive CD3 and CD56 staining, and in situ hybridization for EBER can aid in establishing a clear diagnosis. Other tumors, such as small-cell carcinoma of the lung, also need to be carefully differentiated from small-cell ENKTL.
Medium-cell pulmonary ENKTL should be differentiated from granulomatosis with polyangiitis (GPA), previously referred to as Wegener’s granulomatosis. Both conditions share many morphological characteristics, such as mixed inflammatory cell infiltration and extensive lung tissue necrosis, with vasculitis of the arteries and veins present. Neutrophils are prevalent and often generate microabscesses. However, GPA is characterized by positive anti-neutrophil cytoplasmic antibodies and lacks CD56 and cytotoxic molecule-positive and EBER-positive atypical T/NK cells.
Distinguishing large-cell pulmonary ENKTL from lymphomatoid granulomatosis (LYG) and EBV-positive diffuse large B-cell lymphoma (EBV + DLBCL) is crucial. LYG is a distinct form of extranodal T-cell-rich large B-cell lymphoproliferative disorder, with lung involvement in more than 90% of cases.[16] LYG is commonly associated with EBV, demonstrating angiocentric and angiodestructive atypical lymphocyte infiltration amidst a marked inflammatory background, reminiscent of ENKTL. However, in LYG, atypical cells express B-cell markers but not T/NK-cell markers.
EBV + DLBCL typically presents with scattered large tumor cells expressing EBV in a background rich in T lymphocytes, particularly in biopsy samples. Importantly, the number of tumor cells may be lower in biopsy tissues, and there may be significant tissue extrusion. The identification of these large cells through immunohistochemistry is crucial, as they can be classified as either B-derived or T-derived cells, which is essential because the treatment and prognosis of the two are completely different.
The prognosis for patients with ENKTL is generally unfavorable. In recent years, the application of concurrent chemoradiotherapy and L-asparaginase/pegaspargase-based chemotherapy has significantly improved the survival of ENKTL patients. The SMILE regimen (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) and the P-GEMOX regimen have been recommended by the National Comprehensive Cancer Network guidelines as first-line chemotherapy regimens for ENKTL.[17] High doses of chemotherapy in conjunction with hematopoietic stem cell transplantation (HSCT) represent a standard method for treating aggressive lymphomas in advanced stages that respond well to chemotherapy. In a retrospective study, the complete remission (CR) rate of 14 advanced-stage ENKTL patients increased from 51.6% to 65.5% after receiving autologous HSCT.[18] In another retrospective analysis, 14 of 18 allogeneic HSCT patients received SMILE chemotherapy before HSCT, and 13 patients were in advanced stages at presentation, with 89% of patients achieving remission. After a median follow-up period of 20.5 months, the projected 5-year rates for progression-free survival (PFS) and overall survival (OS) were 51% and 57%, respectively.[19]
While L-asparaginase-based chemotherapy and HSCT have been successful in saving the lives of certain patients, only 30–40% of patients achieve favorable treatment outcomes.[20] Therefore, for relapsed and refractory patients, more effective treatment strategies are critical.
Pulmonary ENKTL cells highly express PD-1. In an interesting study, the humanized monoclonal antibody anti-PD-1, known as pembrolizumab, was administered to a total of seven patients suffering from refractory ENKTL who did not respond to treatment with L-asparaginase-based regimens (n = 7) and had undergone allogeneic HSCT (n = 2). After a median of seven cycles of pembrolizumab, five patients achieved CR.[21] In our study, a patient with advanced disease was treated with a PD-1/PD-L1 inhibitor (tislelizumab) combined with the Gemox regimen for six courses, resulting in disease progression, as shown on chest CT scans. The therapeutic effects of EBV-CTLs in ENKTL are noteworthy. In a study involving 10 patients with ENKTL who received autologous EBV-CTL following the achievement of CR with induction therapy, the 4-year OS and PFS rates were 100% and 90%, respectively.[22] Other treatment options, including daratumumab (an anti-CD38 antibody) and brentuximab vedotin (an anti-CD30 antibody), have been reported to increase survival in advanced ENKTL patients.
Patients with ENKTL presenting initially with lung involvement often have inconspicuous clinical symptoms, leading to frequent misdiagnosis. These patients are generally in stages III and IV when diagnosed, significantly decreasing their prognosis. In this study, all five patients were in stage IV at the time of initial diagnosis, and four out of five patients received first-line chemotherapy, but the treatment effect was poor. One out of five patients who received only supportive therapy had a survival time of only 1 month. Therefore, the accurate diagnosis of early-stage pulmonary ENKTL is of paramount importance. However, the number of lung ENKTL patients was relatively small. In addition, the characteristics of puncture biopsy samples, such as extensive necrosis and a high number of miscellaneous cellular components, greatly increase diagnostic difficulty, making lung puncture biopsy samples prone to be misdiagnosed. Some cases even require multiple biopsies or tissue samples from other sites to confirm the diagnosis. For early detection of pulmonary ENKTL, clinicians should conduct comprehensive examinations and perform puncture biopsies as soon as possible. Pathologists should consider ENKTL when evaluating lung puncture specimens to identify clues that may help reduce the risk of misdiagnosis and missed diagnosis. For patients with advanced disease, it is essential to actively utilize radiotherapy, chemotherapy, and novel treatments to prolong survival.
SUMMARY
Pulmonary ENKTL is typically diagnosed at an advanced clinical stage, lacks specific imaging manifestations, and is challenging to diagnose through biopsy. There is an urgent need for large sample size data to facilitate analysis of its characteristics and the development of innovative treatment strategies.
ACKNOWLEDGMENT
Not applicable.
AVAILBILITY OF DATA AND MATERIALS
The data and materials are available upon reasonable request. All data are available from the corresponding author.
ABBREVIATIONS
EBER: EBV-encoded RNA
EBV: Epstein–Barr virus
ENKTL: Extranodal NK/T-cell lymphoma
GPA: Granulomatosis with polyangiitis
PET/CT: Positron emission tomography/computed tomography
AUTHOR CONTRIBUTIONS
QL: Designed the research study; XW and HS: Data curation; QL and YXZ: Performed the research; DW: Provided help and advice on the experiments; DW: Analyzed the data and wrote. All the authors contributed to editorial changes in the manuscript. All the authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by Xuzhou Central Hospital biomedical research Ethics review Committee (approval no.XZXY-LK-20240830-0137). This study has obtained all appropriate patient consent.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of the CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors were blinded to the reviewers and vice versa) through an automatic online system.
FUNDING: This project is funded by the 14th 5-year Medical Key Discipline Fund of Jiangsu Province (ZDXK202237).
References
- WHO classification of tumors of hematopoietic and lymphoid tissues, 2022 (5th edition): Lymphoid tumors. Arkh Patol. 2023;85:24-31.
- [CrossRef] [PubMed] [Google Scholar]
- The pathology of NK-cell lymphomas and leukemias. Adv Anat Pathol. 2005;12:27-34.
- [CrossRef] [PubMed] [Google Scholar]
- NK/T-cell lymphomas 'nasal type': An Italian multicentric retrospective survey. Ann Oncol. 2006;17:794-800.
- [CrossRef] [PubMed] [Google Scholar]
- Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol. 2007;43:4-14.
- [CrossRef] [PubMed] [Google Scholar]
- T-cell and NK-cell lymphomas in the lung. Semin Diagn Pathol. 2020;37:273-82.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous dissemination of nasal NK/T-cell lymphoma with bone marrow, liver and lung involvement. Clin Exp Dermatol. 2002;27:120-2.
- [CrossRef] [PubMed] [Google Scholar]
- Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): An update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep. 2016;11:514-27.
- [CrossRef] [PubMed] [Google Scholar]
- Extranodal natural killer T-cell lymphoma, nasal-type: A prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary extranodal natural killer/T-Cell lymphoma (Nasal Type): A case report and radiological image review. Medicine (Baltimore). 2015;94:e1527.
- [CrossRef] [PubMed] [Google Scholar]
- Primary extranodal NK/T-cell lymphoma of the lung: Mimicking bronchogenic carcinoma. Thorac Cancer. 2014;5:93-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acute respiratory distress syndrome emerging after surgical debridement in a patient with extranodal natural killer/T cell lymphoma. BMC Pulm Med. 2021;21:27.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: A potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66:877-90.
- [CrossRef] [PubMed] [Google Scholar]
- The predictive implication of programmed cell death ligand 1 expression in extranodal natural killer/T-Cell lymphoma and its correlation with clinicopathological features: A systematic review and meta-analysis. Transl Cancer Res. 2023;12:2115-27.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of Ki-67 antigen expression in extranodal natural killer/T-cell lymphoma, nasal type. MED Oncol. 2014;31:218.
- [CrossRef] [PubMed] [Google Scholar]
- A phase II study of ifosfamide, methotrexate, etoposide, and prednisolone for previously untreated stage I/II extranodal natural killer/T-cell lymphoma, nasal type: A multicenter trial of the Korean Cancer Study Group. Oncologist. 2014;19:1129-30.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary lymphomatoid granulomatosis. Evidence for a proliferation of Epstein-Barr virus infected B-lymphocytes with a prominent T-cell component and vasculitis. Am J Surg Pathol. 1994;18:753-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics and prognostic analysis of extranodal NK/T-Cell lymphoma patients treated with pegaspargase based chemotherapy. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29:735-40.
- [Google Scholar]
- Clinical outcomes and prognostic factors of Up-front autologous stem cell transplantation in patients with extranodal natural killer/T cell lymphoma. Biol Blood Marrow Transplant. 2015;21:1597-604.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic haematopoietic SCT for natural killer/T-cell lymphoma: A multicentre analysis from the Asia lymphoma study group. Bone Marrow Transplant. 2014;49:902-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834-9.
- [CrossRef] [PubMed] [Google Scholar]
- PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437-42.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome of extranodal NK/T cell lymphoma patients treated with postremission therapy using EBV LMP1 and LMP2a-specific CTLs. Mol Ther. 2015;23:1401-9.
- [CrossRef] [PubMed] [Google Scholar]









