Translate this page into:
A mechanism study of tripartite motif 10 modulating septic cardiomyopathy

*Corresponding author: Jie Su, Department of Emergency, Fourth Hospital of Hebei Medical University, Shijiazhuang, China. 18531117582@163.com
-
Received: ,
Accepted: ,
How to cite this article: Yang Z, Su J. A mechanism study of tripartite motif 10 modulating septic cardiomyopathy. CytoJournal. 2024;21:73. doi: 10.25259/Cytojournal_155_2024
Abstract
Objective:
Septic cardiomyopathy (SCM), as a complication of the septic process, severely affects the myocardial function of patients, but its pathogenesis remains unclear. The article aims to explore the mechanism of tripartite motif 10 (TRIM10) in rats with SCM and provide animal experimental basis for the treatment and prevention of SCM.
Material and Methods:
An SCM rat model was constructed by intraperitoneal injection of lipopolysaccharide (LPS). Sh-NC and sh-TRIM10 groups were injected with sh-NC and sh-TRIM10 in the tail vein for 3 consecutive days before SCM modeling. The expression of TRIM10 was detected by Western blot and reverse transcription–polymerase chain reaction analyses. Hematoxylin–eosin staining was performed to observe pathological changes in myocardium. Cardiomyocyte apoptosis was detected by flow cytometry. Serum levels of cardiac troponin I, myohemoglobin, creatine kinase-MB, interleukin-18 (IL-18), interleukin-1 β (IL-1β), tumor necrosis factor-α (TNF-α), superoxide dismutase, and glutathione peroxidase (GSH-Px) were detected by enzyme-linked immunosorbent assay. Apoptosis-related proteins and toll-like receptor 4 (TLR4)/nuclear transcription factor-κB (NF-κB) pathway-related proteins were explored by Western blot assay.
Results:
TRIM10 expression increased in the LPS group (P < 0.0001). Myocardial tissue injury in SCM rats was improved after TRIM10 reduction compared with that in the LPS group. Knockdown of TRIM10 decreased the levels of MDA (P < 0.01), IL-18 (P < 0.0001), IL-1β (P < 0.0001), and TNF-α (P < 0.0001) and increased the contents of SOD (P < 0.001) and GSH-Px (P < 0.001) compared with those in the LPS group. TRIM10 reduced the apoptosis of H9C2 cells (P < 0.0001). After TRIM10 interference, the expression of p-P65/P65 (P < 0.0001) and TLR4 (P < 0.0001) was decreased.
Conclusion:
TRIM10 knockdown can reduce inflammation, oxidative stress, and apoptosis in SCM rats and has a protective effect on cardiomyocytes, which may be attributed to the regulation of the TLR4/NF-κB pathway.
Keywords
Tripartite motif 10
Septic cardiomyopathy
Apoptosis
Toll-like receptor 4/nuclear transcription factor-κB pathway
INTRODUCTION
Sepsis is a multi-organ dysfunction syndrome induced by immune regulation disorder caused by pathogenic microorganisms.[1] It is characterized by severe morbidity, high mortality, and poor prognosis. The heart is the most vulnerable target organ in the course of sepsis, and cardiomyopathy caused by sepsis is called septic cardiomyopathy (SCM), which is a complication of severe sepsis and septic shock.[2,3] The mortality rate of patients with SCM can be as high as 70%, which is higher than that of patients with simple sepsis.[4] The pathophysiological mechanism of SCM is very complex and includes inflammatory response, apoptotic injury, mitochondrial dysfunction, autophagy, abnormal regulation of intracellular calcium ion transporters, and energy metabolism disorders.[5,6] However, the specific pathogenesis of SCM remains unclear.
The Tripartite motif (TRIM) family is a new member of E3 ligases with the structure and function of ubiquitin ligases. The TRIM protein family is a large group of proteins with more than 80 members. Functionally, TRIM family proteins participate in the progression of many human diseases by regulating various biological processes of cells.[7,8] The TRIM family can regulate autophagy, signal transduction, apoptosis, and inflammation and is associated with cardiovascular diseases, such as ventricular hypertrophy, myocardial ischemia, and heart failure.[9] Recombinant TRIM72 protects myocardial function by preserving mitochondria in ischemiareperfusion-induced cardiomyocytes.[10] Trim65 inhibited cardiomyocyte hypertrophy in mice with cardiac hypertrophy by increasing mitochondrial density and membrane potential.[11] TRIM8 knockdown inhibited the apoptosis of H9c2 cells induced by H/R and alleviated oxidative stress, thereby reducing myocardial cell damage.[12] Tripartite motif 10 (TRIM10) has the function of E3 ligase, which is mainly involved in the terminal differentiation of erythroid cells and plays a specific role in hematopoietic function and apoptosis.[13] TRIM10 dysfunction may be closely associated with some autoimmune diseases; in particular, low expression of TRIM10 was found in patients with systemic lupus erythematosus.[14] In addition, overexpression of TRIM10 induces cisplatin resistance in osteosarcoma cells.[15] Downregulating TRIM10 can alleviate reactive oxygen species (ROS) generation and cell apoptosis in Parkinson’s disease cell models.[16] Nonetheless, the role of TRIM10 in SCM has not been explored.
In this research, an SCM model was established in rats induced by lipopolysaccharides (LPS). After interfering the expression of TRIM10, the apoptosis of rat cardiomyocytes, the levels of inflammatory factors and oxidative stress response factors, and the effect on toll-like receptor 4 (TLR4)/nuclear transcription factor-κB (NF-κB) pathway were observed. Results provide a theoretical basis for TRIM10 knockdown as an effective therapeutic target for improving myocardial cell damage in SCM.
MATERIAL AND METHODS
Experimental animals
Male standard deviation (SD) rats (281 ± 27 g) were supplied by the animal experiment center of our hospital for routine feeding and were divided into four groups: Control, LPS, LPS + sh-NC, and LPS+sh-TRIM10 groups, with 10 rats in each group. The control group was injected with normal saline into the abdominal cavity of rats, while the sepsis model was accomplished by intraperitoneally injected with LPS (L2880, Sigma–Aldrich, St. Louis, USA) at a dose of 12 mg/kg.[17] Before LPS modeling, the sh-NC group and the sh-TRIM10 group were injected with adenovirus-mediated sh-NC (SHC016, Sigma-Aldrich, St. Louis, USA) and sh-TRIM10 (5’-GCATCCTCTTAGCACAATT-3’) in the tail vein for 3 consecutive days, respectively. The 24 h left ventricular ejection fraction (LVEF) of rats was measured by a small animal ultrasound system, and LVEF<50% proved that the SIC rat model was successfully constructed. After the experiment began, the rats’ reactions, mental health, diet, and activity were closely observed. The animal experiments were approved by the Animal Care and Use Committee of the Fourth Hospital of Hebei Medical University.
Cell culture
H9C2 cells were purchased from Pricella (CL-0089, Wuhan, China), detected by mycoplasma, and verified by STR analysis. H9C2 cells were cultured in a cell incubator set at 37°C with 5% CO2. H9C2 cells were passed, and cells in the logarithmic phase with good cell growth were selected for subsequent study. H9C2 cells were transfected with sh-NC and sh-TRIM10 and then treated with LPS (10 μg/mL) for 24 h.[18] The above transfection was accomplished through Lipofectamine 2000 (11668019, Invitrogen, Carlsbad, California, USA).
Hematoxylin-eosin (HE) staining
The rats were euthanized by inhalation of CO2. The left ventricular myocardial tissues of rats were fixed with 40 g/L paraformaldehyde (F8775, Sigma-Aldrich, St. Louis, USA), dehydrated, paraffin embedded, and sliced. Myocardium injury of the rats was observed under optical microscope (CKX53, Olympus, Tokyo, Japan) after HE staining (PH0516, Phygene, Fuzhou, China).
Flow cytometry (FCM)
Each group of cell suspension was filtered, centrifuged (800 rpm, 4℃, 4 min), and added with phosphate balanced solution (714001, Servicebio, Wuhan, China) to produce single-cell suspension (5 × 105–10 × 106/mL). About 200 μL of the cell suspension was added with 10 μL of annexin X staining solution (C1062S, Beyotime, Shanghai, China) for reaction at room temperature for 15 min, followed by 10 μL of Propidium Iodide solution for 5 min (C1062S, Beyotime, Shanghai, China). Detection was conducted by a flow cytometer (Canto II, BD, Franklin Lake, New Jersey, USA) within 1 h, and analysis was performed by Flowjo 10.2 software (Flowjo, Ashland, USA).
Enzyme-linked immunosorbent assay (ELISA)
After anesthesia (2% pentobarbital sodium, 40 mg/kg, AM00469, Beijing Chemical Reagent Company, Beijing, China), the rats were fixed supine and the chest cavity was cut open to expose the heart. Blood was collected from the heart cavity by using a blood collection needle, and the upper layer of serum was collected after centrifugation at 3000 rpm for 30 min. The contents of interleukin-18 (IL-18), interleukin-1 β (IL-1β), tumor necrosis factor-α (TNF-α), cardiac troponin I (cTnI), myohemoglobin (Mb), creatine kinase-MB (CK-MB), MDA, superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) were determined according to the ELISA kit instructions (IL-18: MM-0194R2, IL-1β: MM-0047R2, TNF-α: MM-0180R2, cTnI: MM-0426R2, Mb: MM-0515R2, CK-MB: MM-0625R2, SOD: MM-0386R2, GSH-Px: MM-20251R2 all from MeiMian, Yancheng, China; MDA: TW1706, TongWei, Shanghai, China).
Reverse transcription-polymerase chain reaction (RTqPCR)
Total RNA was extracted from the heart tissues of rats or H9C2 cells strictly according to the Trizol method (15596026CN, Invitrogen, Waltham, Massachusetts, USA). Reverse transcription was performed by a reverse transcription reagent (D0401, HaiGene, Harbin, China), and real-time quantitative PCR was conducted by SYBR real-time fluorescence quantitative kit (DRR041A, Takara, Dalian, China). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was served as the internal reference. The primer sequences are TRIM10 forward, 5’-CTTCACTGCCTTCTTCAC-3,’ TRIM10 reverse, 5’- AGAGGAGGGAAACTACAC-3’; and GAPDH forward, 5’-GGCAAGTTCAATGGCACAGT-3,’ GAPDH reverse, 5’-TGGTGAAGACGCCAGTAGACTC-3.’ ABI QuantStudio 6 fluorescence quantitative PCR instrument (ABI, Carlsbad, California, USA) was used for detection, and 2-ΔΔCT method was used for data analysis.
Western blot assay
Myocardial tissue and H9C2 cells were fully lysed with lysate (P0013, Beyotime, Shanghai, China). Total protein was obtained from the supernatant after centrifugation for 10 min at 14000 rpm and 4℃. The concentration of the total protein was tested according to the bicinchoninic acid kit (G2026, JingKe, Shanghai, China). After sodium dodecyl sulfatepolyacrylamide gel electrophoresis, the protein samples were transferred onto polyvinylidene Fluoride (PVDF) membrane (H00010, Millipore, Boston, Massachusetts, USA). The membrane was placed in 5% skimmed milk at 4℃ overnight and incubated with primary antibody solution at room temperature for 1 h. The PVDF membrane was incubated in secondary antibody solution for 40 min. After developing with electrochemiluminescence development kit (ZD310, JingKe, Shanghai, China) on the gel imager (GelDoc Go, BIO-RAD, California, USA), the gray value of the target strip was confirmed by Image–Pro Plus 6.0 (Media Cybernetics, California, USA). The relevant antibodies are as follows: Anti-p-p65, 3039, 1:1000, CST, USA; anti-p65, 8242,1:1000 (CST, USA); anti-TLR4, abs132000, 1:1000 (Absin, Shanghai, China); anti-Bcl-2, 28150, 1:1000 (CST, USA); anti-cleaved caspase-3, 9664, 1:1000 (CST, USA); anti-Bax, 2772, 1:1000 (CST, USA); and anti-GAPDH, 2118, 1:1000 (CST, USA). The second antibody was obtained from Abcam (ab6721, 1:5000, Abcam, Cambridge, UK).
Statistical method
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) 13.0 software (SPSS Inc., Chicago, USA) and GraphPad Prism 8.0 software (GraphPad Software, California, USA). Quantitative data are expressed as mean ± SD and tested using one-way analysis of variance followed by Tukey’s post-hoc test. P < 0.05 is considered to have statistically significant differences.
RESULTS
Expression of TRIM10 in myocardial tissue of SCM rats
The interference rate of sh-TRIM10 was verified by Western blot assay and RT-qPCR. TRIM10 expression in sh-TRIM10 group was successfully reduced compared with that in the sh-NC group [P < 0.01, Figure 1a-c]. After LPS treatment, the protein expression of TRIM10 was significantly higher than that of the control group. The protein expression level of TRIM10 in the LPS+sh-TRIM10 group was lower than that in the LPS+sh-NC group [P < 0.0001, Figure 1d and e]. Unsurprisingly, the same trend was observed in mRNA tests [P < 0.01, Figure 1f]. The results showed that TRIM10 expression interfered successfully.
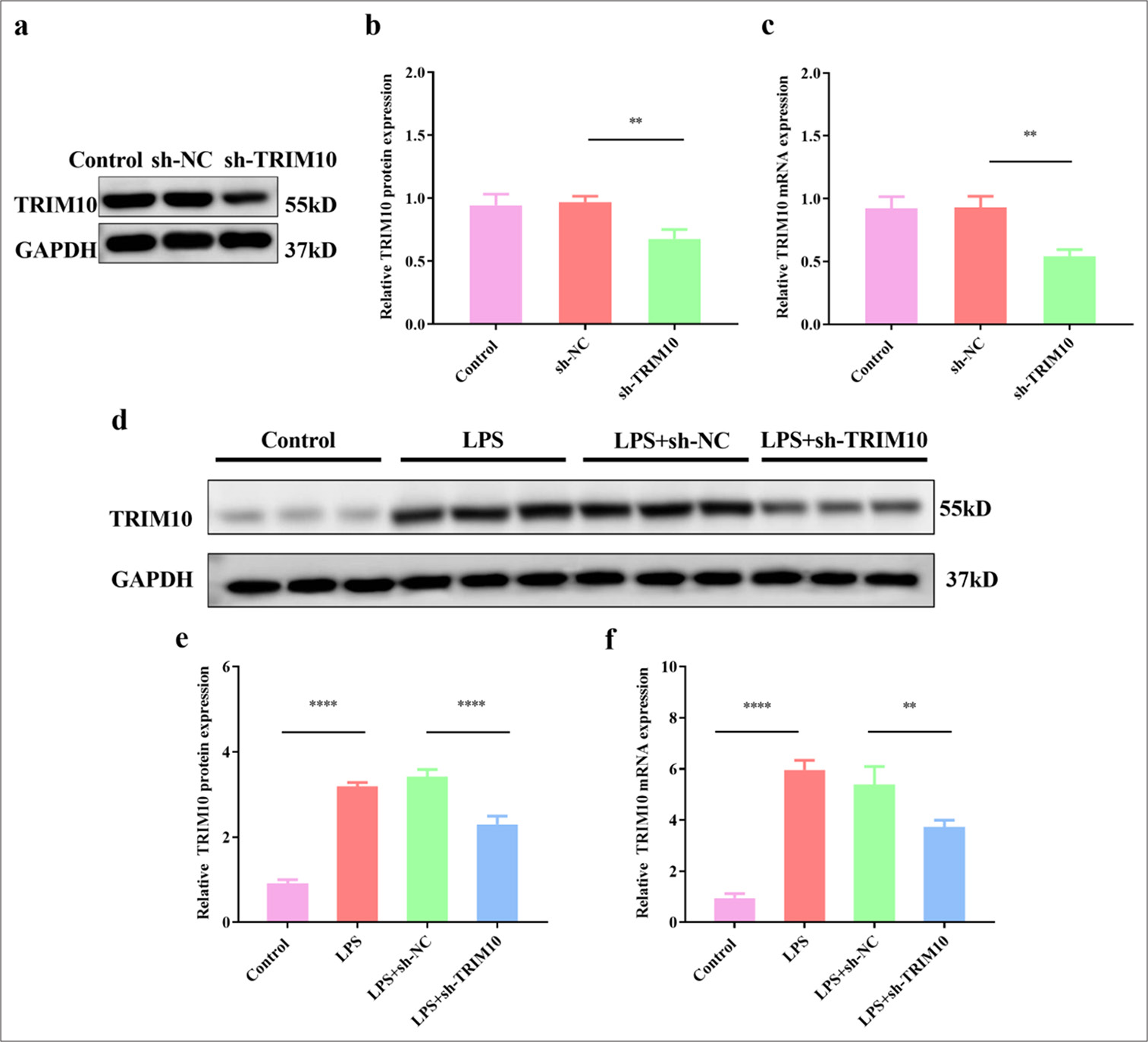
- Expression of TRIM10 in myocardial tissue of rats with SCM. (a and b) The protein expression of TRIM10 was detected by Western blot assay. (c) The mRNA expression of TRIM10 was detected by RT-qPCR. (d and e) The protein expression of TRIM10 after LPS treatment was detected by Western blot assay. (f) The mRNA expression of TRIM10 after LPS treatment was detected by RT-qPCR. ✶✶P<0.01, ✶✶✶✶P<0.0001, n=3. TRIM10: Tripartite motif 10, RT-qPCR: Reverse transcription-polymerase chain reaction, LPS: Lipopolysaccharide, SCM: Septic cardiomyopathy, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, sh-NC: short hairpin-negative control.
Knockdown of TRIM10 repress myocardial damage in SCM model
Rats in the normal group had rapid reaction, stable breathing, normal diet, and urination. All the rats in the LPS, LPS+sh-NC, and LSP+sh-TRIM10 groups showed different degrees of slow movement, diarrhea, rapid heart rate, and mental malaise. After 24 h of modeling, the morphology and structure of myocardial cells in the normal group were complete, the cell arrangement was regular, the edge was clear, and the integrity of myocardial fibers was not damaged. In the LPS group, myocardial fibers were broken, and the cells were deformed and disordered. The myocardial fiber rupture in the LPS+sh-TRIM10 group was reduced, and other pathological changes were improved [Figure 2a]. The ELISA results indicated that the levels of cTnI (P < 0.0001), Mb (P < 0.0001), and CK-MB (P < 0.0001) increased in the LPS group. The levels of cTnI (P < 0.0001), Mb (P < 0.0001), and CK-MB (P < 0.0001) in the LPS+shRNA-TRIM10 group were clearly reduced [Figure 2b-d].
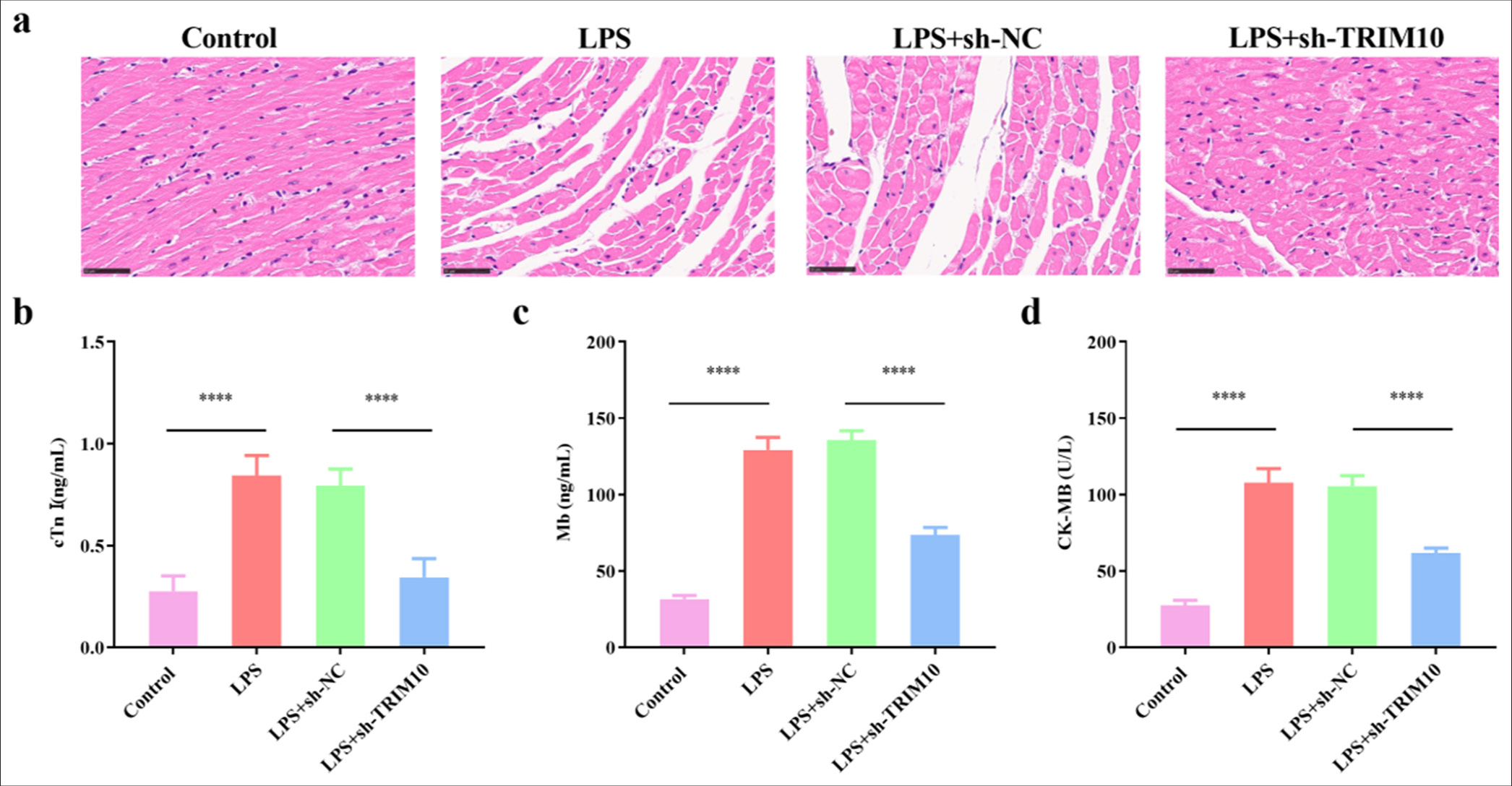
- Knockdown of TRIM10 repress myocardial damage in SCM model. (a) HE staining, magnification 100x, showed the pathological changes of myocardial tissues in each group (scale: 50 μm). The contents of cTnI (b), Mb (c), CK-MB (d) were detected by ELISA. ✶✶✶✶P<0.0001, n=5. SCM: Septic cardiomyopathy, HE: Hematoxylin-eosin, cTnI: Cardiac troponin I, Mb: Myohemoglobin, CK-MB: Creatine kinase-MB, ELISA: Enzyme-linked immunosorbent assay, TRIM10: Tripartite motif 10, LPS: Lipopolysaccharide, sh-NC: short hairpin-negative control.
Knockdown of TRIM10 regulates inflammatory response and oxidative stress in rats with SCM
IL-18 level was notably increased in the LPS group (P < 0.0001) but decreased after TRIM10 was knocked down [P < 0.0001, Figure 3a]. IL-1β level increased in the LPS group (P < 0.0001) but decreased after TRIM10 was knocked down [P < 0.0001, Figure 3b]. TNF-α level was notably increased in the LPS group (P < 0.0001) but decreased after TRIM10 was knocked down [P < 0.0001, Figure 3c]. As shown in [Figure 3d and e], the levels of GSHPx (P < 0.0001) and SOD (P < 0.0001) in the myocardial tissue of rats in the LPS group decreased, while sh-TRIM10 increased the levels of GSH-Px (P < 0.001) and SOD (P < 0.001). MDA content in the LPS group was dramatically increased (P < 0.0001), and TRIM10 knockdown decreased the content [P < 0.01, Figure 3f].
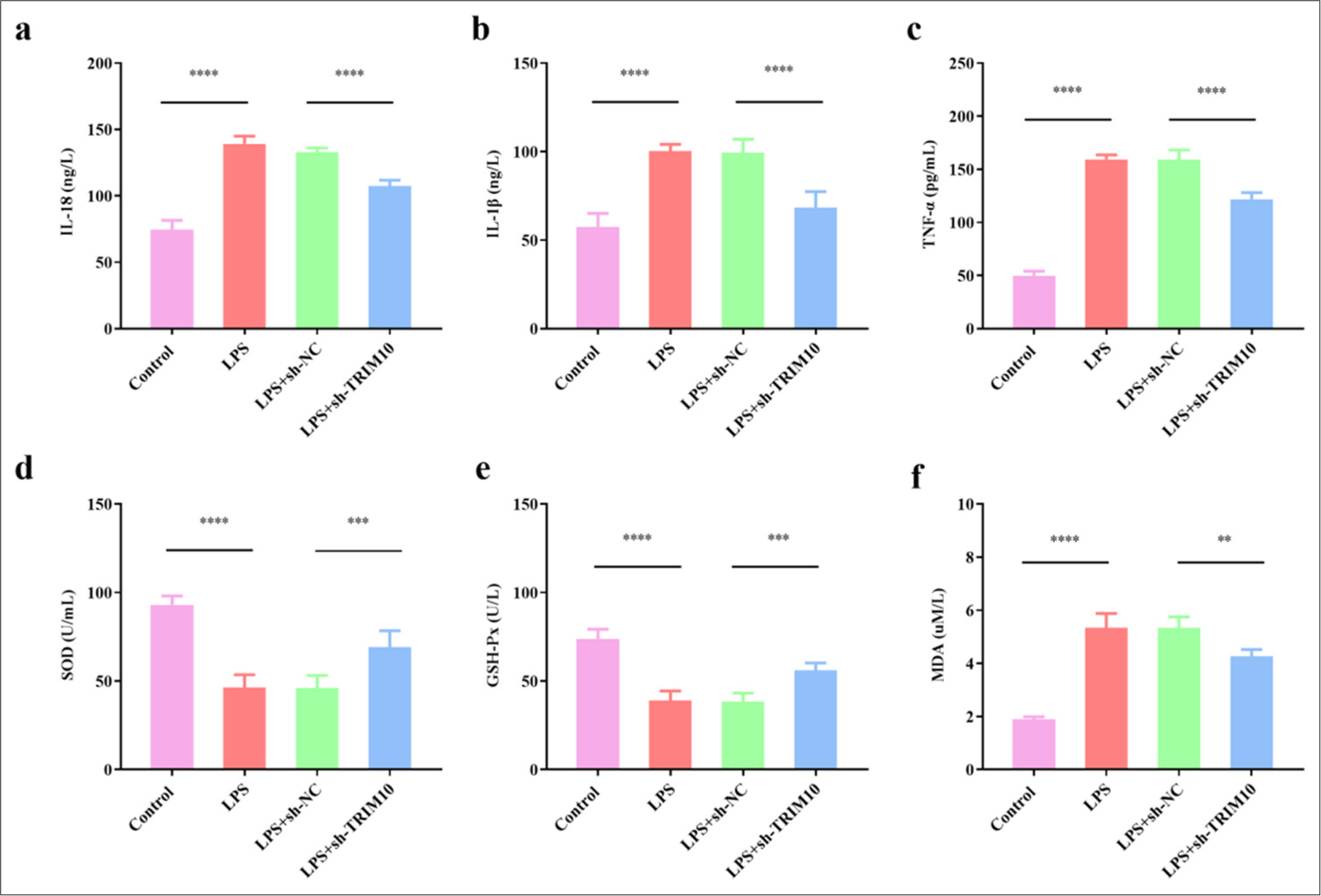
- Knockdown of TRIM10 regulates oxidative stress and inflammatory response in rats with SCM. (a) IL-18 content. (b) IL-1β content. (c) TNF-α content. (d) SOD content. (e) GSH-Px content. (f) MDA content. ✶✶P<0.01, ✶✶✶P<0.001, ✶✶✶✶P<0.0001, n=5. IL-18: Interleukin-18, IL-1β: Interleukin-1 β, TNF-α: Tumor necrosis factor-α, SOD: Superoxide dismutase, GSH-Px: Glutathione peroxidase, MDA: Malondialdehyde, TRIM10: Tripartite motif 10, LPS: Lipopolysaccharide, sh-NC: short hairpin-negative control.
Knockdown of TRIM10 inhibits apoptosis of cardiomyocytes
TRIM10 knockdown was transfected into LPS-treated H9C2 cells to investigate its effect on cardiomyocyte apoptosis [Figure 4a-c]. The FCM results displayed that the apoptosis of cardiomyocytes was obvious in the LPS group (P < 0.0001), but sh-TRIM10 inhibited the apoptosis induced by LPS [P < 0.0001, Figure 5a and b]. The effect of TRIM10 knockdown on apoptosis-associated proteins was verified by Western blot assay. The expression of Bcl-2 decreased in the LPS group (P < 0.0001), while the expression of Bax (P < 0.0001) and cleaved caspase-3 (P < 0.0001) increased. In the LPS+ sh-TRIM10 group, the expression of Bax (P < 0.001) and cleaved caspase-3 (P < 0.0001) decreased, while that of Bcl-2 increased [P < 0.05, Figure 5c-f]. TRIM10 knockdown reduced the apoptosis of cardiomyocytes to a certain extent.
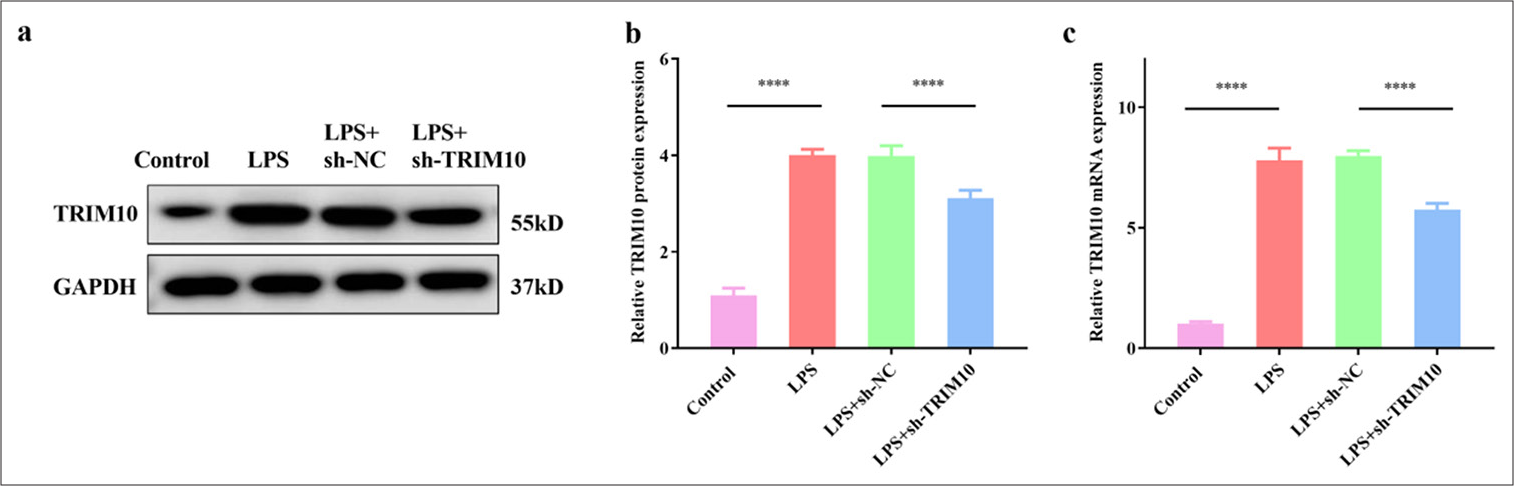
- TRIM10 was knocked down in H9C2 cells. (a and b) Protein expression of TRIM10 was detected by Western blot assay. (c) The mRNA expression of TRIM10 was detected by RT-qPCR. ✶✶✶✶P<0.0001, n=5. RT-qPCR: Reverse transcription-polymerase chain reaction. TRIM10: Tripartite motif 10, LPS: Lipopolysaccharide, sh-NC: short hairpin-negative control.
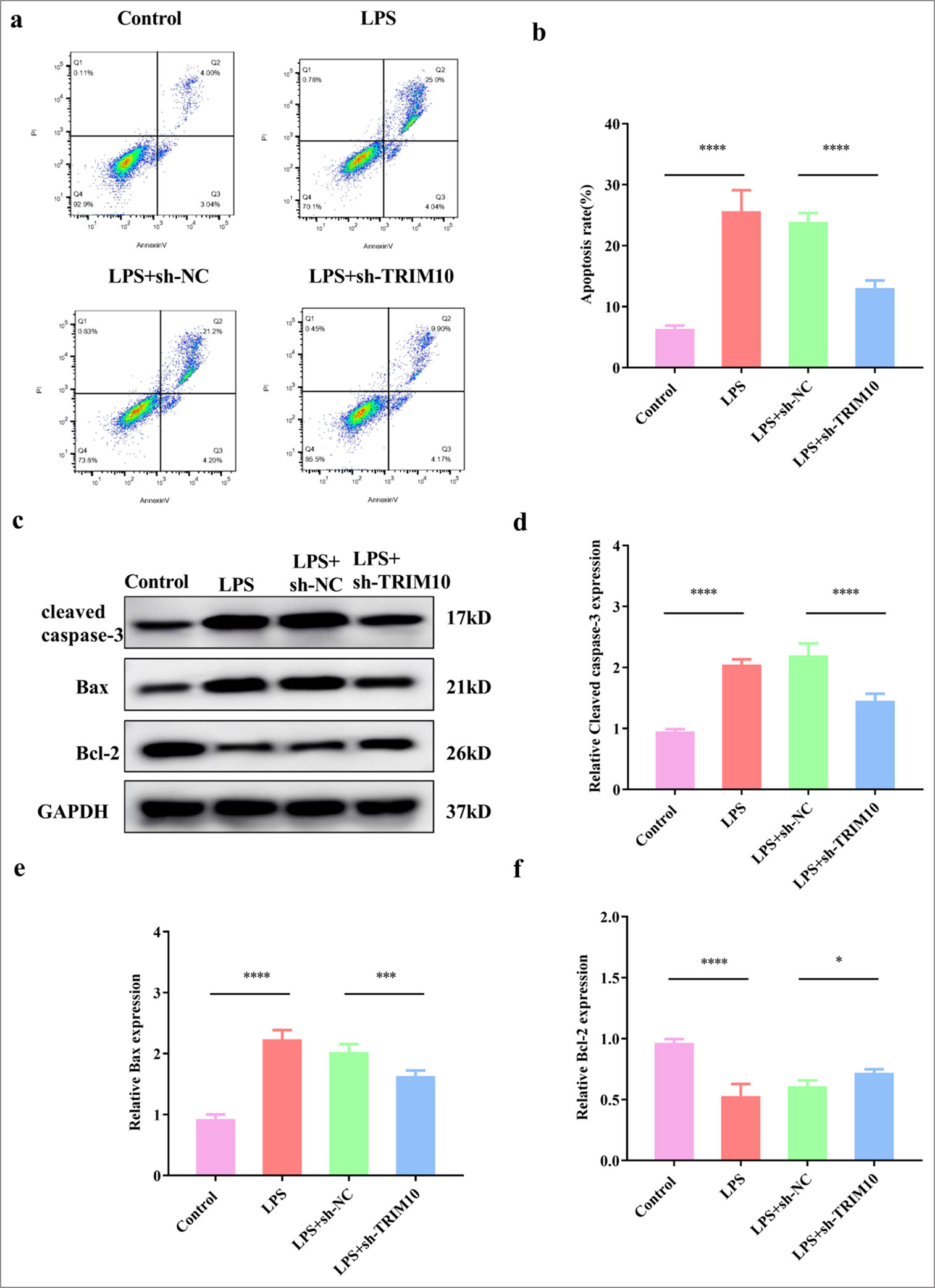
- Knockdown of TRIM10 inhibits apoptosis of cardiomyocytes. (a and b) FCM test was used to detect myocardial apoptosis in each group. (c-f) The expression of apoptosis-related proteins was detected by Western blot assay. ✶P<0.05, ✶✶✶P<0.001, ✶✶✶✶P<0.0001, n=5. FCM: Flow cytometry, TRIM10: Tripartite motif 10, LPD: Lipopolysaccharide, sh-NC: short hairpin-negative control, Bcl-2: B-cell lymphoma 2, Bax: BCL2-associated X protein, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Knockdown of TRIM10 restrains the activation of the TLR4/NF-κB pathway
The effect of TRIM10 on the TLR4/NF-κB pathway was identified by Western Blot assay. LPS activated the expression of TLR4 (P < 0.0001) and p-P65 (P < 0.0001), but TLR4 (P < 0.0001) and p-P65 (P < 0.0001) were inhibited after TRIM10 interference [Figure 6a-c]. TRIM10 affected the myocardial function of rats with SCM may be related to the modulation of the TLR4/NF-κB pathway.
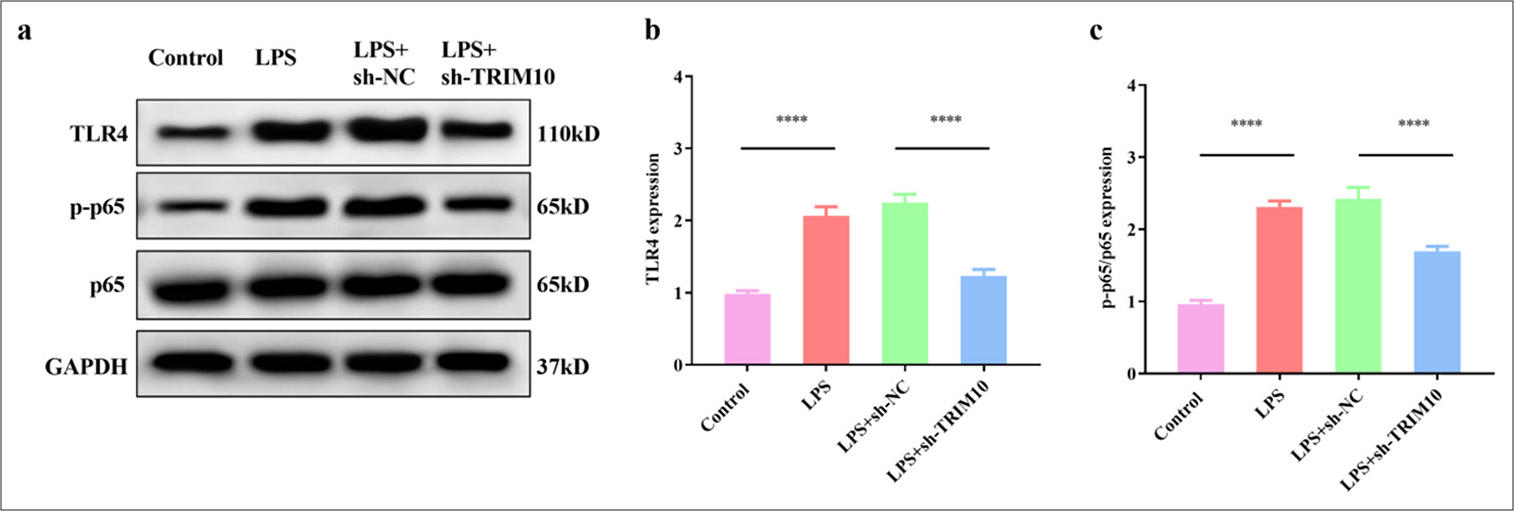
- Knockdown of TRIM10 restrains the activation of the TLR4/NF-κB signaling pathway. (a-c) Expression of p65, p-p65, and TLR4 in each group was detected by Western blot assay. ✶✶✶✶P<0.0001, n=5. TLR4: Toll-like receptor 4, NF-κB: Nuclear transcription factor-κB, TRIM10: Tripartite motif 10, LPS: Lipopolysaccharide, sh-NC: short hairpin-negative control, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
Sepsis can cause damage to myocardial cells after a period of time, which is known as SCM. Restoring heart function or preventing myocardial damage is important to reduce the mortality of patients with sepsis.[19] At present, the main mechanisms of myocardial damage in SCM are induction of endotoxin inflammatory factors and inhibition of mitochondrial function, oxidative stress, microcirculation disturbance, and apoptosis.[20,21] In this work, we investigated the mechanism of TRIM10 on myocardial apoptosis, oxidative stress, and inflammatory response in rats with SCM to provide new evidence for the treatment of SCM.
TRIM10 functions as an E3 ligase and is involved in malignant tumors, neurological diseases, and cardiovascular and cerebrovascular diseases. Our findings displayed that sepsis induced the massive necrosis and apoptosis of cardiomyocytes, while the necrosis of cardiomyocytes was alleviated after TRIM10 knockdown. Similar to our results, silencing TRIM10 reduces cell apoptosis in Parkinson’s disease cell models.[16] TRIM10 also promotes apoptosis and inhibits the proliferation of acute myeloid leukemia cells.[22] Therefore, this study suggested that the degree of myocardial injury induced by sepsis may be related to the abnormal expression of TRIM10.
SCM is a complex, multifactorial pathological process that is often accompanied by the activation of oxidative stress and inflammatory responses in cardiomyocytes. In a Parkinson’s disease cell model, ROS level was reduced after transfection with silencing TRIM10.[16] In the present work, LPS induced oxidative stress and inflammation. In addition, TRIM10 knockdown reduced the levels of oxidative stress and inflammatory factors. TRIM10 knockdown inhibited the expression of IL-6 and TNF-α in peritoneal macrophages of mice; consistently, our study showed that TRIM10 inhibited the inflammatory response in rats with SIC.[23] Our study confirmed that TRIM10 knockdown alleviates oxidative stress and inflammation in rats with SCM.
A number of studies were conducted on the signaling pathways of myocardial dysfunction after sepsis, and these pathways are intertwined to function.[24] The TLR4/NF-κB pathway is one of the most studied pathways. NF-κB is the central mediator of inflammatory response.[25] When sepsis occurs, it can promote the release of inflammatory factors and aggravate the formation of microthrombus.[26] Myocardial damage was alleviated by the suppression of the TLR4/NF-κB pathway in rats with SCM.[27] In the present study, we found that sepsis induced the stress activation of the TLR4/NF-κB pathway in cardiomyocytes, and interference with TRIM10 expression inhibited the increase in TLR4/NF-κB-related proteins induced by sepsis. Interestingly, overexpression of TRIM10 in osteosarcoma activates the NF-κB pathway.[15] Wang et al.[28] found that desocine inhibited H/R-induced apoptosis and oxidative stress of H9C2 cells by downregulating TRIM10 expression. Therefore, the protective effect of TRIM10 knockdown in rats with SCM might be related to the suppression of the TLR4/NF-κB pathway.
However, the above studies have only been preliminarily discussed in animal models and not verified by in vitro experiments. Whether TRIM10 affects other signaling pathways involved in SCM needs to be further explored.
SUMMARY
Interference with TRIM10 expression may inhibit myocardial cell apoptosis, oxidative stress, and inflammatory response and improve myocardial function in rats with SCM, which may be related to the modulation of the TLR4/NF-κB pathway. All results suggested that TRIM10 knockdown might have a cardioprotective effect on sepsis-induced myocardial injury and provide a reference value for targeted therapy of SCM.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
CK-MB: Creatine kinase-MB
cTnI: Cardiac troponin I
ELISA: Enzyme-linked immunosorbent assay
FCM: Flow cytometry
GAPDH: Glyceraldehyde-3-phosphate dehydrogenase
GSH-Px: Glutathione peroxidase
HE: Hematoxylin-eosin
IL-18: Interleukin-18
IL-1β: Interleukin-1 β
LPS: Lipopolysaccharide
Mb: Myohemoglobin
NF-κB: Nuclear transcription factor-κB
RT-qPCR: Reverse transcription-polymerase chain reaction
SCM: Septic cardiomyopathy
SOD: Superoxide dismutase
TLR4: Toll-like receptor 4
TNF-α: Tumor necrosis factor-α
TRIM10: Tripartite motif 10
AUTHOR CONTRIBUTIONS
ZMY: Conducted the research and contributed to data analysis and interpretation; JS: Provided help and advice on the experiments. All authors participated in the drafting and critical revision of the manuscript. All authors have read and approved the final manuscript. All authors were fully involved in the work, able to take public responsibility for relevant portions of the content, and agreed to be accountable for all aspects of the work, ensuring that any questions related to its accuracy or integrity are addressed.
ACKNOWLEDGMENT
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the ethics committee of the Fourth Hospital of Hebei Medical University, approval No. 2020ky327 dated 2020-03-27. Patient’s consent is not required as there are no patients in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
A research grant has been obtained from the Hebei Provincial Health and Wellness Committee, grant No. 20221335.
References
- Sepsis definition: What's new in the treatment guidelines. Acta Clin Croat. 2022;61:67-72.
- [CrossRef] [PubMed] [Google Scholar]
- Heart metabolism in sepsis-induced cardiomyopathy-unusual metabolic dysfunction of the heart. Int J Environ Res Public Health. 2021;18:7598.
- [CrossRef] [PubMed] [Google Scholar]
- Septic cardiomyopathy: From pathophysiology to the clinical setting. Cells. 2022;11:2833.
- [CrossRef] [PubMed] [Google Scholar]
- Sepsis-induced cardiac dysfunction and pathogenetic mechanisms (Review) Mol Med Rep. 2023;28:227.
- [CrossRef] [PubMed] [Google Scholar]
- Current perspectives of mitochondria in sepsis-induced cardiomyopathy. Int J Mol Sci. 2024;25:4710.
- [CrossRef] [PubMed] [Google Scholar]
- Research progress on the mechanism and management of septic cardiomyopathy: A comprehensive review. Emerg Med Int. 2023;2023:8107336.
- [CrossRef] [PubMed] [Google Scholar]
- TRIM proteins in autophagy: Selective sensors in cell damage and innate immune responses. Cell Death Differ. 2020;27:887-902.
- [CrossRef] [PubMed] [Google Scholar]
- TRIM family proteins: Roles in proteostasis and neurodegenerative diseases. Open Biol. 2022;12:220098.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging role of TRIM family proteins in cardiovascular disease. Cardiology. 2020;145:390-400.
- [CrossRef] [PubMed] [Google Scholar]
- MG53 preserves mitochondrial integrity of cardiomyocytes during ischemia reperfusion-induced oxidative stress. Redox Biol. 2022;54:102357.
- [CrossRef] [PubMed] [Google Scholar]
- Trim65 attenuates isoproterenol-induced cardiac hypertrophy by promoting autophagy and ameliorating mitochondrial dysfunction via the Jak1/Stat1 signaling pathway. Eur J Pharmacol. 2023;949:175735.
- [CrossRef] [PubMed] [Google Scholar]
- Knockdown of tripartite motif 8 protects H9C2 cells against hypoxia/reoxygenation-induced injury through the activation of PI3K/Akt signaling pathway. Cell Transplant. 2020;29:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Downregulation of the Spi-1/PU.1 oncogene induces the expression of TRIM10/HERF1, a key factor required for terminal erythroid cell differentiation and survival. Cell Res. 2008;18:834-45.
- [CrossRef] [PubMed] [Google Scholar]
- TRIM10 binds to IFN-α/β receptor 1 to negatively regulate type I IFN signal transduction. Eur J Immunol. 2021;51:1762-73.
- [CrossRef] [PubMed] [Google Scholar]
- Oncogenic gene TRIM10 confers resistance to cisplatin in osteosarcoma cells and activates the NF-κB signaling pathway. Cell Biol Int. 2020;45:74-82.
- [CrossRef] [PubMed] [Google Scholar]
- Silencing of TRIM10 alleviates apoptosis in cellular model of Parkinson's disease. Biochem Biophys Res Commun. 2019;518:451-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nicorandil regulates ferroptosis and mitigates septic cardiomyopathy via TLR4/SLC7A11 signaling pathway. Inflammation. 2024;47:975-88.
- [CrossRef] [PubMed] [Google Scholar]
- Berberine ameliorates septic cardiomyopathy through protecting mitochondria and upregulating Notch1 signaling in cardiomyocytes. Front Pharmacol. 2024;15:1502354.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18:424-34.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccarin alleviates septic cardiomyopathy by potentiating NLRP3 palmitoylation and inactivation. Phytomedicine. 2024;131:155771.
- [CrossRef] [PubMed] [Google Scholar]
- Mitochondrial abnormalities in septic cardiomyopathy. Minerva Anestesiol. 2024;90:922-30.
- [CrossRef] [PubMed] [Google Scholar]
- TRIM10 is downregulated in acute myeloid leukemia and plays a tumor suppressive role via regulating NF-κB pathway. Cancers (Basel). 2023;15:417.
- [CrossRef] [PubMed] [Google Scholar]
- The ubiquitin E3 ligase TRIM10 promotes STING aggregation and activation in the Golgi apparatus. Cell Rep. 2023;42:112306.
- [CrossRef] [PubMed] [Google Scholar]
- Ameliorative effect of anisodamine (654-1/654-2) against myocardial dysfunction induced by septic shock via the NF-kB/NLRP-3 or the PI3K-AKT/NF-κB pathway. Phytomedicine. 2024;123:155277.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct Target Ther. 2020;5:209.
- [CrossRef] [PubMed] [Google Scholar]
- Pretreatment with interleukin-15 attenuates inflammation and apoptosis by inhibiting NF-κB signaling in sepsis-induced myocardial dysfunction. Eur J Histochem. 2024;68:4019.
- [CrossRef] [PubMed] [Google Scholar]
- Berberine attenuates septic cardiomyopathy by inhibiting TLR4/NF-κB signalling in rats. Pharm Biol. 2021;59:121-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of dezocine on cardiac myocytes injury induced by hypoxia and reoxygenation in rats and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2021;37:548-54.
- [Google Scholar]








