Translate this page into:
An unusual neck mass diagnosed by fine needle aspiration: Cytological findings and challenges

*Corresponding author: Neda A. Moatamed, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, Los Angeles, California, United States. nmoatamed@mednet.ucla.edu
-
Received: ,
Accepted: ,
How to cite this article: Lim D, Yu W, Sajed D, Rao J, Rodriguez EF, Moatamed NA. An unusual neck mass diagnosed by fine needle aspiration: Cytological findings and challenges. CytoJournal. 2024; 21:26. doi: 10.25259/Cytojournal_1_2024
QUIZ DESCRIPTION
A 60-year-old woman with a left neck mass was referred for an ultrasound-guided fine needle aspiration (FNA). Ultrasound examination revealed a 2.7 × 2.2 × 1.2 cm, well-circumscribed, solid, hypoechoic lesion with smooth sharp borders in the right neck at level 2A. During the physical examination and interview, the patient reported to have an undiagnosed palate mass. The left neck mass was targeted for FNA. The Diff-Quik stain of the smear showed clusters of papillary-like epithelioid cells with round-to-oval nuclei with wrinkled membranes and extracellular metachromatic stromal fragments. The Papanicolaou stain showed cells with nuclear grooves, small nucleoli, and fine vesicular chromatin. The cells had a moderate amount of delicate cytoplasm, focally intermingled with scant stromal components. The cell block also showed clusters of papillary structures with a cribriform-like architecture [Figure 1a-d].
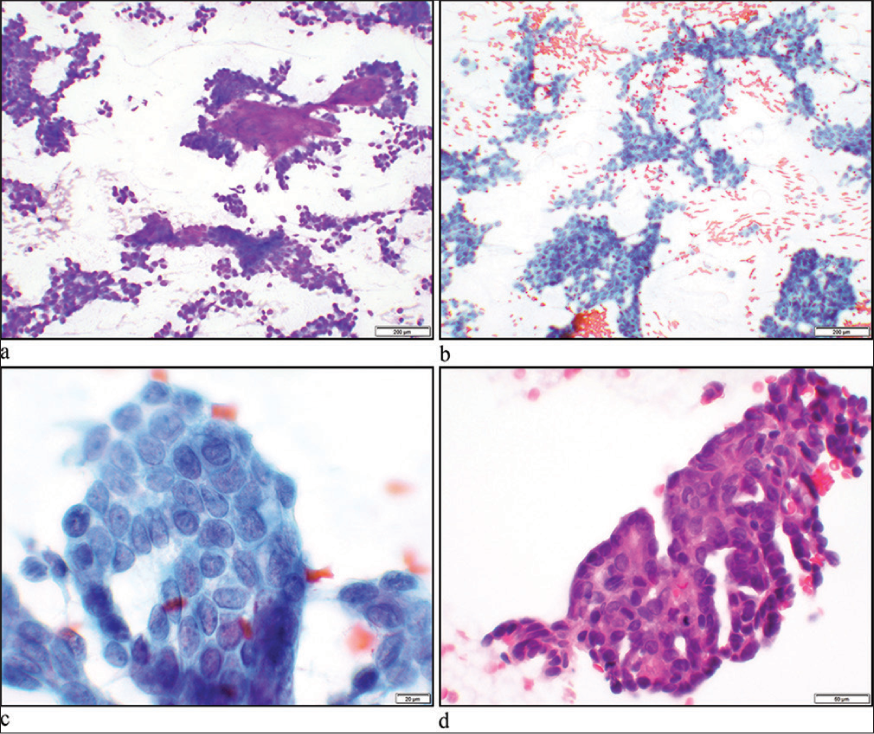
- (a) Hypercellular smears consisting mostly of cohesive cellular aggregates with scattered single cells in the background. Metachromatic stromal fragments are present (Diff-Quik stain, ×10 objective). (b and c) Papillary clusters of neoplastic cells with slightly irregular small to mediumsized nuclei showing pale chromatin and intranuclear grooves (Papanicolaou stain, ×10 and ×60 objectives). (d) Hematoxylin and eosin stain of a cell-block section showing papillary-like structures with lumens consisting of small to medium-sized cells with pale nuclei (×40 objective).
MORPHOLOGY QUIZ
Q1. What is the most likely diagnosis?
Papillary thyroid carcinoma
Salivary gland myoepithelioma
Polymorphous adenocarcinoma (PAC)
Pleomorphic adenoma
Basal cell adenoma.
Answer: C
EXPLANATION
Slightly irregular nuclei with grooves similar to those seen in papillary carcinoma of the thyroid gland are present; however, intranuclear inclusions are absent. The cytological findings combined with clinical information regarding the presence of a mass in the palate are most consistent with metastatic PAC. Pleomorphic adenoma, basal cell adenoma, and myoepithelioma are benign tumors and do not metastasize to the neck lymph nodes.[1]
Q2. Which immunohistochemical panel and findings would be best to confirm the diagnosis of PAC?
Thyroid transcription factor-1 (TTF-1) positive, S100 positive, cytokeratin-7 (CK-7) positive
TTF-1 negative, CK-7 positive, S100 positive
CK-7 negative, paired-box gene 8 (PAX 8) positive, S100 negative
p40 positive, p63 positive, S100 negative.
Answer: B
Immunohistochemistry panel was performed on the cell block which revealed these cells to be positive for CK-7, S100, and Sry-related HMg-Box 10 (SOX10) gene and negative for p40, p63, smooth muscle actin, TTF-1, thyroglobulin, and PAX8 [Figure 2]. Negative TTF-1, thyroglobulin, and PAX8 ruled out metastatic papillary thyroid carcinoma. In the setting of the patient’s palate mass, PAC was considered in this FNA.
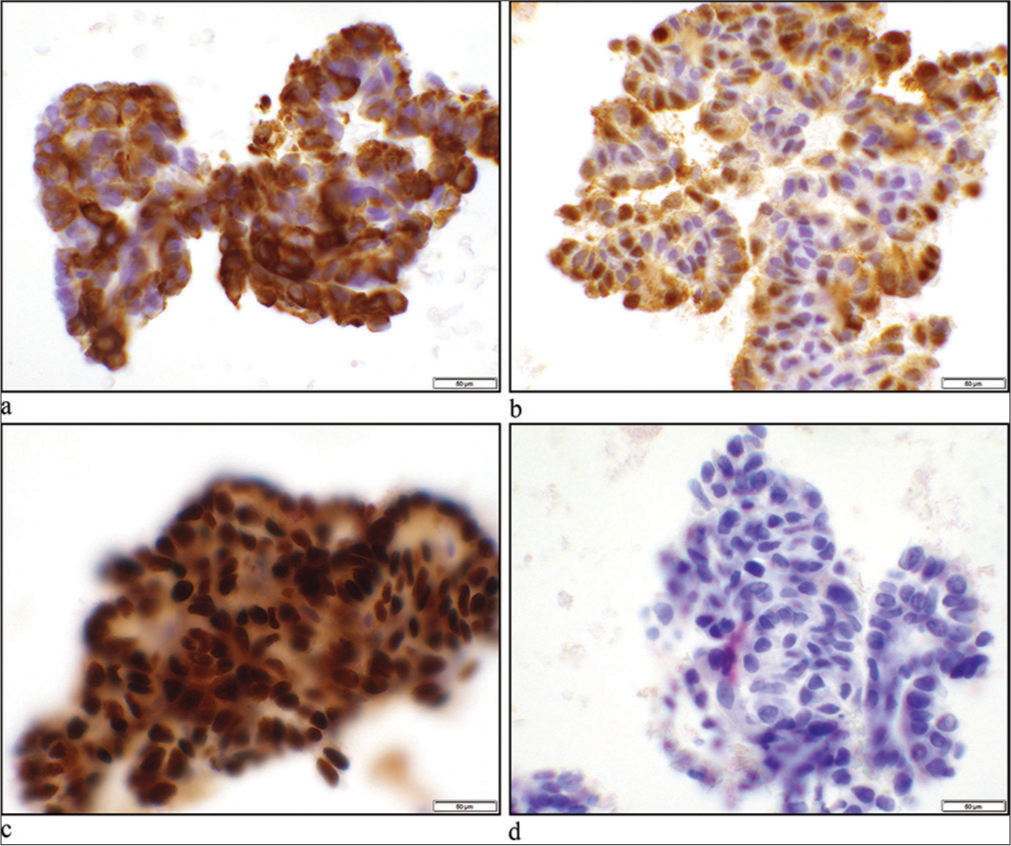
- (a) Cytokeratin-7 showing cytoplasmic stain (×40 objectives). (b) S100 demonstrates nuclear and cytoplasmic staining of neoplastic cells (×40 objectives). (c) SOX10 shows nuclear staining of the tumor cells (×40 objectives). (d) Thyroid transcription factor-1 shows negative nuclear stain (×40 objectives).
EXPLANATION
PAC is immunoreactive for CKs including CK7, in 100% of cases and S100 protein 97–100%. p63 is positive in 78–100% of cases, whereas p40 is usually negative.[1] SOX10 is positive in 86% of PACs.[2]
There is some degree of overlap in the immunohistochemical profile of secretory carcinoma of the salivary glands and PACs. Secretory carcinomas are positive for CK7, S100, SOX10, vimentin, and mammaglobin, and negative for p63, p40, NR4A3, and discovered on GIST-1.[1] However, the cytomorphology of secretory carcinomas varies in that they are composed of microcystic/solid, tubular, and papillary-cystic structures with luminal secretions. The tumor cells in secretory carcinomas have vacuolated pink cytoplasm and granular chromatin.[1]
Q3. Which one of the following is correct about PAC?
PAC is the most common intraoral salivary gland tumor
PAC commonly metastasizes to the neck lymph nodes
PAC has an overall good prognosis
The most common location of PAC is the parotid gland.
Answer: C.
EXPLANATION
PAC is the second most common intraoral salivary gland carcinoma, which rarely metastasizes to the neck lymph nodes. Pleomorphic adenoma is the most common benign tumor and mucoepidermoid carcinoma is the most common malignant tumor of the minor salivary glands of the oral cavity.[3] The palate is the most common site for PAC and the overall prognosis of PAC is excellent with a 10-year survival rate of 94–99%.[1] The main malignant salivary gland tumors that can metastasize to the neck lymph nodes are mucoepidermoid carcinoma, adenoid cystic carcinoma, acinic cell carcinoma, secretory carcinoma, salivary duct carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, hyalinizing clear cell carcinoma, carcinoma ex pleomorphic adenoma, and lymphoepithelial carcinoma.[4]
Q4. What chromosomal abnormality or molecular findings are seen in PAC?
ETS Variant Transcription Factor 6 (ETV6)-Neurotrophic Tyrosine Kinase Receptor Type 3 (NTRK3) fusion
Myelobastosis viral oncogene homolog (MYB) and nuclear factor I/B (NFIB) and MYBL1-NFIB fusions
Mutations in TP53 (55%), Harvey Rat sarcoma virus (HRAS) (23%), Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) (23%)
Protein kinase D (PRKD)1, PRKD2, or PRKD3 fusions.
Answer: D.
EXPLANATION
Most cases of secretory carcinomas show chromosomal translocation, t (12; 15) (p13; q25) resulting in an ETV6-NTRK3 fusion. Adenoid cystic carcinomas have (6;9) or t (8;9) translocations, resulting in MYB-NFIB and MYBL1-NFIB fusions. Mutations in TP53 (55%), HRAS (23%), and PIK3CA (23%) are seen in salivary duct carcinoma. Conventional PAC has predominantly PRKD1 mutation and cribriform subtype has PRKD1,2,3 fusions. Cribriform subtypes are more likely metastasize to the neck lymph nodes.[1,5]
ADDITIONAL DETAILS, FOLLOW-UP
The patient underwent a right maxillectomy with ipsilateral neck dissection 5 months after the FNA procedure, which showed a 5.3 cm high-grade PAC with metastasis to the two lymph nodes. Microscopic examination of the specimen revealed an infiltrative tumor with papillary structures, fibrous connective tissue stroma, and open chromatin nuclei [Figure 3a-c].
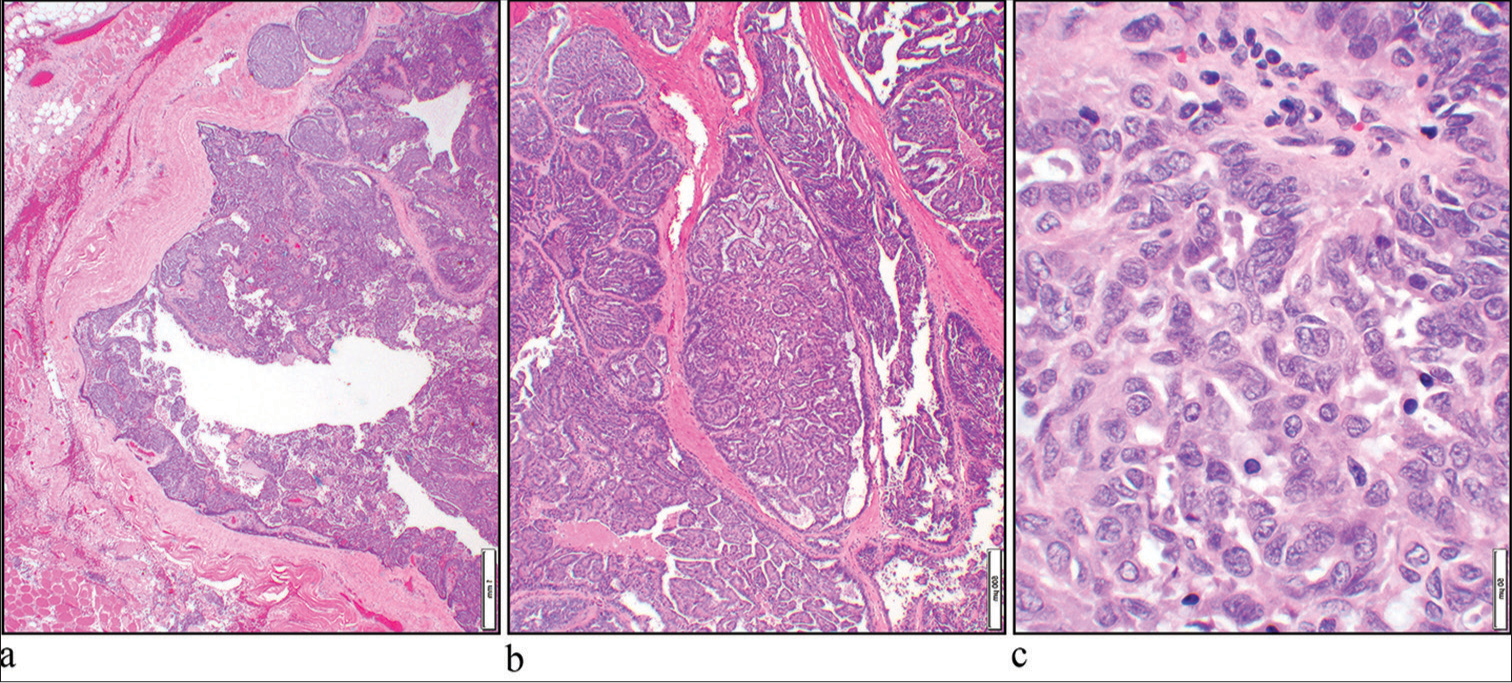
- (a) Hematoxylin and eosin (H&E) stain shows an infiltrative neoplasm (×4 objective). (b) The tumor had a prominent papillary architecture (H&E, ×10 objective). (c) The nuclei show open chromatin (H&E, ×40 objective).
BRIEF REVIEW OF THE TOPIC
PAC of the minor salivary glands is a rare head-and-neck cancer, which generally has a good prognosis. PACs were previously known as “polymorphous low-grade adenocarcinoma”. The recent World Health Organization classification of head-and-neck tumors has characterized it as PAC.[1,6]
There are two subtypes of this tumor, conventional subtype and cribriform subtype. The conventional subtype shows various patterns, including tubules, trabeculae, and microcysts with a mucoid/myxoid background. The cribriform subtype shows papillary structures and glomeruloid bodies in a fibrous stroma. Their nuclei show open chromatin.[1] Recognizing PAC can be challenging due to diverse architectural patterns.[1]
Due to the intraoral location of PAC and rare occasions of metastasizing to the neck lymph nodes, these tumors are hardly sampled by FNA, and therefore, cytopathologists have less experience with the diverse cytomorphology of PAC.[7]
Overall, cytology smears show uniform moderately sized cells with relatively scant cytoplasm and round-to-oval nuclei, forming papillary, tubular, and irregular solid clusters. Dense globular or fibrillary stroma can be seen. Therefore, PAC can resemble adenoid cystic carcinoma or epithelial-rich pleomorphic adenoma. Occasional nuclear grooves and pale chromatin are also seen in PAC and can be misinterpreted as papillary thyroid carcinoma.[1,7]
In our case, TTF-1 and thyroglobulin stains were performed to rule out metastatic papillary thyroid carcinoma.
PAC is immunoreactive for CKs and S100 protein. Staining for p63 is variable. p40 is typically negative. Other positive immunomarkers include mammaglobin (67–100%), CD117 (60%), carcinoembryonic antigen (54%), glial fibrillary acidic protein (15%), melanoma-specific antigen (13%), and epithelial membrane antigen (12%).[1]
Pleomorphic adenomas are immunoreactive with pleomorphic adenoma gene 1 and human high-mobility group protein A2. Cytologically, most of the time, pleomorphic adenomas show the characteristic fibrillary matrix, mixed with myoepithelial cells which merge into the epithelial component. The chromatin of the tumor cells is dark unlike PAC.[1]
Adenoid cystic carcinomas are basaloid tumors. The smears show cohesive clusters of cells forming sheets or show tubular, complex, or cribriform architecture containing hyaline globules. The tumor cells have hyperchromatic nuclei.[1]
Other differential diagnosis includes cribriform-morular thyroid carcinoma (CMTC) which is now considered a distinct malignant thyroid neoplasm. The smears of CMTCs are hypercellular showing papillary and cribriform clusters with slit-like spaces and swirling of nuclei, representing morulae. Nuclei can show peculiar nuclear clearing with thick membranes, grooves, and pseudoinclusions.[8]
Genetic alterations in PRKD 1/2/3 genes have been described in PACs and may be useful in distinguishing PAC from other salivary gland neoplasms.[5]
SUMMARY
In summary, the cytologic features of PAC overlap with other salivary gland neoplasms and papillary thyroid carcinoma. In our case, the immunohistochemical stains in addition to cytomorphology (pale chromatin and papillary-like structures) with the clinical information helped us reach a diagnosis of FNA.
ABBREVIATIONS
FNA – Fine needle aspiration
PAC – Polymorphous adenocarcinoma.
AUTHOR CONTRIBUTIONS
DL, WY, N A.M: Concept, design, data acquisition, and literary Search; DL and N A.M: Manuscript preparation; DL, WY, E F.R, DS and JR: Manuscript editing and review. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
David Geffen School of Medicine University of California Los Angeles, IRB has waived required approval and patient consent since the patient has been anonymized and fully de-identifiied.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Polymorphous adenocarcinoma In: Skalova A, Hyrcza MD, Mehrotra R, eds. WHO classification of tumours: Head and neck tumours Vol 9. (5th ed). Lyon, France: IARC; 2022.
- [Google Scholar]
- Gene expression profiling of head and neck tumors identifies FOXP1 and SOX10 expression as useful for distinguishing ameloblastoma from basaloid salivary gland tumors. Am J Surg Pathol. 2020;44:665-72.
- [CrossRef] [PubMed] [Google Scholar]
- Tumors of the intraoral minor salivary glands: A demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323-33.
- [CrossRef] [PubMed] [Google Scholar]
- High incidence of lymph node metastasis in major salivary gland cancer. Arch Otolaryngol Head Neck Surg. 2003;129:720-3.
- [CrossRef] [PubMed] [Google Scholar]
- The PRKD1 E710D hotspot mutation is highly specific in separating polymorphous adenocarcinoma of the palate from adenoid cystic carcinoma and pleomorphic adenoma on FNA. Cancer Cytopathol. 2018;126:275-81.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphous adenocarcinoma of the salivary glands: Reappraisal and update. Eur Arch Otorhinolaryngol. 2018;275:1681-95.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology of polymorphous adenocarcinoma of the salivary glands: A report of 11 patients and review of the literature. Diagn Cytopathol. 2020;48:1013-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cribriformmorular variant of papillary thyroid carcinoma--cytological and immunocytochemical findings of 18 cases. Diagn Cytopathol. 2010;38:890-6.
- [CrossRef] [PubMed] [Google Scholar]








