Translate this page into:
Application of the international system for reporting serous fluid cytopathology on reporting various body fluids; experience of a tertiary care hospital

*Corresponding author: Sachin Kolte, Department of Pathology, Vardhman Mahavir Medical College & Safdarjung Hospital, New Delhi, India. drsachinkolte@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kolte S, Zaheer S, Aden D, Ranga S. Application of the international system for reporting serous fluid cytopathology on reporting various body fluids; experience of a tertiary care hospital. CytoJournal 2022;19:52.
Abstract
Objectives:
Cytological examination of effusion sample is a preliminary and minimally invasive method for the diagnosis of body fluids. Recently, the International System For Reporting Serous Fluid Cytopathology (ISRSFC) and the Indian Academy of Cytologist (IAC) have published guidelines for reporting effusion cytology and calculating the risks of malignancy (ROMs) for each defined category. We report our 2 years of experience in reclassifying and assessing the feasibility of applying ISRFSC and IAC categories to effusion fluid and to provide an estimate of the risk of malignancy for each diagnostic category.
Material and Methods:
Cytological reports of patients from January 2019 to December 2020 were retrieved and reclassified into a five-tiered classification scheme as per ISRSFC guidelines. Cellblock and immunohistochemistry were performed in selected cases. Clinico radiological and histopathological information were obtained and correlated with the cytological findings wherever available.
Results:
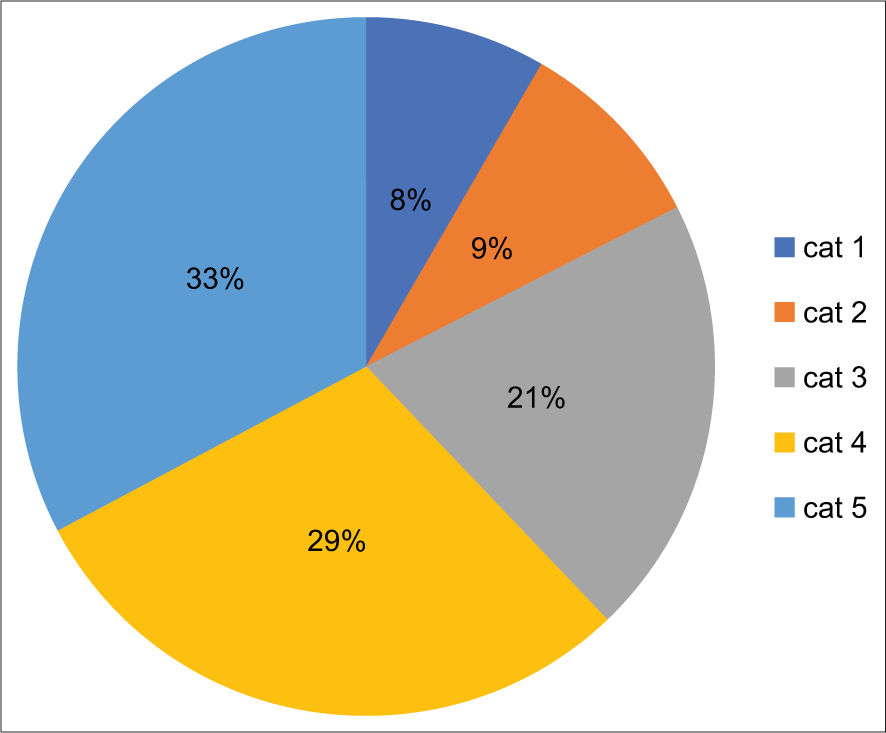
In the study, 652 cases were included during the 2 years. Out of these, 328 (50.3%) were women and 314 (47.3%) were men. Patient’s ages ranged between 2 92 years with a mean age of 47.4 years. There were 366 (56.1%) cases of ascitic fluid followed by 262 (40.1%) cases of pleural fluid and 24 (3.8%) cases of pericardial fluid in the analysis. Of all the cases, 13 (2%) were non-diagnostic (ND), 464 (71.6%) were negative for malignant (NFM) cells, 16 (2.4%) were atypia of uncertain significance, 31 (4.7%) were suspicious of malignancy, and 125 (19.3%) were malignant. Cellblock was prepared in 65 cases. Lung cancer followed by breast cancer was the most common malignancies involving the pleural effusion and ovarian cancer was the most common cause of peritoneal effusion. ROM for each diagnostic category was 23% for ND, 25% for NFM, 56% for the atypical category, 80.6% in suspicious, and 90% were for positive for malignancy category.
Conclusion:
The use of a five-tiered system as per the ISRFC and IAC guidelines are feasible for the standardized reporting of effusion samples, thus avoiding subjective variation of reporting.
Keywords
Effusion
An international system for reporting serous fluid cytopathology
Malignant
Risks of malignancy
INTRODUCTION
Serous effusion is the accumulation of fluid in the body cavities due to various causes and malignancies being one of the important causes of effusions.[1] The various sites from which fluid can be sent for analysis include a pleural, peritoneal, and pericardial cavity. It forms a large part of specimens received in the cytopathology laboratory of many hospitals and is a cost-effective, minimally invasive, and simple procedure that can help categorize fluids A standardized cytological report can be of great help to inpatient management. Effusion is an invariable important diagnostic sample and is an essential landmark in the management roadmap, especially in diagnosing and staging malignancies.[2] Neoplasms are the cause of serious effusion in approximately 10–25% of pleural, pericardial, and peritoneal effusions.[3,4] In many cases, it may be the first manifestation of an unknown primary tumor. Peritoneal effusion is the initial presenting feature in more than 50% of gastrointestinal and gynecological malignancies with peritoneal metastasis.[5]
There are no uniform guidelines for the diagnostic categorization of the fluid samples. Many of the centers are following their reporting system, thus creating a discrepancy in the diagnosis and causing hurdles in reaching a definitive management plan. Following, the usefulness of the Bethesda reporting system for pap smears and thyroid cytology, Paris system for urine cytology, Milan system for reporting salivary gland cytology, etc., the International System For Reporting Serous Fluid Cytopathology (ISRSFC) was proposed, which was endorsed by the International Academy of cytology and American society of cytopathology.[6,8,10,18] The ISRSFC aims to formulate evidence-based standardized reporting system for the diagnosis of effusion fluids to enhance professional communication, increase the reproducibility of the report, and finally improve patient management. It is also helpful in assessing the risk of malignancy (ROM) in all categories. This system proposes five diagnostic categories: Non-diagnostic (ND), negative for malignancy (NFM), atypia of uncertain significance (AUS), suspicious for malignancy (SFM), and malignant (MAL).[5] Accurate cytopathological diagnosis along with the help of ancillary tests in difficult cases serves as a guide to proper patient management. The Indian Academy of Cytologists (IAC) has also published guidelines for the collection, processing, interpretation, and reporting of serous effusion fluids to provide uniformity across all laboratories and to implement a proper reporting format.[11] They have also categorized the reporting of effusion into five recommended categories Category 1, Unsatisfactory for evaluation; Category 2, No malignant cells; Category 3, Atypical cells, NOS; Category 4, Atypical cells, SFM; and Category 5, Malignant cells seen.[12] There are studies in the literature to calculate the ROM for each of these diagnostic categories.[13] To assess the feasibility of these diagnostic categories in effusion samples, we publish our findings by categorizing the effusion fluids into reporting format as prescribed by ISRFRC and IAC.
MATERIAL AND METHODS
After the institutional ethics committee’s approval, data from records of the pathology department were retrieved for the period from January 1, 2019, to December 31, 2020. The patient’s demographic profile, cytology report, radiological diagnosis, ancillary studies, histopathological follow-up, and medical history were collected and analyzed for each case. The two sediment smears were prepared using the cytocentrifugation method. One smear was fixed with alcohol spray for pap stain and the other was air-dried for Giemsa stain. Special staining such as Ziehl–Neelsen and periodic acid Schiff stains was applied wherever required. The leftover samples were stored in the refrigerator at 2–8°C until the case was reported by the pathologist. The cell block was prepared on the next day from the remaining sample using the plasmathromboplastin clot method. Slides were cut and stained with hematoxylin and eosin stain. Immunohistochemistry was performed on selected cases, varying from case to case with standard protocol. The ISRSFC and IAC guidelines were applied and classified into five categories: ND, NFM, AUS, SFM, and MAL.[6,10,18] The cellular component of each category was recorded. Each case was categorized into these five recommended diagnostic categories.
RESULTS
There were 652 cases included in the study [Table 1] during the 2 years. Out of these, 328 (50.3%) were women and 314 (49.7%) were men. The patient’s age was between 2 years and 92 years with a mean age of 47.4 years. Cell blocks were prepared in 65 cases. Immunohistochemistry was applied depending on the site and type of malignancy, wherever required. The majority of the samples were from 366 (56.1%) cases of ascitic fluid followed by 262 (40.1%) cases of pleural fluid and 24 (3.8%) cases of pericardial fluid in the analysis. Of all the cases, 13 (2%) were ND, 464 (71.6%) were NFM cells, 16 (2.4%) were AUS, 31 (4.7%) were suspicious of malignancy, and 125 (19.3%) were malignant. Lung cancer followed by breast cancer was the most common malignancies involving the pleural effusion and ovarian cancer was the most common cause of peritoneal effusion. ROM for each diagnostic category was 23% for ND, 25% for NFM, 56% for the atypical category, 80.6% in suspicious, and 90% were for positive for malignancy category.
| Diagnostic Categories | Total | Percentage | female | % | Male | % | Pleural | % | Peritoneal | % | Pericardial | % | Malignancy | ROM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non diagnostic | 13 | 2 | 9 | 70 | 4 | 30 | 5 | 38.5 | 8 | 61.5 | 0 | 0 | 3 | 23 |
| Negative for malignancy | 464 | 71.6 | 200 | 43 | 264 | 57 | 190 | 40.9 | 260 | 56 | 14 | 3.1 | 76 | 25 |
| Atypia of uncertain significance | 16 | 2.4 | 10 | 63 | 6 | 37 | 7 | 43.5 | 8 | 50 | 1 | 6.5 | 9 | 56 |
| Suspicious of malignancy | 31 | 4.7 | 19 | 61 | 12 | 39 | 10 | 32.2 | 18 | 58.1 | 3 | 9.7 | 25 | 80.6 |
| Malignant | 128 | 19.3 | 90 | 72 | 38 | 24 | 50 | 40 | 72 | 57.7 | 6 | 2.3 | 62 | 90 |
| 652 | 100 | 328 | 50.3 | 314 | 49.70 | 262 | 40.1 | 366 | 56.1 | 24 | 3.8 |
ROM for each diagnostic category [Chart 1 and Table 2] was calculated using histopathological diagnosis as a gold standard. The most common cause of pleural effusion was lung cancer followed by breast cancer and ovarian carcinoma was the most common cause of peritoneal effusion.
- Risk of malignancy.
| Diagnostic categories | Kundu et al. | Farhani et al. | Erika et al. | Hou et al. | Valleria et al. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | % | ROM | Case | % | ROM | Case | % | ROM | Case | % | ROM | Case | % | ROM | ||
| Category 1 | Non diagnostic | 35 | 2.60% | 20% | 52 | 0.20% | 17.40% | 0 | 0 | NA | 0 | 0 | 29 | 5.60% | 50% | |
| Category 2 | Negative for malignancy | 954 | 71.20% | 16.70% | 22202 | 72.20% | 20.70% | 252 | 84.40% | NA | 1000 | 42% | NA | 291 | 56% | 44% |
| Category 3 | Atypia of uncertain significance | 17 | 1.30% | 50% | 194 | 0.60% | 65.90% | 13 | 4.30% | NA | 145 | 6% | 39 | 28 | 5.40% | 50% |
| Category 4 | Suspicious of malignancy | 59 | 4.40% | 94.40% | 711 | 2.30% | 81.80% | 4 | 1.30% | NA | 98 | 4% | 64 | 30 | 5.80% | 83.30% |
| Category 5 | Malignant | 275 | 20.50% | 100% | 6507 | 21.30% | 98.90% | 30 | 10.00% | NA | 1162 | 48% | NA | 141 | 27.30% | 96.20% |
| Total no of cases | 1340 | 100% | 34941 | 100% | 299 | 100% | 2405 | 100% | 519 | 100% | ||||||
The ISRSFC
Category 1 diagnostic (ND)
There were 9 (70%) women and 4 (30%) men in this category [Table 1 and Figure 1]. The majority of the cases 8 (61%) were from ascitic effusion followed by 5(38.5%) cases of pleural fluid. No pericardial fluid was present in this category. There were no diagnostic cells with the presence of a few RBCs, occasional lymphocytes and cells showing degenerative changes.

- Category 1, Cytomorphology shows occasional cells in a proteinaceous background ×100 (a). Cellblock section shows fibrocollagenic tissue with occasional cells not sufficient for categorization, Giemsa stain, ×100 (b)
Category 2, NFM cells
There were 200 (43%) women and 264 (57%) men in this category [Table 1 and Figure 2]. 190 (40.9%) cases were of ascitic fluid, followed by 260 (56%) cases of pleural fluid and 13.1 (4%) cases of pericardial fluid were found. There was the presence of either lymphoid or neutrophilic predominance in the effusion sample along with the presence of few benign and reactive mesothelial cells in these categories. There were no malignant cells observed in the cases reported here.

- (a) Category 2, Pleural effusion showing reactive mesothelial cells along with chronic inflammatory cells and macrophages, Giemsa stain, ×100, Papanicolaou stain, ×400 (b). (c) Category 2, Pleural effusion showing lymphocytic inflammation, Papanicolaou stain, ×100.
Category 3 AUS
There were 10 (63%) women and 6 (37%) men present in this category [Table 1 and Figure 3]. The maximum number of cases was 8 (50%) cases of ascitic fluid, followed by 7 (43.5%) cases of pleural fluid and 1 (6.5%) pericardial fluid. The cases were categorized in this category if they showed reactive atypia of mesothelial cells or lymphoid cells that did not favor malignancy, but were showing atypical changes to characterize them in the benign reactive category.

- Category 3 shows degenerated and the occasional cluster of atypical cells, Giemsa stain, ×400 (a), Papanicolaou stain, ×100 (b).
Category 4 SFM
It comprised 19 (61%) women and 12 (39%) men in this category. 10 (32%) cases of pleural fluid, 18 (58%) cases of ascitic fluid, and 3 (10%) cases of pericardial fluid were observed here [Table 1 and Figure 4]. We observed groups and clusters of epithelial cells with features of malignancies but were falling short of quantitatively or qualitatively for the definitive diagnosis of malignancy. Degenerated cells SFM and a few groups of cells where the morphology was deceptive were included in this category.

- Category 4, Cytomorphology showing aggregates of atypical cells with low nucleocytoplasmic ratio, vesicular chromatin and prominent nucleoli, Giemsa stain, ×400 (a). Cellblock section showing fibrocollagenic tissue with few lymphomononuclear and occasional suspicious cells, Giemsa stain, ×400 (b).
Category 5 malignant (MAL)
There were 90 (72%) women and 38 (24%) men in this category. There were 72 (56.1%) cases of ascitic fluid which was the maximum bulk of cases, preceded by 50 (39.3%) cases of pleural fluid, and 6 (4.6%) case of pericardial fluid in this category [Table 1].[13] Cell blocks were prepared in this category. A panel of immunohistochemistry was performed according to the primary malignancy or suspected malignancy and even in cases where the primary was not known to identify the primary site of a tumor. An algorithm was performed to come to a diagnosis. The definitive features of malignancy beyond doubt were noticed here. The clinical and or histopathological follow-up was available for 62 cases of pleural effusion. The most common primary tumor in the pleural fluid was the lung, primary consisting of adenocarcinoma, squamous cell carcinoma, small cell carcinoma, non-small cell carcinoma, and Non-Hodgkin lymphomas [Figures 5 and 6].

- Category 5, ascitic fluid shows acini, cluster and 3d balls consistent with adenocarcinoma lung Giemsa stain, ×400 (a), papanicolaou stain, ×400 (b).

- Category 6, pleural fluid shows sheets of malignant squamous epithelial cells consistent with squamous cell carcinoma lung Giemsa stain, ×400 (a), papanicolaou stain, ×400 (b).
The most common malignancy in the peritoneal fluid was that of ovarian carcinoma in females and prostatic carcinoma in males. There were many cases of the carcinoma gall bladder, carcinoma colon, carcinoma cervix, and carcinoma rectum.
Estimated risk of malignancy
Based on the information available from the follow-up of the patient as per the clinical record or histopathology, which was considered the gold standard for confirmatory diagnosis. The estimated risk of malignancy (ROM) in serous effusion samples was calculated and results are enumerated in [Table 2 and Chart 1]. ROM for each diagnostic category was 23% for ND, 25% for NFM, 56% for the atypical category, 80.6% for suspicious, and 90% for positive for malignancy category.
DISCUSSION
Cytological diagnosis is one of the first steps in the evaluation of the effusion sample. It is a minimally invasive, simple, and cost-effective diagnostic tool that can help clinicians in patient management. The tumor cells can be detected either only based on cytomorphology or ancillary studies may be needed including immunocytochemistry (ICC) to interpret the second foreign population of MAL cells with the application of subtractive coordinate immunoreactivity pattern in effusion fluid. Ancillary studies like molecular analysis can also be performed.[14] The collection of serous fluids from various effusions is a relatively simple procedure. Although, microscopic evaluation of serous fluids is complex as compared to the evaluation of fine-needle aspiration cytology. Reactive and non-neoplastic etiologies constitute the majority of effusions; cancer is one of the causes of an effusion which manifests the advancement of cancer which shows a bad prognosis. Thus, interpretation of cytopathology as positive for cancer cells is highly critical in planning the clinical management and impact of the prognosis of the patient. There is a cytomorphologic overlap of reactive mesothelial cells with malignant cells, the general cytologic criteria for diagnosis of malignancy in single cells cannot be applied in most of the effusion specimens. It is due to the surface tension related phenomenon that “round up” the cells after exfoliation in serous fluids making it a challenging task. The tumor cells may continue to proliferate after exfoliation in the nutrient-rich effusion fluids and may form proliferation spheres. It is important to keep these factors in mind while interpreting effusion samples.[15]
The results from this study show the efficacy of cytological evaluation in confirming the diagnosis, especially in malignant cases. In cases, where there is a discrepancy in accurately typing the sample into one category, ancillary techniques, such as cell block, ICC, IHC, flow cytometry, and molecular analysis, can be of help in arriving at a definitive diagnosis.[16] Cytopathology has evolved tremendously in the last couple of decades, due to the incorporation of various classification schemes.[6,13] This study categorizes effusion fluids into five diagnostic categories based on the recently proposed ISRSFC and IAC guidelines for effusion cytopathology[6,10,20] It is recommended that the diagnosis should be reported by enumerating the category number along with the accompanying category name or descriptive diagnosis.
A total of 652 cases were included in our study [Table 1]. The majority of the samples were from 366 (56.1%) cases of ascitic fluid followed by 262 (40.1%) cases of pleural fluid and 24 (3.8%) cases of pericardial fluid in the analysis. Rodriguez et al. categorized pericardial effusion in 299 cases, including 252 NFM (84.3%), 30 MAL (10%), 13 AUS (4.3%), and 4 SFM (1.4%) cases. About 96.6% of pericardial fluid was reported as a confirmative diagnosis of benign or malignant categories and only a small number (5.7%) of cases were diagnosed as AUS or SFM. Farhani et al.[13] analyzed eighty studies in their systematic review on 34 941 samples. They categorized them into 52 (0.2%), 22 202 (72.7%), 194 (0.6%), 711 (2.3%), and 6507 (21.3%) as non-diagnostic (ND), NFM, AUS, SFM, and malignant (MAL) category, respectively.[13] Valerio et al., in their study, classified 519 samples from 385 patients into 29 (5.6%) as ND, 291 (56%) as NFM, 28 (5.4%) as atypical, 30 (5.8%) as SFM, and 141 (27.2%) as positive for malignancy.[17] Hou et al. divided the effusion fluid into NFM (42%) or MAL (48%) categories, with 10% of cases falling into AUS and SFM.[24]
There were 328 (50.3%) women and 314 (49.7%) were men in our study similar to the study by Rodriguez et al. where the majority of the patients were females (n = 23, 76.7).[17] Erika et al. found that the ratio of male to female was significantly low in all categories.[15] The mean age in our study was 47.4 which was similar to Rodriguez et al. which was 51 years for all diagnostic categories, with the lowest for MAL with 51 years and highest for SFM with 58 years as mean age of[17] 13 effusion samples (18.8%) were observed in the ND category. There were 8 (61.5%) ascitic, 5 (38.5%) of pleural, and 0% of pericardial fluid in this category. It varied from the study analyzed by Claude et al. where the number of cases in the ND category consisted of 0.8% for pleural, 0% for pericardial, and 0.7% for peritoneal effusions.[17] Other studies found non-diagnostic cases ranging between 0.5% and 1.0% in serous effusion.[24] Only blood was seen in 26 cases by Rodriguez et al. and was put in the ND category.[17] They did not use any specific number of cells to determine the specimen suspicious or positive for malignancy, similar to what we have done.[17] Kundu et al. found 35 effusion samples (2.6%) in the ND category.[12] There was scant material in the serous effusion specimen along with the presence of degenerative changes in the effusion. ROMs may vary between 0 and 100%, with a mean of 17.4% (±8.9%) estimated by a recent meta-analysis.[4] ROM, in our study, was 23%. The incidence rate in this category should range between 0.2 and 1%.[13,17] The cause of increased cases in this category could be due to faulty measures during the pre-analytical stage of tissue handling and transportation.
The majority, of cases of the effusions, were placed in category 2 NFM, similar to Kundu et al. where they studied 954 samples out of 1340 (71%) and classified them in category 2. This constitutes the bulk of most of the patients who have reactive effusion, even in cases of malignancy but malignancy should be ruled out by proper cytological examination. The most common etiology for NFM was infections, cardiac complications, myocardial infarction, uremia, autoimmune disorders, tuberculosis, etc. The various causes reported by Kundu et al. for benign effusions were infections, cirrhosis, hypoalbuminemia, autoimmune diseases, peritoneal dialysis, etc.[12] Cell block was prepared in 15 cases. We found that reactive mesothelial cells were seen in around half of the benign effusions, similar to the observation by Kundu et al.[12] They subcategorized reactive effusions into reactive mesothelial proliferation in 49%, acute inflammation in 8.6%, chronic inflammation in 24.1%, lymphocyte rich effusion in 17.9%, and specific infections in 0.4%[12] as it might help in the diagnosis of the patients more effectively. There are reactive morphological changes in the mesothelial cells that are difficult to diagnose and can be misinterpreted as malignant.[21] Erika found NFM with 84.3% (252/300) of the cases, a percentage higher than that in other reported studies.[20] NFM included mesothelial cells, lymphocytes, histiocytes, and RBCs. Rodriguez et al. suggested that specimens, where no mesothelial cells are seen, can still be considered satisfactory for evaluation since none of the patients developed MAL during the 6 months of follow-up. Tuberculosis is an important cause of lymphocytic pleural effusion globally, especially in developing countries.[12] However, bacilli or granuloma formation in the fluid are rarely noticed. There were two cases of AFB positivity demonstrated by the ZiehlNeelsen staining for AFB similar to the findings of Kundu et al.[12] Among infectious agents, bacterial infections were the most common cause in the pleural effusion, also observed by Erika et al.[20] None of the cases of mycobacterial infections were found by Erika et al. while mycobacterium tuberculosis infection was observed in the literature.[20,21] The risk of malignancy should vary between 0 and 80% with a mean ROM of 21% (± 0.3%).[13,17] ROM in our study in this category was 25%.
Category 3 atypia of unknown significance
There were 10 (63%) women and 6 (37%) men present in this category [Table 1 ]. It constituted 16 (2.4%) cases with 8 (50%) cases of ascitic fluid, followed by 7 (43.5%) cases of pleural fluid, and 1 (6.5%) pericardial fluid. Kundu et al. reported (1.3%) cases in the AUS category[19] Other studies reported the incidence of AUS, 2.2%-3.1%.[3,9,10] We had a slightly increased number of cases. This could occur as a result of diagnostic bias in the interpretation of cytologically atypical cells in the setting of a patient with known malignancy and or distant metastasis.[24] There were a greater number of cases in our study in this category. This could also be due to sample handling and processing error. There were nine cases where cell block was made and IHC applied, but still, the diagnosis was difficult to discern. The ROM should range between 13 and 100% with a mean ROM of 66% (±10.6%).[13,17] The incidence of this category should vary between 0.6 and 1.6%, with more common in pericardial and peritoneal effusion samples compared to pleural effusion.[13,17] ROM observed in this study was 56% for this category.
31 (4.7%) were kept in category 4 SFM. Kundu et al. observed 59 (4.4%) cases in category 4. They had a follow-up in 18 cases with 17 having malignancy while one was benign.[12] SFM was reported to be around 1.7% in various studies.[3,9,10] Category 4 should be viewed seriously by the clinician and managed as malignant until proven otherwise.[22] Erika et al. observed that 64% of SFM cases had cells that were morphologically SFM but quantitatively insufficient for ancillary studies and thus diagnosis. In the cases in which IHC was performed, 36% showed inconclusive results, either because of discordance between IHC or preparation types, similar to what was seen in the AUS cases.[18] IHC was applied in 15 cases, in this study, but the diagnosis remained inconclusive for several reasons. The common reasons for an inconclusive result were discordant staining of IHC markers or the staining results were not concordant. AUS and SFM were categorized as “holding” categories and can be shifted to either benign or malignant categories on repeat sampling with the help of ancillary studies.[23] There is a morphologic spectrum in cases reported as AUS and SFM and those in the SFM group tend to show cytologic features worrisome for malignancies. The risk of malignancy varies between 0 and 100% with a mean ROM of 82% (± 4.8%) in this category.[13] The calculated ROM in this category was 80.6%, similar to others. The expected incidence should be approximately 2%.
Confirmatory malignant diagnosis, MAL category 5 was rendered in 90 (72%) women and 38 (24%) men in this category There were 72 (56.1%) cases of ascitic fluid which was the maximum bulk of cases, preceded by 50 (39.3%) cases of pleural fluid, and 6 (4.6%) case of pericardial fluid in this category [Table 1].[13
Kundu et al. reported malignant pleural effusions constituted 21.1%, followed by pericardial (19.5%) and peritoneal (17.6%) effusions.[12] Claudia found a higher number of cases in the malignant category with 31.9% for pleural, 56.2% for pericardial, and 33% for peritoneal effusions, this may be because the data were collected from an oncology lab.[15]
Other studies reported malignancy rates of 10–20% in serous effusion in LBC samples.[25,26] Mal category in the literature consisted of 10.4% and 20.5%., similar to our study[27-29]Hou et al. had a higher MAL category, as it was a tertiary care cancer center, in which many patients were referred with an established malignant diagnosis and generally at an advanced stage of disease with metastasis. There was a slight variance in the malignancy rates observed from a few studies. Our gentry of patients included both outpatient and hospitalized patients. This could be due to malignant effusion being overwhelmed by infection. The other possibility is that infections such as tuberculosis and other pleural inflammatory effusion could have led to an increased number of cases in non-malignant effusions, and a lesser number in MAL as also observed by Kundu et al.[12] Almost 10% of all PF samples were diagnosed as MAL by Rodriguez et al.[17] This is a much lower percentage than in previous studies.[27] The study of pleural effusion found 23% of MAL,[17] their patient population included only those admitted to the hospital and thus had a higher percentage of cases in this category.
The most common malignancies in our study pleural effusion were from the lung and breast as observed in other studies.[20,31] Claudia et al. and others observed that the most common malignancy in pleural effusions was lung cancer (35.4%) and breast cancer (28.9%). In the case of pericardial effusions, breast cancer (44.4%) and lung cancer (36.1%) were the most frequent diagnosis of malignancies.[3,15,23,36,37]
Cancers of gastrointestinal origin (48%) and ovarian cancer (30%) were the most commonly diagnosed malignancies in peritoneal effusions.[17] Other studies observed 50–75% of malignant pleural effusions were seen due to lung, breast, ovarian cancer, and lymphomas.[30-32] Cancers of unknown primary are reported to cause between 5% and 15% of all malignant serous effusions.[25,26] The differences between the most common etiologies reported in the literature may be due to the variation in the inclusion criteria and method of the study performed and the presence of ancillary techniques[36]
A retrospective was performed and 4684 samples of pleural effusions were reviewed they observed that out of a total of 364 (7.8%) positive for cancer cells, 295 (81%) were classified as adenocarcinoma or carcinoma not otherwise specified (NOS). There were 32 (8.8%) cases of malignant mesotheliomas, 14 (3.8%) cases of small cell carcinomas, 13 (3.5%) cases of hematolymphoid malignancies, and 10 (2.7%)of squamous cell carcinoma.[33-35] Hematolymphoid malignancies consisted of non-Hodgkin lymphoma, multiple myeloma, chronic myeloid leukemia, and acute myeloid leukemia. They concluded that although adenocarcinoma is the most common malignancy seen in pleural effusions, other significant numbers of hematological and nonhematological malignant causes of pleural effusions can also be found should be kept in mind.[38]
ROM, in our study, in pleural effusions for ND, NFM, AUS, SFM, and Mal was 23%, 25%, 56%, 80.6%, and 90%, respectively. This was similar to the ROM in the study by Kundu et al. which was 20% for category 1, 16.7% for category 2, 50% for category 3, 94.4% for category 4, and 100% for category 5. The ROM in peritoneal effusions was similar to 100%, 26.3%, 62.5%, 91.7%, and 100%, respectively. In pericardial effusions, ROM was 0% for NFM and AUS, and 100% for MAL. Ancillary techniques with the help of cell block and ICC for confirming malignancy should be used wherever needed.[12] Farahani et al. reported a mean ROM for ND, NFM, AUS, SFM, and M of 17.4%, 20.7%, 65.9%, 81.8%, and 98.9%, respectively.[13] Valeria et al. observed ROM in 49 (80.3%) specimens.
These results support the role of cytological analysis in serous effusions in confirming the existence of malignancy. Established criteria for the diagnoses of AUS and SFM in effusion cytology are currently not available[31] Although, it is annoying for the pathologist and the clinician to use categories as “atypical” or “suspicious” These categories are essential and need to be used cautiously, to provide meaningful clinical information.[16] The high negative predictive value of the “benign” category is maintained by the “atypical” category. The “suspicious for malignancy” category maintains the high positive predictive value of the “malignant” category.[16] International System for Reporting Serous Fluid Cytology developed a tiered classification system to provide better categorization for reporting.[6,7] Standardized terminologies allow for uniformity across various laboratories for solid FNAC, where cyto-histological correlation is readily available. It is rare, but of benefit to find histological correlation in effusion fluid. Therefore, it is a good practice to have a clinical, radiological, and histopathological correlation and follow-up for accurate cytological diagnosis of effusion fluid.[12]
CONCLUSION
Effusion cytology is an important diagnostic tool in the evaluation of benign and malignant fluids. Standardization of reporting terminologies with negligible interobserver variation ensures an accurate cytological diagnosis needed for proper patient management. These standardized reporting systems might help in making the report more comprehensible. Careful morphological diagnosis, along with the ancillary tests, can provide an accurate diagnosis of effusion. From our research, we concludes that this reporting system is a user-friendly reporting system that can be easily applied on effusion fluid for better patient management and effective communication with clinicians.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
There is no conflict of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors state that they contributed to this publication according to the guidelines of the journal and no part of this manuscript was plagiarized.
ETHICS STATEMENT BY ALL AUTHORS
This material is the authors’ own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere. The paper reflects the authors’ own research and analysis in a truthful and complete manner. The paper properly credits the meaningful contributions of co-authors and co-researchers. The results are appropriately placed in the context of prior and existing research. All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
LIST OF ABBREVIATIONS (In alphabetic order)
AUS - Atypia of uncertain significance
IAC - And the Indian Academy of cytologist
ISRSFC - The international system for reporting serous fluid cytopathology
MAL - Malignant
ND - Non-diagnostic
NFM - Negative for malignancy
ROM - Risks of malignancy
SCIP - Subtractive coordinate immunoreactivity pattern
SFM - Suspicious for malignancy
EDITORIAL/PEERREVIEW STATEMENT
To ensure the integrity and highest quality of cytojournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
References
- The international system for reporting serous fluid cytopathology-diagnostic categories and clinical management. J Am Soc Cytopathol. 2020;9:469-77.
- [CrossRef] [PubMed] [Google Scholar]
- Pleural, Peritoneal and Pericardial Fluids in Comprehensive Cytopathology (3rd ed). Philadelphia, PA: WB Saunders Co; 2008. p. :515-77.
- [CrossRef] [Google Scholar]
- Cytopathology of pericardial effusions: experience from a tertiary centre of cardiology. Herz. 2018;43:543-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of diagnostic accuracy between Cell prep Plus and Thin Prep® liquid-based preparations in effusion cytology. Diagn Cytopathol. 2014;42:384-90.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality among patients with pleural effusion undergoing thoracentesis. Eur Respir J. 2015;46:495-502.
- [CrossRef] [PubMed] [Google Scholar]
- Announcement: The international system for reporting serous fluid cytopathology. Acta Cytol. 2019;63:349-51.
- [CrossRef] [PubMed] [Google Scholar]
- The Bethesda System for Reporting Cervical Cytology: Definitions (3rd ed). New York, Cham, Switzerland: Springer Press; 2015.
- [CrossRef] [Google Scholar]
- The Bethesda System for Reporting Thyroid Cytopathology, Criteria and Explanatory Notes New York: Springer; 2018.
- [CrossRef] [Google Scholar]
- The Paris System for Reporting Urinary Cytology Vol 2016. New York, Switzerland: Springer Press; 2016.
- [Google Scholar]
- The Milan System for Reporting Salivary Gland Cytopathology Vol 2018. Berlin: Springer Press; 2018.
- [CrossRef] [Google Scholar]
- Indian Academy of Cytologists Guidelines for Collection, Preparation, Interpretation, and Reporting of Serous Effusion Fluid Samples. J Cytol. 2020;37:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Application of Indian academy of cytologists guidelines for reporting serous effusions: Aan institutional experience. J Cytol. 2021;38:1-7.
- [Google Scholar]
- Are we ready to develop a tiered scheme for the effusion cytology? A comprehensive review and analysis of the literature. Diagn Cytopathol. 2019;47:1145-59.
- [CrossRef] [PubMed] [Google Scholar]
- A review of uncommon cytopathologic diagnoses of pleural effusions from a chest diseases centre in Turkey. CytoJournal. 2011;8:13.
- [CrossRef] [PubMed] [Google Scholar]
- Approach to diagnostic cytopathology of serous effusions. CytoJournal. 2021;18:32.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous pleural effusions: Aadvances and controversies. J Thorac Dis. 2015;7:981-91.
- [Google Scholar]
- Application of the international system for reporting serous fluid cytopathology (ISRSFC) on reporting pericardial effusion cytology. Acta Cytol. 2020;64:477-85.
- [CrossRef] [PubMed] [Google Scholar]
- Current classification systems and standardized terminology in cytopathology. Rom J Morphol Embryol. 2020;61:655-63.
- [CrossRef] [PubMed] [Google Scholar]
- A 2-year retrospective study on pleural effusions: A cancer centre experience. Cytopathology. 2019;30:607-13.
- [CrossRef] [PubMed] [Google Scholar]
- The International System for Serous Fluid Cytopathology Berlin, Germany: Springer International Publishing; 2020.
- [CrossRef] [Google Scholar]
- Malignant effusions: From diagnosis to biology. Diagn Cytopathol. 2004;31:246-54.
- [CrossRef] [PubMed] [Google Scholar]
- Etiologic diagnosis of 204 pericardial effusions. Medicine. 2003;82:385-91.
- [CrossRef] [PubMed] [Google Scholar]
- Pericardial fluid cytology: An analysis of 128 specimens over 6 years. Cancer Cytopathol. 2013;121:242-51.
- [CrossRef] [PubMed] [Google Scholar]
- The value of a tiered cytology diagnostic reporting system in assessing the risk of malignancy in indeterminate serous effusions. Cancer Cytopathol. 2021;129:75-82.
- [CrossRef] [PubMed] [Google Scholar]
- The role of liquid-based cytology and ancillary techniques in pleural and pericardic effusions: An institutional experience. Cancer Cytopathol. 2015;123:258-66.
- [CrossRef] [PubMed] [Google Scholar]
- The role of liquid-based cytology and ancillary techniques in the peritoneal washing analysis: Our institutional experience. PLoS One. 2017;12:0168625.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20:350-7.
- [CrossRef] [Google Scholar]
- Cytologic detection of malignancy in pleural effusion: A review of 5,255 samples from 3,811 patients. Diagn Cytopathol. 1987;3:8-12.
- [CrossRef] [PubMed] [Google Scholar]
- Cytopathologic diagnosis in pleural effusion and cyto-histopathologic correlation. Turk Patol Derg. 2011;27:12-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cytological evaluation of pericardial fluids: A 5 years experience in a tertiary care centre. Indian J Pathol Microbiol. 2019;62:270-3.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular alterations in patients with pulmonary adenocarcinoma presenting with malignant pleural effusion at the first diagnosis. Acta Cytol. 2017;61:214-22.
- [CrossRef] [PubMed] [Google Scholar]
- Positive pleural cytology is an indicator for visceral pleural invasion in metastatic pleural effusions. Clin Respir J. 2018;12:1011-6.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83:235-50.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant pleural effusion and cancer of unknown primary site: A review of the literature. Ann Transl Med. 2019;7:353.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic yield of cytopathology in evaluating pericardial effusions: Clinicopathologic analysis of 419 specimens. Cancer Cytopathol. 2017;125:128-37.
- [CrossRef] [PubMed] [Google Scholar]
- Pericardial effusions: Causes, diagnosis, and management. Prog Cardiovasc Dis. 2017;59:380-8.
- [CrossRef] [PubMed] [Google Scholar]
- Introduction to the second edition of “diagnostic cytopathology of serous fluids” as cytojournal monograph (CMAS) in open access. In: CytoJournal. Vol 18. 2021. p. :30.
- [CrossRef] [PubMed] [Google Scholar]








