Translate this page into:
Clinicopathological and molecular diagnostic features of liposarcoma: A study of 27 cases

*Corresponding author: Shuo Wang, Cancer Institution, Hangzhou Cancer Hospital, Hangzhou, China. wangs93@zju.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Zhu Y, Chen D, Yu J, Wang S. Clinicopathological and molecular diagnostic features of liposarcoma: A study of 27 cases. CytoJournal. 2025;22:40. doi: 10.25259/Cytojournal_246_2024
Abstract
Objective
Liposarcomas are rare tumors, and it is difficult to collect cases in less densely populated areas. Therefore, we aimed to document more cases over a relatively long period to provide more data about the characteristics of liposarcomas. In this study, the clinicopathological features of liposarcomas were investigated in 27 patients.
Material and Methods
All cases were confirmed by diagnosis through hematoxylin and eosin staining, immunohistochemistry (IHC), and fluorescence in situ hybridization (FISH). Combined IHC analysis was performed for murine double minute 2 (MDM2), cyclin-dependent kinase 4 (CDK4), multiple tumor suppressor 1 (P16), and Cyclin D1. FISH was performed to detect MDM2 amplification in atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDLPS) and dedifferentiated liposarcoma (DDLPS), and DNA damage inducible transcript 3 ( DDIT3) rearrangements in myxoid liposarcoma (MLPS).
Results
Seven cases of liposarcoma were located in the paratesticular region (25.9%, 7/27), 12 in the retroperitoneum (44.4%, 12/27), and eight in the limbs (29.6%, 8/27). Histological analysis showed that there were 13 cases of ALT/WDLPS (48.1%, 13/27), nine cases of DDLPS (33.3%, 9/27), three cases of MLPS (11.1%, 3/27), and two cases of pleomorphic liposarcoma (7.4%, 2/27). IHC analysis revealed that 26 cases were MDM2-positive (96.3%, 26/27), 22 were CDK4-positive (81.5%, 22/27), 26 were P16-positive (96.3%, 26/27), and 27 were cyclin D1-positive (100%, 27/27). FISH analysis revealed 20 cases of MDM2 positivity (90.9%, 20/22) and one case of DDIT3 positivity (50%, 1/2). The clinical outcomes were available for 21 patients. Four patients died (4/21, 19.0%), five experienced recurrence (5/21, 23.8%), and 12 (12/21, 57.1%) survived with no other disease.
Conclusion
A combined IHC examination of the four indicators may be used to diagnose ALT/WDLPS and DDLPS, and FISH is recommended as an important supporting method.
Keywords
Fluorescence in situ hybridization
Immunohistochemistry
Liposarcoma
INTRODUCTION
As the most common soft-tissue tumors, malignant liposarcomas account for approximately one-fifth of all liposarcomas. Liposarcomas are a heterogeneous group of distinct lesions categorized into multiple subtypes.[1] The recently updated World Health Organization (WHO) classification of soft-tissue and bone tumors lists the following five main subtypes: Dedifferentiated liposarcoma (DDLPS), atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDLPS), myxoid liposarcoma (MLPS), pleomorphic liposarcoma (PLPS), and myxoid PLPS (MPLPS).[2] ALT/WDLPS is a locally invasive tumor making up the highest percentage (approximately 40%) of all liposarcomas and with little potential for metastatic spread unless DDLPS occurs. In addition, sclerosing, adipocytic, inflammatory, and spindle cell variants are included in ALT/WDLPS.[3] DDLPS can evolve from WDLPS, which accounts for approximately 60% of all cases.[4] Metastasis occurs in almost 20% of DDLPS cases, resulting in a 5-year disease-specific mortality rate of <30%. Thus, DDLPS is not necessarily a high-grade liposarcoma. There is also a type of liposarcoma with low-grade dedifferentiation that is somewhat controversial but does exist.[5] MLPS accounts for approximately 30% of all liposarcomas and often arises in the deep soft tissues of the extremities, which is determined mainly by the degree of hypercellularity.[6] High-grade MLPS exhibits a significant tendency to metastasize to deep soft tissue. PLPS is the rarest subtype, being observed in <5% of all liposarcomas, and is associated with poor survival.[7] Despite being a high-grade sarcoma, the extent of lipogenic differentiation in PLPS is sometimes overlooked. MPLPS is a subtype with combined histological features of MLPS and PLPS, and it appears to be linked to conventional PLPS.[8]
The five liposarcoma subtypes have distinct morphological and genetic characteristics. Both ALT/WDLPS and DDLPS have murine double minute 2 (MDM2) and cyclin-dependent kinase 4 (CDK4) amplifications in chromosome 12q13-15, suggesting that they share a common genetic origin.[9] The main genetic characteristics of MLPS are karyotypic aberrations, in which DDIT3 and fused in sarcoma (FUS) fusions occur. The identification of DDIT3 rearrangements is an important diagnostic criterion, and immunohistochemistry (IHC) assays are an emerging alternative approach.[10] PLPS exhibits complex karyotypic aberrations and an aggressive clinical course. Although both PLPS and DDLPS are high-grade liposarcomas in most cases, PLPS contains lipoblasts, whereas DDLPS is a non-lipogenic sarcoma characterized by amplification of MDM2 and CDK4.[11] Published genetic analyses of MPLPS have not reported FUS/EWSR1-DDIT3 fusions or genomic inactivation of RB1.[12,13] In this study, we performed IHC and fluorescence in situ hybridization (FISH) assays for accurate diagnosis.
Different liposarcoma subtypes have different anatomical location tendencies, which are the main predictors of survival. Liposarcomas typically occur in the paratesticulum, retroperitoneum, or the limbs.[14] WDLPS is predominantly found in the retroperitoneum and the limbs,[15] while DDLPS occurs most frequently in the retroperitoneum. MLPS occurs predominantly in the lower limbs and tends to spread to distant bones and soft tissue.[16] Cases of PLPS are rare and most commonly occur in the soft tissues of the limbs.[17] In a limited number of cases, MPLPS shows a predilection for being located in the mediastinum.[18]
We collected and recorded information from 27 patients who attended our hospital between 2016 and 2023. To explore the clinicopathological features of liposarcomas, we performed hematoxylin and eosin (H&E) staining, IHC, and FISH assays for every case. In addition, we performed a survival analysis. Notably, liposarcomas at different locations have different clinicopathological features, a key insight worthy of further exploration.
MATERIAL AND METHODS
Collection of cases
We performed an 8-year retrospective review of our pathology database from 2016 to 2023 and investigated 27 confirmed cases of liposarcoma. In December 2023, we obtained access to information that could help identify individual participants for research purposes. Specific clinical information is shown in Table 1. All methods were performed in accordance with relevant guidelines and regulations.
| No. | Age/Gender | Location | Size (cm) | Tumor cohort | Composition | IHC | ||
|---|---|---|---|---|---|---|---|---|
| MDM2 | CDK4 | P16 | ||||||
| 1 | 55/M | Paratesticular | 16.5×10×7.5 | WDLPS | Lipomatous liposarcoma | + | + | + |
| 2 | 72/M | Paratesticular | Three nodules, the largest being 4.8×4.7×4 | DDLPS | Myxoid fibrosarcoma with moderately grade malignancy | + | + | + |
| 3 | 77/M | Paratesticular | 5.2×4.1×4 and 12×9.5×3 | WDLPS | Sclerosing liposarcoma and lipomatoid liposarcoma | + | + | + |
| 4 | 66/M | Paratesticular | 15×5×4.5 | DDLPS | Partly myxoid fibrosarcoma with low-grade malignancy and partly undifferentiated pleomorphic sarcoma | + | + | + |
| 5 | 54/M | Paratesticular | 7×6.5×5 | DDLPS | Fibroid sarcoma | + | + | + |
| 6 | 70/M | Paratesticular | 8×6×6 | WDLPS | Lipomatous liposarcoma | + | + | + |
| 7 | 73/M | Paratesticular | 6.7×6.5×6.2 | WDLPS | Lipomatous liposarcoma with mucinous degeneration | + | + | + |
| 8 | 67/F | Retroperitoneum | 35×24×8 | DDLPS | Fibroid sarcoma and localized ossification with mucinous degeneration in the surrounding area of WDLPS | + | + | + |
| 9 | 78/M | Retroperitoneum | 23.5×19×15 | DDLPS | Myxoid fibrosarcoma | + | + | + |
| 10 | 50/M | Retroperitoneum | 18×16×6.5 | WDLPS | Lipomatous liposarcoma | + | – | Local lesion + |
| 11 | 40/F | Retroperitoneum | 11×8×3 | WDLPS | Lipomatous liposarcoma | + | + | + |
| 12 | 72/M | Retroperitoneum | 11×9×4 | WDLPS | Lipomatous liposarcoma | + | + | + |
| 13 | 66/F | Retroperitoneum | 28×22×9 | WDLPS | Lipomatous liposarcoma | + | + | + |
| 14 | 65/M | Retroperitoneum | 7.3×6×4 | DDLPS | Undifferentiated pleomorphic sarcoma | + | + | + |
| 15 | 73/F | Retroperitoneum | 15×14×10.5 | WDLPS | Lipomatous liposarcoma | + | + | + |
| 16 | 65/M | Retroperitoneum | Punctured treatment | DDLPS | Low-grade fibromyxoid sarcoma | + | + | + |
| 17 | 73/F | Retroperitoneum | Punctured treatment | DDLPS | Fibroid sarcoma | + | + | + |
| 18 | 60/M | Retroperitoneum | 19×18×12 | WDLPS | Lipomatous liposarcoma | + | + | + |
| 19 | 54/F | Retroperitoneum | 7.3×7 × 4.3 | WDLPS | Inflammatory liposarcoma | + | + | + |
| 20 | 69/F | Limbs | 6×4×1.5 | MLPS | Myxoid fibrosarcoma | + | + | + |
| 21 | 52/M | Limbs | 15×11×7 | ALT | Atypical lipomatous tumor | + | + | + |
| 22 | 59/F | Limbs | 3×2×2 | DDLPS | Myxoid fibrosarcoma with low-grade malignancy | + | + | + |
| 23 | 39/M | Limbs | 8×5.5×4.5 | PLPS | Pleomorphic liposarcoma | + | – | + |
| 24 | 35/M | Limbs | 6×5×3 | MLPS | Myxoid fibrosarcoma | + | – | + |
| 25 | 79/M | Limbs | 4×3×2 | PLPS | Pleomorphic liposarcoma | – | – | + |
| 26 | 48/M | Limbs | 7.5×6×2.5 | ALT | Lipomatous liposarcoma | + | – | – |
| 27 | 80/F | Limbs | 4.2×3×3 | MLPS | Myxoid fibrosarcoma | + | + | + |
| 1 | 55/M | Paratesticular | + | – | NP | Surgery | No recurrence | |
| 2 | 72/M | Paratesticular | + | + | NP | Surgery and chemotherapy | Died after 3 years | |
| 3 | 77/M | Paratesticular | + | + | NP | Surgery | No recurrence | |
| 4 | 66/M | Paratesticular | + | + | NP | Surgery, chemotherapy and radiotherapy | No recurrence | |
| 5 | 54/M | Paratesticular | + | + | NP | Surgery | No recurrence occurred by patient self-report | |
| 6 | 70/M | Paratesticular | + | + | NP | Surgery | No recurrence | |
| 7 | 73/M | Paratesticular | + | + | – | Surgery | No recurrence | |
| 8 | 67/F | Retroperitoneum | + | + | NP | Surgery | Recurrence as WDLPS after 5 years | |
| 9 | 78/M | Retroperitoneum | + | + | NP | Surgery | Died after 20 days | |
| 10 | 50/M | Retroperitoneum | + | + | NP | Surgery | Loss to follow-up | |
| 11 | 40/F | Retroperitoneum | + | + | NP | Surgery | Recurrence | |
| 12 | 72/M | Retroperitoneum | + | + | NP | Surgery | No recurrence | |
| 13 | 66/F | Retroperitoneum | + | + | NP | Surgery | No recurrence | |
| 14 | 65/M | Retroperitoneum | Local lesion+ | + | NP | Surgery | Died | |
| 15 | 73/F | Retroperitoneum | + | + | NP | Surgery | No recurrence | |
| 16 | 65/M | Retroperitoneum | + | + | NP | Surgery and targeted chemotherapy | Survive with esophageal squamous cell carcinoma | |
| 17 | 73/F | Retroperitoneum | Local lesion+ | + | NP | Untreated | Died | |
| 18 | 60/M | Retroperitoneum | + | + | NP | Surgery | Loss to follow up | |
| 19 | 54/F | Retroperitoneum | + | + | NP | Surgery | No recurrence | |
| 20 | 69/F | Limbs | + | – | NP | Surgery | Loss to follow-up | |
| 21 | 52/M | Limbs | + | + | NP | Surgery and kidney transplantation | Loss to follow-up | |
| 22 | 59/F | Limbs | + | + | NP | Surgery | No recurrence | |
| 23 | 39/M | Limbs | + | – | NP | Surgery | Loss to follow-up | |
| 24 | 35/M | Limbs | + | – | – | Surgery | Another 1 cm nodule and under observation | |
| 25 | 79/M | Limbs | + | – | NP | Surgery | No recurrence | |
| 26 | 48/M | Limbs | + | – | NP | Surgery | Recurrence after 1 years with 7 cm tumors | |
| 27 | 80/F | Limbs | + | – | + | Surgery | Loss to follow-up | |
WDLPS: Well-differentiated liposarcoma, DDLPS: Dedifferentiated liposarcoma, MLPS: Myxoid liposarcoma, ALT: Atypical lipomatous tumor, PLPS: Pleomorphic liposarcoma, IHC: Immunohistochemistry, MDM2: Murine double minute 2, CDK4: Cyclin-dependent kinase 4, NP: Not performed, +: Positive, −: Negative
The inclusion criteria were as follows: cases with histomorphology from 2016 to 2023 that met the 5th edition of the WHO Liposarcoma Diagnostic Criteria for 2020 and at least two positive immunohistochemical results for MDM2, CDK4, multiple tumor suppressor 1 (P16), and cyclin D1.[19]
The exclusion criteria were as follows: smooth muscle or rhabdomyosarcoma of smooth muscle origin with positive myogenic markers, such as smooth muscle actin and desmin; neurogenic tumors positive for neurogenic markers, such as S100 and neuron-specific enolase ( NSE); and other soft-tissue sarcomas, such as osteosarcoma and chondrosarcoma with bone or cartilaginous components in the H&E-stained sections.
Ethics statement
This study was performed in accordance with the principles of the 2024 Revision of the Declaration of Helsinki and was approved by the Ethics Committee of Ningbo Yinzhou No.2 Hospital.[20] The need for consent was waived by the Ethics Committee.
H&E staining assay
The tissue sections were incubated in an in situ hybridization oven at 70℃ for 30 min to dissolve the paraffin. They were then placed in an automatic tissue stainer (DRS-Prisma-PJCS; Sakura Medical Science Technology, Taizhou, China) and an automatic capping machine (Film-JC2; Sakura Medical Science Technology) and discharged after 1 h.
IHC assay
The Envision method was used to perform IHC analysis of formalin-fixed paraffin-embedded tissues. Primary antibodies against MDM2 (ZM-0425), CDK4 (ZA-0614), P16 (ZM-0205), and cyclin D1 (ZA-0101) were purchased from Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China). These primary antibodies were ready-to-use antibodies, which can be used directly without additional dilution. A two-step test kit (PV-9000) was used for staining. Deparaffinized and hydrated tissue sections were rinsed with distilled water and placed in Tris-buffered saline (TBS). Endogenous peroxidase was blocked, and the samples were rinsed in distilled water, placed in TBS, incubated with primary antibody for 30 min, and rinsed again in TBS. The cells were incubated with EnVision for 30 min, rinsed in TBS, incubated with a color substrate solution for 10 min, and then rinsed in distilled water, re-stained, and sealed. The results were evaluated according to the intensity of positive staining, and the percentage of positive cells was scored as follows: 0, colorless; 1, light yellow; and 2, brown. The percentage of positive cells was scored as 0 for no positive cells, 1 for 1–25%, 2 for > 25–50%, 3 for > 50–75%, and 4 for > 75%. The score for staining intensity was added to the percentage of positive cells; 0 points were considered negative, while more than 3 points were considered positive.[21-24] The results for each sample were confirmed by two experienced pathologists.
FISH assay
FISH assay kits (MDM2 FISH [F.01017-01] and DDIT3 FISH [F.01015-01]) were purchased from Anbipin Pharmaceutical Technology Co., Ltd. (Guangzhou, China). An in situ hybridizer (S500, ThermoBrite) was purchased from Abbott Molecular (Chicago, American). In brief, sections were prepared, DNA was denatured, the probes were hybridized, and the sections were re-stained with 4’, 6-diamidino-2-phenylindole (DAPI) (62248, Invitrogen, Waltham, American). A minimum of 100 cells was counted on each slide. The red and green signals were counted by two independent investigators. A case was judged to be positive when more than 20% of the nuclei showed a signal, and the red/green signal ratio was ≥2, or clusters of red signals appeared in a single nucleus.[25,26]
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 9.0; GraphPad, San Diego, CA, USA).
RESULTS
Clinicopathological features of 27 cases of liposarcoma
The patients in the study included 18 males and nine females. A predilection (male: female = 2:1) for location in the paratesticular region was observed in seven male cases. We classified liposarcomas according to three locations: paratesticular, retroperitoneum, or limbs. There were seven cases of liposarcoma located in the paratesticular region (25.9%, 7/27), 12 cases in the retroperitoneum (44.4%, 12/27), and eight cases in the limbs (29.6%, 8/27). Pathologists histologically characterized the tumor cohort in each case. There were 13 cases of ALT/WDLPS (48.1%, 13/27), nine cases of DDLPS (33.3%, 9/27), three cases of MLPS (11.1%, 3/27), and two cases of PLPS (7.4%, 2/27). Most patients underwent surgical treatment, and some patients received additional chemotherapy. The clinicopathological features of all cases in this study are shown in Table 1.
Examination of the different features of liposarcoma cohorts
The collected cases covered four cohorts of liposarcomas. The highest number of cases was observed in the ALT/WDLPS group. On general examination, the tumors were large, multinodular, or lobulated, with a thin fibrous envelope. Infiltrative growth was also observed in a few cases. The cut surfaces were yellow or grayish-yellow and may be associated with hemorrhage or infarction. Among them, the sclerosing liposarcoma had a grayish-white cut surface and a fibrous-like pattern [Figure 1a]. In DDLPS, the tumors were multinodular with solid grayish-white areas, firm or fish-like in texture, and often necrotic [Figure 1b]. There were few MLPS cases. The tumors were mostly large with clear boundaries. They were multinodular, soft, and jelly-like. The cut surfaces were either yellow or grayish-yellow. When containing a round cell component, they were solid and grayish-white with partial hemorrhage [Figure 1c]. The incidence of PLPS was the lowest, with only two cases in both limbs. The tumors were multinodular or irregular and unenveloped. They were well-defined or infiltrative. The cut surfaces were gray or grayish-yellow, often with necrotic foci [Figure 1d]. More than half of the ALT/WDLPS (53.8%, 7/13) and DDLPS (55.6%, 5/9) cases occurred in the retroperitoneum. All cases of MLPS (three) and PLPS (two) occurred in the limbs. The tumor locations and cohorts of the 27 patients are shown in Table 2.
| Location | Tumor cohort | ||||
|---|---|---|---|---|---|
| ALT/WDLPS | DDLPS | MLPS | PLPS | Totals | |
| Paratesticular | 4 | 3 | 0 | 0 | 7 |
| Retroperitoneum | 7 | 5 | 0 | 0 | 12 |
| Limbs | 2 | 1 | 3 | 2 | 8 |
| Totals | 13 | 9 | 3 | 2 | 27 |
WDLPS: Well-differentiated liposarcoma, DDLPS: Dedifferentiated liposarcoma, MLPS: Myxoid liposarcoma, ALT: Atypical lipomatous tumor, PLPS: Pleomorphic liposarcoma
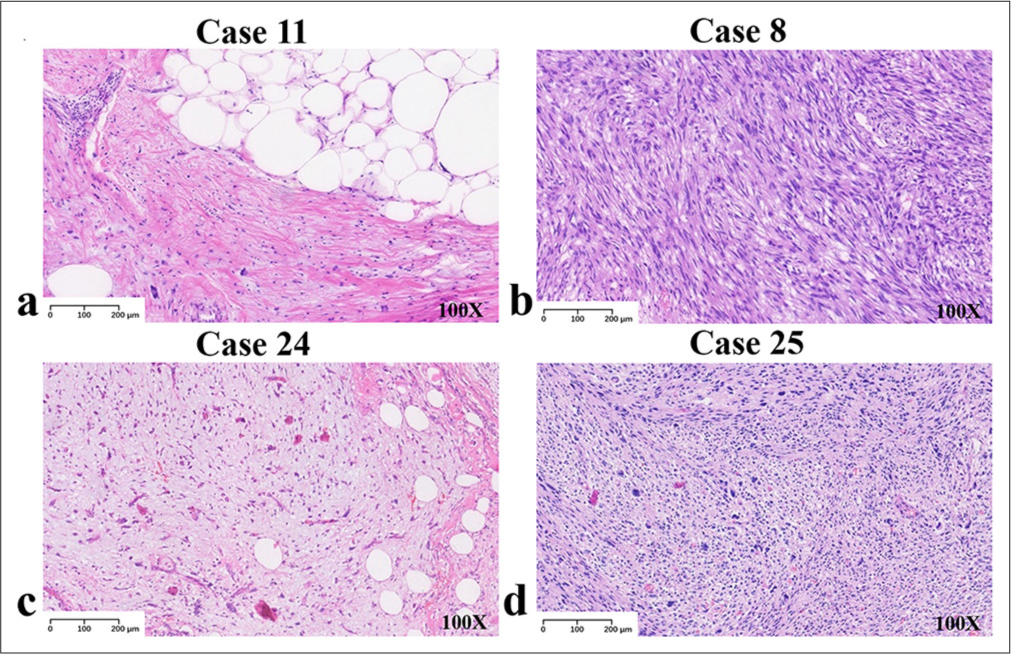
- H&E staining results, 100x. (a) Images displaying well-differentiated components of liposarcoma. (b) Images displaying dedifferentiated components of liposarcoma. (c) Images displaying myxoid components of liposarcoma. (d) Images displaying pleomorphic components of liposarcoma. (H&E: Hematoxylin and eosin)
IHC studies
We performed an IHC analysis on all 27 patients. MDM2, CDK4, P16, and cyclin D1 were combined to allow an accurate diagnosis. IHC analysis revealed that 96.3% (26/27) of cases exhibited MDM2 positivity, with the only negative case being PLPS [Figure 2a]. CDK4 positivity was observed in 81.5% (22/27) of the cases, and four of five cases were located in the limbs [Figure 2b]. The results of P16 detection were similar to those of MDM2 (96.3%, 26/27) [Figure 2c]. Of note, all cases tested positive for cyclin D1 (100%, 27/27) [Figure 2d].
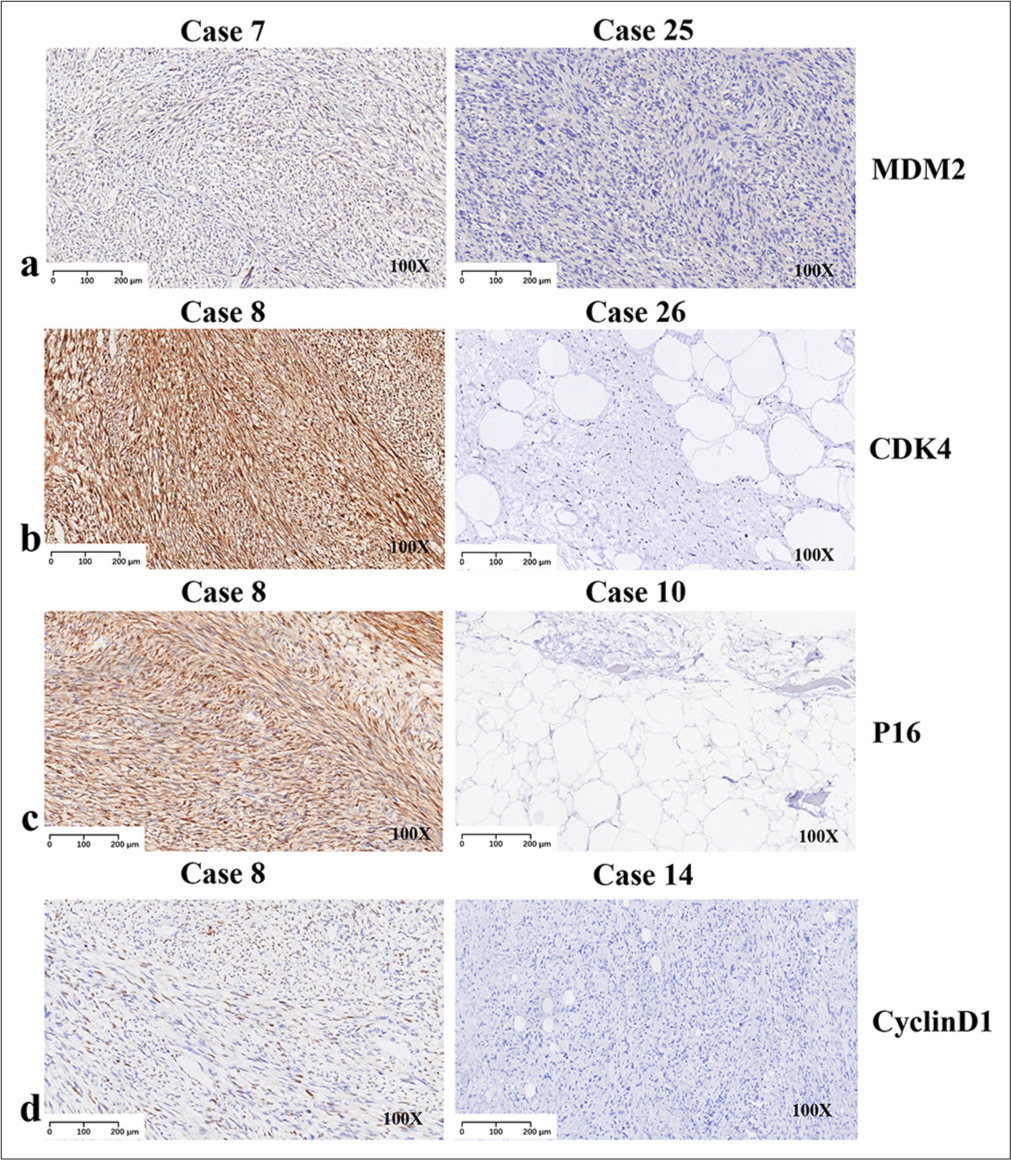
- Immunohistochemistry assays results, 100x, (a) Images displaying positive in murine double minute 2. (b) Images displaying positive in cyclin-dependent kinase 4. (c) Images displaying positive in P16. (d) Images displaying positive in CyclinD1. For each figure, left case is positive while right case is negative. (MDM2: Murine double minute 2, CDK4: Cyclin-dependent kinase 4, P16: Multiple tumor suppressor 1)
FISH studies
We performed FISH on all 27 patients. MDM2 was the most accurate marker for ALT/WDLPS and DDLPS, with positive results in most cases (90.9%, 20/22) [Figure 3a]. Of these, one negative case in the paratesticular region could have been positive because it was too long. Another negative case was observed in the patient’s limbs. However, four of the five cases were negative for MPLS or PLPS. DDIT3 rearrangement was detected in the MPLS, and one case was positive (50%, 1/2) [Figure 3b]. MDM2 is rarely detected in MPLS or PLPS. The signal strength of DDIT3 was significantly weaker than that of MDM2 and needed to be carefully screened before the results were judged. The red signal of DDIT3 appeared mostly as dots and was not as pronounced as that of MDM2, which appeared mostly in clusters.
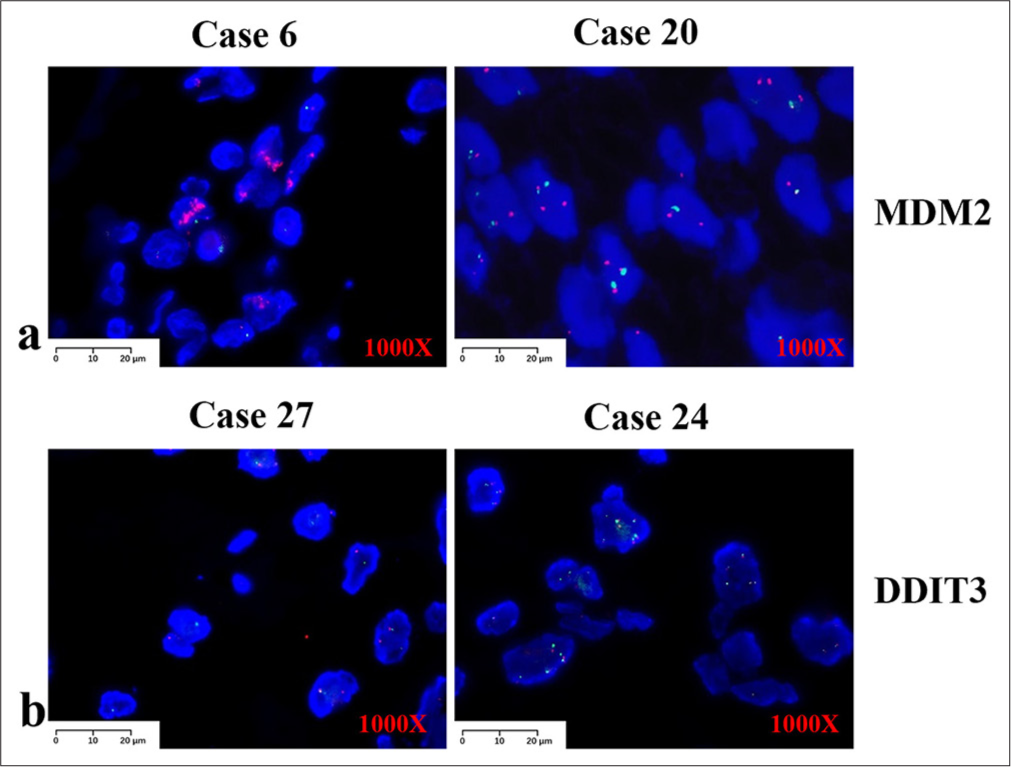
- FISH assays results, 1000x, (a) Images displaying positive in murine double minute 2. (b) Images displaying positive in DDIT3. For each figure, left case is positive while right case is negative. (FISH: Fluorescence in situ hybridization, MDM2: Murine double minute 2, DDIT3: DNA Damage Inducible Transcript 3)
Clinical outcomes
The detailed treatment strategies and outcomes are listed in Table 1. Almost all patients underwent surgery (96.3%, 26/27). Adjuvant chemotherapy and/or radiotherapy were administered in three cases (11.1%, 3/27). Outcome information was available for 21 patients (21/27, 77.8%). Four (4/21, 19.0 %) patients died. Recurrence occurred in five patients (5/21, 23.8%), one of whom survived with esophageal squamous cell carcinoma. One patient diagnosed with DDLPS experienced a recurrence of WDLPS after 5 years. Twelve patients (12/21, 57.1%) survived with no other disease for 5 years. Seventeen patients (17/21, 77.8%) survived for 5 years. The clinical outcome information is shown in Table 3.
| Outcomes | Tumor cohort | Location | Totals | |||||
|---|---|---|---|---|---|---|---|---|
| ALT/WDLPS | DDLPS | MLPS | PLPS | Paratesticular | Retroperitoneum | Limbs | ||
| No recurrence | 8 | 3 | 0 | 1 | 6 | 4 | 2 | 12 |
| With disease | 2 | 2 | 1 | 0 | 0 | 3 | 2 | 5 |
| Died | 0 | 4 | 0 | 0 | 1 | 3 | 0 | 4 |
| Overall survival rate (percentage, number of survivors at follow-up/total number) | 100%, 10/10 | 55.6%, 5/9 | 100%, 1/1 | 100%, 1/1 | 85.7%, 6/7 | 70%, 7/10 | 100%, 4/4 | 77.8%, 17/21 |
| Loss to follow-up | 3 | 0 | 2 | 1 | 0 | 2 | 4 | 6 |
| Totals | 13 | 9 | 3 | 2 | 7 | 12 | 8 | 27 |
WDLPS: Well-differentiated liposarcoma, DDLPS: Dedifferentiated liposarcoma, MLPS: Myxoid liposarcoma, ALT: Atypical lipomatous tumor, PLPS: Pleomorphic liposarcoma
DISCUSSION
Twenty-seven patients with liposarcoma were enrolled at our hospital between 2016 and 2023. Liposarcoma is a rare tumor that accounts for <20% of soft-tissue sarcomas.[27] The number of cases collected here was also small. Male predilection (2.0) was exhibited in this study, though variable predilection (0.5–2.5) has been reported for liposarcomas.[28] It has been reported that liposarcoma is much more common in the retroperitoneum[29] and we found that the retroperitoneum was the most common location (44.4%). The incidence of ALT/WDLPS and DDLPS in the tumor cohort was 81.5% (22/27), whereas an incidence of 60% has been reported in the previous studies. MLPS comprised 11.1% (3/27) of cases, indicating a low incidence. PLPS comprised 7.4% (2/27) of cases, similar to previous reports. Patients with DDLPS had a poor prognosis (55.6%), whereas those with ALT/WDLPS had a good prognosis (100.0%). A poor prognosis was observed for retroperitoneal liposarcoma (70%). Therefore, DDLPS in the retroperitoneum should receive the most attention.
ALT/WDLPS and DDLPS were positive for MDM2, as observed by IHC (96.3%) and FISH (90.9%) analyses. During the observation of the resected sections, some components may go unnoticed. However, MDM2 positivity on IHC is not a specific indicator of MDM2 amplification because it is observed in almost all tumors. Thus, the confirmation of MDM2 expression by IHC alone is of limited clinical value. CDK4 is often amplified in patients along with MDM2. CDK4 staining was more diffuse and intense but less sensitive than MDM2 staining. Approximately 10% of ALT/WDLPS and DDLPS cases did not show CDK4 amplification.[30] MDM2 had high sensitivity and low specificity, whereas CDK4 had low sensitivity and specificity.[31] We found similar results for CDK4 positivity (81.5%), suggesting that its sensitivity was slightly worse than that of MDM2. Molecular analysis of MDM2 and CDK4 amplification may also be useful when diffuse strong nuclear staining is observed. If MDM2 and CDK4 amplification are insufficient to support a diagnosis, the combination of P16 and cyclin D1 can be used. Cyclin D1 is involved in tumorigenesis and tumor development as a cell cycle regulator, and P16 can inhibit cell cycle progression by binding to the CDK4/cyclin D1 complex.[32] Therefore, the P16 expression level is believed to correlate with the CDK4 expression level, which is considered a useful indicator for the diagnosis of liposarcoma.[33] In our study, P16 positivity was 96.3%, and cyclin D1 positivity was 100%. Therefore, ALT/WDLPS and DDLPS may be extremely highly predicted by combination analysis with the three indicators CDK4, P16, and cyclin D1. It is necessary to resort to molecular pathology for the diagnosis of tumors with a dense collagenous stroma.
Here, we report three cases of MLPS and two cases of PLPS. Approximately 95% of MLPS samples exhibited DDIT3 rearrangement.[34] In this study, one case was negative and recurred after surgery, but the existence of other molecular defects requires further investigation. Cases of PLPS are very rare, and we found both P16 and cyclin D1 positivity by IHC in only two cases. There are few reports of PLPS, and the combination of P16 and cyclin D1 may be useful in the diagnosis of PLPS.
The survival rate of patients with ALT/WDLPS was 100% in our study, which is consistent with previous reports, but the survival rate of patients with DDLPS (55.6%) was low. However, one case of DDLPS combined with esophageal squamous cell carcinoma was treated with combined chemotherapy and targeted therapy after surgery and had a good prognosis. The survival rate was the lowest when the tumor was located in the retroperitoneum (70%). It has been reported that the survival rate for retroperitoneal liposarcoma is 54%[35] which is consistent with the results of our study. Tumors located in the paratesticular space seemed to be less prone to recurrence (0%, 0/6). Despite the small number of cases, the prognosis was poor for patients with retroperitoneal liposarcoma and good for the limited number of patients with liposarcoma located in the extremities.
In summary, we present the detailed clinicopathological features of 27 cases of liposarcomas. Further comprehensive studies are necessary for a better understanding of these tumors.
Limitations
There are some limitations of this study that should be noted. First, only 27 cases over an 8-year period were included, which may limit the generalizability of the findings. Second, the number of MLPS and PLPS cases was low due to the low incidence of these two subtypes, and it was difficult to obtain sufficient cases by collecting cases only locally. Third, the follow-up period varied among the patients, and some patients were lost to follow-up. However, we believe that, even in local hospitals, case collection should be maintained, and patient follow-up should be completed as much as possible, which will provide more support for understanding rare diseases.
SUMMARY
Despite the low frequency of liposarcoma cases, exhaustive data collection remains necessary. The results of the analysis of clinicopathological features we collected showed that combined IHC examination of the four indicators could confirm ALT/WDLPS and DDLPS, and FISH is recommended as an important supporting method.
AVAILABILITY OF DATA AND MATERIALS
The datasets and materials used and analyzed during the present study are available from the corresponding author on reasonable request.
ABBREVIATIONS
IHC: Immunohistochemistry
FISH: Fluorescence in situ hybridization
MDM2: Murine double minute 2
CDK4: Cyclin-dependent kinase 4
P16: Multiple tumor suppressor 1
ALT: Atypical lipomatous tumor
WDLPS: Well-differentiated liposarcoma
DDLPS: Dedifferentiated liposarcoma
PLPS: Pleomorphic liposarcoma
MLPS: Myxoid liposarcoma
DDIT3: DNA Damage Inducible Transcript 3
FUS: Fused in sarcoma
H&E: Hematoxylin and eosin
NSE: Neuron-specific enolase
TBS: Tris-buffered saline
ACKNOWLEDGMENT
We thank the editors and the reviewers for their useful feedback that improved this paper.
AUTHOR CONTRIBUTIONS
YZ and DC: Performed the experiments; SW: Analyzed the data; YZ and JJY: Provided resources; SW: Drafted the manuscript; SW: Performed language embellishment; SW: Conceived the study and experimental designed. All authors revised and approved the submitted version. All authors meet ICMJE authorship requirements.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the Ethics Committee of Ningbo Yinzhou No.2 Hospital (Approval No.031 in 2023). The need for consent was waived by the Ethics Committee of Ningbo Yinzhou No.2 Hospital.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: This study was supported by grants from Yinzhou District Health Science and Technology Program (No.2023Y16).
References
- Liposarcomas: Diagnostic pitfalls and new insights. Histopathology. 2014;64:38-52.
- [CrossRef] [PubMed] [Google Scholar]
- Updates from the 2020 World Health Organization classification of soft tissue and bone tumours. Histopathology. 2021;78:644-57.
- [CrossRef] [PubMed] [Google Scholar]
- Well differentiated liposarcoma presenting as a duodenal polyp: A case report and review of the literature. Int J Surg Pathol. 2023;31:307-11.
- [CrossRef] [PubMed] [Google Scholar]
- Biology and management of dedifferentiated liposarcoma: State of the art and perspectives. J Clin Med. 2021;10:3230.
- [CrossRef] [PubMed] [Google Scholar]
- Does “Low-Grade” dedifferentiated liposarcoma exist? The role of mitotic index in separating dedifferentiated liposarcoma from cellular well-differentiated liposarcoma. Am J Surg Pathol. 2023;47:649-60.
- [CrossRef] [PubMed] [Google Scholar]
- The expanded histologic spectrum of myxoid liposarcoma with an emphasis on newly described patterns: Implications for diagnosis on small biopsy specimens. Am J Clin Pathol. 2012;137:229-39.
- [CrossRef] [PubMed] [Google Scholar]
- Management of myxofibrosarcoma and undifferentiated pleomorphic sarcoma. Surg Oncol Clin N Am. 2022;31:419-30.
- [CrossRef] [PubMed] [Google Scholar]
- Myxoid pleomorphic liposarcoma-a clinicopathologic, immunohistochemical, molecular genetic and epigenetic study of 12 cases, suggesting a possible relationship with conventional pleomorphic liposarcoma. Mod Pathol. 2021;34:2043-9.
- [CrossRef] [PubMed] [Google Scholar]
- Similarity in genetic alterations between paired well-differentiated and dedifferentiated components of dedifferentiated liposarcoma. Mod Pathol. 2009;22:1477-88.
- [CrossRef] [PubMed] [Google Scholar]
- DDIT3 Immunohistochemistry is a useful tool for the diagnosis of myxoid liposarcoma. Am J Surg Pathol. 2021;45:230-9.
- [CrossRef] [PubMed] [Google Scholar]
- Liposarcomas with mixed well-differentiated and pleomorphic features: A clinicopathologic study of 12 cases. Am J Surg Pathol. 2010;34:837-43.
- [CrossRef] [PubMed] [Google Scholar]
- Liposarcomas of the mediastinum and thorax: A clinicopathologic and molecular cytogenetic study of 24 cases, emphasizing unusual and diverse histologic features. Am J Surg Pathol. 2012;36:1395-403.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive genetic analysis of a paediatric pleomorphic myxoid liposarcoma reveals near-haploidization and loss of the RB1 gene. Histopathology. 2016;69:141-7.
- [CrossRef] [PubMed] [Google Scholar]
- Soft tissue sarcoma, Version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:815-33.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of dedifferentiated liposarcoma: A multidisciplinary position statement. Cancer Treat Rev. 2024;131:102846.
- [CrossRef] [PubMed] [Google Scholar]
- Metastatic patterns of extremity myxoid liposarcoma and their outcome. J Surg Oncol. 2002;80:89-93.
- [CrossRef] [PubMed] [Google Scholar]
- Pleomorphic liposarcoma: Updates and current differential diagnosis. Semin Diagn Pathol. 2019;36:122-8.
- [CrossRef] [PubMed] [Google Scholar]
- Myxoid pleomorphic liposarcoma is distinguished from other liposarcomas by widespread loss of heterozygosity and significantly worse overall survival: A genomic and clinicopathologic study. Mod Pathol. 2022;35:1644-55.
- [CrossRef] [PubMed] [Google Scholar]
- Soft tissue and bone tumours (5th ed). Lyon: International Agency for Research on Cancer; 2020. p. :34-46.
- [Google Scholar]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human participants. JAMA. 2025;333:71-4.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of MDM2 gene amplification or protein expression distinguishes sclerosing mesenteritis and retroperitoneal fibrosis from inflammatory well-differentiated liposarcoma. Mod Pathol. 2009;22:66-70.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23:1279-88.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of p16 protein in lesional and perilesional condyloma acuminata and bowenoid papulosis: Clinical significance and diagnostic implications. J Am Acad Dermatol. 2013;69:444-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic significance of CyclinD1 and D2-40 expression for follicular neoplasm of the thyroid. Pathol Res Pract. 2022;229:153739.
- [CrossRef] [PubMed] [Google Scholar]
- Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol. 2008;21:943-9.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of the 12q amplicons in lipomatous soft tissue tumors by multiplex ligation-dependent probe amplification-based copy number analysis. Anticancer Res. 2015;35:1835-42.
- [Google Scholar]
- Pharmacotherapy for liposarcoma: Current and emerging synthetic treatments. Future Oncol. 2021;17:2659-70.
- [CrossRef] [PubMed] [Google Scholar]
- Liposarcoma in children and young adults: A clinicopathologic and molecular study of 23 cases in one of the largest institutions of China. Virchows Arch. 2021;479:537-49.
- [CrossRef] [PubMed] [Google Scholar]
- Updates in pathology for retroperitoneal soft tissue sarcoma. Curr Oncol. 2022;29:6400-18.
- [CrossRef] [PubMed] [Google Scholar]
- Beyond targeting amplified MDM2 and CDK4 in well differentiated and dedifferentiated liposarcomas: From promise and clinical applications towards identification of progression drivers. Front Oncol. 2022;12:965261.
- [CrossRef] [PubMed] [Google Scholar]
- MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing Well-differentiated and dedifferentiated liposarcoma subtypes: A comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29:1340-7.
- [CrossRef] [PubMed] [Google Scholar]
- The p16-cyclin D1/CDK4-pRb pathway and clinical outcome in epithelial ovarian cancer. Clin Cancer Res. 1999;5:4152-7.
- [Google Scholar]
- Targeting CDK4 (cyclin-dependent kinase) amplification in liposarcoma: A comprehensive review. Crit Rev Oncol Hematol. 2020;153:103029.
- [CrossRef] [PubMed] [Google Scholar]
- Nuclear expression of DDIT3 distinguishes high-grade myxoid liposarcoma from other round cell sarcomas. Mod Pathol. 2021;34:1367-72.
- [CrossRef] [PubMed] [Google Scholar]
- Giant retroperitoneal liposarcoma: Correlation between size and risk for recurrence. World J Oncol. 2022;13:244-8.
- [CrossRef] [PubMed] [Google Scholar]








