Translate this page into:
Comparative accuracy of fine-needle aspiration cytology between larger and smaller size thyroid nodules

*Corresponding author: Mohammed Abdulrahman Alshaikh, Department of Internal Medicine, King Abdulaziz University Hospital, Jeddah, Saudi Arabia. shmohd51@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Samargandy S, Khedher YZ, Alzahrani GA, Nahhas HT, Alshaikh MA, Alzahrani KA, et al. Comparative accuracy of fine-needle aspiration cytology between larger and smaller size thyroid nodules. CytoJournal. 2025;22:44. doi: 10.25259/Cytojournal_206_2024
Abstract
Objective
Thyroid nodules are frequently encountered in medical practice. Fine needle aspiration cytology (FNAC) is used to rule out malignant nodules, but few studies have questioned the accuracy of FNAC in larger thyroid nodules compared to smaller ones. We, therefore, aim to compare the diagnostic performance of FNAC based on nodule size and whether larger nodule size increases the possibility of obtaining indeterminate or non-diagnostic results.
Material and Methods
Adult patients with thyroid nodules who underwent thyroid biopsy and surgery from 2016 to 2022 were included in the study. We assessed the proportion of benign, malignant, indeterminate, and non-diagnostic FNAC in relation to the nodule size. We then divided cytology into true positive (malignant FNAC and histology), and true negative (benign FNAC and histology) and examined whether the proportion of true FNAC would be affected by different thyroid nodule cutoffs. The study used mean and frequency to describe continuous and categorical variables. t-test and Chi-square tests were used to compare statistics.
Results
Three hundred and forty-five patients were included in the study. The majority were female (86.7%) and older than 40 years. Half had a benign histology; the other 50% were malignant. The majority (49.3%) had indeterminate thyroid cytology. The proportion of indeterminate or non-diagnostic FNAC was the same (58%) in nodules ≥4 cm and <4 cm. The proportion of true FNAC was similar between different nodule size categories. It was 35% in ≥4 cm, and 34.3% in <4 cm nodules.
Conclusion
The study found that the diagnostic performance of FNAC in thyroid nodules did not significantly differ based on nodule size, with similar rates of indeterminate or non-diagnostic results across different size categories. The proportion of true positive FNAC results also remained consistent regardless of nodule size.
Keywords
Fine-needle biopsy
Thyroid cancer
Thyroid nodule
Ultrasonography
INTRODUCTION
In clinical practice, thyroid nodules are common, with a higher prevalence in women. These nodules are found in approximately 50% of healthy individuals and, in most cases, do not result in substantial symptoms. Approximately 80–90% of thyroid nodules are estimated to be benign. Patients at risk for developing thyroid cancer are those with a history of head-and-neck radiation exposure and a family history of thyroid cancer.[1]
The primary goal of managing these nodules is to exclude malignancy; this is often achieved through neck ultrasonography (US) and thyroid fine-needle aspiration cytology (FNAC). The US is a valuable, non-invasive tool for identifying nodules harboring possible malignancy. Radiological findings such as microcalcifications, taller-than-wide nodule dimensions, solid irregular nodule borders, and hypoechogenicity can raise suspicion of thyroid cancer.[2]
Thyroid cytology is a simple diagnostic tool for thyroid nodules, thereby decreasing unnecessary surgeries in most benign cases, with a reported mean sensitivity and specificity of 83% and 92%, respectively.[3] However, there are conflicting data about the accuracy of FNAC in larger thyroid nodules, particularly those larger than 4 cm. Several researchers have shown similar FNAC performance between larger and smaller nodules,[4] while other studies have found higher rates of false-negative FNAC in larger thyroid nodules, and some have even recommended immediate surgery for nodules larger than 4 cm regardless of the FNAC results.[5]
Based on these inconsistent findings, we aim in this study to evaluate the accuracy of thyroid FNAC in detecting malignancy in smaller nodules compared to larger nodules and to determine whether the probability of indeterminate or non-diagnostic thyroid FNAC is higher when the size of the nodule is larger.
MATERIAL AND METHODS
Design and patients
Our study is a retrospective analysis that took place at King Abdul-Aziz University Hospital, Jeddah, Saudi Arabia. Ethical approval was obtained from the Biomedical Research Ethics Committee at King Abdulaziz University, with a reference number of 81–23, with a reference number of 81–23 and following the Declaration of Helsinki.[6] Due to the retrospective and non-interventional nature of the study, participants’ informed consent was waived.
We included all adult patients who underwent total or hemithyroidectomy from 2016 to 2022 and had pre-operative evidence of thyroid nodules on US imaging. Included patients should have available pre-operative nodule FNAC and postoperative histology. Patients with missing US or FNAC or histopathology data or pediatric patients were excluded from the study.
Various patient details were collected, including age, gender, pre-operative thyroid-stimulating hormone levels, the size of the nodule in the US in cm, the US risk category according to the 2015 American Thyroid Association (ATA) guidelines for thyroid nodules,[7] results of the FNAC, and the postoperative histopathology outcome specifying if it was benign or malignant. Age was divided into 40 years or less versus above 40 years based on the mean age of thyroid cancer in Saudi Arabia.[8,9]
FNA characterization
FNAC findings were labeled based on the Bethesda system for reporting thyroid cytopathology. They were divided into non-diagnostic, benign, follicular lesion of undetermined significance/atypia of undetermined significance (FLUS/AUS), follicular neoplasm (FN) or suspicious for FN, suspicious for malignancy (SUSP), and malignant.[10] This addition provides readers with essential criteria, aiding in the interpretation and compatibility of our FNAC results with standardized classifications.[11]
True negative FNAC cases are benign FNAC that yielded a benign histopathology. True-positive FNAC cases are the malignant FNAC that yielded malignant histopathology. True positive and true negative cases were collectively labeled as true FNAC tests. All FNAC were collected under US guidance, the obtained thyroid US-guided DNA sample was used to prepare conventional smears beside liquid base preparations. The cell block was added whenever floating materials were detected.
Statistical analysis
Means with standard deviations and medians with Q1 and Q3, and frequencies with percentages were used to describe continuous and categorical variables, respectively. The overall study sample was categorized based on the sample’s histopathology into malignant or benign tumors. We used the Shapiro–Wilk test to determine the normality of the continuous variables. To compare descriptive statistics between malignant and benign tumors, we used the t-test and the Chi-square test for means of normally distributed variables and frequency distributions of categorical variables, respectively. To determine whether the probabilities of indeterminate, non-diagnostic, and true FNAC vary by nodule size, we calculated the overall probabilities. Then, we calculated different probabilities at smaller and larger nodule sizes using cutoffs at 2, 3, and 4 cm values. All analyses were performed with Statistical Analysis Software (SAS) 9.4 (SAS Institute, Cary NC) with a significance level of 0.05.
RESULTS
Baseline characteristics of the study sample
We had 345 patients who fulfilled the inclusion criteria; the majority were female (86.7%) and older than 40 years of age (53.9%). Approximately half of the patients had benign post-operative histology, and the other half were found to have thyroid cancer. The median nodule size was higher for benign nodules (3.1 cm), whereas for malignant nodules, the median size was 2.7 cm. Most benign nodules had low suspicion US features (49.6%), and most of the malignant sample had high-risk US features (48.1%). The majority of the cohort had indeterminate (FLUS/AUS, FN, SUSP) thyroid cytology results, accounting collectively for 49.3% of the cohort, followed by benign cytology in 29.3%, malignant cytology in 12.8%, and finally non-diagnostic cytology in 8.7%. Table 1 illustrates the baseline characteristics of the study sample. Figures 1, 2a, and b illustrate examples of thyroid nodules of different sizes and characteristics with the corresponding cytology.
| Label | Total n=345 | Histopathology | Test statistic value | P-value | |
|---|---|---|---|---|---|
| Benign n=172 (49.9) | Malignant n=173 (50.1) | ||||
| Age, years, Mean±SD | 42.97±13.83 | 43.52±13.30 | 42.43±14.36 | t=0.73 | 0.4653 |
| Pre-operative TSHa, Median (Q1, Q3) | 1.6 (0.8, 2.7) | 1.3 (0.7, 2.3) | 1.8 (1.1, 3.0) | Z=3.81 | 0.0001 |
| Size of the nodule, cm, Median (Q1, Q3) | 3.0 (1.9, 4.2) | 3.1 (2.0, 4.5) | 2.7 (1.7, 4.0) | Z=2.22 | 0.0266 |
| Age categories, n (%) | χ2=0.85 | 0.3564 | |||
| ≤40 years | 159 (46.1) | 75 (43.6) | 84 (48.6) | ||
| >40 years | 186 (53.9) | 97 (56.4) | 89 (51.4) | ||
| Sex, n (%) | χ2=1.55 | 0.2128 | |||
| Female | 299 (86.7) | 153 (89.0) | 146 (84.4) | ||
| Male | 46 (13.3) | 19 (11.0) | 27 (15.6) | ||
| Type of surgery, n (%) | χ2=25.09 | <0.0001 | |||
| Hemithyroidectomy | 82 (24.0) | 59 (34.3) | 23 (13.5) | ||
| Hemithyroidectomy followed by total thyroidectomy | 17 (5.0) | 3 (1.7) | 14 (8.2) | ||
| Total thyroidectomy | 243 (71.1) | 110 (64.0) | 133 (78.2) | ||
| Size of nodule, n (%) | χ2=4.97 | 0.0833 | |||
| <2 cm | 88 (25.5) | 35 (20.3) | 53 (30.7) | ||
| 2–4 cm | 168 (48.7) | 88 (51.2) | 80 (46.2) | ||
| >4 cm | 89 (25.8) | 49 (28.5) | 40 (23.1) | ||
| USb features, n (%) | χ2=31.54 | <0.0001 | |||
| Benign | 11 (4.0) | 10 (7.1) | 1 (0.8) | ||
| Very low suspicion | 9 (3.3) | 5 (3.5) | 4 (3.0) | ||
| Low suspicion | 112 (40.9) | 70 (49.6) | 42 (31.6) | ||
| Intermediate suspicion | 52 (19.0) | 30 (21.3) | 22 (16.5) | ||
| High suspicion | 90 (32.8) | 26 (18.4) | 64 (48.1) | ||
| Bethesda, n (%) | χ2=88.87 | <0.0001 | |||
| Non-diagnostic or unsatisfactory | 30 (8.7) | 15 (8.7) | 15 (8.7) | ||
| Benign | 101 (29.3) | 77 (44.8) | 24 (13.9) | ||
| AUSc | 82 (23.8) | 49 (28.5) | 33 (19.1) | ||
| FNd or suspicious of FN | 49 (14.2) | 24 (14.0) | 25 (14.5) | ||
| Suspicious of Malignancy | 39 (11.3) | 5 (2.9) | 34 (19.7) | ||
| Malignant | 44 (12.8) | 2 (1.2) | 42 (24.3) | ||
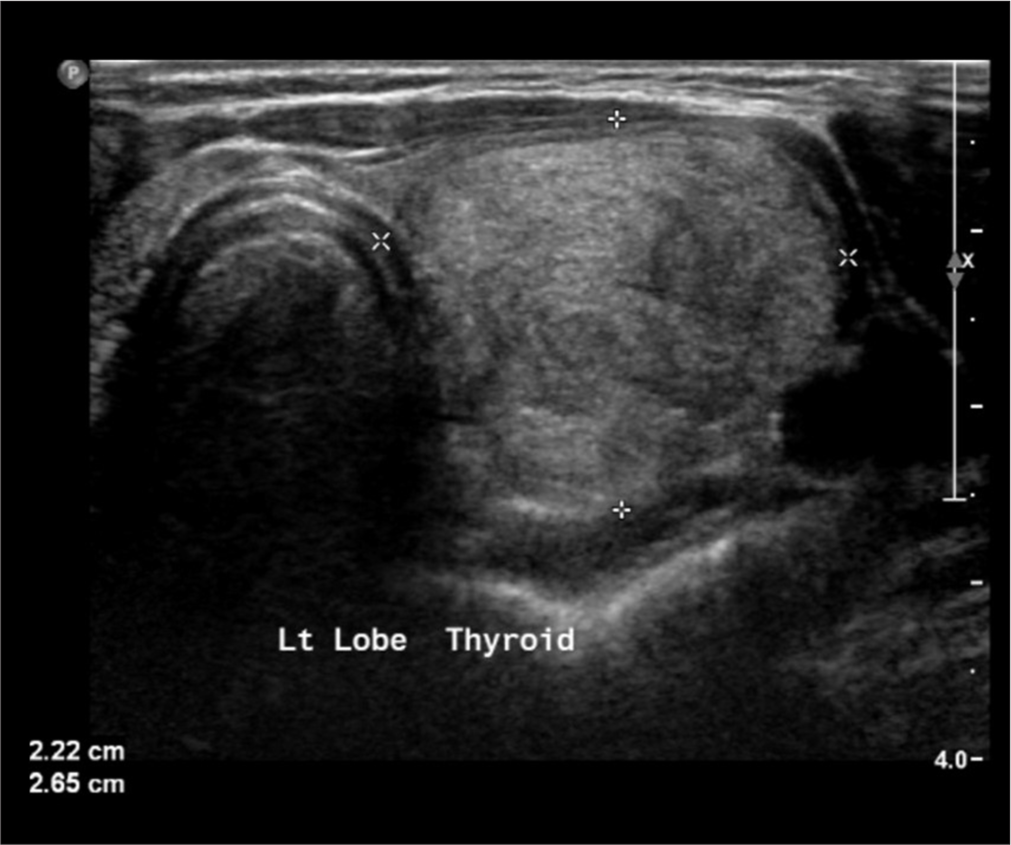
- Thyroid ultrasound image displays a low-risk nodule, occupying the left lobe. Cytology analysis was Bethesda 3. Postoperative histopathology was benign.
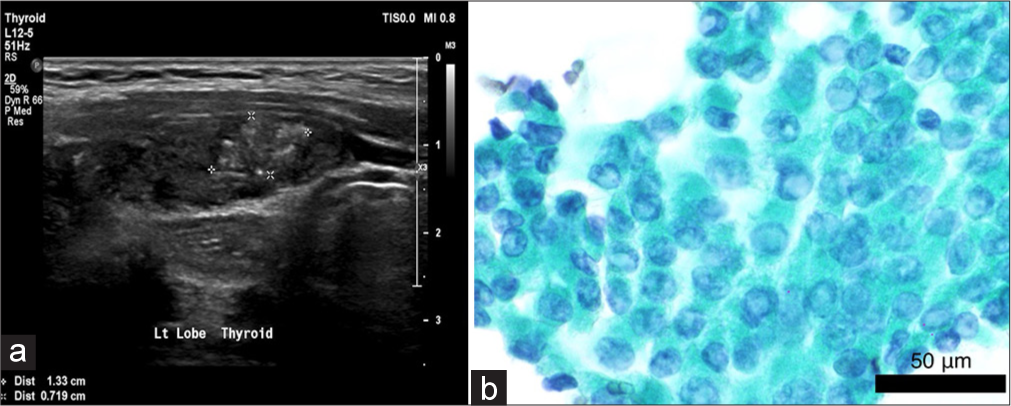
- (a) Thyroid ultrasound image displays a high-risk 1.3 cm nodule at the left lobe. (b) Thyroid cytology:Intranuclear inclusions and nuclear grooves classic feature of papillary thyroid carcinoma are apparent in this image (×60, Papanicolaou stain, Scale bar=50 μm).
Correlation between the nodule size and the probability of obtaining an indeterminate or non-diagnostic cytology
Indeterminate and non-diagnostic FNAC results were found in 200 cases (30 non-diagnostic or unsatisfactory + 170 indeterminate cytology). Therefore, out of the total sample size of 345 patients, 200 (58.0%) had indeterminate or non-diagnostic FNAC.
When assessing if a larger size per se increases the likelihood of obtaining a non-diagnostic or an indeterminate sample, we looked into the overall proportion of indeterminate or non-diagnostic FNAC through different cutoff nodule sizes. Nodule size cutoffs are less than, more than, or equal to 2 cm, 3 cm, and 4 cm. We found that the proportion of indeterminate or non-diagnostic FNAC was relatively constant across the different nodule sizes, as illustrated in Table 2. The proportion of patients with indeterminate or non-diagnostic cytology was 58% in both patients <4 cm and ≥4 cm. Furthermore, patients with nodule size <2 cm had a marginally similar probability of indeterminate or non-diagnostic nodules to nodules ≥2 cm at 60.2% and 57.2%, respectively. Thus, there is no relationship between the nodule size and the probability of obtaining an indeterminate or non-diagnostic cytology.
| The overall proportion of indeterminate or non-diagnostic FNACa. (n=200) 58.0 (%) | Cutoff at 2 cm | Cutoff at 3 cm | Cutoff at 4 cm | Cutoff at 2 and 4 cm | |||||
|---|---|---|---|---|---|---|---|---|---|
| <2 | ≥2 | <3 | ≥3 | <4 | ≥4 | <2 | 2–4 | >4 | |
| 60.2 | 57.2 | 60.2 | 55.8 | 58.0 | 58.0 | 60.2 | 56.6 | 58.4 | |
FNAC performance according to the nodule size
The frequency of FNAC results that were true was 119 subjects (77 benign FNAC and histopathology + 42 malignant FNAC and histopathology). Therefore, out of the total sample size of 345, 119 (34.5%) had true and accurate FNAC results based on final histopathology. The proportion of true FNAC while varying the cutoff thresholds for nodule size did not differ between the different nodule size categories, indicating similar performance of the FNAC throughout the small and large sizes, as seen in Table 3. The proportion of patients with true FNAC results was 34.3% in patients with nodules <4 cm in size and 35.0% in patients with nodules ≥4 cm in size. In addition, the same probability of true FNAC was found when comparing nodules that are 2–4 cm in size versus nodules that are >4 cm in size, at 34.5% and 36%, respectively.
| The overall proportion of true FNACa. (n=119) 34.5 (%) | Cutoff at 2 cm | Cutoff at 3 cm | Cutoff at 4 cm | Cutoff at 2 and 4 cm | |||||
|---|---|---|---|---|---|---|---|---|---|
| <2 | ≥2 | <3 | ≥3 | <4 | ≥4 | <2 | 2–4 | >4 | |
| 33.5 | 35.0 | 33.3 | 35.6 | 34.3 | 35.0 | 33.0 | 34.5 | 36.0 | |
DISCUSSION
Thyroid cytology is a simple diagnostic tool for thyroid nodules, with an overall reported sensitivity and specificity 72% and 99%.[12] Thereby, it decreases unnecessary surgeries in most benign cases. In this study, our results indicated that nodule size does not affect the probability of obtaining an indeterminate or non-diagnostic FNAC. In addition, our results indicated no difference in the accuracy of the FNAC between smaller and larger thyroid nodules.
Similarly, multiple other studies concluded that greater nodule diameter is not linked to the diagnostic utility of US-guided FNAC.[13,14] Moreover, many other more recent studies have had similar results, with no relation found between different nodule sizes and the false-negative rate (FNR) of FNAC. In one study, the FNR for FNAC was low at (4.1%).[15] Furthermore, another study compared the FNR between nodules ≥4 cm and smaller nodules <4 cm. FNAC FNR was 6.6% in nodules ≥4 cm compared to 4.2% in nodules <4 cm. The FNR was mainly driven by the follicular and Hurthle cell carcinomas in nodules ≥4 cm in size.[16]
At the same time, several other studies have suggested that the diagnostic accuracy of FNAC in nodules larger than 3–4 cm is significantly lower compared to smaller nodules. In a study comprised of 323 nodules with benign pre-operative FNAC, nodules ≥3 cm in size had an FNR of 11.7%, and the FNR was 4.8% for nodules <3 cm.[17] The former-mentioned study and also another similar study by Kim et al. suggested direct surgery for any thyroid nodule above 3 and 4 cm in size, respectively.[5]
A study by Koo et al. on 690 thyroid nodules concluded that the diagnostic accuracy for FNAC drops as the size of the nodule increases. In their study, nodules between 1 and 4 cm had a diagnostic accuracy ranging between 94.4% and 99%, while in nodules above 4 cm, the diagnostic accuracy dropped to 87.5%.[18] A systematic review that included studies up to July 2013 concluded that larger nodule sizes reduce cytological accuracy.[19] In contrast, a similar –however – more recent systematic review that collected studies up to December 2017, concluded the opposite, where nodule size did not influence the FNAC accuracy.[20]
We believe that studies proposing lower FNAC accuracy in larger nodules are limited by many factors. First, some were conducted among different populations with different epidemiology of thyroid cancer and different surgical practices.[21] Second, some of these studies, especially the older ones, included FNAC performed by palpation, not ultrasound-guided. Based on a systematic review and meta-analysis comparing the diagnostic accuracy of US-guided versus palpation-guided thyroid FNAC, the accuracy of US-guided FNA was found to be significantly superior. The diagnostic sensitivity and specificity by palpation were 76% and 77%, respectively, while the sensitivity and specificity using US guidance were 90% and 80%, respectively.[22]
Third, a lot of these studies were before the era of standardized US risk stratification systems, such as the 2015 ATA nodule guidelines and TIRADS-ACR guidelines. These clinical guidelines and stratification systems have assisted immensely in better diagnostic decision-making and fine-tuning management planning. Similarly, the Bethesda system for thyroid cytology reporting has evolved remarkably over the past decade. In fact, an analysis conducted to compare the FNAC performance before and after the implementation of the Bethesda cytology system found that it decreased the frequency of non-diagnostic, FN, and SUSP. It also increased the diagnoses of benign cytology.[23]
Fourth, some studies have concluded that lower sensitivity of FNAC in larger nodules might be related to sampling error or an incidental thyroid microcarcinoma.[24] Indeed, when Zhu et al. retrospectively reexamined their discordant FNAC, that is, the false-negative and the false-positive cases, they found that the chief etiology of false-negative diagnoses was sampling error in 86.7% of the cases, whereas interpretation error led to most of the false-positive diagnoses.[25] To avoid such error, it was suggested by some experts that multiple passes (2–5) should be executed from all parts of large nodules to diminish the risk of false-negative results. Alternatively, a core-needle biopsy can be utilized for thyroid nodules >2 cm with intermediate to high-risk US risk stratification as it was found to be diagnostically superior to FNAC in these circumstances and also with lower non-diagnostic rates.[26-28]
Finally, with the introduction of NIFTP nomenclature in 2016[29] the malignancy rates of benign and malignant FNAC specimens decreased significantly. This reduction was particularly pronounced in three indeterminate Bethesda categories.[30,31] Furthermore, the implementation of gene expression classifiers (GEC) on indeterminate cytology, namely, AUS/FLUS and FN categories, resulted in 61% less diagnostic surgeries, regardless of the nodule size.[32,33] Due to the high negative predictive value of these tests, such as Afirma GEC and Thyroseq V3 (96% and 97%, respectively), the management of thyroid nodules has evolved dramatically over the past few years.[34]
Our study is strengthened by being a more contemporary view of thyroid nodules that implements the standardized US and cytology reporting systems within the same population. In this study, we utilized the gold standard of diagnosis, which is post-surgical histopathology. To the best of our knowledge, no previous study has investigated the correlation between nodule size and the probability of indeterminate or non-diagnostic cytology. Conversely, the limitations of our study are that it is retrospective and that not all thyroid cytology was read by an independent cytologist; hence, inter-observer variability is possible.
SUMMARY
We found no correlation between thyroid nodule size and the probability of indeterminate or unsatisfactory FNAC. The diagnostic accuracy of FNAC is comparable among different nodule sizes. FNAC can reliably guide the decision for surgery for larger and smaller thyroid nodules along with the diagnostic and clinical data.
AVAILABILITY OF DATA AND MATERIALS
The datasets used in this study are available on request.
ABBREVIATIONS
ATA: American Thyroid Association
FLUS/AUS: Follicular lesion of undetermined significance/atypia of undetermined significance
FN: Follicular neoplasm
FNAC: Fine needle aspiration cytology
FNR: False-negative rate
GEC: Gene expression classifiers
SAS: Statistical Analysis Software
SD: Standard deviation
SUSP: Suspicious for malignancy
TSH: Thyroid-stimulating hormone
US: Ultrasonography
ACKNOWLEDGMENT
Not applicable.
AUTHOR CONTRIBUTIONS
All authors were involved in the creation and development of the article, data collection, data analysis, and interpretation, along with article composition and critical revision for significant intellectual content. All authors are eligible for ICMJE authorship.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Research Ethics Committee in King Abdulaziz University Hospital, where the study was conducted (Reference no. 81-23). Participants’ consent was waived due to the retrospective and non-interventional nature of the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: Not applicable.
References
- Thyroid nodules: Diagnosis and management. Med J Aust. 2018;209:92-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of ultrasound reporting for thyroid cancer diagnosis and surveillance. Head Neck. 2017;39:1756-60.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration of the thyroid gland In: Endotext. South Dartmouth, MA: MDText. com, Inc.; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK285544/
- [Google Scholar]
- Thyroid nodules over 4 cm do not have higher malignancy or benign cytology false-negative rates. Endocrine. 2019;66:249-53.
- [CrossRef] [PubMed] [Google Scholar]
- Suspicious thyroid nodules 4 cm require a diagnostic lobectomy regardless of their benign fine needle aspiration results. Asian J Surg. 2022;45:1113-6.
- [CrossRef] [PubMed] [Google Scholar]
- The 2024 revision to the declaration of Helsinki: Modern ethics for medical research. JAMA. 2025;333:30-1.
- [CrossRef] [PubMed] [Google Scholar]
- 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological characteristics of thyroid cancer in a Saudi academic hospital. Cureus. 2020;12:e8044.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid cancer in Saudi Arabia: A histopathological and outcome study. Int J Endocrinol. 2017;2017:8423147.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of updates in new the Bethesda system for reporting of thyroid cytopathology using the latest world health organization thyroid tumor classification terminology. Endocr Pract. 2023;29:1020-2.
- [CrossRef] [PubMed] [Google Scholar]
- The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid. 2023;33:1039-44.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of diagnostic accuracy of thyroid cancer with ultrasound-guided fine-needle aspiration and core-needle biopsy: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:44.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy for thyroid nodules three centimeters or larger in size. Diagn Cytopathol. 2015;43:622-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32:77-82.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of malignancy and diagnostic accuracy of fine-needle aspiration biopsy in thyroid nodules with diameters greater than 4 centimeters. Arch Endocrinol Metab. 2023;67:e000644.
- [CrossRef] [PubMed] [Google Scholar]
- Large thyroid nodules: Should size alone matter? Eur Arch Otorhinolaryngol. 2022;279:3139-46.
- [CrossRef] [PubMed] [Google Scholar]
- False negative cytology in large thyroid nodules. Ann Surg Oncol. 2015;22:152-7.
- [CrossRef] [PubMed] [Google Scholar]
- Does tumor size influence the diagnostic accuracy of ultrasound-guided fine-needle aspiration cytology for thyroid nodules? Int J Endocrinol. 2016;2016:3803647.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of thyroid nodule size on prevalence and post-test probability of malignancy: A systematic review. Laryngoscope. 2015;125:263-72.
- [CrossRef] [PubMed] [Google Scholar]
- Large cytologically benign thyroid nodules do not have high rates of malignancy or false-negative rates and clinical observation should be considered: A meta-analysis. Thyroid. 2018;28:1595-608.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of thyroid cancer. Cancer Epidemiol Biomarkers Prev. 2022;31:1284-97.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of palpation versus ultrasound-guided fine needle aspiration biopsy for diagnosis of malignancy in thyroid nodules: A systematic review and meta-analysis. BMC Endocr Disord. 2022;22:181.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration to diagnose primary thyroid lymphomas: A systematic review and meta-analysis. Eur J Endocrinol. 2019;180:177-87.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of fine-needle biopsy in the detection of thyroid malignancy: A systematic review and meta-analysis. JAMA Surg. 2022;157:1105-13.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of misdiagnoses by thyroid fine-needle aspiration cytology (FNAC): Our experience and a systematic review. Diagn Pathol. 2020;15:1.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid nodules: Diagnosis and management. Nat Rev Endocrinol. 2024;20:715-28.
- [CrossRef] [PubMed] [Google Scholar]
- Core-needle biopsy in thyroid nodules: Performance, accuracy, and complications. Eur Radiol. 2019;29:4889-96.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative evaluation of thyroid nodules-diagnosis and management strategies. Pathol Res Pract. 2023;246:154516.
- [CrossRef] [PubMed] [Google Scholar]
- Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023-9.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of noninvasive follicular variant of papillary thyroid carcinoma on rates of malignancy for fine-needle aspiration diagnostic categories. Thyroid. 2015;25:987-92.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in the Bethesda system for reporting thyroid cytopathology. Cancer Cytopathol. 2016;124:181-7.
- [CrossRef] [PubMed] [Google Scholar]
- Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: A prospective blinded multicenter study. JAMA Oncol. 2019;5:204-12.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical factors influencing the performance of gene expression classifier testing in indeterminate thyroid nodules. Thyroid. 2016;26:916-22.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical use of molecular data in thyroid nodules and cancer. J Clin Endocrinol Metab. 2023;108:2759-71.
- [CrossRef] [PubMed] [Google Scholar]









