Translate this page into:
Cytological variations and typical diagnostic features of endocervical adenocarcinoma in situ: A retrospective study of 74 cases
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The sensitivity of Papanicolaou smears for detecting endocervical adenocarcinoma in situ (AIS) is very low. A comprehensive cytological analysis of endocervical AIS is necessary to increase diagnostic accuracy.
Methods:
The subjects were 74 patients with pathologically-diagnosed AIS. A total of 140 Papanicolaou smears were reviewed to calculate the sensitivity of the Papanicolaou smears for detecting AIS and the incidence of sampling/screening/diagnostic errors. The cytological review was performed by 6 cytotechnologists, and the final cytological diagnosis was obtained at the consensus meeting. We classified the cases into three differentiation types; typical type (well-differentiated AIS), polymorphic type (poorly differentiated AIS), and mixed typical and polymorphic type. Three cytological subtypes (endocervical, endometrioid and intestinal subtypes) of AIS were also analyzed.
Results:
The sensitivity of the original Papanicolaou smears for the detection of AIS was 44.6%, while that for the detection of AIS and adenocarcinoma was 63.5%. The diagnostic accuracy of AIS increased to 78.5% in the final diagnosis. The common characteristic features were microbiopsies/hyperchromatic crowded groups (HCG) (82.0%) and mitotic figures (72.2%). The appearance of single cells (2.8%) was rare, and all the cervical cytology smears showed no evidence of necrotic tumor diathesis. The most common AIS was the typical type (41 cases, 67.2%) among all cytologically-diagnosed AIS or adenocarcinoma cases (61 cases). Although mixed typical and polymorphic AIS existed in 17 cases (27.9%), pure polymorphic AIS was very rare (3 cases, 4.9%). The endocervical subtype was the most predominant subtype (67.2%), followed by a few mixed subtypes. The important diagnostic keys for AIS cytology are as follows: (1) The appearance of microbiopsies/HCG (single-cell pattern is rare), (2) mitotic figures in the microbiopsies/HCG, (3) a lack of necrotic tumor diathesis in cases with polymorphic AIS, and (4) recognition of typical cytological subtypes.
Conclusions:
The relatively low diagnostic accuracy AIS was caused by the underestimation of microbiopsies/HCG and the overestimation of polymorphic components. The typical cytological features of AIS are the presence of microbiopsies/HCG with mitotic figures in the absence of necrotic tumor diathesis in specimens containing endocervical samples. The recognition of infrequent AIS subtypes (endometrioid and intestinal subtypes) is also important.
Keywords
Adenocarcinoma in situ
cytobrush
hyperchromatic crowded cell groups
microbiopsies
papanicolaou test
uterine cervix
INTRODUCTION
Cervical cytological screening is an important procedure for the detection of adenocarcinoma in situ (AIS) and cervical intraepithelial neoplasia (CIN) 2/3, both of which are precursors of invasive adenocarcinoma or squamous cell carcinoma (SCC) of the uterine cervix. The detection of these lesions leads to early treatment, and eventually a surgical cure with uterine preservation, enabling patients to become pregnant in the future.[12] Cervical conization or a simple hysterectomy without pelvic lymphadenectomy is the standard surgery and results in a favorable prognosis for patients with AIS.[12] Östör reported no recurrences during an 8-year follow-up of 53 AIS patients who were treated with cervical conization.[2]
As AIS arises in an inner cervical glandular area and is usually not associated with abnormal genital bleeding, detection by colposcopy or clinical manifestation is quite difficult, and a diagnosis usually relies on cervical cytological screening.[2] Endocervical AIS is classified as an independent category in the 2001 Bethesda system (TBS2001).[34] The diagnostic accuracy of AIS is significantly lower than that of the high-grade squamous intraepithelial lesion (HSIL) or SCC because of a low sensitivity resulting from sampling and screening errors.[567891011121314] Schoolland et al. reported a low sensitivity of cervical smear detection for AIS of approximately 50%, based on two large-scale studies of AIS.[9] The retrospective rescreening of 31 negative smears of AIS showed a high rate (55%) of abnormal findings.[7] Very small numbers of AIS cells and blood contamination in the specimens can contribute to false-negative results.[789101114] The AIS cells are often misdiagnosed as normal endocervical cells, HSIL, or SCC.[5810] Since many cytological features overlap between endocervical AIS and well-differentiated invasive cervical adenocarcinoma, the Bethesda 2001 Workshop recommended a special caution against the diagnosis of AIS.[3]
Krumins et al. first reported the cytologic features of uterine cervical AIS in 1977,[13] and Ayer et al. then reported its cytological variations in 1987.[5] They classified AIS into two subtypes according to cytological and nuclear features: Well-differentiated AIS with typical cytological patterns, and poorly differentiated AIS with marked cytological atypia.[5] Since carcinoma in situ (intraepithelial neoplasm) is usually not classified according to the degree of differentiation, we designated well-differentiated AIS as typical AIS and poorly differentiated AIS as polymorphic AIS in this article. The AIS can also be also classified to four subtypes: Endocervical, intestinal, endometrioid, and a rare Paneth cell subtype.[5]
We retrospectively analyzed 74 AIS cases to clarify the incidence of sampling/screening/diagnostic errors, factors related to cytological variations, and the diagnostic accuracy of uterine cervical AIS, and finally found that the recognition of cytological variation was crucial for accurate diagnosis of cervical AIS.
METHODS
Subjects
We retrospectively recruited 74 cases of cervical conization or hysterectomy materials with stage 0 cervical AIS that were diagnosed during a 20-year period from 1993 to 2012 at the Department of Pathology, Jikei University Hospital, Tokyo, Japan. The mean age (range) of the 74 subjects was 41.2 years (21–69 years). The surgical treatments were as follows: Cervical conization alone in 47 cases, simple hysterectomy in 22 cases, and conization and subsequent hysterectomy in five cases. Cases with microinvasive or invasive adenocarcinoma were excluded from the present study. Endocervical smears were usually taken using a cytobrush by gynecologists before surgery. A total of 140 Papanicolaou smears were reviewed. All the smears were prepared using the conventional method: The smears were fixed in 95% ethyl alcohol and were stained with the standard Papanicolaou stain. No liquid-based preparations were used.
Cytological review
All the cervical papanicolaou smears were first blindly rescreened individually by six cytotechnologists including the authors without knowing the final histological diagnosis. If there was discordance among the cytological diagnosis, the consensus meeting was held to reach the final diagnosis. Initial and review papanicolaou smears were diagnosed according to the TBS2001 system.[34] We analyzed the diagnostic accuracy of AIS and the factors influencing the sampling/screening/diagnostic errors. Sampling errors were defined as cases with smears that were initially and finally reported as negative for intraepithelial lesion or malignancy (NILM). Screening errors were defined as cases with possible or definite high-grade epithelial abnormalities initially reported as NILM. Diagnostic errors were defined as AIS cases initially reported as atypical glandular cells (AGC; underdiagnosis), low-grade squamous intraepithelial lesions (LSIL/HSIL; underdiagnosis), adenocarcinoma (overdiagnosis) or SCC (overdiagnosis).
The tissue fragments of abnormal cells were called microbiopsy or hyperchromatic crowded groups (HCG).[1516] HCG are three-dimensional clusters of crowded cells with hyperchromatic nuclei and a high nuclear/cytoplasmic ratio. We analyzed the presence or absence of detailed cytological features, such as feathering, mitosis, polymorphism, endocervical samples, and necrotic diathesis. We designated well-differentiated AIS as typical AIS and poorly differentiated AIS as polymorphic AIS.[5] When features of both typical and polymorphic were observed, the term mixed typical and polymorphic AIS was applied. Thus, three distinct differentiation patterns of AIS were applied to our series of samples: Typical AIS, mixed typical and polymorphic AIS, and polymorphic AIS. We also classified the AIS into three subtypes: Endocervical, intestinal and endometrioid subtypes. No Paneth cell subtypes were included among the samples.
RESULTS
Sampling/screening/diagnostic errors of the cytological diagnosis
Normal endocervical samples were included in all the Papanicolaou smears; thus, the specimens were suitable for the cytological diagnosis of the endocervix. The overall flow chart of the cytological diagnosis before and after the review is shown in Figure 1. Glandular abnormality was absent in two cases (one each case of HSIL and NILM). The review showed AGC in one case of NILM. Thus, sampling and screening errors were only present in one case each (1.4%). The final cytological diagnosis of glandular abnormality included AIS in 58 cases (78.4%), AGC in 11 cases (14.1%), and adenocarcinoma in 3 cases (4.1%). A total of 25 cases were initially misdiagnosed as other cytological diagnoses than AIS. These misdiagnosed cases are shown in red boxes in Figure 1. The diagnostic error rate was 33.8% and the total error rate was 36.5%.
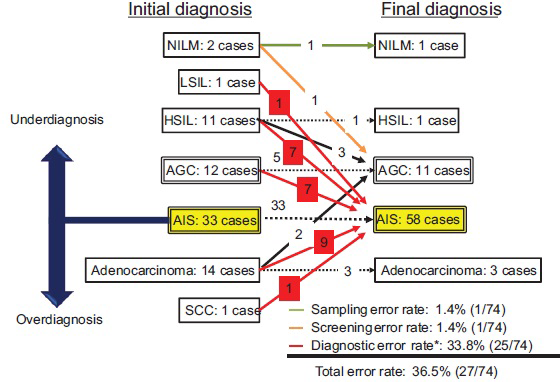
- Flow chart of 74 pathologically-confirmed adenocarcinoma in situ cases before and after a cytological review. The left column shows the distribution of the initial cytological diagnoses, and the right column shows that of the final cytological diagnoses after the review. A few sampling and screening errors occurred, but diagnostic errors predominated. AGC: Atypical glandular cells, AIS: Adenocarcinoma in situ, HSIL: High-grade squamous intraepithelial lesion, LSIL: Low-grade squamous intraepithelial lesion, NILM: Negative for intraepithelial lesion or malignancy, SCC: Squamous cell carcinoma
Cytological details of glandular abnormalities
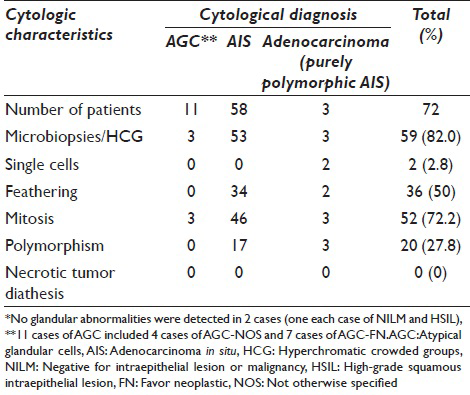
The cytological features of 72 cases with glandular abnormality are shown in Table 1. The common characteristic features were microbiopsies/HCG (82.0%) and mitotic figures (72.2%). The appearance of single cells (2.8%) was rare, and all the cervical cytology smears lacked necrotic tumor diathesis.
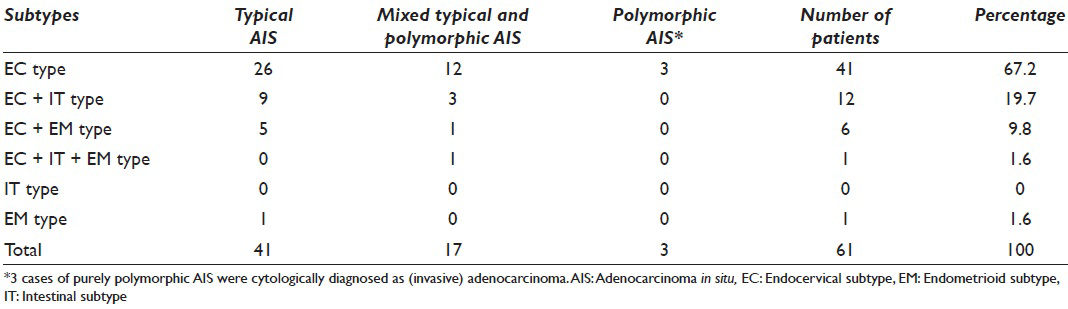
Among 58 cases with AIS, 41 cases showed a typical AIS pattern (67.2%). The remaining 17 cases (32.8%) of AIS showed the apparent polymorphism in some cancer cells, then diagnosed as mixed typical and polymorphic AIS. Typical histological and cytological figures of polymorphic AIS are shown in Figures 2 and 3. Three cases of purely polymorphic AIS were cytologically diagnosed as (invasive) adenocarcinoma. The distribution of the AIS subtypes is shown in Table 2 and typical cytological figures of AIS subtypes are shown in Figure 4. The endocervical subtype was the most predominant subtype, followed by a few mixed subtypes. The pure endometrioid subtype was present only in one case.
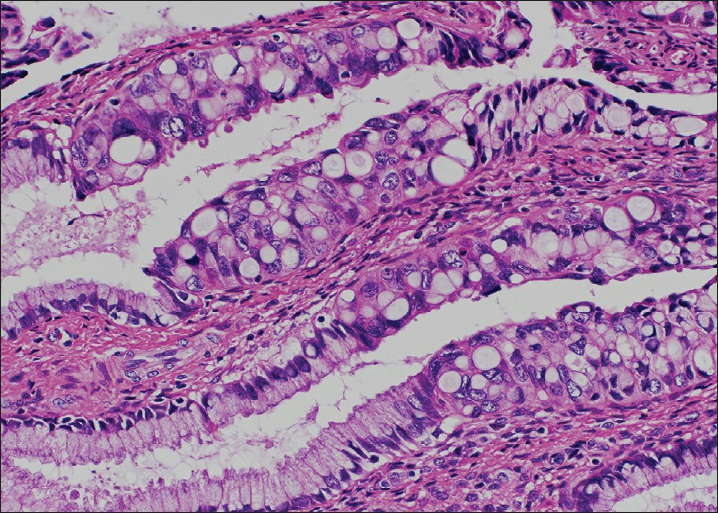
- Histological picture of endocervical polymorphic adenocarcinoma in situ. Note large polymorphic nuclei with conspicuous nucleoli. No stromal invasion is present (H and E stain, ×400)
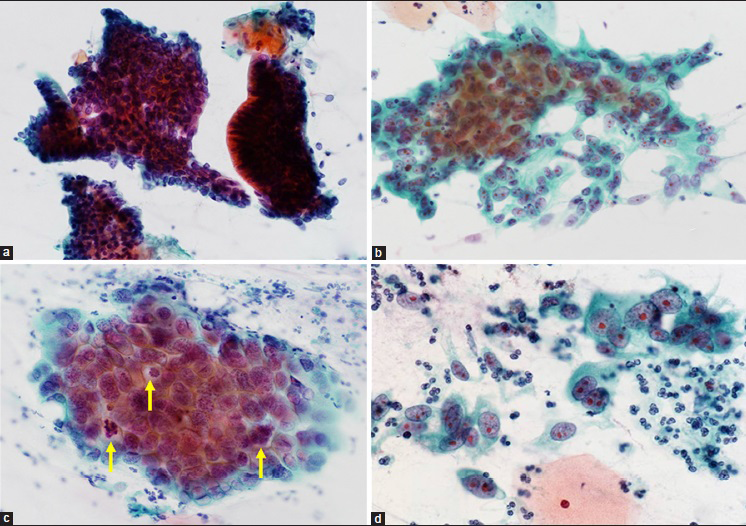
- Cytological pictures of endocervical polymorphic adenocarcinoma in situ (AIS). (a) The tissue fragments of typical endocervical AIS. Dense clusters of darkly stained AIS cells in microbiopsies/hyperchromatic crowded groups (HCG) are present. The AIS cells at the margin of the clusters show no feathering (Papanicolaou stain, ×400). (b) Polymorphic AIS. The AIS cells have large polymorphic nuclei with prominent nucleoli (Papanicolaou stain, ×400). (c) Polymorphic AIS. The AIS cells often exhibit mitotic activity (arrows) (Papanicolaou stain, ×600). (d) Single or loosely arranged AIS cells in polymorphic AIS. Microbiopsies/HCG or three-dimensional clusters are scarce in this case (Papanicolaou stain, ×600)
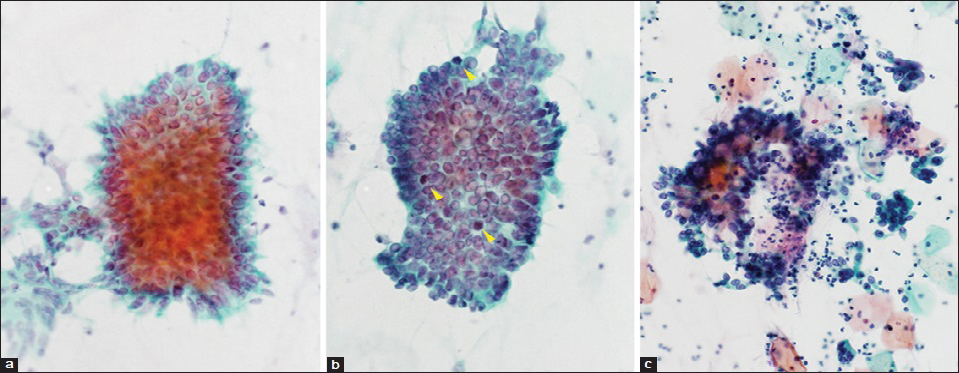
- Typical cytological features of adenocarcinoma in situ. (a) Endocervical subtype. Pseudostratified strip of cells demonstrating crowding, nuclear enlargement and peripheral feathering (Papanicolaou stain, ×600). (b) Endometrioid subtype. Pseudostratified cluster with nuclear crowding, irregular internuclear distances, and scattered mitotic figures (yellow arrow heads) (Papanicolaou stain, ×600). (c) Intestinal subtype. Note large mucin droplets in the clusters (Goblet cells) (Papanicolaou stain, ×400)
Among 11 cases with a final cytological diagnosis of AGC, cytological features favoring a diagnosis of AIS were infrequent; microbiopsies/HCG and frequent mitosis (3 cases each, 27.3%). Abnormal clusters were often obscured by large amounts of blood (one case) or mucus (three cases) and severe inflammation (two cases). Numerous HSIL cells coexisted with AGCs in two AGC favor neoplastic (FN) + HSIL cases, masking glandular abnormalities.
Cytological features of adenocarcinoma in situ with diagnostic errors
The cytological features of 25 AIS cases with diagnostic errors are shown in Table 3. The polymorphism was frequently marked among nine cases with an initial diagnosis of (invasive) adenocarcinoma (5/9 cases). In eight cases with the initial diagnosis of SIL (one case of LSIL and seven cases of HSIL), HSIL frequently coexisted (6/8 cases).
DISCUSSION
Characteristic cytological pictures of adenocarcinoma in situ
The present study showed that the characteristic cytological features of AIS included microbiopsies/HCG, mitotic figures of the cancer cells, and a lack of necrotic tumor diathesis in polymorphic AIS.
Microbiopsies/hyperchromatic crowded cell groups
The appearance of microbiopsy/HCG was a key cytological feature for the AIS diagnosis and was present in 82.0% (59 cases) of the subjects in our study. The cancer tissue seems to be fragmented during sampling with a cytobrush and appears as dense clusters of darkly-stained cells.[1516] Benign microbiopsy/HCG are also observed in cases of squamous atrophy or metaplasia, or in samples originating from normal endocervical and endometrial cells. Malignant microbiopsy/HCG appear in various cases with HSIL, SCC, AIS, invasive adenocarcinoma, or metastatic carcinoma. False-positive or false-negative results have been noted in these cases.[1516] The present study showed that microbiopsy/HCG of an AIS origin were often misdiagnosed as HSIL, indicating a need for the careful assessment of microbiopsies/HCG.[51012] Thiryayi et al. reported a similar result regarding misdiagnosis, stating that the differentiation of microbiopsies/HCG with a glandular nature from those with a squamous nature was often difficult.[17] The microbiopsies/HCG of an AIS origin had a high proportion of columnar cells at the peripheries of the clusters, while the microbiopsies/HCG of HSIL origin had flattened cells at the periphery, the loss of cell polarity within the clusters and the presence of isolated HSIL cells in the background of the specimens.[4] The presence of mitotic figures within the clusters is also an important feature for the diagnosis of AIS, since the epithelial cells of normal cervical glands lack mitotic figures.[411121819] The mitotic figures within the clusters were actually present in 72.2% of the AIS cases in our study. The microbiopsies/HCG with mitotic figures in the specimens containing endocervical samples are typical cytological features of AIS.
Polymorphic adenocarcinoma in situ and necrotic tumor diathesis
Ayer et al. reported a high ratio (50%) of the misdiagnosis of AIS as (invasive) adenocarcinoma.[5] Subsequent studies concluded that a differential diagnosis between AIS and adenocarcinoma was very difficult.[810] Adenocarcinoma usually shows marked cytological atypia, with the appearance of single cells and necrotic diathesis.[1216] The present study revealed the frequent presence of polymorphic subtype and lack of necrotic tumor diathesis among the cases with AIS. The abnormal glandular cells with polymorphic components lacking necrotic tumor diathesis should be diagnosed as polymorphic AIS rather than (invasive) adenocarcinoma.[51011] Single abnormal glandular cells were present in only two cases in the present study.
Subtypes of adenocarcinoma in situ
Our study showed that the endocervical subtype was the most prevalent and cases with intestinal or endometrioid subtypes coexisted with the endocervical subtype. Therefore, the cytological variation due to infrequent subtypes (endometrioid and intestinal subtypes) needs to be recognized for the accurate diagnosis of AIS.
Causes of misdiagnosis
When abnormal glandular cells in a sample are scarce, the diagnosis of AIS is often difficult and may result in a diagnosis of NILM, rather than AGC.[51011] These cases should be diagnosed as AGC-FN to prevent AIS from being overlooked.[4] The Update on ASCCP Consensus Guidelines recommends an excisional procedure for AIS if the initial cytology is AGC-FN or AIS and no invasion is identified.[20] Only seven cases in this study exhibited AGC-FN. Faraker and Boxer indicated that the false-negative factors in cervical cancer screening were (1) scant abnormal cells and (2) abundant dyskaryotic cells presenting as microbiopsies/HCG.[21]
Limitation of the study
The retrospective nature of this study limits the implication of the results. As we have introduced the liquid-based cytology (LBC) system in our lab shortly after this study, we could not reproduce our results in a prospective way. This study adopted the conventional Papanicolaou method rather than LBC. The LBC system has already been widely introduced in US and other western countries, but the conventional method is still widely used in developing countries, because they could not introduce the LBC system due to the financial limitation. The introduction of LBC system has been delayed in Japan as well (<10%). As the cytological details are slightly different between the conventional method and LBC, we safely conclude our results are at least applicable to the cytology using the conventional Papanicolaou method.
CONCLUSIONS
The present study showed that the main causes of diagnostic errors are the underdiagnosis of microbiopsies/HCG, the overdiagnosis of polymorphic components, and recognition of subtypes of AIS. Recent studies reported that p16INK4a and Ki-67 immunocytochemistry was useful for predicting CIN2/3 and AIS/adenocarcinoma.[22] However, sampling and diagnostic skills should be first improved to maximize the diagnostic competency for AIS. Cytotechnologists and pathologists are required to be familiar with the typical cytological features and cytological variations of AIS, especially polymorphic AIS and AIS subtypes (endometrioid and intestinal types), to increase the diagnostic accuracy.
COMPETING INTERESTS
All the authors and their families have no personal conflicts of interest to disclose.
AUTHORSHIP STATEMENT BY ALL AUTHORS
TU perceived the design of the study, acquisition of data, and drafting the article. MU and AH carried out the acquisition of cytological data. KN, HT, and MI participated in the acquisition of pathological data. KY, KO, and AO carried out the acquisition of clinical data. MS drafted and revised the article for important intellectual content. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was retrospectively designed using the usual Pap smears obtained from the gynecological examinations; therefore we didn’t obtain any specific oral or written informed consents from the patients. This study was approved by the ethical committee of the Jikei University School of Medicine (24-102 [6868]).
LIST OF ABBREVIATIONS
AGC = Atypical Glandular Cells
AIS = Adenocarcinoma in situ
CIN = Cervical Intraepithelial Neoplasia
FN = Favor Neoplastic
HCG = Hyperchromatic Crowded Groups
HSIL = High-Grade Squamous Intraepithelial Lesion
LBC = Liquid-Based Cytology
LSIL = Low-Grade Squamous Intraepithelial Lesions
NILM = Intraepithelial lesion or Malignancy
SCC = Squamous Cell Carcinoma
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGEMENTS
We are greatly thankful to all the staff members of the Department of Pathology, Jikei University Hospital for the preparation of slides. We also thank to Ms. Mio Nakagawa for English editing. The present study did not receive any grant support.
REFERENCES
- The safety of conization in the management of adenocarcinoma in situ of the uterine cervix. J Gynecol Oncol. 2011;22:25-31.
- [Google Scholar]
- Adenocarcinoma in situ of the uterine cervix: An experience with 100 cases. Gynecol Oncol. 2000;79:207-10.
- [Google Scholar]
- The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-9.
- [Google Scholar]
- The Bethesda System for Reporting Cervical Cytology: Endocervical/Transformation Zone Component, and Explanatory Notes. (2nd ed). New York: Springer; 2003. p. :12-5.
- [Google Scholar]
- The cytologic diagnosis of adenocarcinoma in situ of the cervix uteri and related lesions. I. Adenocarcinoma in situ. Acta Cytol. 1987;31:397-411.
- [Google Scholar]
- Detection of adenocarcinoma in situ of the cervix in Papanicolaou tests: Comparison of diagnostic accuracy with other high-grade lesions. Arch Pathol Lab Med. 2004;128:153-7.
- [Google Scholar]
- Papanicolaou smear sensitivity for adenocarcinoma in situ of the cervix. A study of 34 cases. Am J Clin Pathol. 1997;107:30-5.
- [Google Scholar]
- Papanicolaou smear sensitivity for the detection of adenocarcinoma of the cervix. A study of 49 cases. Cancer Cytopathol. 2001;93:8-15.
- [Google Scholar]
- Adenocarcinoma in situ of the cervix. Sensitivity of detection by cervical smear. Cancer Cytopathol. 2002;96:330-7.
- [Google Scholar]
- Adenocarcinoma in situ of the uterine cervix. Screening and diagnostic errors in Papanicolaou smears. Cancer Cytopathol. 2004;102:280-7.
- [Google Scholar]
- Comparative cytologic features of adenocarcinoma in situ of the uterine cervix. Acta Cytol. 1991;35:117-26.
- [Google Scholar]
- Severe cervical glandular cell lesions with coexisting squamous cell lesions. Cancer Cytopathol. 2004;102:218-27.
- [Google Scholar]
- The cytologic diagnosis of adenocarcinoma in situ of the cervix uteri. Acta Cytol. 1977;21:320-9.
- [Google Scholar]
- Endocervical glandular dysplasia, adenocarcinoma in situ, and early invasive (microinvasive) adenocarcinoma of the uterine cervix. Semin Diagn Pathol. 1990;7:190-204.
- [Google Scholar]
- Hyperchromatic crowded cell groups in gynaecological liquid-based cytology samples. Br J Biomed Sci. 2010;67:154-63.
- [Google Scholar]
- Hyperchromatic crowded groups: Pitfalls in pap smear diagnosis. Am J Clin Pathol. 2000;114(Suppl):S36-43.
- [Google Scholar]
- An audit of liquid-based cervical cytology screening samples (ThinPrep and SurePath) reported as glandular neoplasia. Cytopathology. 2010;21:223-8.
- [Google Scholar]
- Endocervical adenocarcinoma in situ: An analysis of cellular features. Diagn Cytopathol. 1997;17:326-32.
- [Google Scholar]
- Cytologic features of endocervical glandular lesions: Comparison of SurePath, ThinPrep, and conventional smear specimen preparations. Diagn Cytopathol. 2008;36:232-7.
- [Google Scholar]
- Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. Am Fam Physician. 2009;80:147-55.
- [Google Scholar]
- Rapid review (partial rescreening) of cervical cytology. Four years experience and quality assurance implications. J Clin Pathol. 1996;49:587-91.
- [Google Scholar]
- Immunocytochemical colocalization of P16(INK4a) and Ki-67 predicts CIN2/3 and AIS/adenocarcinoma. Cancer Cytopathol. 2012;120:26-34.
- [Google Scholar]








