Translate this page into:
Cytomorphological features as predictors of epidermal growth factor receptor mutation status in lung adenocarcinoma
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Epidermal growth factor receptor mutation-positive (EGFR-p) lung adenocarcinomas are sensitive to tyrosine kinase inhibitors. Although histopathological subtype is an independent predictor of mutation status, there is a paucity of data on the cytomorphological features correlating with the EGFR mutation status. Therefore, the aim of this study was to determine whether certain cytomorphological features correlate with EGFR mutation in lung adenocarcinoma.
Materials and Methods:
A retrospective analysis of 48 lung adenocarcinoma cases diagnosed on fine needle aspiration cytology with known EGFR mutation status was conducted. All cytology smears with cellblock sections were reviewed. The cytomorphological features including tumor pattern, stromal features, nuclear and cytoplasmic features, and tumor grade were evaluated. Clinicoradiological features such as age, sex, smoking, tumor size, clinical stage, metastases, and presence of mass, nodule, lymphadenopathy, pleural effusion, and clinical outcome were also assessed.
Results:
Of 48 cases, 19 were EGFR-p and 29 were negative. EGFR-p cases showed a positive and significant correlation with flat monolayered sheets and acini, mild nuclear atypia, fine chromatin and smooth nuclear margins and these tumors were well differentiated. EGFR-negative tumors were moderate to poorly differentiated with predominance of solid clusters, moderate to marked nuclear atypia, with irregular nuclear margins and coarse chromatin. Clinically, female sex, nonsmoking status, smaller tumor size, and good clinical outcome correlated with EGFR-p status.
Conclusion:
Certain cytomorphological features correlate with and may suggest EGFR mutation status in advanced lung adenocarcinoma in an appropriate clinical context.
Keywords
Cytomorphological features
epidermal growth factor receptor mutation
fine needle aspiration cytology
lung adenocarcinoma
INTRODUCTION
In the present era, new oncogenic driver mutations are being discovered in nonsmall cell lung carcinoma (NSCLC). Epidermal growth factor receptor (EGFR) mutation is the most common with a prevalence of 5%–15% in Caucasians and 30%–60% in Asian population.[12] In India, the reported frequency varies from 22% to 40%.[2] Identification of EGFR mutations has improved the overall survival in patients with advanced lung adenocarcinoma, being sensitive to tyrosine kinase inhibitors (TKIs) that target the intracellular tyrosine kinase domain of EGFR.[3] Majority of these mutations are reported in adenocarcinoma and involve exons 18–21, the most common being exon 19 deletions and exon 21 L858 R missense point mutations.[3] The molecular studies are increasingly being performed to detect these mutations on cytology samples so as to select the patients who may be benefitted from TKIs.[4] Cellblocks obtained by fine needle aspiration cytology (FNAC), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and effusion samples are being used.[5] The histopathological subtype can predict not only the overall survival but also the mutation status.[678] However, there is a scarcity of data on cytomorphological features that could reliably predict the EGFR mutation status. Majority of the histopathological studies comprise of surgically resectable early-stage disease (I-IIIA).[78] FNAC and/or effusion cytology remains the primary modality for diagnosis and molecular testing in approximately 70% cases of lung cancer that are unresectable and at an advanced stage (IIIB–IV). Therefore, the aim of the present study was to evaluate the correlation between cytomorphological features and EGFR mutation status in advanced stage lung adenocarcinoma. In addition, the association of clinical and radiological characteristics with EGFR mutation was also assessed.
MATERIALS AND METHODS
This was a retrospective study, which was approved by the Institutional Ethics Committee. NSCLC-adenocarcinoma cases diagnosed on FNAC (computed tomography [CT]/ultrasonography/palpation-guided FNAC and EBUS-TBNA) with known EGFR mutation status were retrieved. Lung cancer database was searched for clinical and radiological details including treatment and clinical outcome. Cytological diagnosis was recorded in each case. Cases with low cellularity, inadequate cells for mutation testing, and squamoid differentiation were excluded from the study.
Image-guided FNAC was performed from primary and/or metastatic sites using a 23-gauge needle by a team of interventional radiologists and cytopathologists. Two to three passes were taken for obtaining adequate sample. Palpation-guided FNAC was performed from cervical lymph nodes using a 22-gauge needle by the cytopathologists, whereas EBUS-TBNA was performed by interventional pulmonologists. In each case, 5–6 direct cytology smears were prepared, of which 3–4 were air-dried and stained with May–Grünwald–Giemsa (MGG) and others were fixed in 95% alcohol and stained with hematoxylin and eosin (H and E). The aspirated material was also transferred to a15-ml falcon tube containing 1% of 1 ml ammonium oxalate and later, fixed in 10% neutral buffered formalin, and processed into formalin fixed paraffin embedded cellblocks. H and E-stained cell block sections were assessed for adequacy/tumor cellularity.
EGFR mutation testing was performed in the cases with the cell blocks had at least 100 tumor cells. DNA extraction was performed using Qiagen DnAeasy kit. EGFR mutation testing was performed by conventional polymerase chain reaction (PCR) followed by Sanger sequencing. PCR was performed for the known EGFR mutations involving exons 18, 19, 20, and 21 using forward and reverse primers.
In the present study, based on EGFR mutation status, the cases were categorized into two groups - EGFR mutation-positive (EGFR-p) and EGFR mutation-negative (EGFR-n). The cytomorphological features were evaluated in each case by two independent cytopathologists, and finally, a consensus diagnosis was made. Cytologically, adenocarcinoma is characterized by various architectural patterns including three-dimensional clusters, sheets, papillae, acinar clusters and “picket-fence though pattern heterogeneity may be present within the same tumor.” The cytomorphological features evaluated included (a) predominant tumor aggregation or pattern (monolayered/flat sheets, papillary clusters, acini, solid overlapping clusters and discrete tumor cells) with each pattern quantified from 0 to 1 (0: absent to few; 1: moderate to predominant); (b) tumor stroma including necrosis (0: absent; 1: present), acute/chronic/mixed inflammatory infiltrate (0: absent; 1: present), extracellular mucin (0: absent; 1: present) and multinucleated giant cells (0: absent; 1: present); (c) nuclear features including nuclear atypia (0: mild; 1: moderate; 2: marked); nuclear margins (0: smooth; 1: irregular); chromatin (0: fine; 1: granular; 2: coarse); nucleoli (0: inconspicuous; 1: conspicuous/prominent) and intra-nuclear cytoplasmic inclusions (INCIs) (0: absent; 1: present); (d) intracellular mucin (0: absent; 1: present) and (e) overall tumor grade/differentiation (0: well; 1: moderate; and 2: poor). The presence of signet ring tumor cells was also noted. Histopathological correlation was sought, wherever available.
Clinical variables such as mean age, sex, smoking status, tumor stage, distant metastasis, and clinical outcome based on radiological response assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) and performance status, and contrast-enhanced CT (CECT) features such as mean size, pulmonary mass, nodules, lymphadenopathy (mediastinal/hilar), and pleural effusion were also analyzed. The correlation of EGFR mutation status with clinical and cytomorphological features was assessed using Fisher's exact test (P ≤ 0.05 was considered statistically significant).
RESULTS
A total of 61 cases of lung adenocarcinoma diagnosed on FNAC over a period of 2 years who underwent EGFR mutation testing were initially recruited. Of these, 13 cases were excluded due to low cellularity and inadequate samples for EGFR mutation testing.[67] Finally, 48 cases with a cytodiagnosis of adenocarcinoma (n = 36), NSCLC favor adenocarcinoma (n = 4), and metastatic pulmonary adenocarcinoma (n = 8) were included in the study, of which19 cases (39.6%) were EGFR-p and 29 cases (60.4%) were EGFR-n.
Table 1 summarizes the FNAC details, cytodiagnosis, and mutation status with treatment details. FNAC was done from metastatic sites in six cases including liver (n = 2), supraclavicular lymph node (n = 2), para-tracheal lymph node (n = 1), and mediastinal lymph node (n = 1) in EGFR-p group, and two cases in EGFR-n group including liver (n = 1) and para-tracheal lymph node (n = 1). Majority of the patients in EGFR-p group received TKIs including gefitinib (53%), erlotinib (32%), and afatinib (10.52%), whereas EGFR-n cases received pemetrexed- and cisplatin-based chemotherapy (96%) combined with radiotherapy for distant metastases. Immunohistochemistry (IHC) was used for diagnosis (TTF-1, CK7 and/or napsin A positive and p63 and/or CK 5/6 negative) in seven EGFR-p (2 NSCLC-favor adenocarcinoma and 5 metastatic adenocarcinoma) and eight EGFR-n cases (4 adenocarcinoma, 2 NSCLC-favor adenocarcinoma, and 2 metastatic adenocarcinoma).
Correlation of epidermal growth factor receptor mutation status with demographic and clinicoradiological variables
Clinicoradiological and demographic data are summarized in Table 2. The mean age was comparable in both groups, with majority in the age group of 61–80 years (52.6%) in EGFR-p and 55.2% between 41 and 60 years in EGFR-n group. EGFR-p cases were predominantly females and nonsmokers. All EGFR-p cases had Stage IV cancer along with metastatic disease, most commonly involving contralateral lung (63.2%), and pleural fluid being least common (10.5%). In EGFR-n cases, metastases most frequently occurred in brain and opposite lung (33.3% each) and least in adrenals and liver (22.2% each). Based on the RECIST scoring, EGFR-p status was associated with an overall good clinical outcome in 84.2% cases including partial response (PR) in five cases and stable disease (SD) in 11 cases. Progressive disease (PD) was seen in only 3 (15.8%) cases with a mean follow-up of 6.9 months (2–18 months). However, in EGFR-n group, 50% showed poor outcome (PD in 4; poor performance in 6; and death in 3 cases), while 50% showed good response (SD in 6 and PR in 7 cases) with a mean follow-up of 10 months (1 month–2 years).

On CECT, EGFR-p tumors had an overall smaller mean size, majority being <5 cm. However, no significant difference was observed when radiological parameters such as mass, nodules, lymphadenopathy, or pleural effusion were compared between the two groups.
Correlation of epidermal growth factor receptor mutation status with cytomorphological features
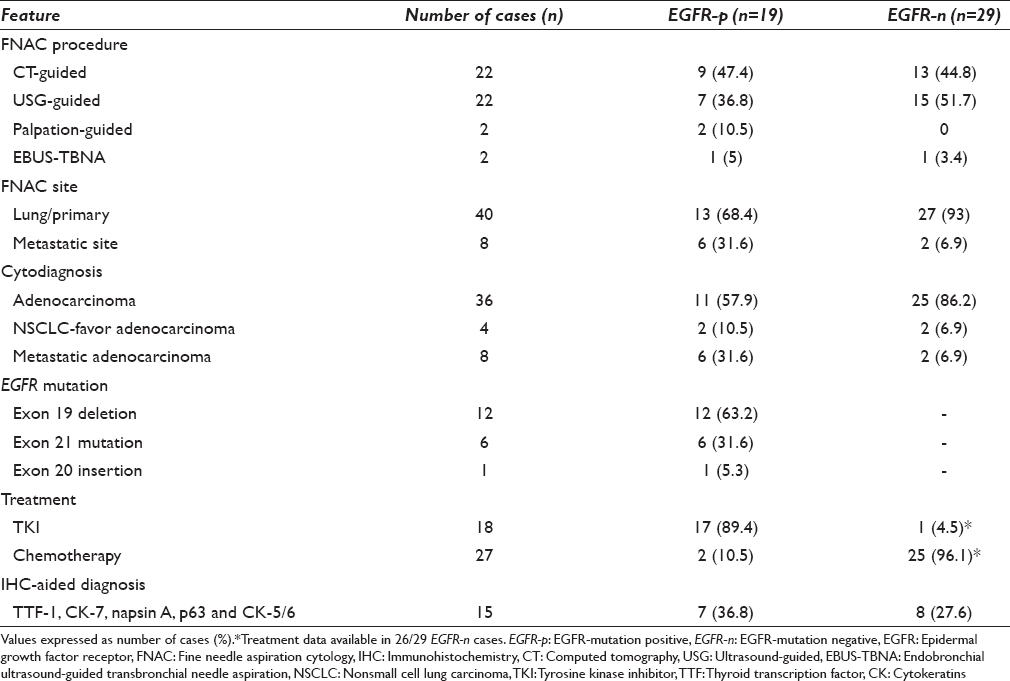
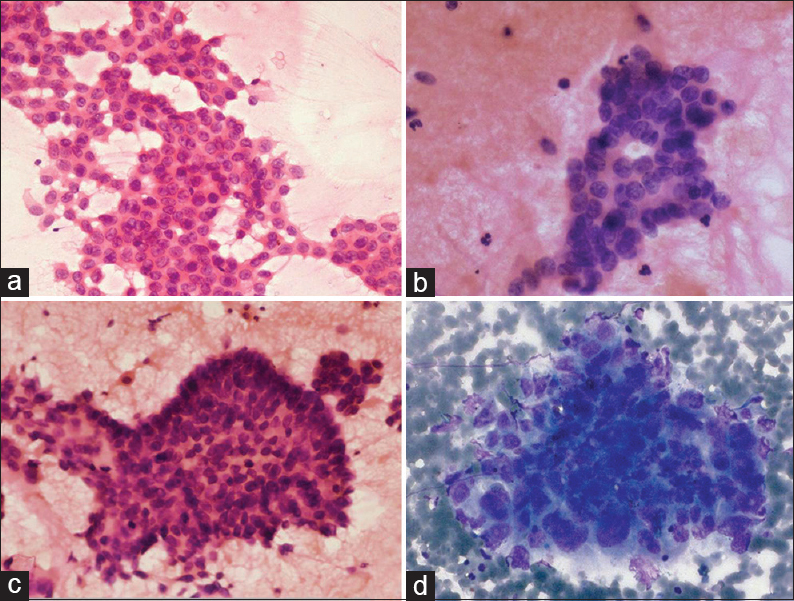
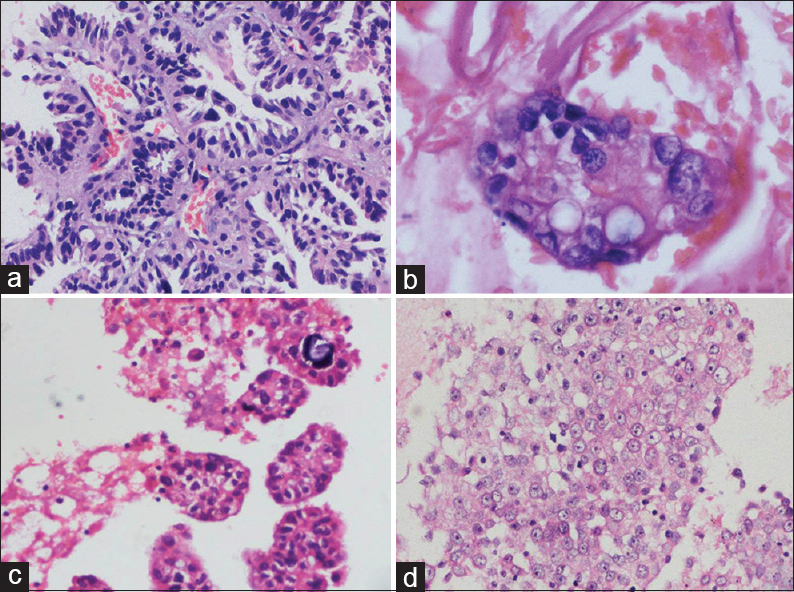
Table 3 summarizes the cytomorphological features of EGFR-p and EGFR-n groups. In each case, tumor patterns/aggregation were quantified from 0 to 1 [Figures 1 and 2a–d] and the predominant pattern was noted. There was preponderance of monolayered flat sheets (52.6%) and acini (42%) in EGFR-p group (P < 0.01, 0.041, respectively), whereas overlapping solid clusters predominated in EGFR-n group (75.9%, P = 0.01). Though papillary clusters were more frequent in EGFR-p group and discrete tumor cell pattern was more common in EGFR-n group, these were not statistically significant. Stromal features such as necrosis (44.8%), acute/chronic/mixed inflammatory infiltrate (58.6%), and giant cells (20.7%) were higher in EGFR-n group [Figure 3a, b and d]. There was no significant difference for extracellular mucin between the two groups, though it was slightly higher in EGFR-p group [21% vs. 13.8%, Figure 3c]. For the nuclear features [Figure 4a-d], EGFR-p cases revealed a significant association with low nuclear grade in the form of mild nuclear atypia (52.6%), smooth nuclear margins (68.4%), and fine chromatin (57.9%), whereas EGFR-n cases had moderate to marked nuclear atypia (44.8% and 55.2%), irregular nuclear margins (93%), and coarse chromatin (65.5%). INCIs were noted in almost similar percentage in the two groups [Figure 4]. Intracellular mucin and inconspicuous nucleoli were also found to be higher in EGFR-p group than EGFR-n group; however, these were not statistically significant. A highly significant difference was noted when overall tumor grade was compared (P < 0.01). EGFR-p tumors were predominantly well-differentiated (52.63%) as compared to EGFR-n tumors, which were moderate and poorly-differentiated (44.8% and 55.2%, respectively). Two EGFR-n cases showed few signet ring tumor cells.
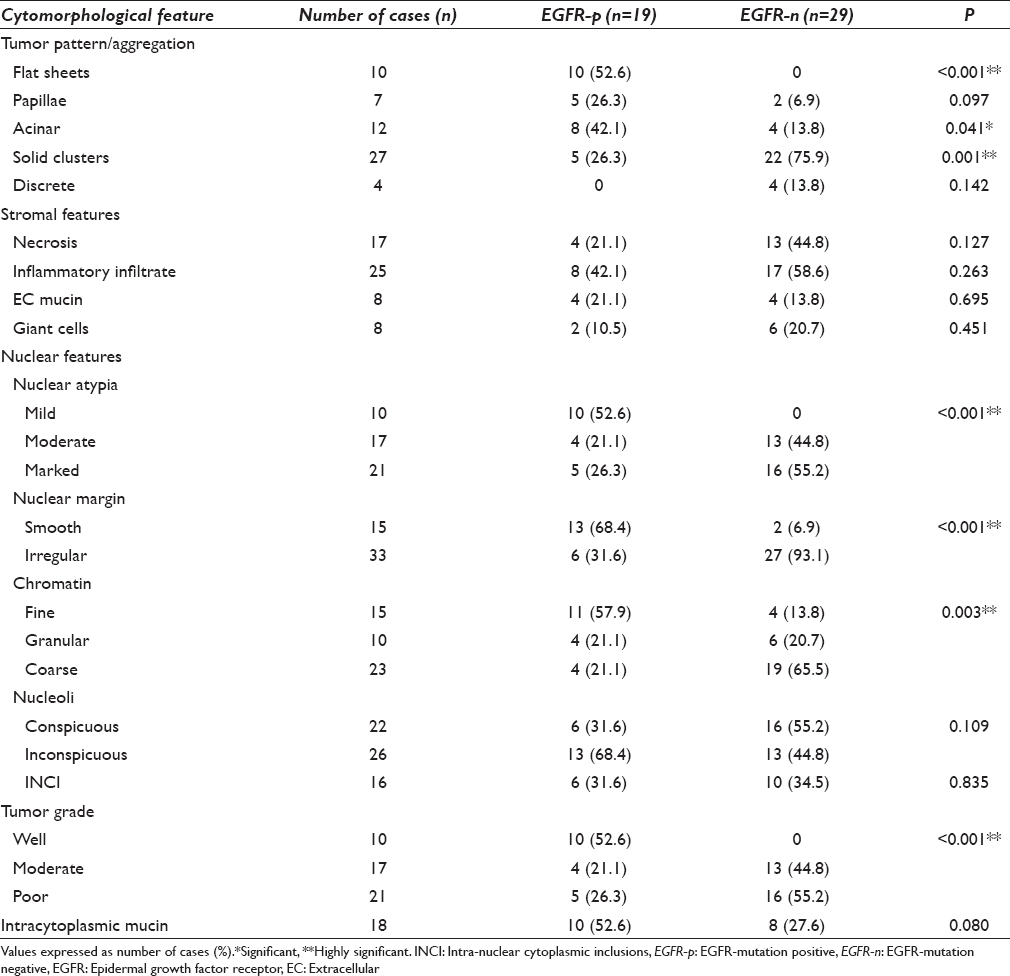
- Tumor pattern: Epidermal growth factor receptor mutation positive cases showing flat monolayered sheets (a), acini (b), and papillary fragment of tumor cells (c) (H and E, ×400); (d) A solid overlapping cluster in epidermal growth factor receptor mutation negative case (MGG, ×400)
- Cellblock sections: epidermal growth factor receptor mutation positive cases tall columnar tumor cells with intervening stroma (a), glandular pattern (b), and papillary fragments (c); (d) a solid sheet-like pattern in epidermal growth factor receptor mutation negative case (H and E, ×400)
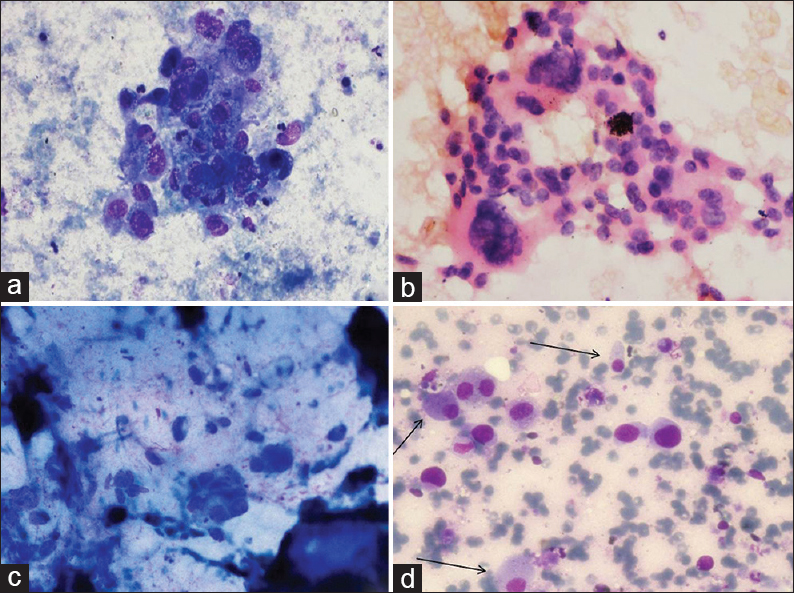
- Stromal features: (a) Necrosis in epidermal growth factor receptor mutation negative case; (b) giant cells in epidermal growth factor receptor mutation negative case; (c) abundant extracellular mucin in epidermal growth factor receptor mutation positive case; (d) foamy macrophages (arrows) interspersed with tumor cells in epidermal growth factor receptor mutation positive case (MGG, ×400)

- Nuclear features: (a) Epidermal growth factor receptor mutation-positive tumor exhibiting mild nuclear atypia with relatively monomorphic nuclei (H and E, ×400); (b) epidermal growth factor receptor mutation-positive tumor with regular and smooth nuclear margins with fine chromatin (MGG, ×400); (c) epidermal growth factor receptor mutation negative tumor showing moderate nuclear atypia (H and E, ×400); (d) epidermal growth factor receptor mutation negative tumor with tumor cells showing irregular nuclear margins and coarse chromatin (MGG, ×400). Intra-nuclear cytoplasmic inclusions identified in both the groups (MGG, ×400; (3b and 4d-inset))
DISCUSSION
There is a limited data describing the cytomorphological features that could suggest mutation status in lung adenocarcinoma, though histopathological subtype is an independent predictor of mutation status.[367] EGFR mutations have been linked to lung adenocarcinoma with lepidic pattern.[910] With the advent of molecular techniques and targeted therapies, cytology has become an indispensable tool in guiding the diagnosis and management of mutation-positive advanced stage adenocarcinoma.[511] International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society proposed a new classification of lung adenocarcinoma in 2011 that strongly recommended the strategic use of cytology/small biopsy specimens for diagnosis and molecular testing in cases presenting with locally advanced or metastatic disease.[12] It recommended more specific categorization of NSCLC on cytology/small biopsies into adenocarcinoma or squamous cell carcinoma. The therapeutic implication of this categorization lies in the fact that the patients with EGFR-p adenocarcinoma are the potential candidates for TKIs and for pemetrexed and bevacizumab-based chemotherapy if EGFR-n.[12] Thus, EGFR mutation, an independent predictor of response to TKIs and survival, should be tested in cytologically diagnosed adenocarcinoma, NSCLC-favor adenocarcinoma or NSCLC-NOS.[12] FNAC is a minimally invasive, relatively safe, cost-effective and rapid test as compared to lung biopsy and is highly recommended with the intent of early diagnosis and molecular analysis. Cell blocks prepared from aspirated material may have more tumor cells than a small biopsy. In a study by Rekhtman et al., 98% (126/128) of the cell block specimens were found to be adequate for EGFR (25%) and KRAS (20%) mutation testing.[13] Ma et al. performed EGFR mutation testing by PCR followed by direct sequencing on cell blocks that were subjected to the enrichment of tumor cells to >30% by microdissection or at least 50 tumor cells and on surgical specimens. They reported a positive EGFR mutation detection rate of 39.4% as compared to 48% in surgical specimens.[14]
It is important to have good cell blocks with at least 50 tumour cells to extract good quantity and quality DNA material for molecular studies. Previous publications have highlighted various methods to concentrate diagnostic material.[15] In the absence of cell blocks, air-dried MGG stained cytosmears can also be scraped for DNA extraction.
In lung adenocarcinoma, the independent clinical prognostic factors determining the disease progression or recurrence include age, sex, stage, performance status and smoking.[12] The predictive factors determining the response to specific therapies include histological subtypes and EGFR mutations, each predicting a good response to TKIs and progression-free survival.[12]
Various studies have reported the association of EGFR mutated lung adenocarcinoma with female sex, nonsmoking status and Asian ethnicity.[16] The present study also corroborates with these findings with preponderance of nonsmokers and females in EGFR-p group. The most common mutations reported are exon 19 in-frame deletions and exon 21 L858R missense mutations. In the present study, exon 19 deletions were most frequent (63%) with a ratio of exon 19 to exon 21 mutations being 2:1, in line with the previous studies (1.3:1–4.6:1).[17] Exon 20 mutations are usually resistant to TKIs, though rarely, better clinical responses have been reported.[18] We also found one case with exon 20 insertion (9 bp) who received Gefitinib and had SD. Brachtel et al. reported 77% of EGFR mutated cases to be alive with no evidence of disease at last follow-up as compared to 59% of EGFR non-mutated cases.[19] An Indian study by Maturu et al. reported higher median overall survival in cases with EGFR mutated type than EGFR wild-type and EGFR unknown type.[20] In the present study, EGFR-p status was associated with overall good clinical outcome than EGFR-n group.
Several studies have shown that lepidic, papillary, micropapillary and acinar subtypes correlate positively and solid subtype has a negative correlation with EFGR mutation, though most of these studies included the resected specimens of stage I-IIIA patients.[367] Maturu et al. reported a positive association of EGFR mutation status with lepidic predominant adenocarcinoma (36.4%) and negative association with solid predominant subtype.[20] However, only a handful of studies have attempted to correlate the cytomorphological features with EGFR mutation in the literature. A cytological study by Brachtel et al. comprising of 37 cases of lung adenocarcinoma reported 13 EGFR-p (35%) and 24 EGFR-n (65%) cases (similar to our study, 39.6% and 60.4%, respectively), wherein the combination of flat architecture, presence of intra-nuclear inclusions, absence of macronucleoli and absence of extracellular mucin correlated significantly with the presence of EGFR mutation with a positive predictive value of 69% and a negative predictive value of 92%.[19] Though presence of overlapping clusters, irregular nuclear membranes and degree of nuclear pleomorphism was higher in the control group, while nuclear grooves and fine chromatin were more common in mutation positive group, these features did not reach a statistical significance. None of the mutation positive cases had extracellular mucin (in contrast to our study).[19] Marotti et al.[21] in a retrospective analysis of 50 cases of lung adenocarcinoma found 12% cases with EGFR mutations and showed a significant correlation of INCIs, papillary fragments, and lepidic predominant adenocarcinoma histology with EGFR mutation status (similar to our study).
The present study also supports presence of flat monolayered sheets, acini, less stromal desmoplastic response, mild nuclear atypia, smooth nuclear margins, fine nuclear chromatin, and well-differentiated grade being significantly associated with EGFR-p status, whereas EGFR-n status is strongly associated with solid overlapping clusters, high desmoplastic stromal response, moderate to marked nuclear atypia, irregular nuclear margins, coarse chromatin, and moderate-to-poor differentiation. In contrast to previous studies, INCIs were not found to have positive correlation with EGFR-p tumors.
CONCLUSION
The present study supports the indispensable role of cytology as a minimally invasive diagnostic test in lung adenocarcinoma. As documentation of EGFR mutation is a prerequisite for initiating TKI therapy in such patients, molecular analysis could be performed on cytological cell blocks. Certain cytomorphological features in an appropriate clinical context may suggest EGFR mutation, thereby acting as potential surrogate markers in predicting mutation status.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS'
The authors declare that we have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
To give appropriate credit to each author of a paper, the individual contributions of all authors to the manuscript have been specified.
According to International Committee of Medical Journal Editors (ICMJE http://www.icmje.org) “author” is generally considered to be someone who has made substantive intellectual contributions to a published study.
Authorship credit is based on (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published. Authors meet conditions 1, 2, and 3.
We declare that each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
All authors of this article declare that we qualify for authorship as defined by ICMJE. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article.
SS, NG and RC were instrumental in collection and interpretation of data. NG and RC did technical work including PCR for EGFR mutations. SS and NG did the slide review and prepared the first draft of the manuscript along with literature search. Clinical data were provided by NS and DB. Initial acquisition of data, PubMed search, and modification in the manuscript was done by NG and SS. The manuscript was finally edited by NS, DB, and AR.
All authors read and approved the final manuscript.
Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of the institution associated with this study as applicable. This study was a retrospective study not directly involving the patients. Authors take responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
CECT – Contrast enhanced computed tomography
EBUS-TBNA– Endobronchial ultrasound guided transbronchial needle aspiration
EGFR – Epidermal growth factor receptor
EGFR-n – EGFR mutation negative
EGFR-p – EGFR mutation positive
FFPE – Formalin fixed paraffin embedded
FNAC – Fine needle aspiration cytology
H and E – Hematoxylin and eosin
INCI – Intra nuclear cytoplasmic inclusions
MGG– May–Grünwald–Giemsa
NSCLC – Non small cell lung carcinoma
PCR – Polymerase chain reaction
PD – Progressive disease
PR – Partial response
RECIST – Response Evaluation Criteria in Solid Tumors
SD – Stable disease
TKI – Tyrosine kinase inhibitors
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82:179-89.
- [Google Scholar]
- Aprospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154-62.
- [Google Scholar]
- Molecular pathology of lung cancer: Key to personalized medicine. Mod Pathol. 2012;25:347-69.
- [Google Scholar]
- Seize the opportunity: Underutilization of fine-needle aspiration biopsy to inform targeted cancer therapy decisions. Cancer Cytopathol. 2009;117:289-97.
- [Google Scholar]
- Correlation of cytomorphology and molecular findings in EGFR+, KRAS+, and ALK+ lung carcinomas. Am J Clin Pathol. 2014;141:420-8.
- [Google Scholar]
- Clinicopathological predictors of EGFR/KRAS mutational status in primary lung adenocarcinomas. Mod Pathol. 2010;23:159-68.
- [Google Scholar]
- Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma. Onco Targets Ther. 2014;7:1423-37.
- [Google Scholar]
- Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496-504.
- [Google Scholar]
- Epidermal growth factor receptor mutation in lung cancer are linked to bronchioloalveolar differentiation. Am J Surg Pathol. 2006;30:1309-15.
- [Google Scholar]
- Acorrelation between EGFR gene mutation status and bronchioloalveolar carcinoma features in Japanese patients with adenocarcinoma. Jpn J Clin Oncol. 2006;36:69-75.
- [Google Scholar]
- Mutational analysis in cytological specimens of advanced lung adenocarcinoma: A sensitive method for molecular diagnosis. J Thorac Oncol. 2007;2:1086-90.
- [Google Scholar]
- International association for the study of lung cancer/American thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-85.
- [Google Scholar]
- Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: High accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol. 2011;6:451-8.
- [Google Scholar]
- Cell block preparation from cytology specimen with predominance of individually scattered cells. J Vis Exp 2009 pii: 1316
- [Google Scholar]
- EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-500.
- [Google Scholar]
- Epidermal growth factor receptor mutation subtypes and geographical distribution among Indian non-small cell lung cancer patients. Indian J Cancer. 2013;50:107-11.
- [Google Scholar]
- Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877-82.
- [Google Scholar]
- Cytomorphological correlates of epidermal growth factor receptor mutations in lung carcinoma. Diagn Cytopathol. 2007;35:257-62.
- [Google Scholar]
- Relationship of epidermal growth factor receptor activating mutations with histologic subtyping according to International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society 2011 adenocarcinoma classification and their impact on overall survival. Lung India. 2016;33:257-66.
- [Google Scholar]
- Cytomorphologic features of advanced lung adenocarcinomas tested for EGFR and KRAS mutations: A retrospective review of 50 cases. Diagn Cytopathol. 2013;41:15-21.
- [Google Scholar]








