Translate this page into:
Development of a clinical prediction model for pathological upgrading in low-grade squamous intraepithelial lesions following cervical conization

*Corresponding author: Liqun Wang, Department of Gynecology, The First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, China.wangliqqun@163.com
-
Received: ,
Accepted: ,
How to cite this article: Peng X, Wan J, Wang Y, Wang L. Development of a clinical prediction model for pathological upgrading in low-grade squamous intraepithelial lesions following cervical conization. CytoJournal. 2024;21:37. doi: 10.25259/Cytojournal_7_2024
Abstract
Objective:
This study aimed to identify key factors influencing post-operative pathologic escalation in Chinese women with histologic cervical low-grade squamous intraepithelial lesions (LSILs) undergoing cervical conization and construct a predictive nomogram model.
Material and Methods:
A retrospective analysis was conducted on 107 patients with LSIL from Bengbu City, Anhui Province, China, who underwent cervical conization at the First Affiliated Hospital of Bengbu Medical College from January 2019 to January 2023. Patients were categorized into groups based on post-operative pathological upgrade. Univariate and multivariate logistic regression analyses identified independent risk factors. A nomogram model was developed and evaluated for clinical predictive ability using calibration curves, the Hosmer–Lemeshow test, and decision curve analysis (DCA).
Results:
Post-operative pathological upgrades were experienced by 39.3% of patients with LSIL. Independent risk factors for escalation included positive human papillomavirus (HPV)16/18/52/53/58 high-risk types (P < 0.05, OR = 4.95, 95% CI = 1.32–18.46), ThinPrep Cytology Test (TCT) results indicating high-grade squamous intraepithelial lesion (HSIL)/atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion (ASC-H)/atypical glandular cells ( AGC) (P < 0.01, OR = 13.12, 95% CI = 3.10–55.50), and cervical transformation zone (TZ) type III (P < 0.05, OR = 6.10, 95% CI = 1.65–22.56). Based on these factors, the nomogram demonstrated good differentiation and calibration (area under the curve [AUC]: 0.744, 95% CI: 0.674–0.839). DCA indicated high clinical predictive value.
Conclusion:
HPV16/18/52/53/58 high-risk types, TCT HSIL/ASC-H/AGC, and colposcopic cervical TZ type III are independent risk factors for post-operative pathologic escalation in LSIL. Consideration of pre-operative HPV, TCT results, and cervical TZ type is crucial for effective triage and patient management. The constructed nomogram provides a practical tool for risk assessment of patients with LSIL undergoing cervical conization.
Keywords
Low-grade squamous intraepithelial lesion
Cervical conization
Pathologic progression
Clinical prediction model
INTRODUCTION
Cervical cancer (CC) is well known to be among the top cancers among women; according to the latest report, half of cases of CC have a mortality rate.[1] The occurrence of CC is caused by the development of precursor lesions called cervical intraepithelial neoplasia (CIN), and the process of disease progression is very slow, which can be intervened and treated before the disease progresses to cancer.[2] If intervention and treatment can be applied before the disease develops into cancer or even before the disease progresses, it can greatly reduce the incidence of CC. Thus, in all current research, CC must be promptly addressed. As outlined by the 2014 World Health Organization (WHO) guidelines, CIN I, identified through cervical biopsy, is categorized as a low-grade squamous intraepithelial lesion (LSIL), whereas CIN II and CIN III are classified as high-grade squamous intraepithelial lesions (HSILs).[3] According to relevant literature, the influencing factors of post-operative pathological progression in patients with LSIL may include age, cytology, detection of human papillomavirus (HPV), type of transformation zone (TZ), pregnancy.[4-6]
Cytological screening is the first step in CC screening. As a routine screening method, ThinPrep Cytology Test (TCT) classifies and diagnoses cervical cells by storing them in cell preservation solutions and centrifuging them. Compared with traditional cervical scraping and Pap smear examinations, it significantly improves specimen satisfaction and the detection rate of cervical abnormal cells. Therefore, TCT has high diagnostic accuracy and can be effectively used for early cancer screening .[7] In general, vaginoscopy is an important and powerful auxiliary screening tool for CC, which can be used to study the size and location of pre-cancerous lesions in the cervix. However, colposcopy results in diagnosing cervical pathology may vary among observers depending on the environment, colposcopy physicians’ experience, and the surgery duration.[8]
This classification introduces complexities, particularly highlighted in the 2001 edition of the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines, where it is noted that “histologic LSIL is somewhat heterogeneous”. This heterogeneity in histological LSIL poses a challenge, leading to a phenomenon of missed diagnosis of high-grade lesions or even invasive cancer in affected patients. Understanding the potential risk of progression to CINIII+ in individuals with histological CINI is crucial. Data from the Caesar Medical Institution in Northern California (KPNC) reveal intriguing insights: if the previous cytology was LSIL- or HPV-positive in atypical squamous cells of undetermined significance (ASC-US), the risk of CINIII+ occurrence within five years is 3.8%; but if the previous cytology was HSIL, the risk escalates to 15%.[9] Meanwhile, the 2019 version of the ASCCP guidelines proposes the concept of thresholds, which can be refined with equal risk and management.[10] The inherent heterogeneity in histological LSIL poses a significant challenge, leading to the phenomenon of missed diagnosis of high-grade lesions or even invasive cancer in affected patients. This challenge is further underscored by findings that the accuracy of direct colposcopic biopsy is reported at 71.9%.[3] It highlights the limitations of cervical tissue biopsy, which has a certain misdiagnosis rate and cannot fully replace the comprehensive evaluation of the entire cervix. In clinical practice, cervical circular cone resection is often performed after evaluation to mitigate the risk of missed diagnoses. However, uniform standards for stratifying and managing patients with histologic LSIL are lacking. This gap in knowledge prompts exploration into strategies that reduce the missed diagnosis rate of patients with LSIL while minimizing the harm associated with overtreatment.
To address these challenges, this study retrospectively analyzed 107 patients with histological LSIL who underwent cervical conization surgery. The findings from this analysis aim to lay the groundwork for the development of reasonable and accurate personalized diagnosis and treatment plans for patients with histological LSIL. This section aims to describe the intricacies of CIN classification, exploring its clinical implications and the challenges posed by histologic LSIL heterogeneity. By elucidating the potential risks associated with different cytological findings, we aim to underscore the importance of refining our understanding of cervical neoplasia for enhanced diagnostic accuracy and patient care.
MATERIAL AND METHODS
Collection of materials
Based on relevant literature,[4,5,11] the following indicators were included: Age, type of HPV infection, TCT results, and type of cervical TZ, representing a critical anatomical region situated at the junction of squamous and columnar epithelia in cervical pathology. This zone assumes pivotal significance in CC screening and evaluation and is the predominant site of origin for most CCs and pre-cancerous lesions. A comprehensive understanding of the characteristics and dynamics of TZ is essential for accurate diagnosis and management in the field of cervical pathology, number of pregnancies, duration of HPV infection, type of HPV infection (single type and mixed type), and compatibility of pathologic findings with colposcopic images. This study received approval from the Clinical Medical Research Ethics Committee of the First Affiliated Hospital of Bengbu Medical College, with approval number 2023YJS166.
Conization
For clinical considerations, conization was performed on women diagnosed with LSIL to assess and control potential risks associated with disease progression. LSIL, being a histopathological diagnosis, encompasses a range of abnormalities, with concerns about the potential presence of higher-grade lesions or even invasive cancer. Conization serves diagnostic and therapeutic purposes by excising abnormal tissue for further pathological examination. The procedure aids in identifying occult high-grade lesions that initial biopsy may not fully capture to facilitate a comprehensive evaluation of cervical pathology. The purpose of selecting conization is to ensure accurate diagnosis, control potential disease progression, and minimize the risk of missed diagnoses that could lead to adverse outcomes.
In clinical practice, there are two commonly used techniques for cervical conization: the loop electrosurgical excision procedure (LEEP) and the cold knife conization (CKC). The choice of excision technique was based on factors such as the extent of the lesion, depth of infiltration, and clinical considerations. LEEP and CKC are commonly used clinical excision techniques due to their efficacy in removing abnormal tissue while allowing for histopathological examination. The specific technique chosen for each patient was determined after carefully considering individual clinical characteristics and lesion features.
Patient information and data collection
In our clinical practice, the choice between LEEP or CKC is often determined by the lesion’s extent and the infiltration depth. All patients in this study underwent LEEP surgery for further diagnosis of cervical diseases to minimize errors arising from surgical methods.
Inclusion criteria
Patients who met the following criteria were included
Diagnosis of LSIL by cervical biopsy before cervical conization
No history of other medications or physical therapy before the operation
Complete pre-operative auxiliary examination data
Patients who had signed the informed consent for the operation
Exclusion criteria
Patients meeting any of the following criteria were excluded from the study:
Use of other auxiliary treatments before surgery
Lack of complete information
Pregnancy and childbirth requirements
Coagulation disorders, hepatic, immune dysfunction, and renal disorders that could not tolerate the surgery
Undergone surgical treatments in outside hospitals
The training and validation sets were not split due to this study’s limited number of cases. All data were analyzed as the training set.
Statistical analysis
After grouping and assigning values, the indicator data were analyzed using SPSS (Version R27.0.1.0, IBM Corp., Chicago, IL, USA) with the optimal scale, where count data were represented as cases (%). A meticulous variable selection process was employed to construct a predictive model, guided by a comprehensive review of relevant literature, clinical significance, and statistical considerations.
Univariate and multivariate (forward method) regression analyses were conducted using logistic regression (P < 0.05) to determine independent risk factors. Variables with odds ratios (ORs) >1 were identified as independent risk factors and considered crucial selections.
The chosen independent risk factor indicators were imported into R language (version 4.3.0, R Foundation, Vienna, Austria) for model development. The “rms” package and other pertinent tools were utilized to construct a nomogram prediction model due to its capacity to effectively visualize the results of the independent risk factor analysis in the model. The calculation of sample size was performed by SPSS (Version: R27.0.1.0) before the study was conducted. At the same time, receiver operating characteristic (ROC) curves were drawn to test the model’s discrimination. As TCT results are currently used as indicators for further cervical treatment in clinical practice, ROC curves were drawn for TCT indicators for comparison to test the model’s discrimination further and demonstrate the advantages of this model.
Given that the number of cases collected by this model did not reach the split training set and validation set, the bootstrap internal autonomous sampling method was used to obtain 1000 samples of the model data for internal validation, resulting in a 95% confidence interval (CI) of ROC, to test the credibility of the model’s discrimination. The calibrate function in the “rms” package was used to draw the calibration curve of the model and conduct H-L tests to evaluate the consistency between the predicted model probability and the actual probability. Finally, the “rmda” package was used to draw a clinical DCA for further validation of the clinical utility of the model.
RESULTS
A total of 107 patients with histological LSIL underwent clinical evaluation for cervical conization surgery. The overall data analysis process is illustrated in Figure 1. Baseline analysis indicates that the rate of post-operative pathological upgrades among patients with LSIL at The First Affiliated Hospital of Bengbu Medical College reached 39.3% from January 2019 to January 2023 [Table 1].
- Inclusion and exclusion table for all patients.
| (All) | 0 | 1 | P-value | |
|---|---|---|---|---|
| n=107 (%) | n=65 (%) | n=42 (%) | ||
| Age | 0.027 | |||
| ≤45 years | 47 (43.9) | 23 (35.4) | 24 (57.1) | |
| >45 years | 60 (56.1) | 42 (64.6) | 18 (42.9) | |
| TCT | <0.001 | |||
| NILM/ASC-US/LSIL | 86 (80.4) | 60 (92.3) | 26 (61.9) | |
| HSIL/ASC-H/AGC | 21 (19.6) | 5 (7.7) | 16 (38.1) | |
| HPV | 0.004 | |||
| Other low-risk types of HPV | 26 (24.3) | 22 (33.8) | 4 (9.5) | |
| HPV16/18/52/53/58 | 81 (75.7) | 43 (66.2) | 38 (90.5) | |
| Transformation-zone | 0.009 | |||
| TZ 1/TZ 2 | 46 (43.0) | 34 (52.3) | 12 (28.6) | |
| TZ 3 | 61 (57.0) | 31 (47.7) | 30 (71.4) | |
| Times of pregnancy | 0.306 | |||
| ≤1 time | 82 (76.6) | 52 (80.0) | 30 (71.4) | |
| >1 times | 25 (23.4) | 13 (20.0) | 12 (28.6) | |
| Type of HPV infection | 0.756 | |||
| Single type | 85 (79.4) | 51 (78.5) | 34 (81.0) | |
| Mixed type | 22 (20.6) | 14 (21.5) | 8 (19.0) | |
| Years of HPV infection | 0.539 | |||
| <1 year | 70 (65.4) | 44 (67.7) | 26 (61.9) | |
| ≥1 years | 37 (34.6) | 21 (32.3) | 16 (38.1) | |
| Match | 0.434 | |||
| Consistent | 71 (66.4) | 45 (69.2) | 26 (61.9) | |
| Inconsistent | 36 (33.6) | 20 (30.8) | 16 (38.1) |
“0”represents pathology did not escalate after conization surgery, “1” represents pathological upgrade after conization, “transformation zone,” type of cervical transformation zone, “TZ 1” represents type I of cervical transformation zone, “TZ 2” represents type II of cervical transformation zone, “TZ 3” represents type III of cervical transformation zone, “type of HPV infection,” single or mixed infection of HPV, and “Match,” pathology and colposcopy results. ASCUS: Atypical squamous cells of undetermined significance, LSIL: Low-grade squamous intraepithelial lesion, HSIL: High-grade squamous intraepithelial lesion, HPV: Human papillomavirus, TCT: ThinPrep cytology test, TZ: Transformation zone, N represents the number of cases.
Based on data baseline analysis
Age
In this study, patients were aged between 33 and 66 years, with a median age of 47.
TCT
Pathological upgrade: No intraepithelial lesion cells and malignant cells ( NILM) (n = 8), ASC-US(n = 7), LSIL (n = 11), ASC-H (n = 6), AGC (n = 0), and HSIL (n = 10); pathology not upgraded: NILM (n = 20), ASC-US (n = 12), ASC-H (n = 3), LSIL (n = 28), AGC (n = 0), and HSIL (n = 2).
HPV
Pathological upgrade: HPV16/18/52/53/58 (n = 38) and other low-risk types of HPV (n = 4); pathology not upgraded: HPV16/18/52/53/58 (n = 43) and other low-risk types of HPV (n = 22).
TZ
Pathological upgrade: TZ 3 (n = 32) and TZ 1/TZ 2 (n = 10); pathology not upgraded: TZ 3 (n = 33) and TZ 1/TZ 2 (n = 32).
The data underwent one-way logistic regression analysis, yielding statistically significant results (P < 0.05) for HPV 16/18/52/53/58 high-risk types. TCT results indicated HSILs/atypical squamous cells cannot be excluded from HSILs (ASC-H)/(AGC), and TZ 3. No statistically significant differences were found in the number of pregnancies, duration of persistent HPV infection, type of HPV infection (single type and mixed type), and concordance of pathologic findings with colposcopic images (P > 0.05). Multivariate logistic regression analysis (forward method) of the high-risk indicators identified in the univariate analysis revealed that TCT > LSIL (HSIL/ASC-H/AGC), HPV16/18/52/53/58 high-risk types, and TZ 3 were independent risk factors for pathologic upgrading after conization for LSIL [Table 2]. In this study, the median age was 47 years old. It is worth noting that, contrary to existing literature and guidelines, age>45 years (P<0.05, OR<1) becomes a protective factor. Therefore, this indicator was excluded from the model. Simultaneously, for all variables in this experiment, the minimum forward regression (Akaike information criterion; [AIC]) method was employed to determine the optimal model and independent risk factors. The AIC values for the three high-level factors in the aforementioned multivariate regression analysis were the smallest (128.2).
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | ||||||
| >45 years vs. <45 years | 0.41 | 0.19-0.91 | 0.028 | 0.06 | 0.01-0.24 | <0.01 |
| TCT | ||||||
| HSIL/ASC-H/AGC vs. NILM/ASC-US/LSIL | 7.38 | 2.44–22.31 | <0.01 | 13.12 | 3.10–55.50 | <0.01 |
| HPV16/18/52/53/58 | ||||||
| Yes vs. No | 4.86 | 1.54–15.36 | 0.007 | 4.95 | 1.32–18.46 | 0.014 |
| TZ | ||||||
| TZ 3 vs. TZ 1/2 | 2.74 | 1.20–6.27 | 0.017 | 6.10 | 1.65–22.56 | 0.007 |
| Type of HPV infection | ||||||
| Mixed vs. Single | 0.86 | 0.32–2.26 | 0.756 | |||
| Years of HPV infection | ||||||
| >1 vs. ≤1 | 1.29 | 0.57–2.90 | 0.539 | |||
| Times of pregnancy | ||||||
| ≥1 vs. <1 | 1.6 | 0.65–3.95 | 0.308 | |||
| Match | ||||||
| Inconsistent vs. consistent | 1.38 | 0.61–3.13 | 0.434 | |||
ASCUS: Atypical squamous cells of undetermined significance, LSIL: Low-grade squamous intraepithelial lesion, HSIL: High-grade squamous intraepithelial lesion, HPV: Human papillomavirus, TCT: ThinPrep cytology test, TZ: Transformation zone, CI: Confidence interval, OR: Odds ratio, vs: versus.
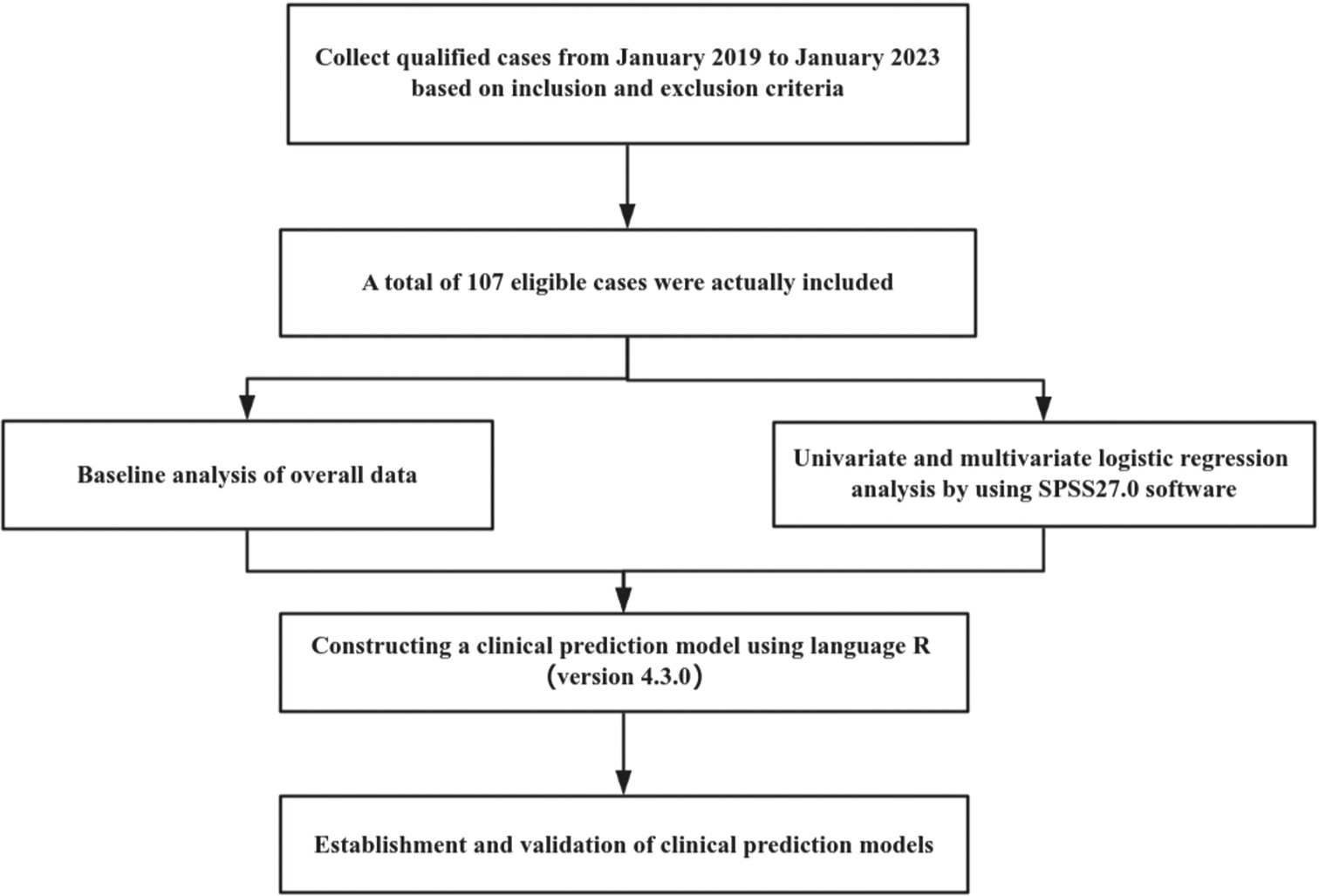
Utilizing the three independent risk factors, the data were input into language R (version 4.3.0) to construct a clinical prediction model for pathologic escalation after LSIL. A nomogram was plotted, and scores were assigned by placing vertical lines at the points corresponding to the axes of the variables in the graph at the scoring scale. The scores for each variable were then aggregated, and the total score value at the corresponding point on the predictive probability axis represented the risk of pathologic escalation after LSIL [Figure 2].
- Single-factor and multifactor logistic regression analyses. (HPV: Human papillomavirus, TCT: ThinPrep Cytology Test, ASC-US: HPV-positive inatypical squamous cells of undetermined significance, LSIL: Low-grade squamous intraepithelial lesion, HSIL: High-grade squamous intraepithelial lesion.)
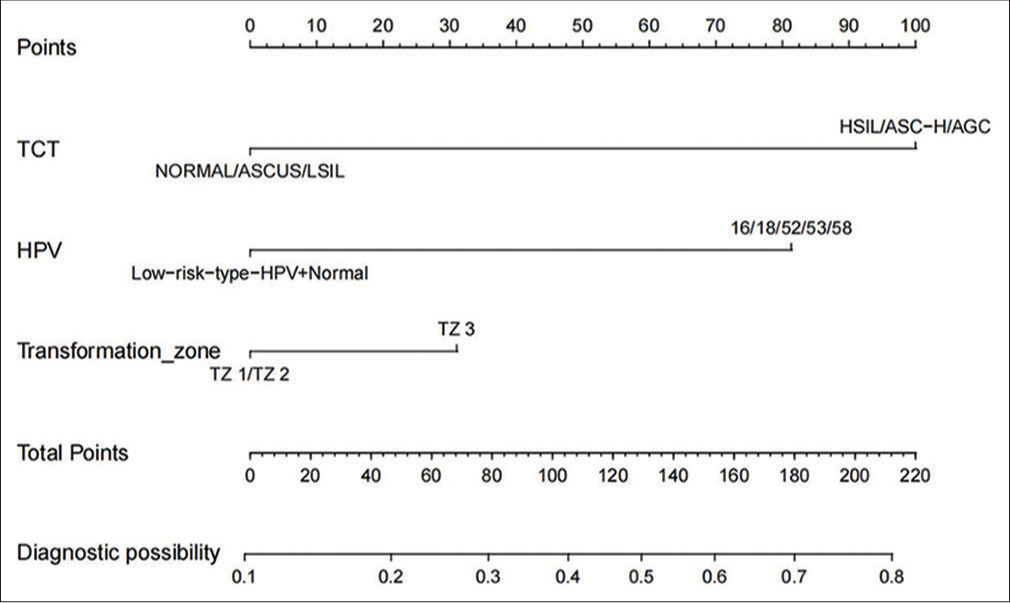
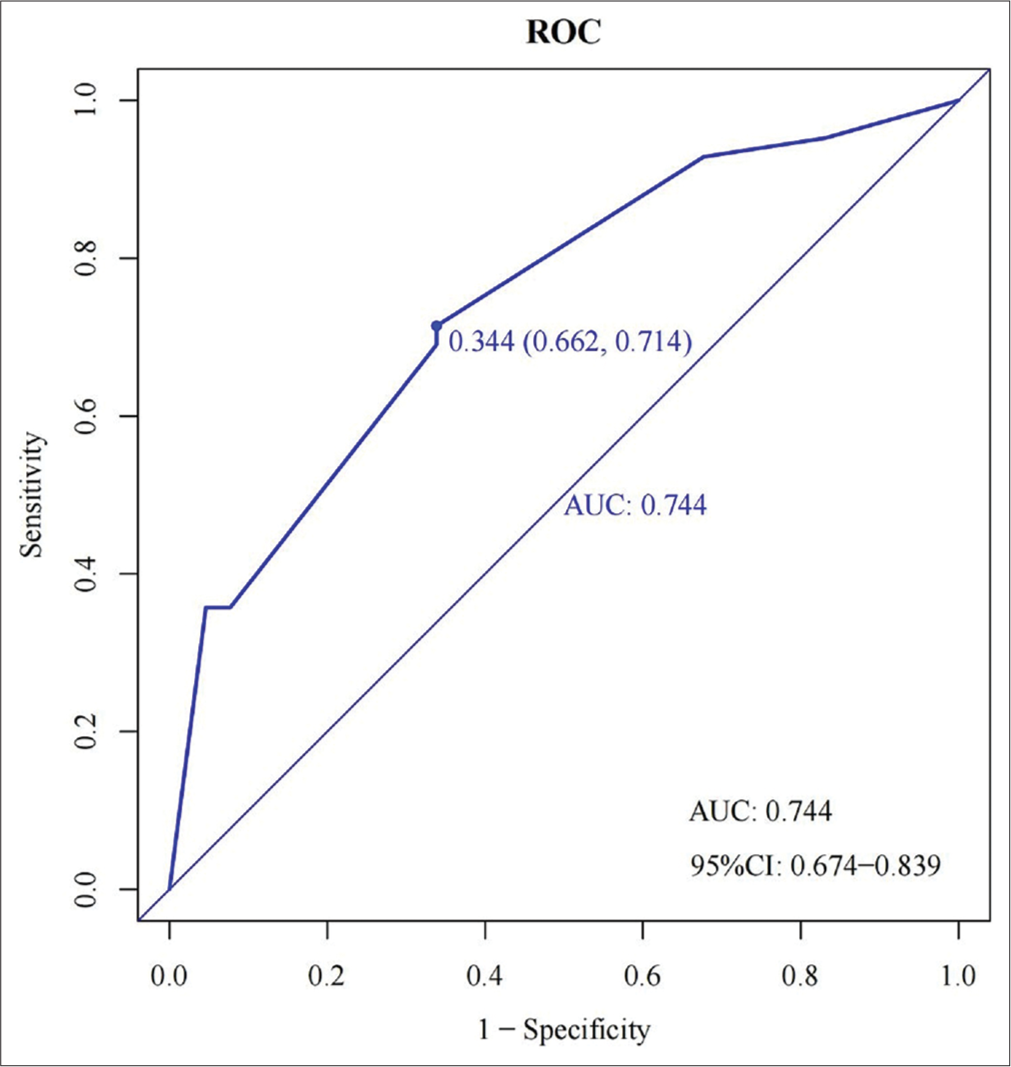
Area under the ROC curve of the model: 0.744 (cutoff value of 34.4%, sensitivity: 66.2%, and specificity: 71.4%; [Figure 3]). The model’s designated cutoff value of 34.4% implies that patients with LSIL with a predicted probability of post-operative pathologic escalation exceeding 34.4%, as determined by this model, may benefit from further diagnostic and therapeutic intervention with cervical conization. The pre-operative TCT results serve as a crucial indicator in clinical practice for determining the need for subsequent cervical conization. Comparing the present model with TCT results, we observed that the area under the ROC curve of TCT was 0.652 (cutoff value of 53.2%, sensitivity: 38.1%, and specificity: 92.3%). This comparison indicated that the present model exhibited superior clinical differentiation compared with the standalone TCT test (TCT data were derived from the data of the present model).
- Receiver operating characteristic (ROC) curve of the model. The area under the ROC curve of the model: 0.744 (cutoff value of 34.4%, sensitivity: 66.2%, specificity: 71.4%); the resulting ROC curve yielded a 95% confidence interval of 0.674–0.839. (AUC: Area Under the Curve, CI: Confidence Interval.)
Considering the limited data points collected for this model, we opted not to split the dataset into traditional training and testing sets. We employed the bootstrap resampling method to assess the model’s discrimination further. Specifically, 1000 samples were randomly drawn from the dataset for internal validation. The resulting ROC curve yielded a 95% CI of 0.674–0.839. This approach was adopted to assess the internal validity of the model, which demonstrated its robust discrimination capability.
Traditional cross-validation was not utilized in this study, but bootstrap resampling was employed as a viable alternative for internal validation. The chosen methodology was aligned with the constraints imposed by the small dataset, and the obtained CI provided confidence in the internal validity of the model. This methodology aligned with the unique challenges presented by the limited data available. The obtained CI enhanced our confidence in the internal validity of the model and underscored its potential clinical applicability [Figure 4]. The calibration curve generated by this model closely approximated the ideal curve (where the X-axis denotes the predicted probability of pathological escalation after LSIL by the nomogram, and the Y-axis represents the actual probability of pathological escalation of LSIL post-surgery [Figure 5]). In addition, the Hosmer– Lemeshow deviation test was conducted to assess the model’s accuracy, yielding results of χ2 = 1.156, P = 0.998, signifying excellent calibration. Decomposing the DCA curves plotted by the model [Figure 6], a net benefits range of approximately 0–22% was evident when the Normoton model high-risk threshold probability value for patients was set between 10% and 70%. The net benefit of the model, at approximately 12% (corresponding to the cutoff value), indicated the proportion of LSIL beneficiaries requiring cervical conization, accounting for the predicted population. The model demonstrated a broad range of clinically valuable net benefits, with smaller values within this range signifying increased clinical significance (where the X-axis of the decision curve represents the threshold probability, and the Y-axis denotes the net benefit corresponding to each threshold probability).
- Receiver operating characteristic (ROC) curve versus thinprep cytology test (TCT). ROC curve in a clinical prediction model for postoperative pathologic escalation in low-grade squamous intraepithelial lesion. The black line represents the ROC curve of this model, the red line represents the ROC curve of TCT, and the area under the ROC curve of TCT was 0.652 (cutoff value of 53.2%, sensitivity: 38.1%, specificity: 92.3%). In the DCA curve, the black gray “ALL” line represents that all patients have undergone cervical conization surgery; the black “None” line represents that all patients have not been treated.
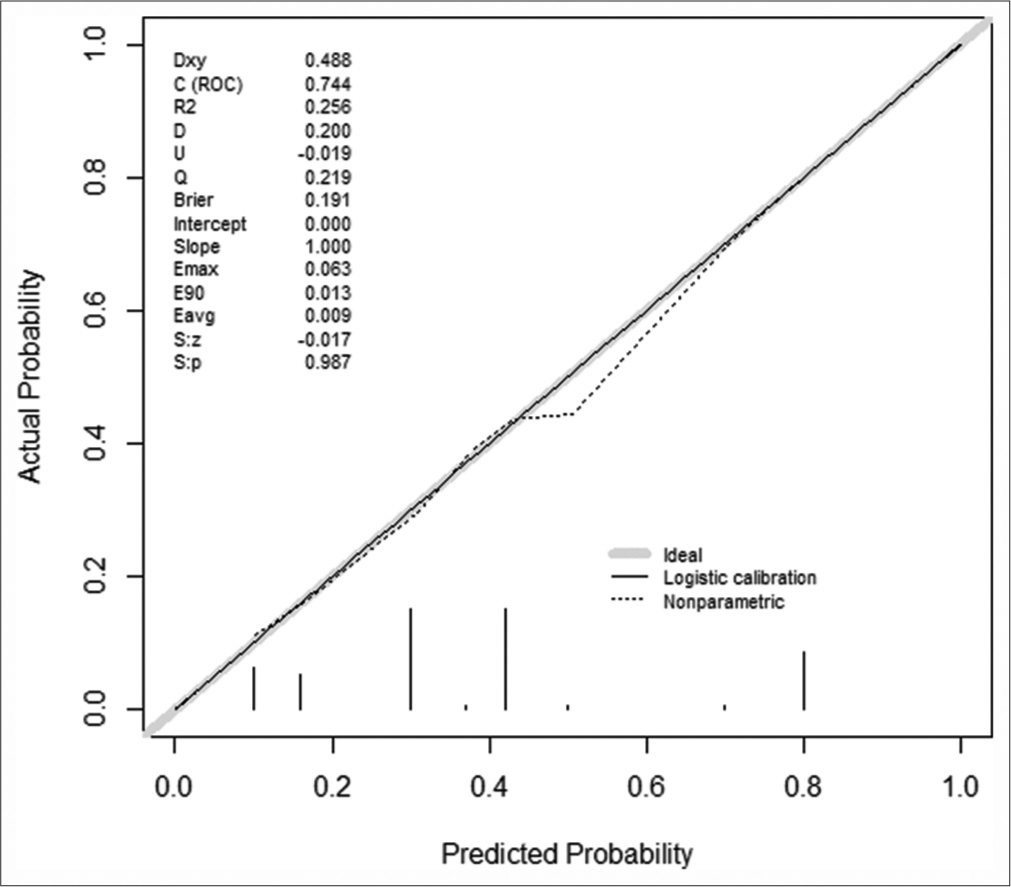
- Calibration curves for the clinical prediction model for post-operative pathology escalation in low-grade squamous intraepithelial lesion. (The solid line in the figure represents the ideal curve, whereas the dashed line represents the calibration curve of the model. “Dxy” is the rank correlation between the predicted probability and the observed value, equal to 2C-0.5/“C “is the C index, which is the area under the ROC curve, equal to 1+Dxy/2/“R2” is the complex correlation coefficient of the model/“D” is the distinguishing index/“U” is the unreliable index/“O” is the quality index/“Q” represents quality index/“Emax” is the maximum absolute difference between the predicted value and the actual value/“E90” is the 90th percentile of the difference between the predicted value and the true value/“Eavg” is the average difference between the predicted and actual values/“S: Z” and “S: r” are the Z and P values of Spiegelhalter Z-test, with a P value greater than 0.05, indicating no difference and a high degree of fit between the fitted line and the standard reference line).
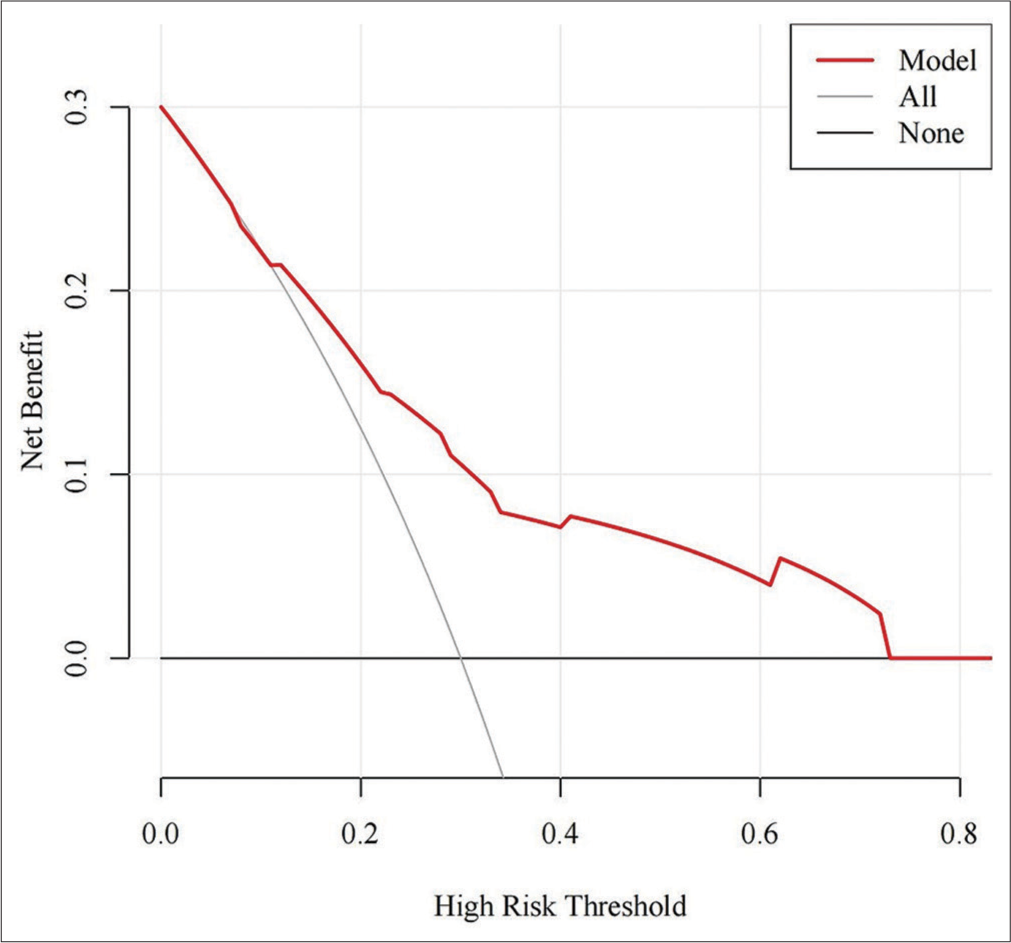
- Clinical decision curve for clinical predictive modeling of pathologic escalation after low-grade squamous intraepithelial lesion surgery. The net benefit value of the model is meaningful above the icons “All” and “None.” The decision curve analysis curve of the model indicates that when the probability value of the high-risk threshold is set between 10% and 70%, the net return range of about 0–22% is obvious.
DISCUSSION
With the increasing awareness of CC screening, the population of individuals with LSIL at the base of the pyramid is expanding. The demand for stratified management is becoming increasingly pressing. This study addressed this imperative by conducting a comprehensive analysis of 107 cases of patients with histological LSIL who underwent cervical conization (LEEP). We established a clinical predictive model to identify the factors associated with pathological progression after LEEP, providing a timely solution to this urgent issue. The model demonstrated robust performance with an ROC of 0.744, and its calibration curve exhibited excellent concordance. In addition, the DCA curve illustrates significant clinical implications. Certain guidelines in the USA and Europe advocate a principle of non-treatment for LSIL and emphasize clinical follow-up after the exclusion of occult HSIL due to the inherent variability in the natural course of LSIL, characterized by clearance and regression rates driven by their immune response.[12,13] However, LSIL exhibits significant heterogeneity, and existing literature highlights the inaccuracy and high false-negative rates associated with colposcopic biopsies.[14,15] In our study involving 107 patients with histological LSIL, we observed a notable post-operative pathological upgrade rate of 39.3%. This finding underscored the limitations of visible vaginal biopsy and emphasized the need for careful consideration of the pathological escalation rate following cervical biopsy under colposcopy. The elevated rate observed in our study may be attributed to the preliminary screening of patients with LSIL before cervical conization during clinical examination. In conclusion, this study comprehensively analyzed and predicted relevant risk factors in 107 cases of histological LSIL post-cervical conization, providing a valuable tool for accurately identifying hidden HSIL and facilitating stratified management. The remarkable predictive performance of the model further emphasizes its utility in addressing the challenges posed by LSIL heterogeneity and refining clinical management strategies. In previous clinical practice, the primary determinant for proceeding with diagnostic conization in patients with LSIL rested on pre-operative TCT results. However, emerging literature suggested that post-operative pathologic escalation in patients with histologic LSIL results from a multifaceted interplay of factors, including TCT outcomes, HPV status, age, and TZ.[16] Consistent with our study’s findings, HPV status, TCT results, and cervical TZ type were identified as independent risk factors for pathologic escalation. In the results of this study, age>45 years old became a protective factor (OR<1, P<0.05). However, according to relevant guidelines, follow-up is more preferred for young patients with LSIL.[3] Relevant literature pointed out that the incidence rate of CC ranks sixth, and the mortality rate ranks tenth among women. The average age of patients is 52.6 years old, with most cases occurring between 40 and 44 years old, as well as between 55 and 59 years old.[17] Contrary to the protective outcomes observed in this study, age was omitted from the model’s construction. This omission could be attributed to two main factors: first, the relatively small sample size in this model introduced a certain degree of deviation; second, the growing awareness of CC screening has led to higher compliance among younger patients (≤45 years old) with medical interventions compared with older women (>45 years old), resulting in a higher proportion seeking medical treatment. In addition, research has established that high-risk HPV infection is a pre-requisite for CC and its pre-cancerous lesions.[18,19] The presence of high-risk HPV (hrHPV) is also associated with HSIL, as CIN and cancer may occur due to hrHPV infection. HPV16 and HPV18 are the two main carcinogenic subtypes of hrHPV.[20] In addition, several other subtypes of hrHPV, including 52, 58, and 53, are associated with a high frequency of CC development.[9] In this study, the results indicating HPV16/18/52/53/58 high-risk type as an independent risk factor (OR = 4.95, P < 0.05) aligned with findings in the aforementioned literature. For TCT results, individuals with results of ≤ LSIL (NILM/inflammation/ASC-US/LSIL) and > LSIL (HSIL/ASC-H/AGC) have a risk of progressing to HSIL within five years at approximately 3.8% and 15%, respectively.[21] At the same time, TCT plays an important role in the early detection of cervical abnormalities and is also the foundation for clinical workers to manage patients further. The present study highlighted TCT > LSIL (HSIL/ASC-H/AGC) as an independent risk factor (OR = 13.12; P < 0.01), serving as an evaluation indicator for determining the need for further cervical conization surgery in patients with LSIL. Concurrently, TZ is closely associated with cervical lesions. Previous research has identified TZ 3 as a contributing factor to pathological upgrades following LSIL surgery.[14] Consistently, TZ 3 emerges as an independent risk factor in this study (OR = 6.10, P < 0.05). To ensure the accuracy of the optimal model and independent risk factors, this article employs the AIC minimum stepwise regression method, yielding the minimum AIC for this study: 128.2. Establishing a nomogram model for LSIL post-operative pathological progression, based on the identified high-risk factors, represents a significant advancement. Previously, LSIL diagnostic conization decisions relied on simplistic TCT results. Compared with TCT (AUC: 0.652), our model showed superior discrimination with an AUC of 0.744, affirming its enhanced predictive performance.
This evidence underscores our model’s potential in clinical applications and refines the classification of patients with LSIL for effective management. Despite the limited cases, not splitting the training and validation sets does not diminish the model’s robust performance, emphasizing its efficacy for clinical integration.
Strengths and limitations of this study
While acknowledging the significance of our study, it is important to address its limitations. The relatively small number of clinical cases collected and the absence of a distinct division between modeling and validation sets pose challenges to the robustness and generalizability of the clinical model developed in this study. Due to the limited dataset size, this study refrained from traditional model training and validation set division.
By recognizing these limitations, the model was first established based on retrospective data, and there may be some unknown or overlooked confounding factors. The sample size was relatively small, and the results may be biased when the model modeling and validation sets were not split for testing, such as increased sampling error and poor accuracy. We found that the practicality and real-world performance of the clinical model must be evaluated. Given the constraints of the dataset, there is a possibility of deviations in the experimental results. To enhance the reliability and generalizability of our findings, we should conduct further external validation, preferably with larger datasets or big data.
Clinical significance
This study aimed to establish a nomogram model for simple risk assessment and risk stratification of histologic patients with LSIL to clinically develop effective treatment and reduce the rate of underdiagnosis and the harm of overtreatment to a certain extent, thereby enabling early detection, treatment, and prevention of cervical diseases. Meanwhile, the nomogram can present the degree of influence of the influencing factors in the form of a graph, which is clinically operable.[22]
Promotion of clinical applications
The nomogram model established in this study provides a new approach and method for the clinical decision-making of patients with LSIL. To promote the application of this model in clinical practice, we must strengthen the training of doctors and improve their understanding and ability to use the model. Second, model-based decision support systems or mobile applications can be developed to help doctors quickly and accurately obtain prediction results in clinical practice. Additional clinical research and practical validation are needed to confirm the effectiveness and reliability of the model and gain wide clinical recognition and application.
Clinical impact
First, regarding the impact on patients, the column chart prediction model established in this study is expected to impact patients with LSIL positively. By predicting the model’s results, doctors can accurately assess the patient’s condition and prognosis, formulating personalized and accurate treatment plans. This helps reduce the occurrence of overtreatment or missed diagnosis and improves the treatment effectiveness and quality of life of patients. Furthermore, promoting and applying the model can help improve patients’ awareness and understanding of the disease and enhance their awareness and ability to self-care. Second, the promotion and application of the model are expected to improve the quality and efficiency of medical services, enhance patient satisfaction and trust, and influence society and economy. This work significantly enhances doctor-patient relationships and promotes social harmony and stability. In addition, by developing personalized and precise treatment plans for patients, their quality of life and health status can be improved, promoting the healthy development of society.
SUMMARY
High-risk factors influencing pathological progression after cervical conization in patients with histological LSIL are associated with HPV16/18/52/53/58 high-risk type infections, TCT > LSIL (HSIL/ASC-H/AGC), and TZ 3. This model holds clinical applicability for assigning values to the pertinent data of histological LSIL patients and facilitating a clear distinction between follow-up and those requiring further surgical intervention.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
ABBREVIATIONS
LSILs - Low-grade squamous intraepithelial lesions
DCA - Decision curve analysis
HPV - Human papillomavirus
TCT - ThinPrep Cytology test
HSIL - High-grade squamous intraepithelial lesion
ASC-H - Atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion
AGC - Atypical glandular cells
TZ - Transformation zone
AUC - Area under the curve
CC - Cervical cancer
CIN - Cervical intraepithelial neoplasia
ASCCP - American Society for Colposcopy and Cervical Pathology
ASC-US - Atypical squamous cells of undetermined significance
LEEP - Loop electrosurgical excision procedure
CKC - Cold knife conization
NILM - No intraepithelial lesion cells and malignant cells
AIC - Akaike information criterion
AUTHOR CONTRIBUTIONS
XP: Performed trial, data curation, and analysis; wrote the original draft; designed and performed trial; supervised the study; and edited manuscript draft. LW: Performed trial and supervised the study. YW and JW: Performed trial and data collection. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research/study was approved by the Institutional Review Board at The First Affiliated Hospital of Bengbu Medical College, number Approval no.: 2023YJS166, dated 2023. This research was conducted in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Real-world safety profile of the 9-valent human papillomavirus vaccine: A study in Zhejiang, China from 2019 to 2021. Hum Vaccin Immunother. 2022;18:2152256.
- [CrossRef] [Google Scholar]
- Quality assessment of cervical cytology practices in Estonia from 2007 to 2018. Cancer Control. 2022;29:10732748221141794.
- [CrossRef] [Google Scholar]
- Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: Systematic review and meta-analysis. BMJ. 2018;360:k499.
- [CrossRef] [Google Scholar]
- Clinical impact of age-specific distribution of combination patterns of cytology and high-risk HPV status on cervical intraepithelial neoplasia grade 2 or more. Oncol Lett. 2023;26:384.
- [CrossRef] [Google Scholar]
- The risk factors for cervical cytological abnormalities among women infected with non-16/18 high-risk human papillomavirus: Cross-sectional study. JMIR Public Health Surveill. 2022;8:e38628.
- [CrossRef] [Google Scholar]
- The role of PAX1 methylation in predicting the pathological upgrade of cervical intraepithelial neoplasia before cold knife conization. Front Oncol. 2022;12:1064722.
- [CrossRef] [Google Scholar]
- Value of TCT combined with HPV and CA125 in early cervical cancer screening in a medical examination population. Cell Mol Biol (Noisy-le-grand). 2022;68:160-4.
- [CrossRef] [Google Scholar]
- Improving colposcopic accuracy for cervical precancer detection: A retrospective multicenter study in China. BMC Cancer. 2022;22:388.
- [CrossRef] [Google Scholar]
- Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol Oncol. 2015;136:178-82.
- [CrossRef] [Google Scholar]
- 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-31.
- [CrossRef] [Google Scholar]
- 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-46.
- [CrossRef] [Google Scholar]
- Clinical outcomes after conservative management of cervical intraepithelial neoplasia grade 2 (CIN2) in women ages 21-39 years. Cancer Prev Res (Phila). 2018;11:165-70.
- [CrossRef] [Google Scholar]
- 2023 Canadian colposcopy guideline: A risk-based approach to management and surveillance of cervical dysplasia. Curr Oncol. 2023;30:5738-68.
- [CrossRef] [Google Scholar]
- Endocervicoscopy and biopsy to detect cervical intraepithelial squamous neoplasia in nonvisible squamocolumnar junction with unsatisfactory colposcopy: A pilot study. Technol Cancer Res Treat. 2018;17:1533034617753811.
- [CrossRef] [Google Scholar]
- A retrospective analysis on 1901 women with high grade cervical intraepithelial neoplasia by colposcopic biopsy. Eur J Obstet Gynecol Reprod Biol. 2017;217:53-8.
- [CrossRef] [Google Scholar]
- Discrepancy between colposcopy, punch biopsy and final histology of cone specimen: A prospective study. Arch Gynecol Obstet. 2018;297:1271-5.
- [CrossRef] [Google Scholar]
- Cervical cytology-histology correlation based on the American Society of Cytopathology guideline (2017) at the Russian National Medical Research Center for obstetrics, gynecology, and perinatology. Diagnostics (Basel). 2022;12:210.
- [CrossRef] [Google Scholar]
- Follow up in women with biopsy diagnosis of cervical low-grade squamous intraepithelial lesion (LSIL): How long should it be. Arch Gynecol Obstet. 2017;295:997-1003.
- [CrossRef] [Google Scholar]
- Comparison of primary cytology, primary HPV testing and co-testing as cervical cancer screening for Chinese women: A population-based screening cohort. BMJ Open. 2022;12:e063622.
- [CrossRef] [Google Scholar]
- Chapter 1: Human papillomavirus and cervical cancer--burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;31:3-13.
- [CrossRef] [Google Scholar]
- Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: A prospective cohort study. Int J Cancer. 2011;128:2898-910.
- [CrossRef] [Google Scholar]
- Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ. 2015;350:g7594.
- [CrossRef] [Google Scholar]








