Translate this page into:
Diagnostic utility of cell block preparations from liquid-based cytology in cervical lesions: A comparative retrospective analysis

*Corresponding author: Ceren Canbey, Department of Medicine Pathology, Bagcilar Education and Research Hospital, University of Health Sciences Istanbul, Türkiye. drcerencanbey@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Canbey C, Şen S, Özcan TB. Diagnostic utility of cell block preparations from liquid-based cytology in cervical lesions: A comparative retrospective analysis. CytoJournal. 2025;22:48. doi: 10.25259/Cytojournal_3_2025
Abstract
Objective
Cervical cancer ranks as the fourth most prevalent cancer among women globally; it originates in the cervix and has a significant association with human papillomavirus (HPV) infection. The purpose of this study was to investigate the diagnostic utility of cell block (CB) preparations from liquid-based cytology samples in identifying cervical lesions among Turkish patients with HPV. This approach was intended to supplement conventional Pap smear tests and HPV testing.
Material and Methods
A retrospective analysis was conducted on 60 HPV-positive cervical smear samples processed through the ThinPrep Pap test. CBs were prepared from liquid-based residues, stained with hematoxylin and eosin, and analyzed. Cytological diagnoses were compared with histopathological findings from colposcopy-guided biopsies. The relationships between the Pap smear, CB, and biopsy results were statistically analyzed.
Results
Pap smear cytology identified 1.6%, 16.6%, 43.3%, and 3.3% as high-grade squamous intraepithelial lesion (HSIL), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells of undetermined significance, and atypical squamous cells - HSIL cannot be excluded + LSIL, respectively. The CB evaluations classified 6.6% of the samples as cervical intraepithelial neoplasia (CIN)1, 1.6% as CIN2, and 1.6% as squamous cell carcinoma (SCC), with 78.3% deemed negative. Histopathological biopsy revealed CIN1 in 11.7%, CIN2 in 1.7%, and CIN3 in 8.3% of the patients. High concordance was observed between the Pap smear and CB diagnoses for negative and low-grade lesions, although discrepancies occurred in higher-grade lesions. HPV testing revealed 65% high-risk positivity, predominantly for HPV16 and HPV18. Significant correlations were found among HPV subtype positivity, CB, and biopsy diagnosis (P < 0.05).
Conclusion
CB preparations provide enhanced diagnostic accuracy for high-grade lesions and SCC, thus complementing Pap smear cytology and HPV testing. This approach supports their integration into the routine cervical cancer screening protocols in Türkiye. Further global, multicenter studies are recommended to validate these findings.
Keywords
Cervical cancer
Human papillomavirus
Pap smear
Screening
INTRODUCTION
According to the Surveillance, Epidemiology, and End Results (SEER) 2023 database, cervical cancer is most commonly diagnosed in women aged 35-44 years, with an average age at diagnosis of 50 years.[1] Early detection significantly improves survival, with a 5-year survival rate of 91.1%. The fact that 42% of cases are diagnosed at an early stage highlights the importance of timely screening and intervention. The primary cause of cervical cancer is the transformation of cells into precancerous and invasive lesions by high-risk human papillomavirus (HPV) genotypes.[2] The overexpression of viral oncoproteins disrupts cellular functions, affecting cell proliferation, cell cycle regulation, and apoptosis.[3] In a global study involving 10,575 cervical cancer cases from 38 countries, the most common HPV types were identified as 16, 18, 31, 33, 35, 45, 52, and 58, with HPV 16 and 18 accounting for 71% of all cases.[4] Understanding these processes is crucial for unraveling the disease mechanism and developing targeted therapies. The cervix, located in the lower part of the uterus, connects to the vagina through the endocervical canal. The “squamocolumnar junction,” where stratified squamous and columnar epithelia meet, is a region frequently associated with premalignant transformation and is often linked to high-risk HPV types 16 and 18.[5] Premalignant changes in the cervical epithelium are referred to as “cervical intraepithelial neoplasia” (CIN) and, if left untreated, can progress to carcinoma in situ or invasive cancer. It is believed that certain viral proteins of HPV induce dysplastic changes in infected cells, leading to the progression of pre-cancerous lesions to cancerous lesions.[6] CIN is classified based on the thickness of the affected epithelium: CIN1 (mild dysplasia), CIN2 (moderate dysplasia), and CIN3 (severe dysplasia). CIN2 and CIN3 are collectively known as high-grade CIN. While the normal epithelium is orderly, HPV infection can lead to dysplasia.[1,7,8]
The cervical Pap test is an effective screening tool for detecting cervical precancerous lesions and is best reported through The Bethesda System (TBS). This system classifies squamous intraepithelial lesions (SILs) from the low-grade squamous intraepithelial lesions (LSIL) to high-grade squamous intraepithelial lesions (HSIL) and invasive carcinomas. Ambiguous findings are categorized as “Atypical Squamous Cells” (ASC), divided into atypical squamous cells of undetermined significance (ASCUS) (possible LSIL) and atypical squamous cells - HSIL cannot be excluded (ASCH) (cannot exclude HSIL).[9] The distribution of TBS categories in PAP smear tests and the high-risk HPV (HR-HPV) positivity rates are as follows: The negative for intraepithelial lesion malignancy category is the most frequent, comprising approximately 94% of all samples, with an HR-HPV positivity rate of 4%. ASCUS accounts for 3.6% of cases, with a 54% HR-HPV positivity rate. The frequency of LSIL was 1.7%, and the HR-HPV positivity rate was 87%. The ASC-H and HSIL categories each had a frequency of 0.3%, with HR-HPV positivity rates of 82% and 95%, respectively.[10]
HPV testing detects HR-HPV subtypes associated with cervical cancer, typically covering the 13 most common types.[5] HPV genotyping usually includes HPV 16, 18, and, in some tests, HPV 45.[11] The clinical performance and sensitivity of HPV tests may vary between laboratories, but all tests are generally effective in detecting HPV. The development of new tests and extended approvals for existing tests may necessitate updates to cervical screening guidelines.[9] At present, the smear test is used as the standard method for screening for cervical dysplasia and cancer.[12] Smear evaluation can be performed through liquid-based or conventional methods.[13] For HPV testing, samples are collected from the endocervix through a spatula, brush, or swab and placed into a transport medium. Some liquid-based cytology systems allow the same sample to be used for both HPV testing and cytological analysis.[13,14] In our center, liquid-based thin-layer technology is preferred for screening cervical lesions.[15] This method reduces the rate of inadequate samples, enables higher-quality evaluations, and allows for the molecular examination of the presence, absence, or types of HPV from the same liquid.[16] The analyzed materials are stored for a certain period and then disposed of. In our study, we aimed to obtain tissue-like materials by preparing cell block (CB) from liquid-based smear samples that were stored for routine evaluation and subsequently disposed of in our laboratory. This approach seeks to provide additional diagnostic contributions beyond the current smear diagnosis.
MATERIAL AND METHODS
This study was approved by the local ethics committee. Informed consent was obtained from all participants in accordance with the principles of the 75th World Medical Association Declaration of Helsinki (2024),[17] which states that participation by individuals capable of providing informed consent in medical research must be voluntary. While consultation with family members or community leaders may be appropriate, no individual capable of providing informed consent was enrolled in the study unless they freely agreed. The privacy and confidentiality of patient data were strictly maintained.
This retrospective study included smear cases with HPV positivity identified in 60 routine screenings that were routinely evaluated through an HPV assay kit (Aptima®, Hologic, Inc., UK). The selection of patients in this study was based on the analysis of HPV-associated cytopathological evaluations. The inclusion criteria required patients to have an HPV-positive smear diagnosis and cytological assessments. The exclusion criteria included patients whose previously evaluated liquid-based smear results were negative for HPV, patients assessed by specialists, and individuals under the age of 18. These criteria were established to ensure the reliability and accuracy of the study’s findings. All methods were carried out by a team of two expert pathologists, ensuring the reliability and accuracy of the results. All evaluations and citations were carried out in accordance with the World Health Organization 2021 guidelines.[18]
A ThinPrep 2000 (LOT: 70097-083) (Hologic, Inc., UK) was used for gynecological cytology examination. Pap smear samples were collected by the gynecology department through spatulas, brushes, or brooms supplied with PreservCyt. The CBs were prepared from the remaining cell sediment of previously diagnosed Pap smear bottles. A total of 60 PreservCyt bottles (LOT: 70097-083) (Hologic, Inc. UK) were selected for CB preparation.
The liquid cell suspensions were centrifuged at 400 × g for 5 min in 50 mL tubes, after which the supernatant was carefully removed. Samples with sediment volumes under 1 mL were excluded from further processing. The remaining sediment was washed by resuspending it in 5 mL of normal saline, followed by an additional round of centrifugation. To resuspend the cell pellet, small amounts of human plasma (obtained as unused plasma from a blood bank) were added dropwise. For sediment volumes under 3 mL, 2-3 drops of plasma were used, while 4-10 drops were added for sediment exceeding 4 mL. The cells were thoroughly mixed into the plasma suspension. Next, thrombin (Jones Pharma, Inc., St. Louis, MO) was added dropwise, and the mixture was gently rotated in the tube until clotting occurred, typically within 5 min. Once clotted, formalin was added along the inner wall of the tube until it submerged the clot and formed a floating layer above it. The sample was allowed to fix for 10 min before being transferred to a labeled tissue cassette for histological processing. If clotting did not occur, the washing, plasma addition, and thrombin steps were repeated up to two additional times.
A total of 60 block sections were prepared and stained with hematoxylin and eosin (H&E) (Cat #14-5983-82) (Thermo Fisher, USA) to examine each block thoroughly. Ten fields were analyzed microscopically at appropriate magnification scales with a U-DO3 microscope (Model: CX43RF, serial number: 9F47009) (Olympus Corporation, Japan). The scale bars are automatically generated and measured by the imaging software of the Olympus Soft Imaging Solutions GMBH system and the Olympus EP50 model (Olympus Corporation, Japan), ensuring accuracy in each magnification setting. The degree of cellular adequacy was categorized as >50%, 25-50%, or <25%.[19] The results were recorded. The Pap smear results were then reviewed, and where available, ThinPrep slides were examined for each case. All patients underwent further tissue analysis to assess the predictive value of the CB compared with that of the Pap smear alone and the predictive value of combining the two methods. In each CB, the presence of inflammation and endocervical cells was also observed and recorded. These findings were compared to the original ThinPrep slide or case to determine if they were consistent or had discrepancies.
Statistical analyses were performed through the Statistical Package for the Social Sciences (SPSS) statistical software version 30.0 (SPSS Inc., USA). The Pearson Chi-square test was used to compare categorical variables, and Fisher’s exact test was applied when expected frequencies were less than 5 in any cell of a 2 × 2 table. For contingency tables larger than 2 × 2 with low expected counts, the Monte Carlo simulation method was employed to obtain accurate P-values. A P < 0.05 was considered to indicate statistical significance. When 1 ≤ T < 5 and N ≥ 40, the continuity-corrected Chi-square (Yates’ correction) test was applied. When T < 1, N < 40, or zero samples were present, Fisher’s exact test was conducted. All figures presented in the study, were created using Microsoft Excel (Microsoft Excel for Microsoft 2016, Microsoft Corporation, Redmond, WA, USA).
RESULTS
For the cervical smear samples, CBs were prepared from the liquid component of the smears through a minimally invasive approach and stained with H&E, and the results were documented. Among the 60 Pap smear samples analyzed, 1.6% (1/60) were classified as HSIL, 16.6% (10/60) as LSIL, 43.3% (26/60) as ASCUS, and 3.3% (2/60) as LSIL+ASCH. Cytological evaluation of the CB samples derived from the smear samples revealed CIN1 in 4 cases, CIN2 in 1 case, and SCC in 1 case, and 47 cases were categorized as negative [Figures 1a-f]. In addition, 5 cases were deemed insufficient for evaluation, and 2 cases were classified as insufficient-negative. Histopathological evaluation of tissue samples obtained through colposcopy revealed that 66.6% (40/60) of the samples were negative. Conversely, CIN1 was identified in 11.6% (7/60) of the samples, CIN2 in 1 sample, and CIN3 in 8.3% (5/60) of the samples. Furthermore, chronic cervicitis findings were reported in 11.6% (7/60) of the cases. Among all LSIL samples, 80% (8/10) were identified as negative, and 20% (2/10) were identified as CIN1 through CB analysis.
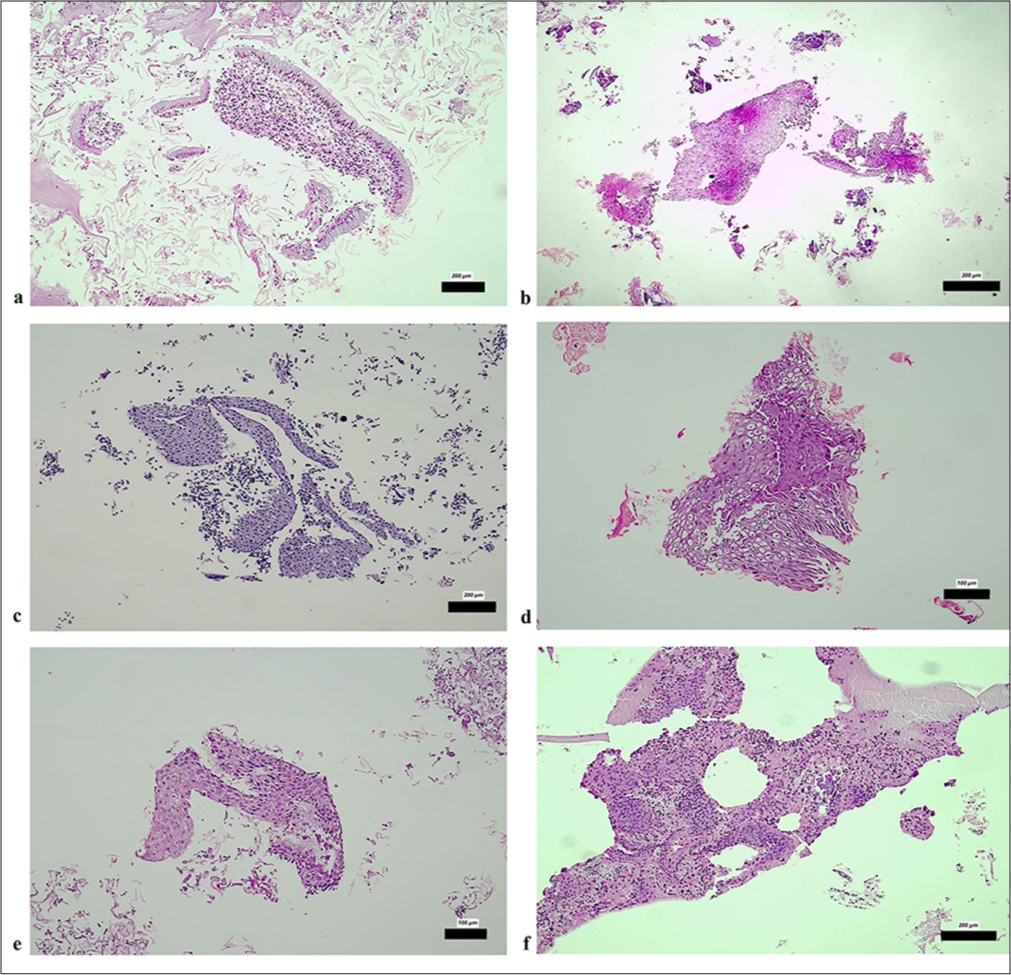
- (a) Epithelial fragments from normal endocervical glands (H&E ×200); (b) Fragment of normal squamous epithelium (H&E ×200); (c) Atrophic squamous epithelium (H&E ×200); (d) Reactive squamous epithelium fragment containing pseudokoilocytes (H&E ×100); (e) Tissue fragment of squamous epithelium from the transformation zone (H&E ×100); (f) Tissue fragment of squamous epithelium from the transformation zone (H&E ×200). The scale bars indicate the actual size at different magnifications (200 µm and 100 µm), as marked in each subfigure. H&E: Hematoxylin and eosin.
Only one HSIL sample was observed in the pathological tissue analysis and was identified as CIN3 [Figures 2a and b]. In addition, two cases exhibited a mix of LSIL and ASCH types in the Pap smear analysis. Histopathological examination of the CB sections revealed the presence of tadpole-shaped cells with large, hyperchromatic nuclei, which are characteristic of squamous cell carcinomas (SCCs). These atypical cells occurred in different cases at different densities and presented morphologic features suggestive of malignant transformation. As shown in Figures 2a and b, at 20× magnification, the tadpole cells presented irregular nuclear contours, an increased nuclear-cytoplasmic ratio, and hyperchromasia, suggesting high-grade dysplasia and invasive potential. Chromatin appeared coarse-grained, and occasional mitotic figures were noted, further supporting the diagnosis of SCC. The cytoplasmic borders were well-defined, and some cells showed keratinization, a feature of squamous differentiation. Figure 2c, taken at 2 20× magnification, provides a more comprehensive view of the cellular architecture and shows the scattered distribution of malignant cells within the sample. The tumor cells appeared to be embedded in a fibrillar extracellular matrix, suggesting stromal involvement. The presence of necrotic debris in certain areas is further evidence of tumor progression and tissue destruction.
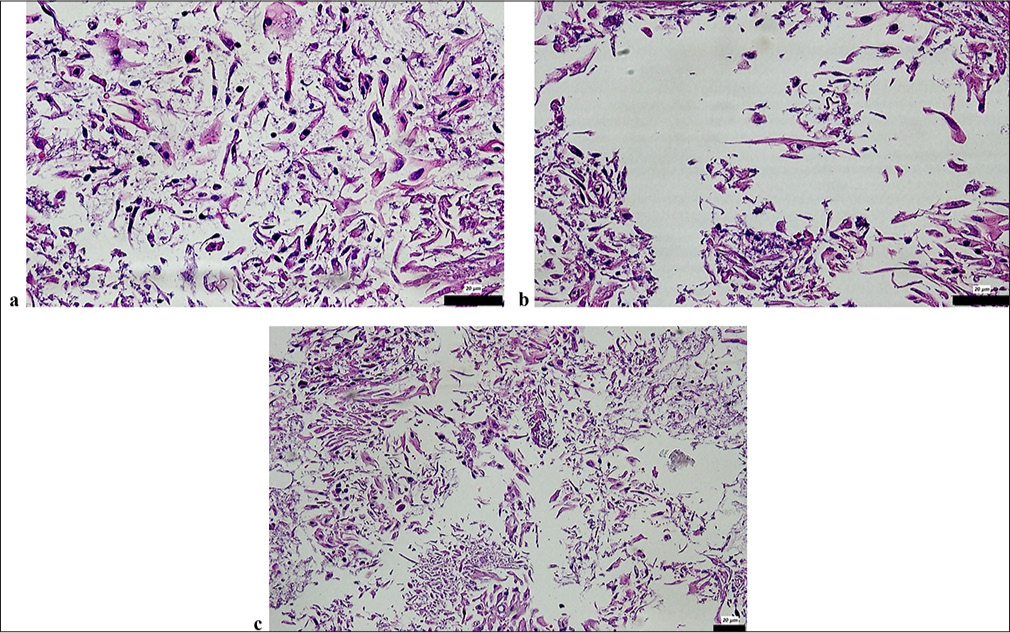
- CB sections revealed: (a and b) Tadpole cells with large, hyperchromatic nuclei characteristic of SCC in CB sections of a case diagnosed as HSIL in the smear (H&E ×20); (c) Tadpole cells with large, hyperchromatic nuclei characteristic of SCC in CB sections of a case diagnosed as HSIL in the smear (H&E ×20). The scale bars indicate the actual size at 20 µm magnifications, as marked in each subfigure. CB: Cell block, SCC: Squamous cell carcinoma, H&E: Hematoxylin and eosin, HSIL: High-grade squamous intraepithelial lesion, LSIL: Low-grade squamous intraepithelial lesion.
CB evaluation through H&E staining revealed CIN1 in one sample and CIN2 in another, whereas colposcopy-derived tissue samples presented a normal cellular composition. Among 18 patients, smear samples were categorized as negative, whereas three could not be evaluated due to insufficient sample quality. The analysis revealed distinct features: fragments of squamous epithelium with LSIL showing koilocytosis and nuclear hyperchromasia [Figures 3a and b] and areas of immature squamous metaplasia from the transformation zone [Figure 3c].
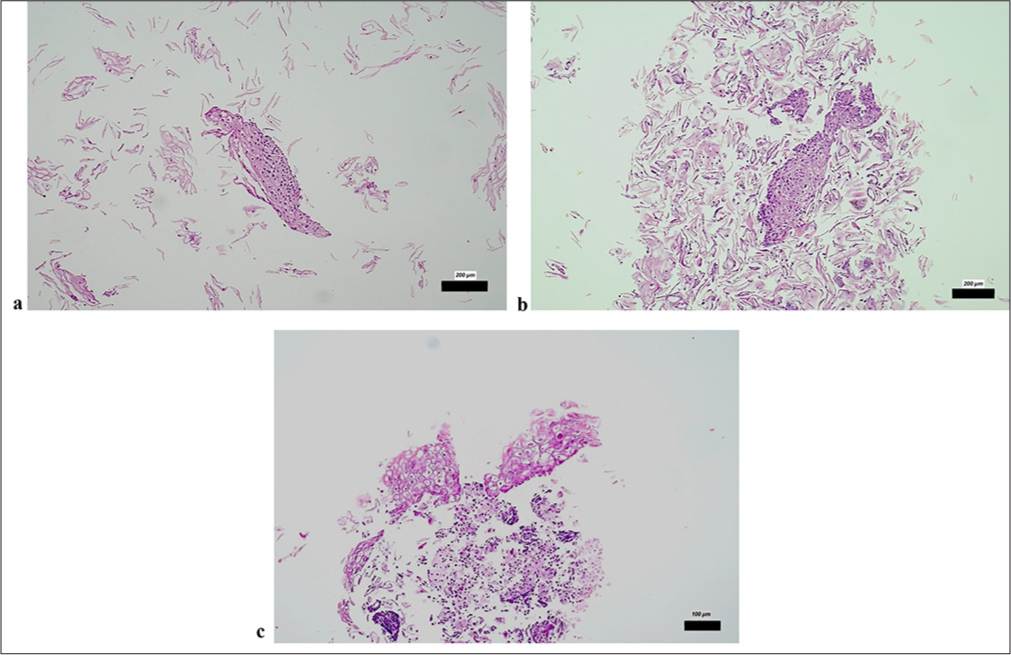
- CB sections revealed (a and b) tissue fragments of squamous epithelium with HSIL/high-grade dysplasia (H&E ×200); (c) Presence of koilocytes and nuclear hyperchromasia in dissociated squamous epithelium fragments with LSIL (H&E ×100). The scale bars indicate the actual size at different magnifications (200 µm and 100 µm), as marked in each subfigure. CB: Cell block, H&E: Hematoxylin and eosin, HSIL: High-grade squamous intraepithelial lesion, LSIL: Low-grade squamous intraepithelial lesion.
Figure 4 shows the distribution of smear and CB diagnoses according to the biopsy category. While significant differences are observed between smear and CB diagnoses in the negative and CIN1 categories, a more balanced distribution is observed in the CIN2, CIN3, and chronic cervicitis groups.
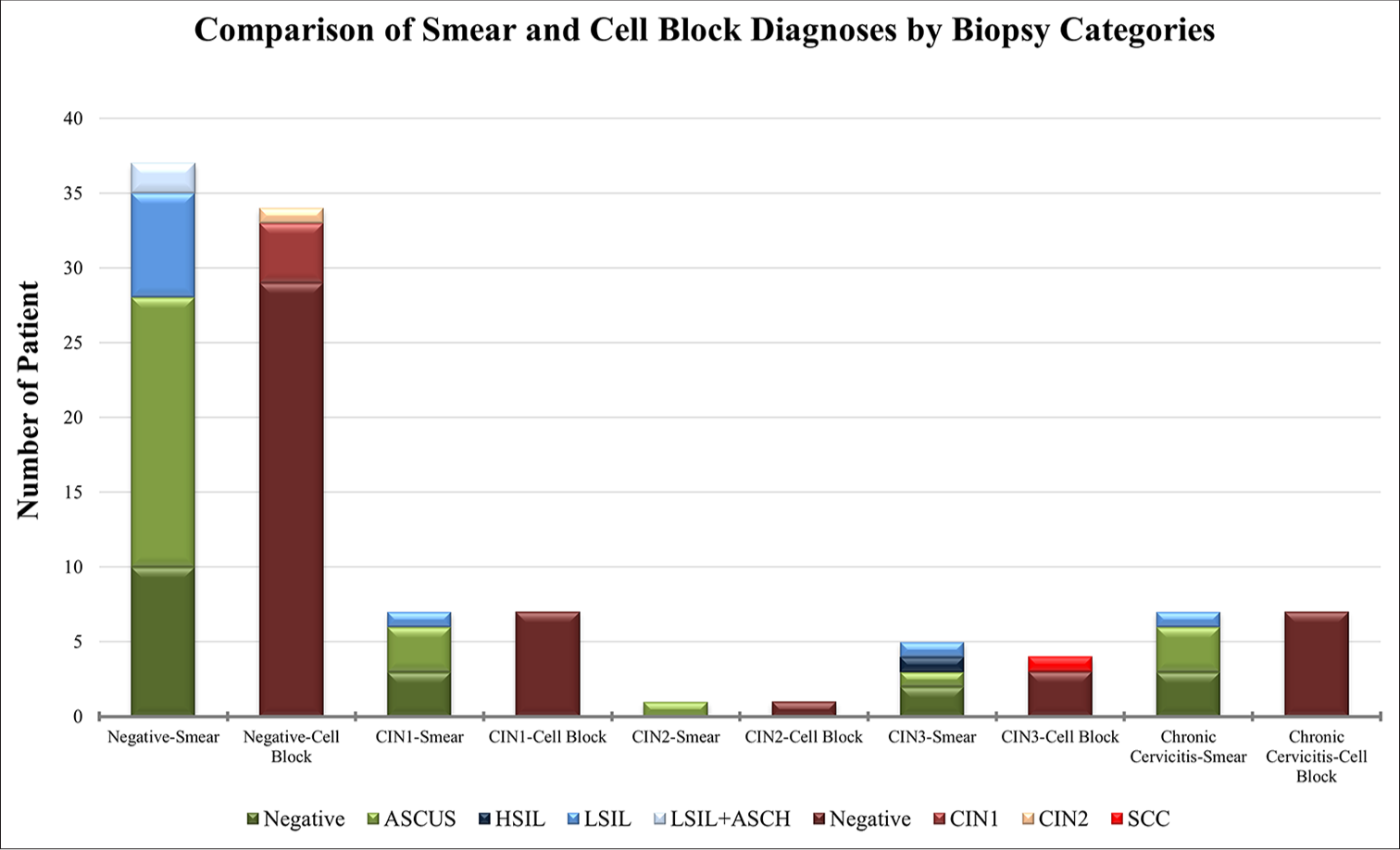
- Comparison of smear and CB diagnoses by biopsy category: Comparison of smear and CB diagnoses according to biopsy category. The chart illustrates the distribution of different diagnoses, including negative, ASCUS, HSIL, LSIL, LSIL+ASCH, CIN1, CIN2, and SCC, across biopsy categories through smear and CB methods. CB: Cell block, ASCUS: Atypical squamous cells of undetermined significance, HSIL: High-grade squamous intraepithelial lesion, LSIL: Low-grade squamous intraepithelial lesion, ASCH: Atypical squamous cells, HSIL cannot be excluded, CIN1: Cervical intraepithelial neoplasia 1, CIN2: Cervical intraepithelial neoplasia 2, SCC: Squamous cell carcinoma.
The Pearson Chi-square test indicated a significant association between cytological evaluation of Pap smears and CBs and pathological diagnoses. However, owing to the small sample sizes in some subgroups, the Monte Carlo simulation method was used. Table 1 provides a comparison of the cytological evaluation of Pap smears and CBs with pathological diagnoses, indicating that there were no statistically significant differences among the groups (P > 0.05).
| Parameters | Biopsy diagnosis | P-value | ||||
|---|---|---|---|---|---|---|
| Negative | CIN1 | CIN2 | CIN3 | Chronic cervicitis | ||
| Smear diagnosis | ||||||
| Insufficient | 3 | 0 | 0 | 0 | 0 | 0.476 |
| Negative | 10 | 3 | 0 | 2 | 3 | |
| ASCUS | 18 | 3 | 1 | 1 | 3 | |
| HSIL | 0 | 0 | 0 | 1 | 0 | |
| LSIL | 7 | 1 | 0 | 1 | 1 | |
| LSIL+ASCH | 2 | 0 | 0 | 0 | 0 | |
| CB diagnosis | ||||||
| Insufficient | 6 | 0 | 0 | 1 | 0 | 0.251 |
| Negative | 29 | 7 | 1 | 3 | 7 | |
| CIN1 | 4 | 0 | 0 | 0 | 0 | |
| CIN2 | 1 | 0 | 0 | 0 | 0 | |
| SCC | 0 | 0 | 0 | 1 | 0 | |
P < 0.05 is statistically significant. CB: Cell block, ASCUS: Atypical squamous cells of undetermined significance, HSIL: High-grade squamous intraepithelial lesion, LSIL: Low-grade squamous intraepithelial lesion, ASCUS: Atypical squamous cells of undetermined significance, ASCH: Atypical squamous cells, HSIL cannot be excluded, CIN1: Cervical intraepithelial neoplasia 1, CIN2: Cervical intraepithelial neoplasia 2, SCC: Squamous cell carcinoma
In Figure 5, the distribution of HR+ HPV and its subgroups across different cytological diagnoses is visualized. Among the HPV types, 65% (39/60) were HPV HR+, including eight patients with HPV 16+, five patients with HPV 18+, and two patients with HPV HR+/HPV 16+/HPV 18+ coinfections. When the HPV status of patients diagnosed with ASCUS through Pap smear testing was examined, 53.8% (14/26) were HPV HR+, 30.8% (8/26) were HPV 16+, 7.7% (2/26) were HPV 18+, and 7.7% (2/26) were coinfected with HPV 16/HPV 18. All 10 patients diagnosed with LSILs were HPVHR positive. Among patients with mixed LSIL and ASCH types, one had HR+ HPV, and the other had HR+ and 16+ HPV coinfection. A single HSIL patient was positive for HPV HR+/HPV 16+/HPV 18+. CIN1 was observed only in HR-positive HPV samples, specifically in 1 out of 3 samples. CIN 2 was detected exclusively in a single patient with a combination of HPV HR and HPV 16+. SCC was identified only in samples with a combination of HR+, HPV 16+, and HPV 18+ HPV.
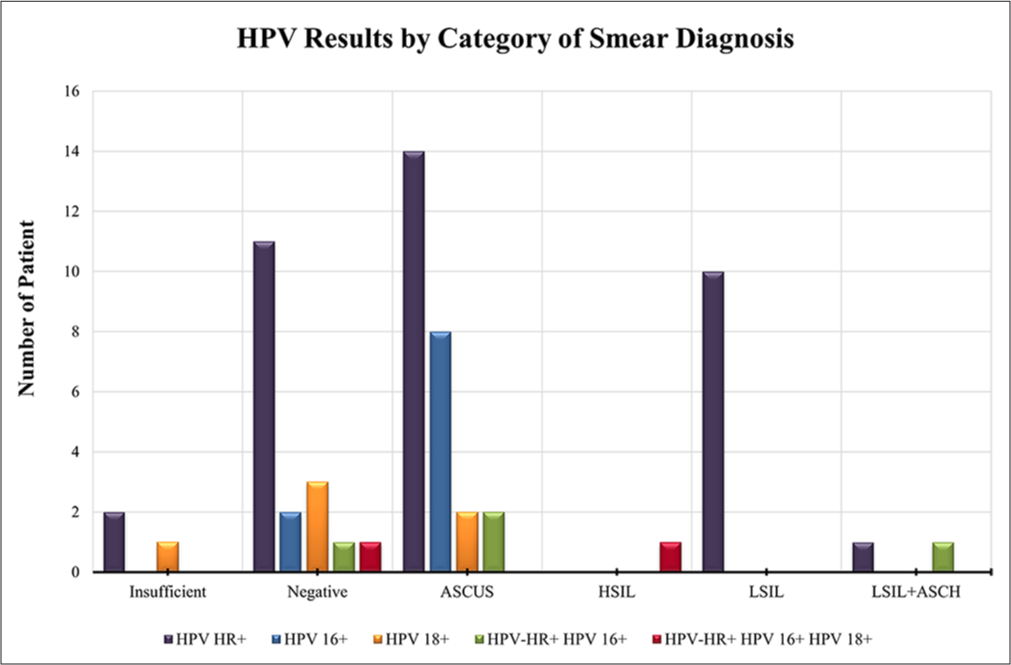
- Distribution of HPV-positive captions: A bar chart showing the distributions of HPV HR+, HPV 16+, HPV 18+, and their combinations across different cytological categories. ASCUS: Atypical squamous cells of undetermined significance, HSIL: High-grade squamous intraepithelial lesion, LSIL: Low-grade squamous intraepithelial lesion, ASCH: Atypical squamous cells, and HSIL cannot be excluded, HR: High-risk, HPV: Human papillomavirus.
Relationships between the Pap smear, CB, and colposcopy biopsy results regarding HPV positivity were analyzed through the Monte Carlo simulation method, and the p values and descriptive statistics are presented in Table 2. A significant association was found between smear diagnosis and HPV status through the Monte Carlo-adjusted Chi-square test (P = 0.005). The highest prevalence of HR+ HPV was observed in the ASCUS group (n = 14), followed by the LSIL (n = 10) and negative cases (n = 11). The presence of HPV 16+ and HPV 18+ was relatively low across smear categories, with only 8 cases of HPV 16+ in ASCUS and 1 case of HPV 18+ in the insufficient category. A statistically significant relationship was observed between CB diagnosis and HPV status (P = 0.012, Monte Carlo). The majority of HPV HR+ cases were in the negative category (n = 29), followed by insufficient samples (n = 6) and CIN1 cases (n = 3). The HPV 16+ and 18+ subtypes were rarely detected, with only a few cases across different diagnostic groups. No statistically significant association was observed between biopsy diagnosis and HPV status at the 0.05 level (P = 0.053, Monte Carlo). However, HR+ HPV was more frequently detected in negative biopsy samples (n = 25) than in the other biopsy samples. Among the high-grade lesions, CIN3 had the highest frequency of multiple HPV infections, with 2 cases positive for both HPV 16 and HPV 18. These findings suggest a statistically significant correlation between smear and CB diagnoses and HPV status, whereas biopsy results revealed a borderline association. The application of Monte Carlo simulations provided more robust statistical estimates due to small sample sizes in some subgroups.
| Parameters | HPV types | P-value | ||||
|---|---|---|---|---|---|---|
| HPV HR+ | HPV 16+ | HPV 18+ | HPV-HR+HPV 16+ | HPV-HR+HPV 16+HPV 18+ | ||
| Smear diagnosis | ||||||
| Negative | 13 | 2 | 4 | 1 | 1 | 0.004✶ |
| ASCUS | 14 | 8 | 2 | 2 | 0 | |
| HSIL | 10 | 0 | 0 | 0 | 0 | |
| LSIL | 0 | 0 | 0 | 0 | 1 | |
| LSIL+ASCH | 1 | 0 | 0 | 1 | 0 | |
| CB diagnosis | ||||||
| Negative | 35 | 9 | 6 | 3 | 1 | 0.008✶ |
| CIN1 | 3 | 1 | 0 | 0 | 0 | |
| CIN2 | 0 | 0 | 0 | 1 | 0 | |
| SCC | 0 | 0 | 0 | 0 | 1 | |
| Biopsy diagnosis | ||||||
| Negative | 25 | 8 | 3 | 4 | 0 | |
| CIN1 | 3 | 2 | 2 | 0 | 0 | 0.053 |
| CIN2 | 1 | 0 | 0 | 0 | 0 | |
| CIN3 | 3 | 0 | 0 | 0 | 2 | |
| Chronic cervicitis | 6 | 0 | 1 | 0 | 0 | |
Figure 6 shows the morphological features of the cervical lesions observed in the CB preparations. The normal cervical epithelium has an organized cell structure with uniform nuclei. CIN1 shows mild nuclear enlargement and hyperchromasia, whereas CIN2 shows increased nuclear atypia and loss of polarity. CIN3 is characterized by severe dysplasia in which the epithelium is affected throughout its thickness. SCC involves invasive, pleomorphic tumor cells whose architecture is significantly disrupted. These histologic differences play crucial roles in the cytopathologic evaluation and grading of cervical lesions.
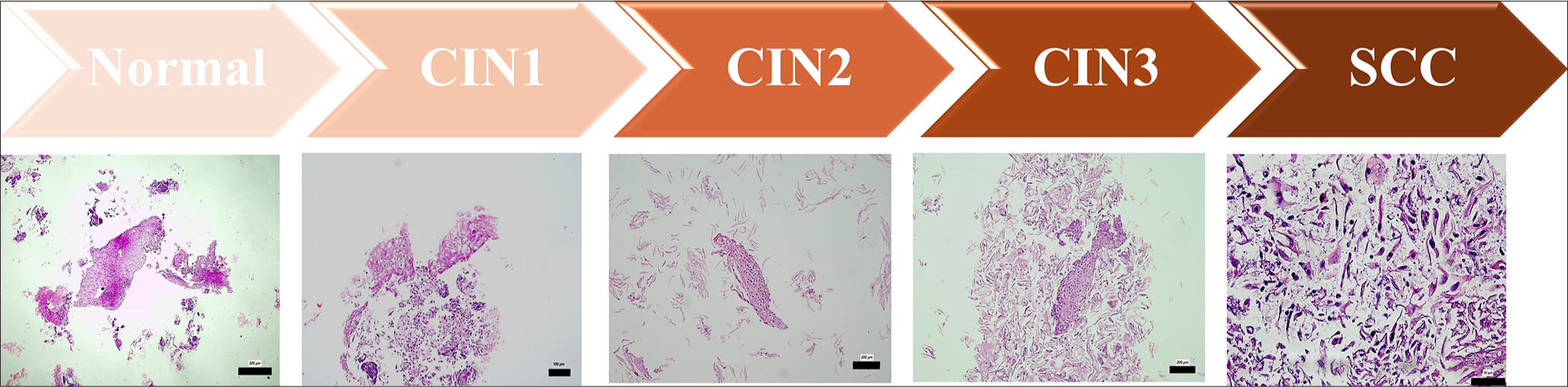
- Morphological features of different cervical lesions in CB preparations: Representative histological images illustrating the typical morphological characteristics of different cervical lesion categories identified in CB preparations. The images depict normal epithelium, CIN grades 1-3 (CIN1, CIN2, CIN3), and SCC, highlighting progressive cellular atypia, increased nuclear-cytoplasmic ratio, and architectural disorganization. The scale bars indicate the actual size at different magnifications (200 µm, 100 µm and 20µm), as marked in each subfigure. CB: Cell block, CIN1: Cervical intraepithelial neoplasia 1, CIN2: Cervical intraepithelial neoplasia 2, CIN3: Cervical intraepithelial neoplasia 3, SCC: Squamous cell carcinoma.
DISCUSSION
This study analyzed the role of CB preparations obtained from liquid-based cytology samples in diagnosing cervical lesions associated with HPV. The pivotal role of HPV in the prevention and early detection of cervical cancer has encouraged efforts to develop additional diagnostic tools alongside existing screening methods.[20,21] HPV, particularly its HR types (e.g., HPV16 and HPV18), has been identified as a key factor in the development of CIN and invasive cervical cancer.[4]
Among the 60 cervical smear cases analyzed, cytological evaluation revealed that 1.7% were classified as HSIL, 16.7% as LSIL, 43.4% as ASCUS, and 3.4% as LSIL+ASCH. Evaluations of CBs derived from the same liquid-based samples identified 6.7% of the samples as CIN1, 1.7% as CIN2, and 1.7% as SCC, whereas 78.3% were categorized as negative. Notably, there was high concordance between Pap smears and CB diagnoses, particularly for negative results and low-grade lesions, although discrepancies were observed in certain cases involving higher-grade lesions. These discrepancies may be due to the sampling technique, variation in cell preservation, and difficulty in detecting nuclear irregularities and chromatin density in Pap smear samples. High-grade lesions such as HSILs and SCCs often have more complex cellular features that can be better visualized in CB preparations. These results demonstrate the importance of combining cytology and CB analysis to improve the accuracy of CC screening.
The preparation of CBs from liquid-based cytology samples offers an opportunity to improve diagnostic evaluations by providing tissue-like material for further analysis.[22] In our study, a patient initially diagnosed with HSIL and reclassified as SCC through CB examination was later confirmed as CIN3 through pathological analysis. Comparison with initial cytological diagnoses of HSIL in the corresponding smears highlighted the correlation between cytological and histopathological findings. The identification of these distinctive malignant features in CB preparations validated the utility of this method in confirming carcinoma diagnoses, particularly in cases where conventional smear cytology raised suspicion of high-grade lesions. In addition, a subset of cases exhibited varying degrees of nuclear pleomorphism, with some cells displaying spindle-like or irregular shapes, reinforcing the heterogeneous nature of SCC. The observed morphological characteristics align with known histopathological criteria for malignancy and emphasize the importance of detailed cytological‒ histopathological correlations in diagnostic pathology. Overall, the results strongly support the malignant nature of the analyzed samples, demonstrating that CB preparations provide valuable architectural and nuclear details crucial for the definitive diagnosis of SCC. This observation highlights the potential of this method to complement routine smear diagnoses. This approach can offer additional insights in cases requiring more detailed cytological and histological correlation. Our findings align with those of previous studies demonstrating the benefits of CB preparations in enhancing diagnostic sensitivity for detecting cervical lesions.[23-26] CB preparations not only improve morphological assessment but also offer additional diagnostic possibilities, such as the ability to perform immunohistochemical staining. This method is particularly valuable for recognizing high-grade lesions and distinguishing them from reactive cellular changes. In addition, CBs serve as a useful adjunct to conventional Pap smear cytology, especially in cases where smear results are inconclusive. Their role in improving screening accuracy suggests that they can be considered for integration into routine cervical cancer screening programs. CB preparations enable more detailed morphological evaluations, including the identification of tissue structure and features indicative of high-grade dysplasia or carcinoma.[27] This method also facilitates auxiliary tests, such as immunohistochemistry, which can further increase diagnostic accuracy.[26] While CB analysis presents an opportunity to improve cytological interpretation, its integration into clinical practice should be approached cautiously and always validated with biopsy results.[28,29]
The histopathological evaluation of biopsy samples provided definitive diagnoses, with more than half of the cases reported as negative, while the remainder demonstrated CIN1 (11.7%), CIN2 (1.7%), CIN3 (8.3%), or chronic cervicitis (11.7%). The significant correlation between CB diagnoses and biopsy results underscores the diagnostic accuracy of CCs in detecting both low-grade and high-grade lesions. Cervical cancer diagnosis continues to rely on colposcopy-guided biopsy as the gold standard for definitive evaluation, as it allows for the direct assessment of tissue type and histological characteristics.[8,20] However, the use of CBs prepared from liquid-based cytology samples has emerged as a promising adjunct method, offering tissue-like material for enhanced diagnostic and prognostic evaluations.[30] In our study, a case initially identified as HSIL through Pap smear was reclassified as SCC through CB examination and subsequently confirmed as CIN3 by biopsy. This reclassification highlights the potential predictive value of CBs. This finding emphasizes the necessity of correlating CB results with colposcopy-guided biopsy findings to ensure diagnostic accuracy.[31-34] The results of this study suggest that CB preparations offer additional diagnostic value, particularly in the detection of high-grade lesions (HSIL, SCC) and invasive diseases. While some high-grade lesions may be missed by conventional Pap smears, the CB method can increase diagnostic accuracy by providing more cellular detail in these cases. The inclusion of CB specimens in cervical cancer screening guidelines may, therefore, optimize the need for colposcopy by providing additional information, particularly in the evaluation of indeterminate or low-grade lesions such as ASCUS and LSIL.
HPV testing revealed that 65% of the cases were HPV HR-positive, with 13.3% showing HPV16, 8.3% showing HPV18, and 3.3% presenting coinfections with HPV16 and HPV18. Among the ASCUS patients, 53.8% were HR-positive for HPV, with the prevalence of HPV16 being greater than that of HPV18. Interestingly, all LSIL cases demonstrated HPV HR positivity, underscoring the strong association between HR-HPV and cytological abnormalities. In the single HSIL case, an HPV HR+/HPV16+/HPV18+ coinfection was identified, which is consistent with previous studies highlighting the role of these genotypes in high-grade lesions and the development of SCC.[35] The strong correlation between HPV positivity and lesion grade supports the utility of HPV testing as a complementary tool in cervical cancer screening. HR-HPV subtypes, particularly HPV16 and HPV18, are closely associated with CIN2 and CIN3 lesions, which aligns with global data identifying these genotypes as primary drivers of cervical carcinogenesis.[36] If HPV testing is positive, further examinations and tests may be warranted, as certain HPV types (especially HR types) can lead to the development of CIN. For individuals with an ASCUS result, monitoring is generally performed, with additional tests (such as HPV testing, colposcopy, or biopsy) conducted if necessary to determine the presence of cervical dysplasia and the CIN stage.
However, this study has several limitations that must be considered. The retrospective design and small sample size limit the generalizability of our findings. In addition, the reliance on a single-center cohort and the exclusion of HPV-negative cases may introduce selection bias. Future studies with larger multicenter cohorts are needed to confirm these results and further investigate the clinical utility of CB preparations in cervical cancer screening and diagnosis. Inter- and intraobserver variability remains a major challenge in cytologic and histologic assessment. In this study, steps, including the use of standardized scoring criteria and regular quality control checks, were taken to ensure diagnostic consistency between pathologists. However, further efforts are needed to minimize observer-related discrepancies in cytological diagnoses. Since only HPV-positive cases were evaluated in this study, the diagnostic performance of CB preparations can only be tested to a limited extent for the entire spectrum of cervical lesions. Cervical lesions can also be found in HPV-negative individuals, and evaluation of these cases may provide more comprehensive information on the diagnostic sensitivity and specificity of CB preparations in the general population. The inclusion of HPV-negative cases would have been useful for determining the discriminatory power of the CB method, especially for low-grade lesions and reactive changes.
Although the ThinPrep Pap test was used in the present study, the effects of different cytologic methods (conventional Pap smear, SurePath, etc.) on the efficacy of CB preparations were not examined. While the conventional Pap smear method results in a rather random distribution of cell material, the ThinPrep method results in a more homogeneous cell distribution. How this affects the CB analysis is not known. As other liquid-based cytology methods use different solvents, future studies should investigate how this may affect CB formation and morphological integrity.
SUMMARY
A combination of Pap smear cytology, CB preparation, and HPV testing provides a comprehensive diagnostic approach for detecting cervical lesions. CB preparations offer significant advantages, particularly in improving the detection of high-grade lesions and SCCs, making them a valuable complement to conventional screening methods. Their integration into routine cervical cancer screening programs may increase diagnostic accuracy and patient outcomes. Future studies are essential to validate the predictive role of CBs in cervical cancer screening, especially in alignment with histopathological gold standards.
AVAILABILITY OF DATA AND MATERIALS
All data and materials used in this study are publicly available.
ABBREVIATIONS
×: The zoom ratio of the lens
ASCH: Atypical squamous cells - HSIL cannot be excluded
ASCUS: Atypical squamous cells of undetermined significance
CIN: Cervical intraepithelial neoplasia
CIN1: Cervical intraepithelial neoplasia 1
H&E: Hematoxylin and Eosin
HPV: Human papillomavirus
HSIL: High-grade squamous intraepithelial lesion
LSIL: Low-grade squamous intraepithelial lesion
n: Sample size
SCC: Squamous cell carcinoma
SEER: Surveillance, Epidemiology, and End Results
TBS: The Bethesda System
χ2: Chi-square value
AUTHORS’ CONTRIBUTIONS
CC: Conceptualization; CC, TBÖ: Methodology; CC, SŞ: Formal analysis and investigation; CC, SŞ, TBÖ: Writing – original draft preparation; CC: Writing – review and editing; CC: Resources; CC: Supervision. All authors meet ICMJE authorship requirements.
ACKNOWLEDGMENT
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Kartal Kosuyolu Training and Research Hospital (Date: 26.12.2019/No: 2018.8/14-278). The authors certify that they have obtained all appropriate patient consent.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: Not applicable.
References
- The prognosis of patients with locally advanced cervical cancer undergoing surgical versus non-surgical treatment: A retrospective cohort study based on SEER database and a single-center data. Int J Surg. 2024;111:1619-23.
- [CrossRef] [PubMed] [Google Scholar]
- Human papillomavirus associated cervical lesion: Pathogenesis and therapeutic interventions. MedComm (2020). 2023;4:e368.
- [CrossRef] [PubMed] [Google Scholar]
- Cell cycle regulation during viral infection. Methods Mol Biol. 2014;1170:165-227.
- [CrossRef] [PubMed] [Google Scholar]
- Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect Dis. 2007;7:453-9.
- [CrossRef] [PubMed] [Google Scholar]
- Human papillomavirus laboratory testing: The changing paradigm. Clin Microbiol Rev. 2016;29:291-319.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical intraepithelial neoplasia In: StatPearls. Treasure Island, FL: StatPearls; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544371/
- [Google Scholar]
- Cancer screening in the United States, 2019: A review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69:184-210.
- [CrossRef] [PubMed] [Google Scholar]
- 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-46.
- [CrossRef] [PubMed] [Google Scholar]
- Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24:132-43.
- [CrossRef] [PubMed] [Google Scholar]
- The combined finding of HPV 16, 18, or 45 and cytologic atypical glandular cells (AGC) indicates a greatly elevated risk of in situ and invasive cervical adenocarcinoma. Gynecol Oncol. 2023;174:253-61.
- [CrossRef] [PubMed] [Google Scholar]
- New insights into cervical cancer screening. J Gynecol Oncol. 2012;23:282-7.
- [CrossRef] [PubMed] [Google Scholar]
- Variables that impact HPV test accuracy during vaginal self collection workflow for cervical cancer screening. Gynecol Oncol Rep. 2024;54:101421.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:CD008587.
- [CrossRef] [PubMed] [Google Scholar]
- Thin-layer liquid-based cervical cytology and PCR for detecting and typing human papillomavirus DNA in Flemish women. Br J Cancer. 2003;88:560-6.
- [CrossRef] [PubMed] [Google Scholar]
- The efficiency of cervical pap and comparison of conventional pap smear and liquid-based cytology: A review. Cureus. 2023;15:e48343.
- [CrossRef] [Google Scholar]
- WMA declaration of Helsinki - ethical principles for medical research involving human participants. Available from: https://www.wma.net/policies/post/wma-declaration-of-helsinki [Last accessed on 2025 Mar 27]
- [Google Scholar]
- Guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention: Use of dual-stain cytology to triage women after a positive test for human papillomavirus (HPV) 2024. Geneva: World Health Organization; Available from: https://www.who.int/publications/i/item/9789240091658
- [Google Scholar]
- Immunohistochemical detection of p16INK4a in liquid-based cytology specimens on cell block sections. Cancer. 2007;111:74-82.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis. Lancet Oncol. 2014;15:172-83.
- [CrossRef] [PubMed] [Google Scholar]
- Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907.
- [CrossRef] [PubMed] [Google Scholar]
- Cell blocks in cytopathology: A review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25:356-71.
- [CrossRef] [PubMed] [Google Scholar]
- Cell block preparation as a diagnostic technique complementary to fluid-based monolayer cervicovaginal specimens. Acta Cytol. 1999;43:69-73.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of the cell block technique in diagnostic cytopathology. J Cytol. 2012;29:177-82.
- [CrossRef] [PubMed] [Google Scholar]
- Cell block preparation from cytology specimen with predominance of individually scattered cells. J Vis Exp. 2009;21:1316.
- [CrossRef] [Google Scholar]
- Cell blocks in cytology: Review of preparation methods, advantages, and limitations. J Am Soc Cytopathol. 2023;12:77-88.
- [CrossRef] [PubMed] [Google Scholar]
- Morphological features of cell blocks prepared from residual liqui-PREP samples can distinguish between high-grade squamous intraepithelial lesions and squamous cell carcinomas. Acta Cytol. 2011;55:245-50.
- [CrossRef] [PubMed] [Google Scholar]
- The value of cell block based on fine needle aspiration for lung cancer diagnosis. J Thorac Dis. 2017;9:2375-82.
- [CrossRef] [PubMed] [Google Scholar]
- Combined use of cell block and smear improves the cytological diagnosis of malignancy in non-palpable breast lesions screened by imaging. Anal Cell Pathol (Amst). 2023;2023:1869858.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of liquid-based cytology and cell blocks prepared from cell remnants for diagnosis of cervical pathology. Ann Diagn Pathol. 2024;69:152265.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnostic accuracy of colposcopy-A review of research methodology and impact on the outcomes of quality assurance. Eur J Obstet Gynecol Reprod Biol. 2019;240:182-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of diagnostic methods in detection of squamous cell abnormalities in Iranian women with abnormal pap's smear test and associated demographic and issues. Iran J Pathol. 2020;15:106-16.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective randomized study on limits of colposcopy and histology: The skill of colposcopist and colposcopy-guided biopsy in diagnosis of cervical intraepithelial lesions. Infect Agent Cancer. 2015;10:47.
- [CrossRef] [PubMed] [Google Scholar]
- Factors correlated with the accuracy of colposcopy-directed biopsy: A systematic review and meta-analysis. J Invest Surg. 2022;35:284-92.
- [CrossRef] [PubMed] [Google Scholar]
- HPV genotypes in high-grade cervical lesions and invasive cervical carcinoma detected in Gabonese women. Infect Agent Cancer. 2023;18:16.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of human papillomavirus subtypes 16 and 18 among Yemeni patients with cervical cancer. Asian Pac J Cancer Prev. 2017;18:1543-8.
- [Google Scholar]









