Translate this page into:
Dual color multiplex TTF-1 + Napsin A and p63 + CK5 immunostaining for subcategorizing of poorly differentiated pulmonary non-small carcinomas into adenocarcinoma and squamous cell carcinoma in fine needle aspiration specimens
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The distinction of lung adenocarcinoma (ADC) from squamous cell carcinoma (SCC) has important therapeutic implications. Napsin A is a recently developed marker, which has shown high specificity for lung tissue in the surgical pathology specimens. In this study, we have evaluated whether the use of a panel of novel multiplex cocktails of TTF-1 + Napsin A and p63 + CK5 for dual color immunostaining will improve the diagnostic accuracy of lung adenocarcinoma and squamous cell carcinoma in fine needle aspiration (FNA) specimens, usually with relatively scant microfragments of diagnostic material.
Materials and Methods:
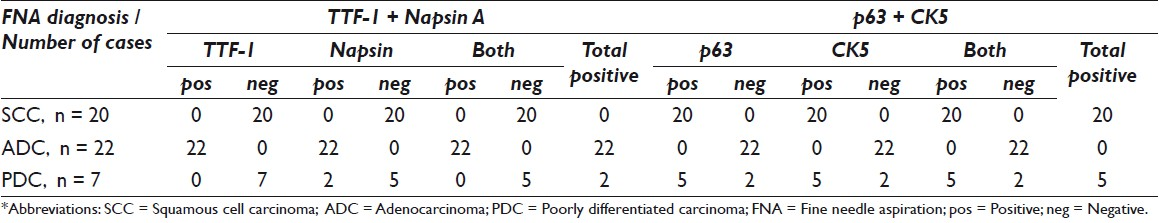
Formalin-fixed, paraffin-embedded, adequately cellular FNA cell blocks with a confirmed diagnosis of either ADC (n = 22), SCC (n = 20) or poorly differentiated carcinoma (PDC; n = 7), from a total of 49 consecutive cases, were studied. All these cases had subsequently confirmed diagnosis in biopsies or resection specimens. The sections were immunostained with two color methods of TTF-1 + Napsin A and p63 + CK5 multiplex cocktails. The presence of one or more unequivocal individual tumor cells with convincing brown nuclear TTF-1 and red cytoplasmic Napsin A staining, and cells with brown nuclear p63 and membranous / cytoplasmic CK5 staining were interpreted as ‘positive’.
Results:
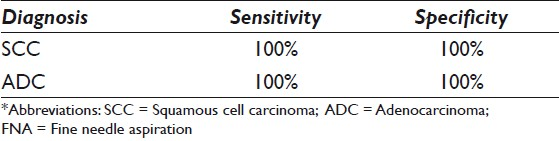
All 20 FNA cell blocks from SCC cases were positive for dual stain p63 + CK5 and negative for dual stain TTF-1 + Napsin A. The sensitivity and specificity of the dual immunoexpressions of p63 + CK5 for SCC of lung FNAs were both 100%. All 22 ADC cases were positive with dual stain of TTF-1 + Napsin A and negative for dual stain of p63 + CK5. On follow-up of the surgical pathology specimens, 22 cases were confirmed as ADC. The sensitivity of the dual immunoexpression of TTF-1 + Napsin A for ADC of lung FNAs was 100% and the specificity was also 100%. Of the seven PDC cases, five cases that were positive for dual stain p63 + CK5 and negative for dual stain TTF-1 + Napsin A could be categorized as SCC. Two of the seven (2 / 7) PDC cases were positive for dual stain TTF-1 + Napsin A and negative for dual stain p63 + CK5, consistent with ADC.
Conclusions:
Simultaneous coordinate or individual immunostaining for Napsin A / TTF-1 in ADC and p63 / CK5 in SCC demonstrated high sensitivity and specificity. The panel with multiplex Napsin A / TTF-1 and p63 / CK5 dual color immunostains could specifically subcategorize PDC into ADC and SCC in lung FNA specimens. Multiplex dual color Napsin A / TTF-1 and p63 / CK5 immunostaining is especially recommended for evaluation of FNA specimens with relatively scant cellularity.
Keywords
Adenocarcinoma
CK5
FNA
lung
Napsin A
p63
squamous cell carcinoma
TTF-1
INTRODUCTION
Historically, all the subtypes of non-small cell lung carcinomas (NSCLC) received similar treatment.[1] With the advent of a more personalized approach in the treatment of NSCLC, based on novel targeted molecular-based therapies, it is becoming increasingly important to accurately sub-classify these cases into squamous cell carcinomas (SCC) versus adenocarcinomas (ADC). The targeted therapies like tyrosine kinase inhibitors are more effective in adenocarcinomas than in squamous cell carcinomas.[2–4] Antiangiogenic modalities like bevacizumab (Avastatin) can be associated with life-threatening pulmonary hemorrhage in squamous cell carcinomas.[56] Similarly, ADC as a category predicts a better response to epidermal growth factor (EGFR) inhibitors.[7]
Fine needle aspiration (FNA) is routinely used in the diagnosis and staging of lung cancers, especially in patients with advanced disease, wherein, invasive surgical biopsies are not feasible. Although the diagnostic accuracy of the FNAs lies in distinguishing between primary versus metastatic lung cancer, and ADC versus SCC, it is relatively good based on the cytomorphological features; some cases may not be categorized further than non-small cell carcinoma or poorly differentiated carcinoma, even after application of the usual single color immunohistochemistry evaluation.[89] This is mainly due to the presence of relatively scant diagnostic material in the FNA specimens, which frequently limits the evaluation of the coordinate immunoreactivity pattern of the diagnostic cells. The immunoactivity pattern in the cell block sections of such specimens may be improved with the application of the SCIP (Subtractive Coordinate Immunoreactivity Pattern) approach.[10] This approach allows the precise location and coordination of the same cells or cell groups in identically oriented and appropriately numbered adjacent level sections of the cell blocks. However, even the SCIP approach may not help in some cases with very scant, singly scattered, diagnostic cells in the cell block sections.
The immunohistochemistry (IHC) antibodies currently used for detecting lung cancer in FNA specimens include Thyroid transcription factor-1 (TTF-1), which is immunoexpressed in the nuclei of lung adenocarcinomas with ~80% sensitivity.[11–13] However, TTF-1 is also expressed in several other tumors, including neuroendocrine neoplasms and some squamous cell carcinomas of the lung, metastatic breast carcinomas, thyroid carcinomas, renal cell carcinomas, and gynecological malignancies like ovarian carcinomas.[1113–15] Immunoexpression of TTF-1 is relatively less dependable when the IHC is a diagnostically more significant component in the differential diagnosis of poorly differentiated ADC of the lung.[12–14]
Napsin A is a recently reported, promising immunomarker for lung ADC.[8] This proteinase, involved in the maturation of surfactant protein B, is expressed in the cytoplasm of type II pneumocytes, Clara cells and alveolar macrophages in the lung, proximal and convoluted tubules in the kidney, lung adenocarcinomas, and renal cell carcinomas.[1116] It has been demonstrated to be a better marker than TTF-1 for detecting well- and moderately-differentiated lung adenocarcinomas in the tissue sections.[14–18] In the surgical pathology specimens Napsin A has shown higher sensitivity and specificity for lung ADC, 79-85%[1] and 100%, [15] respectively, than TTF-1, 54-75% [1] and 97-100%, respectively, and was virtually negative in all lung SCC. Napsin A stains more tumor cells and a higher percentage of lung ADC than TTF-1.[15] Of late, it has been seen to be a good immunomarker for subclassifying poorly differentiated lung adenocarcinomas in cytological specimens.[11]
The clinical application of Napsin A in FNA specimens is evolving.[192021] The immunoreactivity of Napsin A versus TTF-1 in cytologically ‘poorly differentiated’ carcinomas has been reported to have 81% sensitivity and 81% specificity for TTF-1 and 65% sensitivity and 96% specificity for Napsin A.[11] The application of both immunomarkers simultaneously, as dual color immunostaining, should improve the diagnostic accuracy for subclassifying PDC of the lung. [11] Recent histological studies have suggested an immunopanel composed of p63, CK5 / 6, TTF-1, CK7, Napsin A, and a mucin stain, to evaluate coordinate immunoreactivity patterns with these immunomarkers, for subcategorization of most cases of poorly differentiated NSCLC into ADC and SCC, especially in small tumor samples.[1]
Recent advances in immunohistochemistry have resulted in the development of multiplex IHC panels. These panels allow simultaneous evaluation of multiple immunomarkers for improved applicability, especially for specimens with scant clinical samples. Currently Napsin A and TTF-1 are available in a dual color multiplex IHC cocktail, with TTF-1 immunostaining the nuclei and Napsin A immunostaining the cytoplasm of lung adenocarcinomas. p63 and CK5 / 6 are the markers currently used for squamous differentiation with nuclear immunostaining for p63 and cytoplasmic / membranous immunostaining for CK 5.[2122]
The present study is aimed at evaluating whether the use of a panel of novel multiplex TTF-1 + Napsin A and p63 + CK5 dual color immunostaining would improve the subclassification of lung tumors into ADC and SCC in FNA specimens with enhanced accuracy.
MATERIALS AND METHODS
Case selection
After obtaining approval from the Institutional Review Board (IRB), the lung FNA specimen data was retrieved from the computerized archived database at our tertiary care hospital, affiliated with the cancer center. Fifty consecutive lung FNA cases (23 adenocarcinomas, 20 squamous cell carcinomas, seven poorly differentiated carcinomas) with adequate cell block material, for further immunostaining, were originally selected for the study. All cases had concurrent and / or follow-up histopathology. Only histologically confirmed cases of primary lung adenocarcinoma (ADC, n = 22), squamous cell carcinoma (SCC, n = 20), and poorly differentiated carcinoma (PDC, n = 7) were included in the final study group (a total of 49 cases). One FNA case with cytological diagnosis of ADC was excluded from the study, as the surgical pathology follow-up showed adenosquamous carcinoma. Cases were defined as ADC, SCC, and PDC, based histologically on H and E appearance alone, or by a combination of H and E and an immunoprofile. PDC was defined histologically in our laboratory as, ‘non-small cell carcinoma, not otherwise specified’. All FNAs were performed under CT / ultrasound guidance. In each case a cytopathologist performed on-site evaluation, for specimen adequacy. Air-dried and alcohol-fixed slides were prepared for each pass. On an average, three passes were obtained in each case. The needle was rinsed with a Cytolyte fixative for cell block preparation. A dedicated pass for cell block preparation was made if necessary.
Four micron sections (test and negative controls) were cut in each case and placed on positively charged slides for dual color immunostaining with multiplex IHC cocktails, Napsin A + TTF1 and p63 + CK5.
Multiplex immunohistochemical staining
The sections were stained by multiplex IHC, using specific antibody cocktails of Napsin A + TTF1 (Biocare, PM394DSAA) and p63 + CK5 (Biocare, PM391DSAA). The staining protocol used was as per the manufacturer. A known positive tissue for the antigen of interest was stained as the positive control in each batch during immunostaining. Immunohistochemical staining was performed as follows: The tissue sections were deparaffinized, hydrated with phosphate-buffered saline (PBS) buffer (pH 7.4), and pretreated with hydrogen peroxide (3%) for five minutes, to block the endogenous peroxidase. This was followed by antigen retrieval in a steamer for 45 minutes in a citrate. The slides were then incubated with the primary antibody cocktail at room temperature for 30 minutes and then incubated with Mach2 Double Stain 2 (Biocare) for 30 minutes, at room temperature. Finally the slides were developed with 0.05% 3’,3-diaminobenzidine (DAB) tetrahydrochloride, which had been freshly prepared in 0.05 mol / L Tris buffer, at pH 7.6, containing 0.024% H2O2, for five minutes. Then the slides were incubated with freshly prepared Vulcan Fast Red for 12 minutes at room temperature and then counterstained with Mayer hematoxylin for 30 seconds, and rinsed with deionized water; following this the bluing solution was applied for one minute and mounted.
Assessment of immunohistochemical staining
The immunostained slides were blindly (without knowing the diagnosis) evaluated under a transmission light microscope by two cytopathologists (TG, SS) and revaluated by one cytopathologist (VS), as an additional check. Areas with the strongest immunostaining were located, for evaluating the immunoexpression in those sections. Immunostaining was assessed only in the tumor cells, while using the corresponding H and E-stained slide for their detection and utilizing the SCIP approach to exclude staining of the contaminating macrophages or benign pulmonary epithelium. The presence of one or more unequivocal individual tumor cells, with convincing brown nuclear TTF-1 and / or red cytoplasmic Napsin A immunostaining, and cells with brown nuclear p63 and / or red cytoplasmic CK5 immunostaining were interpreted as ‘positive’. Immunoreactivity was defined as negative if none of the immunostaining was present.
RESULTS
Fine needle aspirations from 49 cases of lung tumors were selected (22 primary ADC, 20 squamous cell carcinomas, and seven poorly differentiated carcinomas). All cases had concurrent or follow-up surgical pathology specimens.
The tumor cells of ADC showed brown immunostaining of the nuclei for TTF-1 and / or red, granular cytoplasmic immunostaining for Napsin A [Figure 1]. The tumor cells of SCC showed brown nuclear immunostaining for p63 and / or red, membranous / cytoplasmic immunostaining for CK5 [Figure 2].
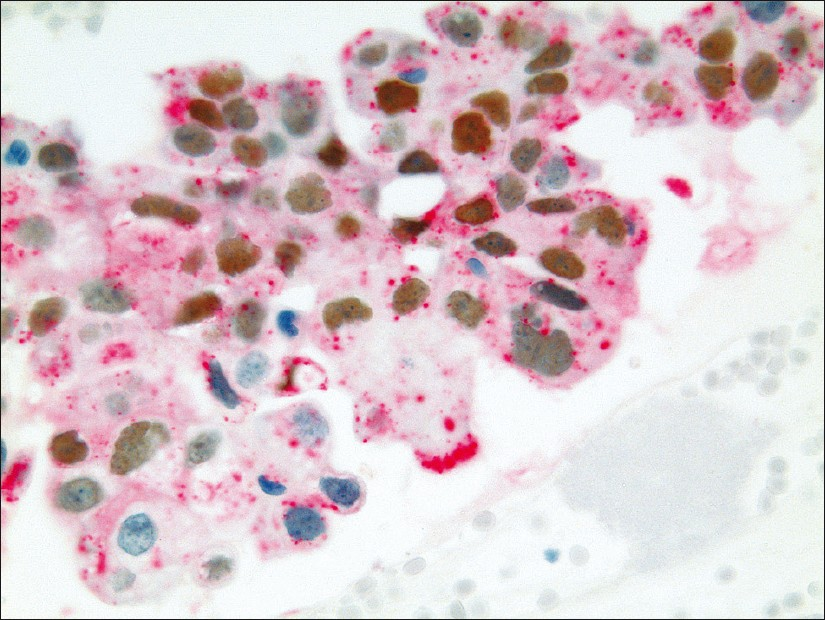
- Pulmonary ADC showing brown, nuclear immunostaining for TTF-1 expression and cytoplasmic immunostaining for Napsin A expression (dual color Multiplex TTF-1 + Napsin A immunostain; ×400)
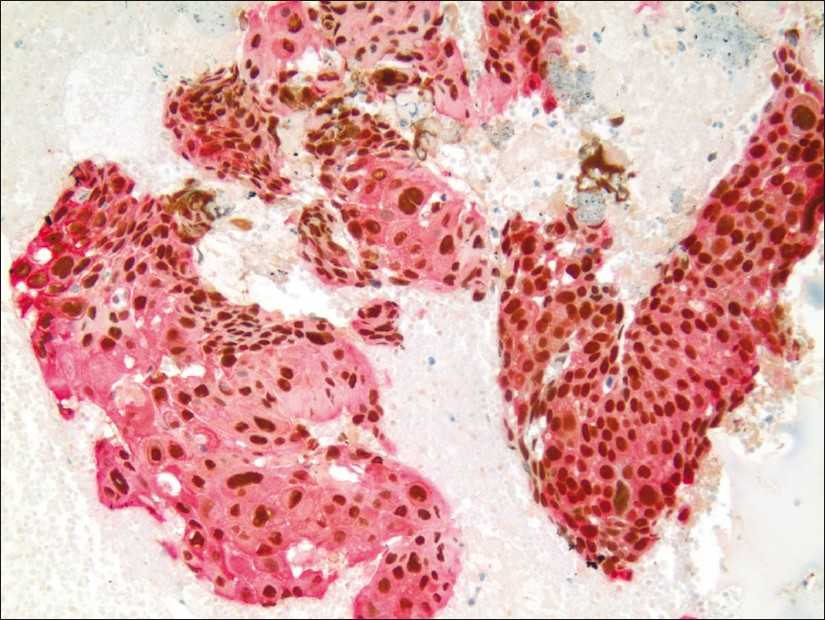
- Pulmonary SCC showing brown, nuclear immunostaining for p63 expression and membranous / cytoplasmic immunostaining for CK5 expression (dual color Multiplex p63 + CK5 immunostain; ×200)
All 20 cell blocks from the FNA of SCC cases were positive (20 / 20; 100%) with the dual stain for p63 + CK5, Figure 3, Table 1, and negative for TTF-1 + Napsin A, (0 / 20; 0%). The follow-up confirmed SCC suclassification in the surgical specimens. The sensitivity and specificity for dual color immunostaining, for p63 + CK5 in the SCC of lung FNA specimens, were 100%.
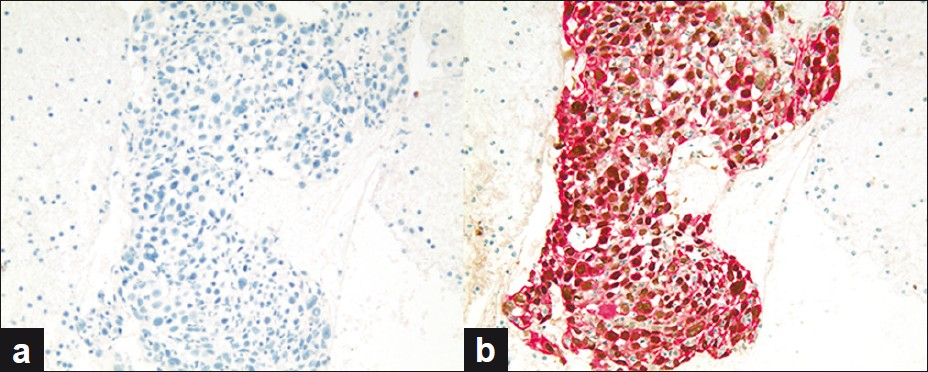
- (a and b) SCC case demonstrating (a) negative immunoreactivity for dual color TTF-1 + Napsin A immunostain and (b) positivity for dual color p63 + CK5 immunostain (dual color Multiplex TTF-1 + Napsin A and p63 + CK5 immunostains; ×200)
All 22 ADC cases were positive, with dual color immunostaining for TTF-1 + Napsin A (22 / 22; 100%), Figure 4, Table 1, and negative for both p63 + CK5 (0 / 22; 0%). On follow-up, all 22 cases were confirmed to be ADC. The sensitivity of the dual color immunostaining for TTF-1 + Napsin A for ADC in the FNA specimens was 100%, with a specificity of 100% [Table 2].
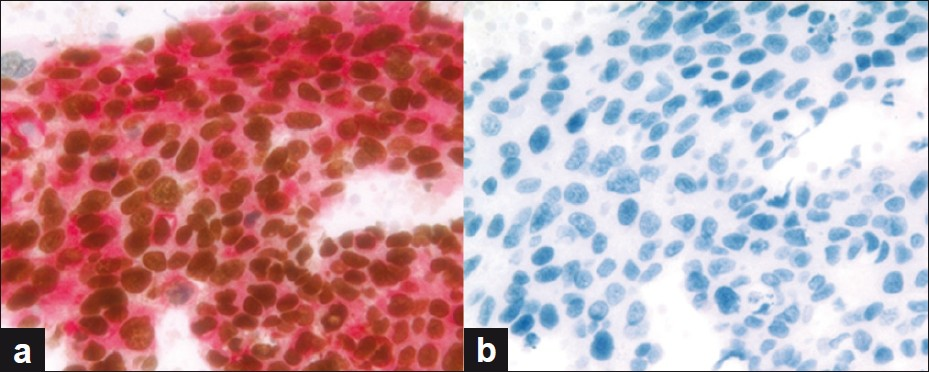
- (a and b) ADC case demonstrating (a) positivity for dual color TTF-1 + Napsin A immunostain and (b) negative immunoreactivity for dual color p63 + CK5 immunostain (dual color Multiplex TTF-1 + Napsin A and p63 + CK5 immunostains; ×200)
Of the seven PDC cases, five were positive for dual color immunostaining for p63 + CK5 and negative for TTF-1 + Napsin A, Figure 5, consistent with SCC. Two out of seven (2 / 7) PDC cases were positive for dual color immunostaining for TTF-1 + Napsin A and negative for p63 + CK5, Figure 6, consistent with ADC. In the two cases consistent with ADC, TTF-1 immunostaining was equivocal; however, Napsin A showed strong cytoplasmic immunoreactivity, supporting the interpretation of poorly differentiated ADC [Figure 7]. All seven cases of PDC (100%) could be subclassified with these two dual color immunopanels [Table 1].
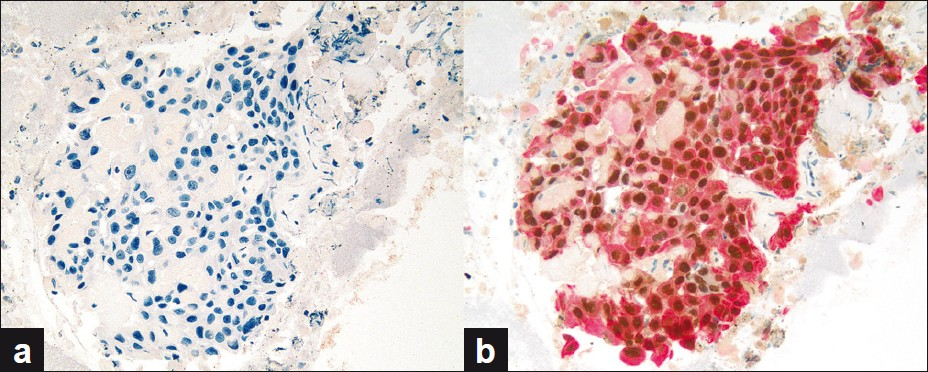
- (a and b) PDC case (a) negative for dual color TTF-1 + Napsin A immunostain and (b) positive for dual color p63 + CK5 immunostain, consistent with SCC (dual color Multiplex TTF-1 + Napsin A and p63 + CK5 immunostains; ×200)
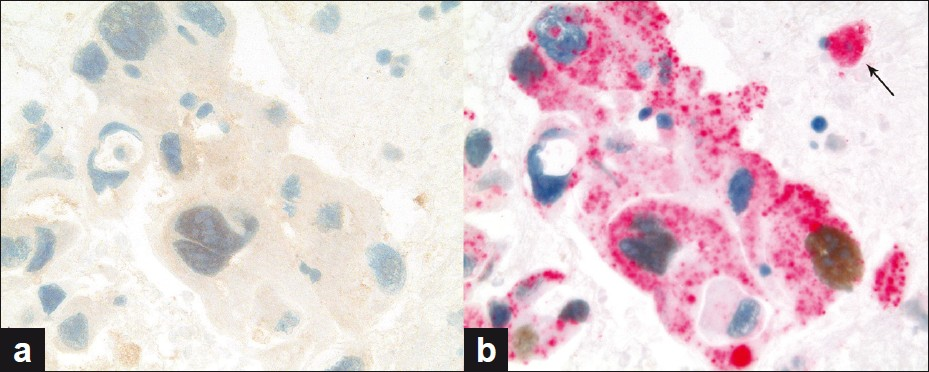
- (a and b) PDC case (a) nonreactive for dual color p63 + CK5 immunostain and (b) showing immunoreactivity for dual color TTF-1 + Napsin A immunostain, consistent with AD; arrow showing cytoplasmic immunoreactivity for Napsin A in a pulmonary macrophage (dual color Multiplex p63 + CK5 and TTF-1 + Napsin A immunostains; ×400)
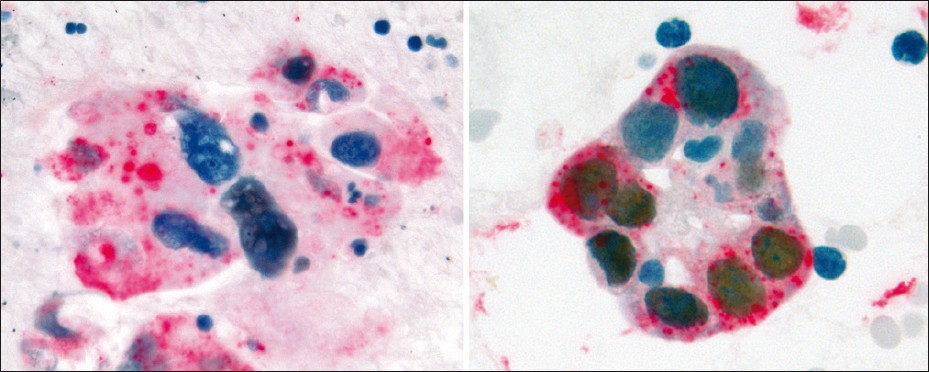
- PDC case revealing variability of nuclear TTF-1 immunostaining and strong cytoplasmic immunoreactivity for Napsin A, supporting the interpretation of poorly differentiated ADC (dual color Multiplex TTF- 1+Napsin A immunostain; ×400)
DISCUSSION
Lung cancer is the leading cause of adult cancer-related deaths in most countries, and non-small-cell carcinomas comprise about 80% of these cases.[23] FNA is a routinely used clinical test to diagnose these tumors. With the recent change in the management of NSCLC, it is important to subcategorize these tumors accurately into ADC and SCC, prior to definitive therapy on the specimens procured, by minimally invasive procedures such as FNA. ADC is more amenable to tyrosine kinase inhibitors than SCC. Additionally antiangiogenic modalities like bevacizumab (Avastatin) can lead to fatal pulmonary hemorrhages in 30% of the SCC cases, precluding their use in this subset of tumors.
Although cytomorphological features are useful in distinguishing between lung ADC and SCC, some PDCs may need immunocharacterization. [8] Cell block sections of FNA specimens usually have relatively scant microfragments of diagnostic tumor material. This may compromise the interpretation of routine one color immunostaining, even after application of the SCIP approach, with extensive immunopanels.[10] Application of the SCIP approximate protocol to prepare cell blocks to align diagnostic microfragments along the cutting surface, may help to overcome this limitation in most of the cases.[24]
Multiplex immunohistochemistry is an additional recent development on the horizon, which has clinically significant advantages in analyzing scant specimens such as those obtained by minimally invasive procedures like FNA. Multiplex immunohistochemistry allows evaluation of coordinate immunoreactivity patterns in a few serial sections. In addition to this interpretation benefit, this approach also saves precious limited diagnostic material, resources, cost, and time. In our study, application of multiplex dual color IHC panels consisting of TTF-1/Napsin A and p63/CK5 on FNA specimens of lung tumors, demonstrated distinct, non-overlapping, dual color immunostaining patterns in lung ADC and SCC.
All 22 unequivocal cases of lung ADC showed TTF-1 and Napsin A immunostaining, with negative p63 and CK5 expression. The sensitivity of the dual color immunostaining for TTF-1 + Napsin A for ADC in the FNA specimens was 100%, with a specificity of 100% [Table 2]. A mixed pattern is not uncommon in lung tumors and the potential of sampling artifact-related pitfalls should be considered while applying the management decisions based on samples obtained by limited procedures, such as those procured by FNA. In fact, one FNA case with a cytological diagnosis of ADC and immunostaining profile TTF+ / Napsin A+ / CCK5 / 6- / p63- was excluded from our study due to a sampling issue, as the surgical pathology follow-up showed adenosquamous carcinoma. These findings are consistent with previously reported studies, which demonstrate the usefulness of TTF-1 + Napsin A dual color immunostain in distinguishing lung ADC versus SCC.[192025]
A study by Bishop JA et al. demonstrated Napsin A as a sensitive immunomarker for lung ADC.[15] Combining TTF-1 with Naspin A further improved the sensitivity and specificity. Stoll LM et al. have also reported that combined application of Napsin A and TTF-1 immunomarkers may be necessary to improve diagnostic accuracy in lung ADC.[11]
All 20 cases of pulmonary SCC in our study showed p63 and CK5 expression, but none for TTF-1 or Napsin A. An immunopanel with a TTF-1-, P63+,CK5+ coordinate immunophenotypic expression pattern was reported to be useful in distinguishing SCC from ADC.[22] Immunostaining for p63 has been described as the single best marker to separate ADC from SCC, with a sensitivity of 84%, and specificity of 85% for SCC.[1]
Characterization of PDC in FNA specimens is particularly challenging based on cytomorphology alone.[22] In this study we selected a small number of PDC cases to evaluate this challenge. All our seven PDCs showed distinct immunostaining patterns with our dual color immunopanels. The immunostaining pattern showed dual immunoreactivity in most tumor cells; however, we did encounter some cells that were immunoreactive for only one antibody or showed equivocal dual immunostaining. Of note, Napsin A immunoreactivity was also noticed in pulmonary macrophages, however, these cells could be easily discriminated from the tumor cells by morphology [Figure 6, arrow]. Variable immunostaining was noted in different groups of tumor cells on the same slide. The dual color IHC could accurately classify 100% of the cases of PDCs in this study. Napsin A was relatively precise for categorizing PDC cases into ADC of the lung. Napsin A assisted in subclassifying two cases of PDC, which were TTF-1 negative. The immunoreactivity for Napsin A helped to identify two cases of ‘PDC,’ which were negative for TTF1. Looking at this from the cost-benefit perspective, the use of dual immunostains is maximal when there is a diagnostic dilemma, especially in PDC cases.
A word of caution, before these immunostains are used for routine diagnostics: The dual immunostaining used in this study is unnecessary when the cytomorphology is diagnostic of adenocarcinoma or squamous carcinoma; it is useful in characterizing cases where the differentiation cannot be achieved, for example, poorly differentiated carcinomas in specimens with scant diagnostic tumor cells, such as in FNA cell block sections.
CONCLUSIONS
In summary, multiplex dual color IHC, utilizing immunopanel with TTF-1 + Napsin A and p63 + CK5 was effective for subclassifying NSCLC of the lung. This approach also saved time, sample, resource, and cost, with improved application for the FNA-procured specimen, with definitive diagnosis, using a minimally invasive approach.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The author(s) declare that they do not have competing commercial interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that they qualify for authorship as defined by ICMJE http://www.icmje.org/#author.. Each author has participated sufficiently in the study and take public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version has been read and approved by them.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board (IRB) of all the institutions associated with this study. The authors take the responsibility of maintaining relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENTS
This study was presented in part as a platform at the Hundredth Annual USCAP meeting (March, 2011, San Antonio, TX). The authors acknowledge the excellent technical support of Ms. Melodee Dudley and Ms. Pamela Tabaczka.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/10/94570.
REFERENCES
- Optimal immunohistochemical markers for distinguishing lung adenocarcinomas from squamous cell carcinomas in small tumor samples. Am J Surg Pathol. 2010;34:1805-11.
- [Google Scholar]
- DOG1 Utility in diagnosing gastrointestinal stromal tumors on fine-needle aspiration. Cancer Cytopathol. 2011;119:202-8.
- [Google Scholar]
- FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced / metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713-8.
- [Google Scholar]
- Bevacizumab / chemotherapy in non-small-cell lung cancer: looking for a few good men? Clin Lung Cancer. 2008;9:75-6.
- [Google Scholar]
- Toxicities of antiangiogenic therapy in non-small-cell lung cancer. Clin Lung Cancer. 2006;8 Suppl 1:S23-30.
- [Google Scholar]
- The prognostic and predictive role of histology in advanced non-small cell lung cancer: a literature review. J Thorac Oncol. 2008;3:1468-81.
- [Google Scholar]
- Respiratory Cytology. In: Atkinson BF, ed. Atkinson Atlas of Diagnostic Cytopathology (2nd ed). Philadelphia, PA: W. B. Saunders Company; 2004. p. :273-356. Chapter 7
- [Google Scholar]
- Immunohistochemistry: Diagnostic and Prognostic Applications. In: Detrick B, Hamilton RG, Folds JD, eds. Manual of Molecular and Clinical Laboratory Immunology (7th ed). Philadelphia, PA: American Society of Microbiology Press; 2006. p. :408-413. (http://online.statref.com/titleinfo/fxid-274.html) Chapter 47
- [Google Scholar]
- Immunocytochemistry of effusion fluids: Introduction to the SCIP approach. In: Shidham VB, Atkinson BF, eds. Cytopathologic Diagnosis of Serous Fluids. Philadelphia, PA: Elsevier (W. B. Saunders Company); 2007. p. :55-78. Chapter 5
- [Google Scholar]
- The utility of napsin-A in the identification of primary and metastatic lung adenocarcinoma among cytologically poorly differentiated carcinomas. Cancer Cytopathol. 2010;118:441-9.
- [Google Scholar]
- Thyroid transcription factor-1: a review. Appl Immunohistochem Mol Morphol. 2002;10:97-102.
- [Google Scholar]
- Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med. 2008;132:384-96.
- [Google Scholar]
- A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23:654-61.
- [Google Scholar]
- Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010;41:20-5.
- [Google Scholar]
- Processing of pulmonary surfactant protein B by napsin and cathepsin H. J Biol Chem. 2004;279:16178-84.
- [Google Scholar]
- Surfactant protein B (SP-B) - / - mice are rescued by restoration of SP-B expression in alveolar type II cells but not Clara cells. J Biol Chem. 1999;274:19168-74.
- [Google Scholar]
- TKTL1 is overexpressed in a large portion of non-small cell lung cancer specimens. Diagn Pathol. 2008;3:35.
- [Google Scholar]
- TTF-1 and p63 for distinguishing pulmonary small-cell carcinoma from poorly differentiated squamous cell carcinoma in previously pap-stained cytologic material. Mod Pathol. 2006;19:1117-23.
- [Google Scholar]
- TTF-1 and Napsin A double stain: a useful marker for diagnosing lung adenocarcinoma on fine-needle aspiration cell blocks. In: Cancer Cytopathol. Vol 119. 2011. p. :127-33. doi: 10.1002/cncy.20135
- [Google Scholar]
- Value of P63 and CK5 / 6 in distinguishing squamous cell carcinoma from adenocarcinoma in lung fine-needle aspiration specimens. Diagn Cytopathol. 2009;37:178-83.
- [Google Scholar]
- TTF-1 and Napsin A double stain: a useful marker for diagnosing lung adenocarcinoma on fine-needle aspiration cell blocks. Cancer Cytopathol. 2011;119:127-33.
- [Google Scholar]
- Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276-99.
- [Google Scholar]
- Cell block preparation from cytology specimen with predominance of individually scattered cells? J Vis Exp. :pii-1316. Available from: http: //www.jove.com/index/Details.stp?ID=1316
- [Google Scholar]
- Distinction of pulmonary small cell carcinoma from poorly differentiated squamous cell carcinoma: an immunohistochemical approach. Mod Pathol. 2005;18:111-8.
- [Google Scholar]








