Translate this page into:
Esculin promotes skin wound healing in mice and regulates the Wnt/β-catenin signaling pathway

*Corresponding author: Mengsi Zhang, Department of Obstetrics and Gynecology, Wenzhou Central Hospital, Wenzhou, China. zms123098@163.com
-
Received: ,
Accepted: ,
How to cite this article: Xu M, Zhang M, Wu J, Wang J, Wu H, Xu X. Esculin promotes skin wound healing in mice and regulates the Wnt/b-catenin signaling pathway. CytoJournal. 2025;22:32. doi: 10.25259/Cytojournal_184_2024
Abstract
Objective
Previous studies reported that esculin could protect against renal ischemia-reperfusion injury and liver injury, but its mechanism of action in skin wound healing is unclear. The Wnt/β-catenin signaling pathway plays a positive role in the wound healing process. This study aimed to investigate the effects of esculin on the rate and quality of skin wound healing in mice and explore its regulatory role in the Wnt/b-catenin signaling pathway.
Material and Methods
Circular full-thickness skin wounds with a diameter of 8 mm were created on the backs of C57BL/6 mice, which were administered with 20 and 40 mg•kg−1 esculin through gastric lavage. Wound healing was monitored, and samples collected on day 14 were analyzed through hematoxylin-eosin and Masson staining to assess granulation tissue formation and collagen deposition. Immunohistochemistry, immunofluorescence, and Western blot evaluated markers of collagen synthesis, proliferation, angiogenesis, and proteins in the Wnt/b-catenin signaling pathway. National institutes of health/3T3 cells treated with esculin (50 and 200 μM) were analyzed for proliferating cell nuclear antigen (PCNA) expression to assess proliferative activity.
Results
Compared with the model group, the esculin-treated groups exhibited significantly enhanced wound healing (P < 0.05), increased skin epithelial thickness (P < 0.01), and promoted extracellular matrix formation in mice. In addition, esculin significantly raised type I collagen alpha-1 chain and type III collagen alpha-1 chain protein levels (P < 0.05), boosted the expression of the cell proliferation marker PCNA and the vascular marker cluster of differentiation 31 in the dermis (P < 0.05), and upregulated proteins related to the Wnt/b-catenin signaling pathway and increased glycogen synthase kinase 3 beta phosphorylation in skin wound and NIH/3T3 cells (P < 0.05).
Conclusion
Esculin could upregulate and activate the Wnt/b-catenin signaling pathway to promote wound healing.
Keywords
Cell proliferation
Esculin
Skin
Wound healing
INTRODUCTION
Wound healing is a biological process aimed at repairing and restoring damaged tissues within the body.[1] It is a natural and dynamic response that occurs in stages.[2] The wound healing process can be broadly divided into three main stages: Inflammation, proliferation, and remodeling, which comprise crucial processes such as inflammation, angiogenesis, extracellular matrix (ECM) remodeling, and cell migration.[3,4] In addition, the healing process involves complex interactions between various cell types and signaling pathways.[5,6] The quality of wound healing plays a key role in ensuring optimal recovery and restoring normal function in damaged tissues, encompassing aspects such as healing speed, minimal scarring, and restoration of tissue integrity.[7-9] The importance of wound healing quality is widely recognized and emphasized in clinical practice and scientific research. Parameters such as wound closure rate and the formation of tissue granulation are crucial in evaluating the speed of wound healing. Furthermore, ECM components, cell proliferation (proliferating cell nuclear antigen [PCNA]), and angiogenesis (cluster of differentiation 31 [CD31]) in skin tissue are essential indicators of wound healing quality.[10-12] Different types of cells, including fibroblasts and myofibroblasts, produce collagen (type I collagen alpha-1 chain [Col1a1] and type III collagen alpha-1 chain [Col3a1]), which is crucial for wound closure and tissue repair.[13,14]
The Wnt/b-catenin signaling pathway is a key mechanism involved in the wound healing process.[15] It plays a crucial role in regulating cell proliferation, migration, and differentiation, which are essential for efficient wound healing.[16] During wound healing, the activation of the Wnt/b-catenin pathway promotes the cell proliferation of epithelial cells at the wound edges, contributing to wound closure.[17,18] It also enhances the migration of endothelial cells toward the wound site, promoting the formation of granulation tissue and angiogenesis.[19] In addition, the Wnt/b-catenin pathway influences the differentiation of fibroblasts into myofibroblasts, which are crucial for wound contraction.[20]
Esculin is a natural compound found in plants such as horse chestnut and seven-leaf clover. Its antibacterial, anti-inflammatory, antioxidant, and angiogenic properties have been studied, hinting at its potential in wound healing.[21] Previous literature has reported that esculin can protect against renal ischemia-reperfusion injury by activating the PI3K/Akt pathway,[22] and escin, a related compound, can protect against lipopolysaccharide/DGal-induced liver injury by suppressing inflammation and oxidative reactions.[23] However, there is still a lack of data supporting the potential and mechanisms of esculin in wound healing. Given the complexity of the wound healing process and the numerous microenvironmental factors involved, this study aimed to explore whether esculin has a pro-healing effect on skin wound healing and its impact on healing quality. The study also investigated the potential of esculin in regulating the Wnt/b-catenin pathway during the wound healing process. These findings provide new insights into the molecular mechanisms underlying the potential of esculin in wound healing.
MATERIAL AND METHODS
Experimental animals
Nine 8-week-old and male C57BL/6JNifdc mice were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. The mice were housed under constant conditions with a temperature of 22°C ± 2°C, humidity ranging from 50% to 60%, and a 12 h light/dark cycle.
Model establishment and drug administration
All animals were anesthetized with 1.0% (w/v) pentobarbital sodium through intraperitoneal injection at a dose of 40 mg/kg, and the backs of the mice were shaved. Two full-thickness skin wounds were created using an 8 mm ONLY disposable punch (#P850, Acuderm, USA).[24] According to previous reports about esculin dosage,[23] the animals were randomly divided into three groups: The model group, the 20 mg/kg esculin group, and the 40 mg/kg esculin group, with three mice in each group. Esculin (#E8230, Beijing Solarbio, China) was administered through oral gavage. We observed the wound healing progress daily and took photographs on days 0, 7, 10, and 14. The wound area was calculated with Image Pro Plus Software (version 6.0, Media Cybernetics, USA) at each time point.[25]
Histopathological staining
The mice were anesthetized and euthanized by intraperitoneal injections of pentobarbital sodium at a dose of 100 mg/kg. Appropriate amounts of wound skin tissue were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned into 5 μm-thick slices. Hematoxylin and eosin (H&E) staining (#G1120, Beijing Solarbio) was performed to observe the formation of wound granulation tissue and measure the thickness of the skin epithelium. Masson staining (#G1346, Beijing Solarbio) was used to assess the deposition of ECM in the tissue. Immunohistochemistry (IHC) staining was performed for the detection of Col1a1 (1:200 dilution, #A1352, ABclonal, China) and Col3a1 (1:200 dilution, #A3795, ABclonal) to evaluate the collagen content in the skin. The slides were deparaffinized, rehydrated, and treated with boiling citrate antigen retrieval buffer (sodium citrate buffer, 0.01 mol/L, pH 6.0) for 15 min. This was followed by blocking non-specific antibody binding with 5% goat serum (#SL038, Beijing Solarbio) at 37°C for 1 h. After overnight incubation with the primary antibody at 4°C, visualization was achieved using the streptavidinbiotin complex-peroxidase (rabbit Immunoglobulin G [IgG]) Kit (#SA1022, BOSTER, China). Images were captured using a Leica positive fluorescence microscope (THUNDER Imager 3D Tissue, Leica, Germany), and quantification was performed using Image-Pro Plus software for semi-quantitative evaluation of the results.
Immunofluorescence (IF)
Paraffin-embedded sections of mouse skin were deparaffinized, rehydrated, and treated with boiling citrate antigen retrieval buffer (sodium citrate buffer, 0.01 mol/L, pH 6.0) for 10 min. The slides were blocked with 5% (v/v) goat serum for 1 h at 37°C and then incubated overnight at 4°C with rabbit anti-CD31 antibody (1:200 diluted in 1% [v/v] goat serum solution, #db15306, Diagbio, China) and rabbit anti-PCNA antibody (1:200 diluted in 1% [v/v] goat serum solution, #db11523, Diagbio). Cells cultured in glass cell creep were fixed in cold 4% (w/v) paraformaldehyde for 20 min and then incubated with 0.5% (v/v) Triton X-100 at room temperature for 10min. Cell creep was blocked with 5% (v/v) goat serum for 1 h at 37°C and then incubated overnight at 4°C with rabbit anti-PCNA antibody. The slides or cell creep were washed and incubated for 1 h with coraLite488-conjugated goat anti-rabbit IgG (1:200 diluted in 1% [v/v] goat serum, #SA00013-2, Proteintech, China). Finally, all samples were mounted with an antifade mounting medium containing diamidino phenylindole (#36308ES20, YEASEN, Shanghai, China). Images were captured and quantified using a Leica-positive fluorescence microscope (THUNDER Imager 3D Tissue, Leica, Germany).
Western blot
After the tissue near the wound healing of the mouse skin was collected, the protein supernatant was extracted after homogenization in lysis buffer (#P0013B, Beyotime, Shanghai, China). The protein concentration was quantified by a bicinchoninic acid protein assay kit (#E112, Vazyme Technology, Nanjing, China), and the sample was prepared for Western blot. Western blot analysis was performed to detect the protein expression levels of the Wnt/b-catenin signaling pathway-related proteins, including Wingless-type MMTV integration site family, member 3A (Wnt3a) (1:1000 dilution, #db14992, Diagbio), glycogen synthase kinase 3 beta (GSK3b) (1:3000 dilution, #22104-1-AP, Proteintech), phosphorylated GSK3b (phosphorylated site Ser9, 1:3000 dilution, #67558-1-Ig, Proteintech), Snai1 (1:1000 dilution, #bs-1371R, Bioss, China), E-Cadherin (1:1000 dilution, #db6304, Diagbio), Vimentin (1:1000 dilution, #db11589, Diagbio), and b-catenin (1:5000 dilution, #66379-1-Ig, Proteintech). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:50,000 dilution, #60004-1-Ig, Proteintech) was used as an internal control. HRP-conjugated Affinipure Goat Anti-Mouse IgG (1:10000 dilution, #SA00001-1, Proteintech) and horseradish peroxidase-conjugated Affinipure Goat Anti-Rabbit IgG (1:10000 dilution, #SA00001-2, Proteintech) were used as secondary antibodies. The blots were developed using a highly sensitive enhanced chemiluminescence detection kit (#E411, Vazyme, China) and captured using a ChemiDoc XRS+ Chemiluminescence gel imager (BIORAD, USA). Protein bands were analyzed with the Image Lab software (version 6.1, BIO-RAD, California, USA), and protein expression was normalized to the expression levels of GAPDH.
Cell culture and assay
The NIH/3T3 cell line (#CL-0171, Pricella, Wuhan, China), fibroblast-like cells derived from mouse embryos, was cultured with 4.5 g/L D-glucose Dulbecco’s modified eagle medium (#C11995500BT, Gibco, USA) containing 10% fetal bovine serum (#10270106, Gibco) and 1% penicillin and streptomycin mixture (#CB010, Epizyme, Shanghai, China). The cell lines used in this study have all undergone short tandem repeat authentication and tested negative for mycoplasma. After starvation, NIH/3T3 cells in a six-well plate were treated with 0, 50, and 200 μM esculin for 24 h, collected, and sampled for further tests.[26] The effect of esculin in promoting cell proliferation was determined through PCNA-positive staining by IF. The Wnt/b-catenin signaling pathway-related proteins in NIH/3T3 cells after esculin treatment were determined by Western blot.
Statistical analysis
One-way analysis of variance (Tukey’s multiple comparisons test) was employed for comparisons among multiple groups. Prism version 9.0 software (version 8.0, GraphPad Software, San Diego, California, USA, https://www.graphpad-prism.cn/) was utilized for statistical analysis of the data. In vivo experiments involved three mice in each group, whereas in vitro experiments involved three replicates in each group. All data were expressed as mean ± standard deviation. P < 0.05 was considered statistically significant.
RESULTS
Esculin accelerates wound healing in mice
To evaluate the potential of esculin in improving skin wound healing in mice, Figure 1a presents a series of representative wound images captured on days 0, 7, 10, and 14 during different treatment periods. As shown in Figure 1b, compared with the model group, the esculin-treated group exhibited significant improvements in skin wound closure rates in C57BL/6 mice on days 7, 10, and 14 (P < 0.05). Specifically, on day 7, the wound closure rate of the group receiving 40 mg/kg esculin demonstrated a significant increase by 17.69% compared with that of the group receiving 20 mg/kg (P < 0.05, 76.893% ± 3.547% vs. 65.337% ± 6.456%). However, on days 10 and 14, the wound closure rate showed no significant difference between the group receiving 20 and 40 mg/kg (P > 0.05).
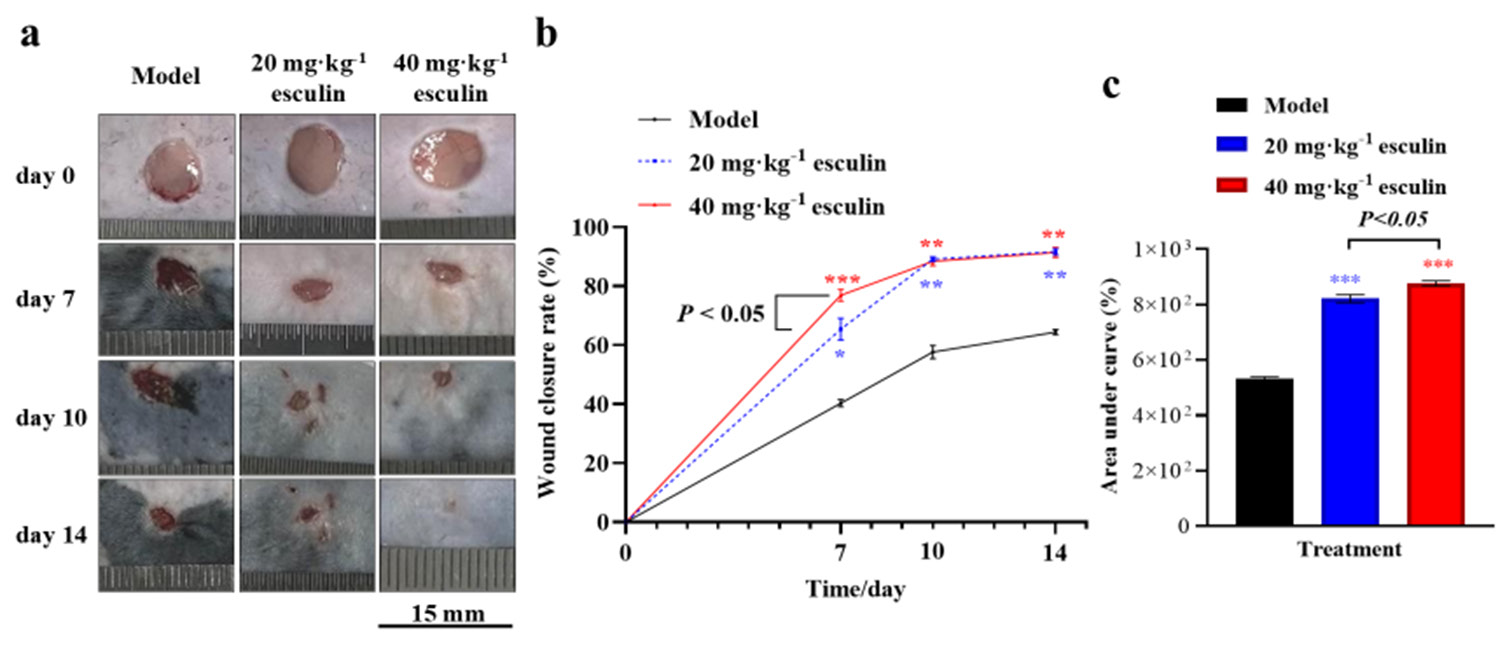
- Wound healing of mice skin. (a) Skin wound of mice (scale bar 15 mm, n = 3 in each time and group). (b) Wound healing rate. (c) Area under the curve for healing rates (n = 3). Compared with the model group, ✶P < 0.05, ✶✶P < 0.01, and ✶✶✶P < 0.001.
Furthermore, analysis of the area under the curve for healing rates revealed that the 20 and 40 mg/kg esculin-treated groups promoted the wound closure rate compared with the model group ([Figure 1c], P < 0.001, 876.6% ± 16.2% and 821.8% ± 25.1% vs. 531.9% ± 13.0%). In addition, the 40 mg/kg esculin-treated group exhibited an overall improvement of 6.67% in promoting the wound closure rate in mice compared with the group receiving 20 mg/kg ([Figure 1c], P < 0.05).
Esculin enhances granulation tissue formation and epithelial regeneration
Moreover, histological analysis was conducted through H&E staining on skin tissues from each group on day 14 to evaluate the improvement in granulation tissue formation in the esculin-treated groups. The results confirmed that the esculin-treated groups exhibited significant advantages in histopathological repair [Figure 2a]. Compared with the model group, the 40 mg/kg esculin-treated group showed an intact epithelium, with orderly arranged collagen in the dermis. In addition, the peripheral region was well-integrated into the surrounding native skin tissue, and numerous hair follicles were observed. By contrast, the dermis in the 20 mg/kg esculin-treated group still exhibited defects.
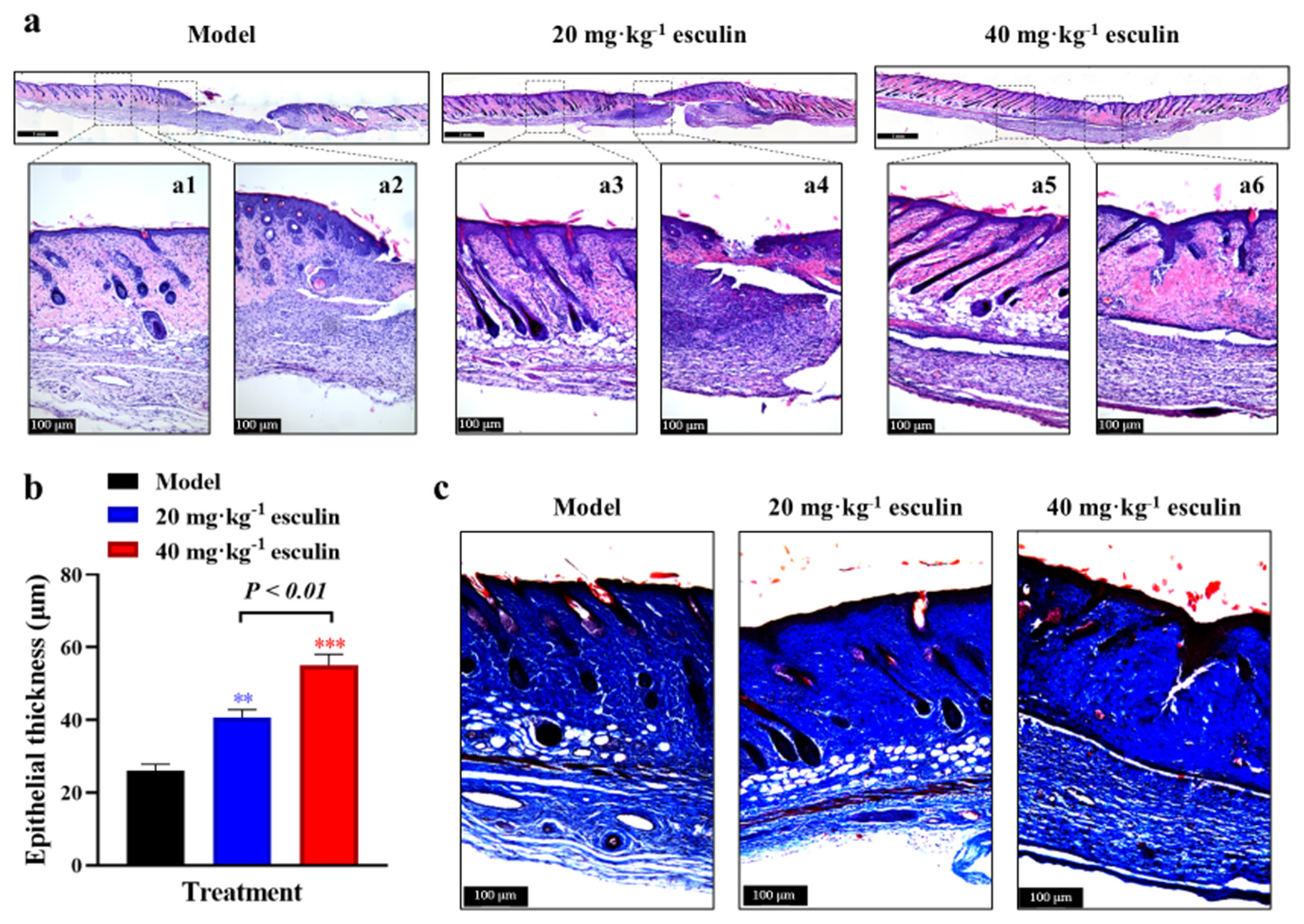
- Granulation tissue formation and epithelial regeneration of mice skin. (a) H&E staining of full thickness of skin on day 14 (10x objective magnification in tile mode with scale bar 1 mm and single capture mode with scale bar 100 μm). (b) Statistical analysis of epidermis thickness (n = 3). (c) Masson staining of skin on day 14 (10 fold objective magnification with scale bar 100 μm). Compared with the model group, ✶✶P < 0.01, ✶✶✶P < 0.001. H&E: Hematoxylin and eosin.
To assess the effect of esculin on epithelial regeneration during wound healing, we measured the thickness of the epithelium on day 14. As shown in Figure 2b, the 20 and 40 mg/kg esculin-treated groups exhibited significantly thicker epidermal layers than the model group (P < 0.01, 40.67 ± 5.346 μm and 55.09 ± 7.437 μm vs. 26.05 ± 4.371 μm). In addition, the 40 mg/kg esculin-treated group exhibited an overall improvement of 35.46% in the 20 mg/kg esculin-treated group (P < 0.01). This finding suggested that esculin treatment effectively promoted epithelial regeneration, contributing to rapid wound healing.
Esculin promotes collagen deposition in wound tissue
Collagen, as the primary component of the ECM, provides structural support to tissues and plays a crucial role in wound healing. Masson staining revealed that esculin significantly promoted the deposition of total collagen, visualized as blue staining, in the skin tissue of the wound area [Figure 2c]. Given that type I and type III collagen play key roles in the formation of new ECM, IHC staining was used to assess the levels of Col1a1 and Col3a1 proteins. As shown in Figure 3a-d, compared with the model group, significant increases in Col1a1 and Col3a1 protein levels were observed in the esculin-treated groups on day 14 (P < 0.05 or P < 0.01). However, the 40 mg/kg esculin-treated group only exhibited a slight increase in the expression of Col1a1 and Col3a1 protein than the 20 mg/kg esculin-treated group, and the difference was not significant (P > 0.05).

- Results of promoted collagen expression. (a) IHC of Col1a1 in dermis (40-fold objective magnification with scale bar 100 μm). (b) Statistical analysis of Col1a1 measured with IHC in dermis (n = 3). (c) IHC of Col3a1 in dermis (40x objective magnification with scale bar 100 μm). (d) Statistical analysis of Col3a1 measured with IHC in dermis (n = 3). Compared with the model group, ✶P < 0.05, ✶✶P < 0.01. IHC: Immunohistochemistry, Col1a1: Type I collagen alpha-1 chain, Col3a1: Type III collagen alpha-1 chain.
Esculin induces cell proliferation and angiogenesis
To evaluate the proliferative effects, we assessed the expression of PCNA in the tissue on day 14. IF staining revealed significantly higher PCNA expression in the 20 and 40 mg/kg esculin-treated groups compared with that in the model group (1.531- and 1.554-fold, respectively, P < 0.01, [Figure 4a and b]). Newly formed blood vessels provide a network of oxygen and nutrient supply to the healing tissue, which is crucial for the survival and function of cells involved in wound healing. IF staining for CD31 demonstrated that esculin significantly promoted angiogenesis in the skin wound, resulting in a significant increase in CD31 positive staining in the 20 and 40 mg/kg esculin-treated groups compared with the model group (1.373- and 1.425-fold, respectively, P < 0.05, [Figure 4c and d]). However, compared with the 20 mg/kg esculin-treated group, the 40 mg/kg esculin-treated group exhibited no statistical significance in the expression levels of PCNA and CD31 proteins (P > 0.05).
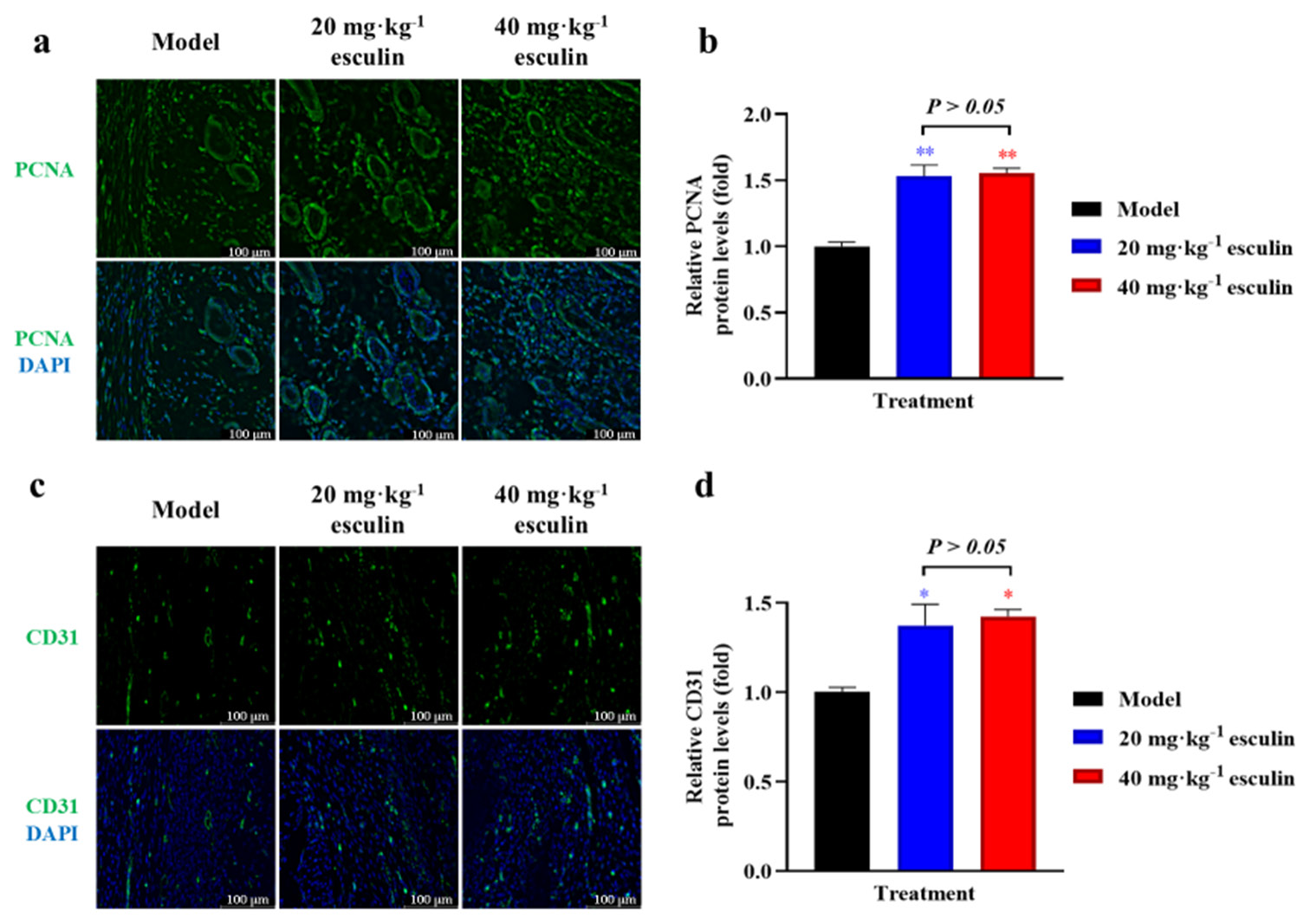
- Results of promoted cell proliferation and angiogenesis. (a) IF of PCNA in dermis (40x objective magnification with scale bar 100 μm). (b) Statistical analysis of PCNA measured with IF in dermis (n = 3). (c) IF of CD31 in dermis (40x objective magnification with scale bar 100 μm). (d) Statistical analysis of CD31 measured with IF in dermis (n = 3). Compared with the model group, ✶P < 0.05, ✶✶P < 0.01. PCNA: Proliferating cell nuclear antigen, CD31: Cluster of differentiation 31, also known as platelet/endothelial cell adhesion molecule 1, IF: Immunofluorescence.
Esculin significantly activates the Wnt/β-catenin signaling pathway
In this study, we aimed to investigate whether esculin exerts its wound-healing effects through the Wnt/b-catenin signaling pathway. Therefore, we examined the expression levels of proteins associated with the Wnt/b-catenin signaling pathway in the wound tissue. As shown in Figures 5a and b, compared with the model group, the esculin-treated groups significantly upregulated the protein expression levels of Wnt3a, GSK3b, Snail, E-cadherin, Vimentin, and b-catenin in the mouse wounds (P < 0.05 at least). Furthermore, the 40 mg/kg esculin-treated group exhibited an overall improvement of 69.6% in protein expression of Wnt3a in mice compared with the group receiving 20 mg/kg ([Figure 5b], P < 0.01). The transmission of the Wnt pathway relies on GSK3b, and Western blot results demonstrated that esculin significantly promoted the phosphorylation level of GSK3b ([Figure 5], P < 0.05). These findings confirmed that esculin significantly upregulated and activated the Wnt/b-catenin signaling pathway during the wound healing process in mice.
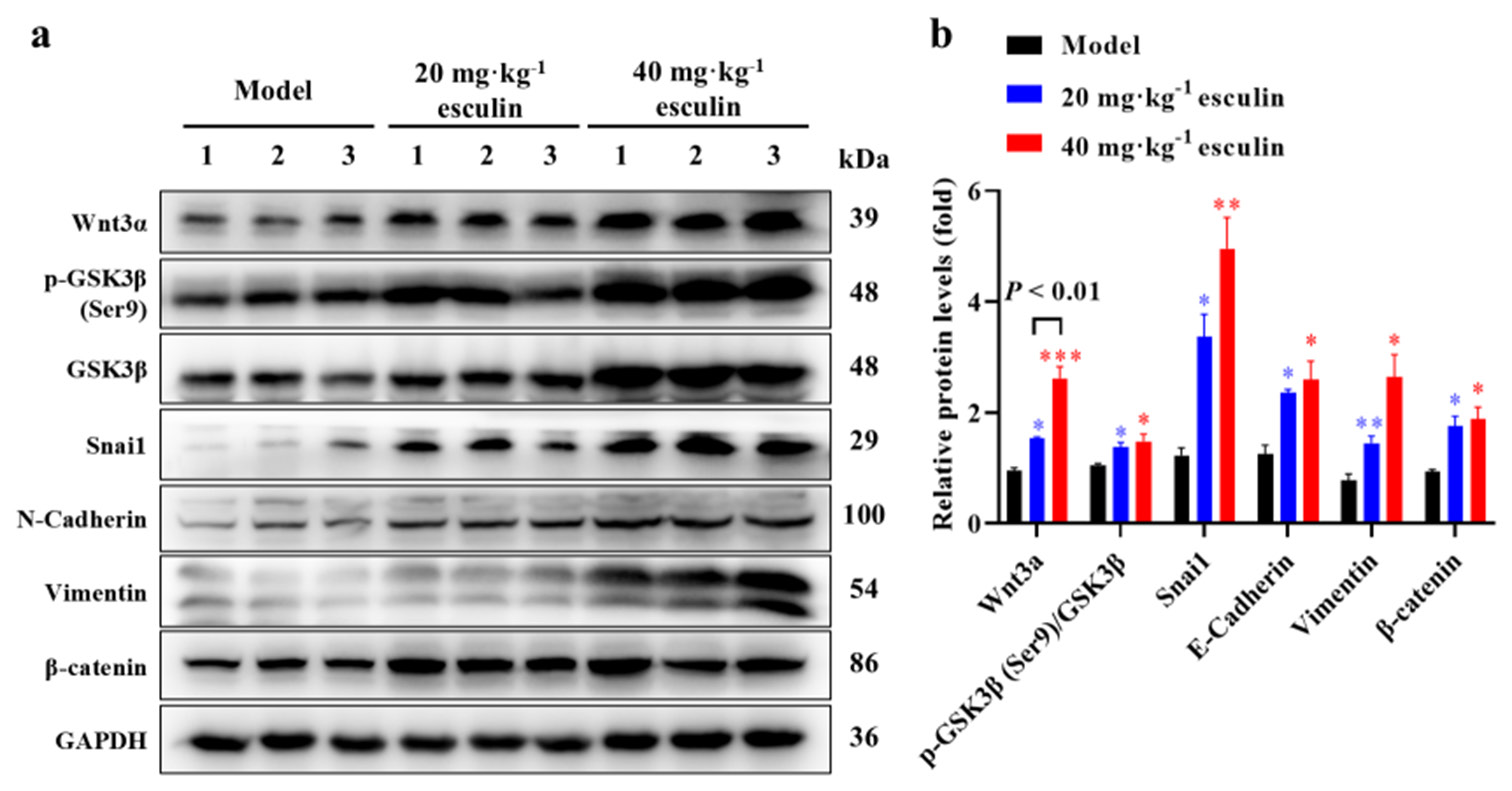
- Results of the activated Wnt/b-catenin signaling pathway. (a) Western blot of Wnt3a, GSK3b, phosphorylated GSK3b, Snai1, N-Cadherin, Vimentin, and b-catenin in wound section on day 14. (b) Statistical analysis of the above protein expression measured with Western blot (n = 3). Compared with the model group, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. Wnt3a: Wingless-type MMTV integration site family, member 3A, GSK3b: Glycogen synthase kinase 3 beta, p-GSK3b: Phosphorylated glycogen synthase kinase 3 beta, Snail1: Snail family transcriptional repressor 1.
Esculin significantly promotes fibroblast cell proliferation and activation of the Wnt/b-catenin signaling pathway
In the results depicted in Figure 4a, we observed that the positive staining for PCNA was predominantly concentrated in the dermal layer and around the hair follicles in the esculin-treated group. Considering that fibroblasts are the primary constituent cells of the dermal layer, we hypothesized whether esculin exerts a proliferative effect on fibroblasts. As illustrated in Figures 6a and b, compared with the control group, the 50 and 200 μM esculin-treated groups significantly upregulated the mean intensity of PCNA positive staining in NIH/3T3 cells (P < 0.05 at least). Furthermore, compared with the 50 μM esculin-treated group, the esculin intensity of PCNA positive staining in the 200 μM esculin-treated NIH/3T3 cells increased by 1.26-fold ([Figure 6b], P < 0.05). Considering that the Wnt/b-catenin signaling pathway is involved in promoting fibroblast proliferation, we examined the expression levels of proteins related to the Wnt/b-catenin signaling pathway in NIH/3T3 cells treated with esculin. Western blot results revealed that the protein expression levels of Wnt3a, Snail, N-Cadherin, Vimentin, and b-catenin in NIH/3T3 cells, as well as the phosphorylation level of GSK3b (Ser9), were significantly upregulated after 50 and 200 μM esculin treatment ([Figure 6c and d], P < 0.05 at least) when compared with the control group.
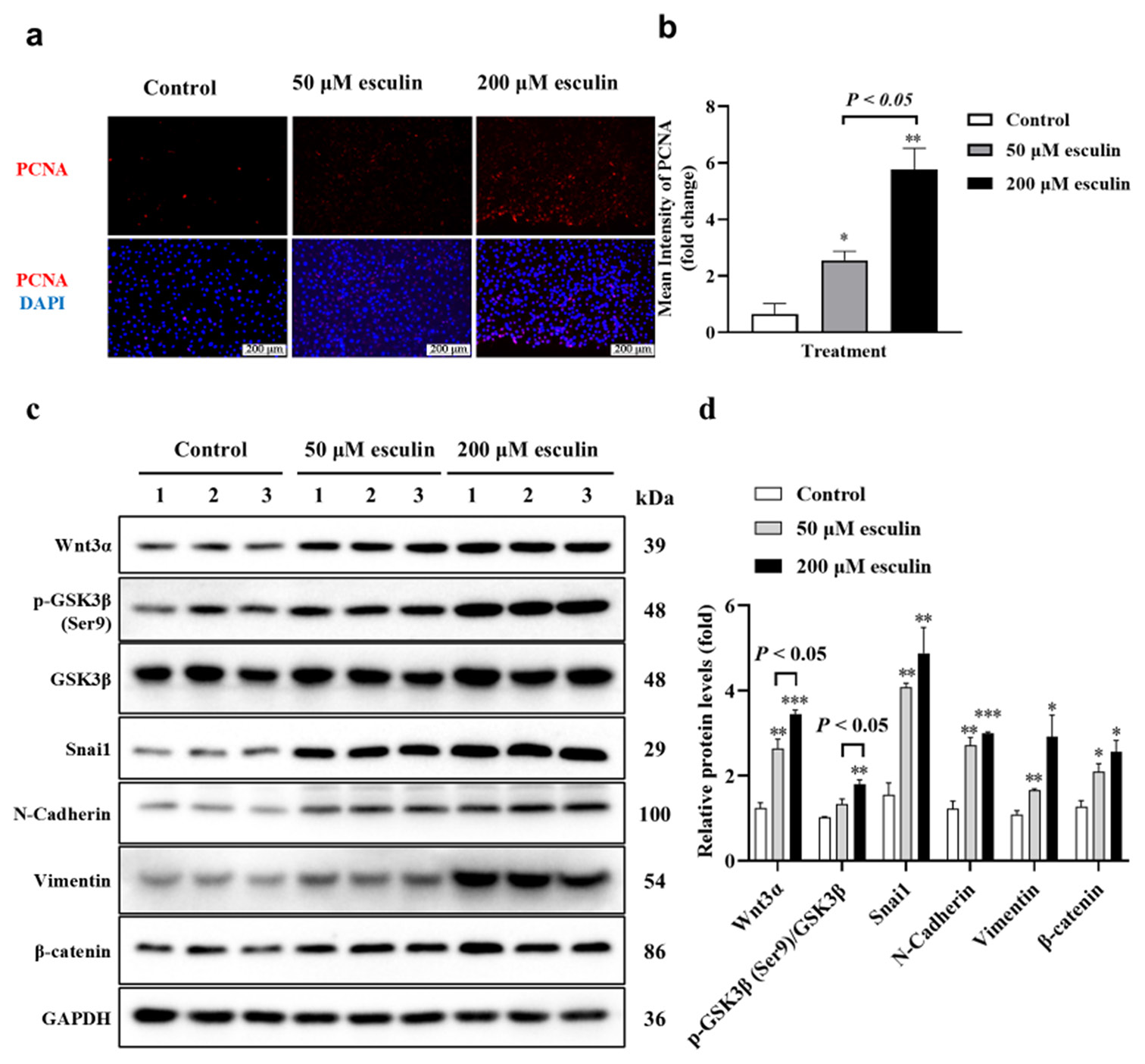
- Results of effect of esculin treatment on promoted cell proliferation and activated Wnt/b-catenin signaling pathway in NIH/3T3 cells. (a) IF of PCNA in NIH/3T3 cells (20 fold objective magnification with scale bar 200 μm). (b) Statistical analysis of mean intensity of PCNA measured with IF in NIH/3T3 cells (n = 3). (c) Western blot of Wnt3a, GSK3b, phosphorylated GSK3b, Snai1, N-Cadherin, Vimentin, and b-catenin in NIH/3T3 cells (d) Statistical analysis of above protein expression measured with Western blot (n = 3). Compared with the control group, ✶P < 0.05, ✶✶P < 0.01, ✶✶✶P < 0.001. PCNA: Proliferating cell nuclear antigen, Wnt3a: Wingless-type MMTV integration site family, member 3A, GSK3b: Glycogen synthase kinase 3 beta, p-GSK3b: Phosphorylated glycogen synthase kinase 3 beta, Snail1: Snail family transcriptional repressor 1, IF: Immunofluorescence.
DISCUSSION
Wound healing is a complex biological process that involves a series of interconnected events aimed at repairing damaged skin tissue.[4] The quality of wound healing refers to the degree and effectiveness of the healing process, encompassing factors such as healing speed, appearance of scars, and restoration of normal skin function.[3,27,28] In this study, we comprehensively demonstrated that 20 and 40 mg/kg esculin treatment significantly promoted skin wound healing in mice from multiple aspects.
Initially, healthy granulation tissue, composed of neovascularization, fibroblasts, and ECM filling the wound bed, serves as a scaffold for tissue regeneration and provides a foundation for re-epithelialization. Our results confirmed that esculin significantly promoted the formation of granulation tissue, enhanced the speed and thickness of epithelialization, and accelerated wound closure. However, in terms of dosage, 40 mg/kg esculin treatment only showed a slight advantage in promoting wound healing on day 7, so the difference between the two doses must be increased in the future. Moreover, on day 14, the wounds of the model group still showed significant unhealed areas, which easily affected the integrity of the experimental skin and interfered with the experimental results.
During the wound-healing process, collagen is synthesized and organized to restore skin integrity.[29,30] Correct collagen synthesis and remodeling are crucial for achieving strong and flexible scars, thereby improving the quality of healing. Col1a1 and Col3a1 play different physiological functions in the strength and toughness of the later stage of skin wound healing. However, in our study, esculin significantly promoted the expression of both collagen proteins. Thus, our findings only confirmed that esculin treatment significantly increased collagen deposition in the dermis. Unfortunately, our results could not fully reflect the relative difference between the two kinds of collagen, omitting the difference in the final quality of wound healing. This study also indicated that the healing time of mice in the experiment was insufficient to accurately reflect the skin healing process.
Cell proliferation and angiogenesis are two critical processes in skin wound healing that play essential roles in achieving successful and effective tissue repair.[31] Cell proliferation is responsible for the rapid growth and multiplication of cells during wound healing, whereas angiogenesis involves the formation of new blood vessels, crucial for supplying oxygen, nutrients, and immune cells to the wound. Our results demonstrated that esculin administration significantly increased the expression of PCNA in the epidermis and dermis. The vascular marker CD31 is primarily distributed in the dermis, and esculin treatment significantly promotes the process of angiogenesis. However, as a result of limitations in sample size and timing, this study did not investigate the dynamic regulation of esculin on inflammatory responses during skin wound healing, which remains a future research direction.
Another contribution of this study is the discovery that esculin potentially promoted cell proliferation by upregulating proteins related to the Wnt/b-catenin pathway and activating the phosphorylation levels of these proteins. However, the proliferating cells during wound healing include fibroblasts and keratinocytes, among others.[32] During proliferation, fibroblasts proliferate and migrate to the wound bed, synthesizing and depositing collagen to strengthen the wound.[33,34] Keratinocytes migrate from the wound edges to close the wound and restore the protective barrier function of the skin.[6,35] In this study, the results presented in Figure 6 demonstrated that esculin significantly enhanced the proliferative capacity of mouse fibroblasts, accompanied by the activation of the Wnt/b-catenin signaling pathway. Further investigation is required to elucidate the effects of esculin on other aspects of fibroblast function, as well as its impact on the functionality of other types of cells.
SUMMARY
This study clarified the potential therapeutical effect of esculin on promoting skin wound healing, which might benefit from accelerated granulation tissue formation, epithelial regeneration, collagen deposition, cell proliferation, and vascular regeneration through regulating the Wnt/b-catenin signaling pathway in mice. Our results provide new insights for the development of esculin, directing its clinical application.
AVAILABILITY OF DATA AND MATERIALS
The data and materials that support the findings of this study are available from the corresponding author on reasonable request.
ABBREVIATIONS
CD31: Cluster of differentiation 31
Col1a1: Type I collagen alpha-1 chain
Col3a1: Type III collagen alpha-1 chain
GSK3β: Glycogen synthase kinase 3 beta
PCNA: Proliferating cell nuclear antigen
p-GSK3β: Phosphorylated glycogen synthase kinase 3 beta
Snail1: Snail family transcriptional repressor 1.
Wnt3α: Wingless-type MMTV integration site family, member
AUTHOR CONTRIBUTIONS
MX and MSZ: Designed the study, all authors conducted the study; JJW, JMW, and HZW: Collected and analyzed the data; MX, MSZ, and XTX: Participated in drafting the manuscript, and all authors contributed to critical revision of the manuscript for important intellectual content. All authors gave final approval of the version to be published. All authors participated fully in the work, take public responsibility for appropriate portions of the content, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or completeness of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENT
Not Applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the Animal Ethics Committee of Jinzhou Medical University (Ethical Approval No.2023109 date: 8 June 2023). This study does not involve patients, so informed consent is not required.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: Not Applicable.
References
- Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370-8.
- [CrossRef] [PubMed] [Google Scholar]
- Wound healing: A cellular perspective. Physiol Rev. 2019;99:665-706.
- [CrossRef] [PubMed] [Google Scholar]
- Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25:599-616.
- [CrossRef] [PubMed] [Google Scholar]
- Innate immune cell-epithelial crosstalk during wound repair. J Clin Invest. 2019;129:2983-93.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21:18-24.
- [CrossRef] [PubMed] [Google Scholar]
- Breakthrough treatments for accelerated wound healing. Sci Adv. 2023;9:eade7007.
- [CrossRef] [PubMed] [Google Scholar]
- Plasticity of epithelial cells during skin wound healing. Cold Spring Harb Perspect Biol. 2023;15:a041232.
- [CrossRef] [PubMed] [Google Scholar]
- Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther. 2019;10:247.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cell aggregation-released extracellular vesicles induce CD31+ EMCN+ vessels in skin regeneration and improve diabetic wound healing. Adv Healthc Mater. 2023;12:E2300019.
- [CrossRef] [PubMed] [Google Scholar]
- Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12:257.
- [CrossRef] [PubMed] [Google Scholar]
- Diversity of fibroblasts and their roles in wound healing. Cold Spring Harb Perspect Biol. 2023;15:a041222.
- [CrossRef] [PubMed] [Google Scholar]
- Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161-80.
- [CrossRef] [PubMed] [Google Scholar]
- Regulatory processes of the canonical Wnt/β-catenin pathway and photobiomodulation in diabetic wound repair. Int J Mol Sci. 2022;23:4210.
- [CrossRef] [PubMed] [Google Scholar]
- Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb Perspect Biol. 2013;5:a008029.
- [CrossRef] [PubMed] [Google Scholar]
- Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol Ther. 2019;196:79-90.
- [CrossRef] [PubMed] [Google Scholar]
- Wnt signaling in adult epithelial stem cells and cancer. Prog Mol Biol Transl Sci. 2018;153:21-79.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress-induced endothelial cells-derived exosomes accelerate skin flap survival through Lnc NEAT1-mediated promotion of endothelial progenitor cell function. Stem Cell Res Ther. 2022;13:325.
- [CrossRef] [PubMed] [Google Scholar]
- Skin fibrosis and recovery is dependent on Wnt activation via DPP4. J Invest Dermatol. 2022;142:1597-606.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological activities and synthesis of esculetin and its derivatives: A mini-review. Molecules. 2017;22:387.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of esculin on streptozotocin-induced diabetic renal damage in mice. J Agric Food Chem. 2014;62:2069-76.
- [CrossRef] [PubMed] [Google Scholar]
- Esculin prevents Lipopolysaccharide/D-Galactosamine-induced acute liver injury in mice. Microb Pathogen. 2018;125:418-22.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of heparin-poloxamer hydrogel modified bFGF and aFGF for in vivo wound healing efficiency. ACS Appl Mater Interfaces. 2016;8:18710-21.
- [CrossRef] [PubMed] [Google Scholar]
- Higher biostability of rh-aFGF-Carbomer 940 hydrogel and its effect on wound healing in a diabetic rat model. ACS Biomater Sci Eng. 2018;4:1661-8.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of compounds that inhibit the binding of Keap1a/Keap1b Kelch DGR domain with Nrf2 ETGE/DLG motifs in zebrafish. Basic Clin Pharmacol Toxicol. 2019;125:259-70.
- [CrossRef] [PubMed] [Google Scholar]
- Wound healing and skin regeneration. Cold Spring Harb Perspect Med. 2015;5:a023267.
- [CrossRef] [PubMed] [Google Scholar]
- Nematic fibrin fibers enabling vascularized thrombus implants facilitate scarless cutaneous wound healing. Adv Mater. 2023;35:e2211149.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harbor Perspect Biol. 2011;3:a005124.
- [CrossRef] [PubMed] [Google Scholar]
- A strain-programmed patch for the healing of diabetic wounds. Nat Biomed Eng. 2022;6:1118-33.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose mesenchymal stem cell derived exosomes promote keratinocytes and fibroblasts embedded in collagen/platelet-rich plasma scaffold and accelerate wound healing. Adv Mater. 2023;35:e2303642.
- [CrossRef] [PubMed] [Google Scholar]
- Fibroblast state switching orchestrates dermal maturation and wound healing. Mol Syst Biol. 2018;14:e8174.
- [CrossRef] [PubMed] [Google Scholar]
- Mechano-activated cell therapy for accelerated diabetic wound healing. Adv Mater. 2023;35:e2304638.
- [CrossRef] [PubMed] [Google Scholar]
- Reversing stratification during wound healing. Nat Cell Biol. 2017;19:595-7.
- [CrossRef] [PubMed] [Google Scholar]









