Translate this page into:
Expression and clinical significance of programmed death ligand-1 evaluated by 22C3 antibody in pleural effusion metastatic non-small-cell lung cancer

Lingchuan Guo

Shan Huang
*Corresponding author: Lingchuan Guo, Department of Pathology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China. szglc@hotmail.com
Shan Huang, Department of Pathology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China. goatsz@163.com
-
Received: ,
Accepted: ,
How to cite this article: Gu D, Hu L, Huang S, Guo L. Expression and clinical significance of programmed death ligand-1 evaluated by 22C3 antibody in pleural effusion metastatic non-small-cell lung cancer. CytoJournal. 2024;21:70. doi: 10.25259/Cytojournal_59_2024
Abstract
Objective:
Programmed death ligand-1 (PD-L1) is involved in tumor immune escape and is an important target molecule for the immunotherapy of non-small-cell lung cancer (NSCLC). The expression of PD-L1 affects NSCLC invasion, metastasis, and patient survival. This study aims to explore the levels of PD-L1, as identified by the 22C3 antibody, in the malignant pleural effusion of patients suffering from advanced NSCLC, and to determine its clinical implications.
Material and Methods:
A two-step immunohistochemical EnVision assay was used to evaluate the expression of PD-L1 by the 22C3 antibody in 149 malignant pleural fluid cell wax clots of NSCLC. The relationship between PDL1 expression and clinicopathological characteristics, anaplastic lymphoma kinase (ALK) expression, epidermal growth factor receptor (EGFR) mutation, and overall survival (OS) time of patients with NSCLC was analyzed.
Results:
Positive expression of PD-L1 in malignant pleural fluid of NSCLC was observed as follows: Positive (<1%: 11.4%), positive (1–49%: 19.5%), and positive (≥50%: 11.4%), with a total positive rate of 42.3%. There was a significant association between PD-L1-positive expression and factors such as tumor differentiation, lymph node metastasis, and metastasis to other organs (P < 0.05). Furthermore, PD-L1 expression showed a positive correlation with ALK expression (rs = 11.49, P < 0.05) but did not correlate with EGFR mutations (rs = 0.004, P > 0.05). Significant differences in median OS were observed between patients exhibiting positive PD-L1 expression and those without, according to survival follow-up data (P < 0.05).
Conclusion:
Immunohistochemical detection of PD-L1 expression in malignant pleural fluid of advanced NSCLC provides a basis for clinical tumor immunotherapy. Immunohistochemical detection of PD-L1 expression in malignant pleural fluid of advanced NSCLC is minimally invasive, simple, and fast, particularly for metastatic NSCLC where malignant pleural fluid is the first symptom, offering significant clinical application value.
Keywords
Pleural effusion
Non-small-cell lung carcinomas
Immune checkpoint inhibitors
Immunohistochemistry
INTRODUCTION
Lung cancer ranks as one of the most common cancers, leading in both incidence and mortality rates globally. In 2020, an estimated 2.2 million new cancer cases and 1.8 million deaths were attributed to lung cancer, making it the second most common cancer and the leading cause of cancer death. Lung cancer accounted for approximately 11.4% of all cancer diagnoses and 18.0% of cancer deaths.[1] Non-small-cell lung cancer (NSCLC) represents 80–85% of lung cancer cases. Of these, 20% of newly diagnosed lung cancer cases are in stages I and II, and 30% are in stage III.[2] After the diagnosis of NSCLC, only about 26% of patients survive for more than 5 years, and for advanced patients, the 5-year survival rate is only 6% with traditional chemotherapy.[3]
Lung cancer stands as the most prevalent malignant tumor, with NSCLC constituting over 80% of cases.[4] Due to its unique anatomical structure, tumors are predisposed to hematologic or lymphatic metastases. Moreover, mid to late-stage NSCLC frequently presents with malignant pleural fluid, resulting in a dismal clinical prognosis. Despite the successful integration of clinically targeted drugs, which has enhanced patients’ quality of life and moderately prolonged survival, individuals with intermediate and advanced NSCLC still encounter challenges such as restricted gene-targeted therapy options or drug resistance in later stages of treatment. Immunotherapy represents a burgeoning area in cancer research. Investigating the mechanisms of the tumor immune checkpoint (Programmed death receptor-1 [PD-1]/Programmed death ligand-1 [PD-L1])-related pathways holds promise for furnishing novel theoretical underpinnings for the immunotherapy of clinical NSCLC.[5]
PD-1, also referred to as CD279, is a member of the B7/CD28 receptor superfamily. T-cell hybridoma assay conducted by Ishida et al. in 1992, utilizing the ablative hybridization technique, it earned the designation of the programmed death molecule.[6] PD-L1, the principal ligand of PD-1, is commonly expressed on the cell membrane surfaces of various immune cells including T-cells, B-cells, NK T-cells, macrophages, as well as non-immune cells such as mesenchymal stem cells and cancer cells. Serving as an immunosuppressive agent, PD-L1 regulates its expression and is notably abundant on the surfaces of lymphohematopoietic or cancer cells, predominantly induced by interferon-γ. Current research underscores the pivotal role of the PD1/PD-L1 pathway in enabling tumor immune evasion.[7] The active PD-1/PD-L1 axis within the tumor microenvironment facilitates immune escape by attenuating the anti-tumor immune response. Many tumors exhibit growth and progression correlated with this immune evasion mechanism mediated through the PD1/PD-L1 pathway.
Malignant serous effusion is a common complication of NSCLC, indicating advanced disease progression.[8] Malignant pleural effusion can cause lung compression, leading to dyspnea and impaired respiratory function.[9] The prognosis for patients with malignant pleural effusion is poor, significantly reducing survival time.[10] Traditional treatment methods have limited efficacy against malignant serous effusion, necessitating the search for effective drug treatments to control disease progression and improve patient quality of life.[11]
Immunotherapy has become a focal point in cancer research. Immunotherapies, particularly those involving PD-1 and PDL1 monoclonal antibodies, have become important treatment options for the first-line and subsequent treatment of NSCLC. Studies suggest that malignant pleural effusion creates a tumor tolerance environment complicated by immunosuppressive factors. Immunotherapy may benefit patients with malignant pleural effusion by stimulating tumor-specific immune responses in the pleural cavity to counteract the tumor tolerance environment.[12] However, clinical research and application of immunotherapy in metastatic NSCLC patients with malignant pleural effusion remain limited. PD-L1 is currently the most valuable predictor of NSCLC immunotherapy response.
This study aims to detect the expression of PD-L1 in 149 cases of NSCLC malignant pleural effusion cell paraffin block samples using immunochemistry (IHC). It also seeks to analyze the relationship between PD-L1 expression and clinicopathological characteristics, anaplastic lymphoma kinase (ALK) expression, epidermal growth factor receptor (EGFR) mutation, and overall survival (OS) time of NSCLC patients. The goal is to provide a theoretical basis for the immunotherapy of metastatic NSCLC patients with pleural effusion.
MATERIAL AND METHODS
Materials
One hundred and forty-nine specimens with malignant pleural fluid confirmed by IHC of cellular wax blocks as metastatic NSCLC were collected from December 2017 to December 2019 at the First Affiliated Hospital of Soochow University. The study included 72 males and 77 females (male-to-female ratio approximately 1:1.07), with patient ages ranging from 37 to 92 years (median age 65 years). Ninety-three cases had tumor diameters ≤3 cm, while 56 cases had tumor diameters >3 cm. Among these, 55 cases were smokers, and 94 cases were non-smokers. Two experienced pathologists classified each case according to the World Health Organization classification criteria for respiratory tumors: [13] 144 adenocarcinomas and 5 squamous cell carcinomas; 79 high- and moderately differentiated carcinomas and 70 low-grade carcinomas; 76 cases with lymph node metastases, 73 cases without lymph node metastases, 61 cases with distant organ metastases; 74 cases with pleural involvement; and 75 cases without pleural involvement.
The inclusion criterion for this study was as follows: Diagnosis and treatment of 149 cases with malignant pleural effusion confirmed by IHC of cellular wax blocks as metastatic NSCLC between December 2017 and December 2019 at the First Affiliated Hospital of Soochow University.
The exclusion criteria were as follows: Patients with benign pleural effusion, patients with metastatic small-cell lung cancer and pleural effusion other than metastatic NSCLC, or patients with metastatic non-lung cancer pleural effusion.
Cytological smears and sediment embedding
Clinical samples consisted of 200 mL of pleural fluid, from which 10 mL was taken into a centrifuge tube, centrifuged at 2,500 r/min for 5 min, and the supernatant discarded. The precipitate, excluding red blood cells, was treated and centrifuged again. Two smears of the precipitate were taken and stained with hematoxylin and eosin (HE) for observation under light microscopy (Olympus Corporation, Japan). The remaining specimens were processed similarly, with the sediment embedded into cell wax blocks, fixed in 10% formalin (Shanghai Haling Biotechnology Co., Ltd, China), dehydrated, paraffin-embedded, and sectioned at 4 μm. These sections underwent corresponding HE and IHC staining for light microscopy observation.
HE staining
The specific operation steps of HE dyeing are as follows: (1) Baking sheet: Bake the cell wax block slices at 60 fixed in 10% formalin, (2) Dewaxing: Dewaxing with xylene twice, 5 min each time. (3) Water washing: Wash xylene with anhydrous ethanol, and then wash with ethanol of different concentrations (95%, 85%). (4) Nuclear staining: Stain the sections in hematoxylin dye solution for 5 min, then wash off the excess dye with water, then differentiate with 1% hydrochloric acid ethanol for a few seconds, then rinse with warm water for 5 min for bluing. (5) Cytoplasmic staining: Put the sections into eosin stain solution for a few seconds, then wash with ethanol of different concentrations (85%, 95%), and finally dehydrate with anhydrous ethanol. (6) Clearing: clear twice with xylene, 1 min each time. (7) Mounting: Mount with neutral gum and finish HE dyeing.
Immunohistochemistry
The main antibodies tested included: ALK (clone number: D5F3; Ready-to-use antibody), PD-L1 (clone number: 22C3; Ready-to-use antibody), Epithelial cell adhesion molecule (EpCAM) (clone number: UMAB131; Ready-touse antibody), cytokeratin 7 CK7 (clone number: UMAB161; Ready-to-use antibody), Thyroid transcription factor 1 (TTF1) (clone number: 8G7G3/1; Ready-to-use antibody), Napsin A (clone number: OTI85A; Ready-to-use antibody), CK5/6 (clone number: OTI1C7; Ready-to-use antibody), P40 (clone number: BC28; Ready-to-use antibody), Calretinin, and Ki-67 (clone number: MIB-1; Ready-to-use antibody). The above antibodies were purchased from ZSGB-BIO in Guangzhou, China. The ALK antibody was purchased from VENTANA, Switzerland, the PD-L1 antibody from DAKO, America, and other reagents from Beijing Zhongshan Jinqiao Biotechnology Co., LTD, China. ALK and common antibodies were tested using the automatic IHC staining apparatus (VENTANA Benchmark XT, VENTANA, Switzerland) according to the kit instructions.
For cell block sectioning, sections (4–6 μm) were placed on anti-shedding glass slides and dried in an oven at 60°C for at least 1–2 h. Staining procedures for antibodies were programmed and saved in the IHC/in situ hybridization staining module (VENTANA Medical System-BenchMark XT, VENTANA, Switzerland). For the ALK antibody, the repair mode was hot repair, with a repair time of 92 min at 99°C. The primary antibody was incubated for 16 min at 37°C, secondary antibody for 8 min at 37°C, and diaminobenzidine DAB color development for 8 min at 37°C. Common antibodies were repaired thermally for 32 min at 99°C, with primary antibody incubation for 32 min at 37°C, secondary antibody for 8 min at 37°C, and DAB color development for 8 min at 37°C. Following this, all test kits compatible with the system were scanned and positioned on the corresponding slots of the instrument. Subsequently, the waterproof barcode slice label was carefully edited, printed, and affixed to the side of each slice. The slices were then meticulously placed in the designated, temperature-controlled slots within the staining module. To initiate the staining process, the “RUN” button was clicked on the computer operating software. The number of stainings was set accordingly, followed by clicking “OK” to commence the program. The machine then autonomously executed all staining procedures. On completion of automatic staining, the sections were delicately removed, inserted into the slice holder, and cleansed with clean water. Subsequently, they underwent dehydration with gradient alcohol and were finally sealed with neutral glue.
The PD-L1 antibody was detected using the automatic IHC staining machine (DAKO Omnis, Agilent Technologies Inc., America) according to the PD-L1 kit instructions. The PDL1 staining procedure included thermal repair for 40 min at 97°C, primary antibody incubation for 30 min at 32°C, and secondary antibody incubation for 30 min at 32°C. DAB color development was performed for 8 min at 32°C. Remaining steps were similar to those for ALK and common antibodies.
Genetic testing
The amplification refractory mutation system (ARMS) technique was used to identify mutations within exons 18 through 21 of the EGFR gene in wax-embedded cells of malignant pleural fluid. Following deoxyribonucleic acid extraction, EGFR gene mutations were analyzed using the human EGFR base mutation detection kit (fluorescent polymerase chain reaction, ARMS) from Xiamen Aide Biomedical Technology Co, China.
Interpretation of results
IHC detection showed Ep-CAM, CK7, and Napsin-A positive expression in the cytoplasm as brownish yellow, and TTF-1, P40, and Ki-67 positive expression in the nucleus as brownish yellow. ALK (D5F3) was interpreted according to the Ventana anti-ALK (D5F3) scoring guide, using a binary classification (positive or negative).[14] Strong granular cytoplasmic staining in tumor cells indicated ALK positivity; otherwise, it was ALK negative.
For PD-L1 (22C3), only live tumor cells were considered. At least, 100 well-formed tumor cells were analyzed. Any visible linear cell membrane staining (partial or complete) and cytoplasmic and membrane staining were considered positive. No cell membrane staining with only cytoplasmic staining was negative. Two experienced diagnostic pathologists interpreted the results, excluding non-specific staining factors such as lymphocytes, macrophages, background staining in necrosis areas, and mucus-secreting areas. Tumor cell PD-L1 positive and negative expression values were carefully examined for accurate calculation. The percentage of tumor positive cells (TPS) was calculated as: TPS = (number of PD-L1 positive tumor cells/total number of tumor cells) × 100%.
Statistical methods
Data analysis was conducted using statistical package for social sciences (SPSS) 16.0 statistical software (SPSS Inc., Chicago, IL, USA). Differences in expression levels were examined using the χ2 test and its corrected version, while correlations were evaluated using the Spearman rank correlation test. Survival rates across different groups were depicted using Kaplan–Meier curves for cumulative survival functions, and the log-rank test assessed differences between survival curves. Statistical significance was set at P < 0.05.
RESULTS
Identification of cancer cells in malignant pleural fluid of NSCLC patients
Specimens sent for examination underwent pleural fluid exfoliation cytology smear, HE staining, and IHC detection of pleural fluid cell wax blocks. Tumors were diagnosed through microscopic examination using immune markers related to lung cancer [Figure 1 a-l and Table 1].
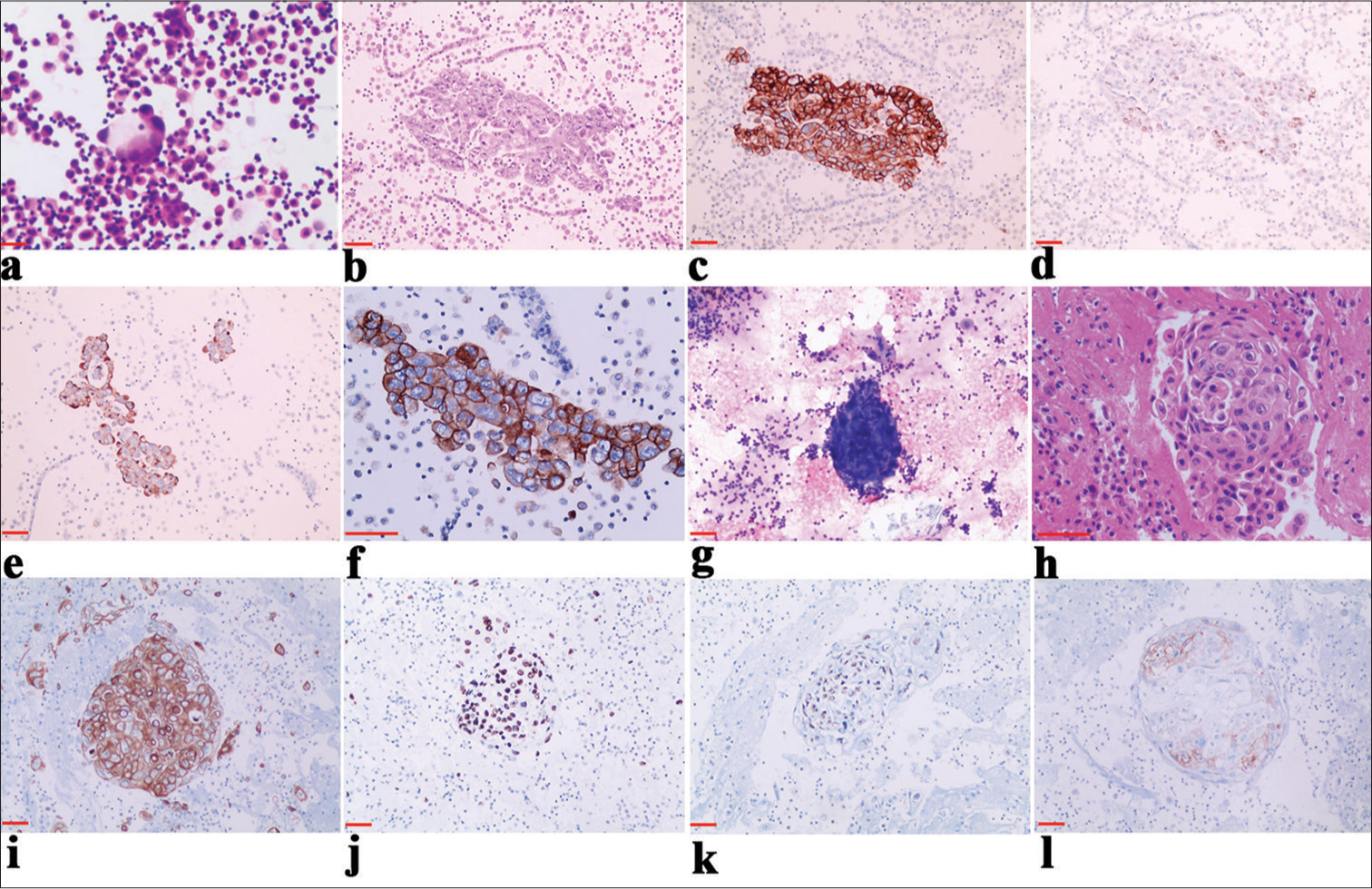
- The expression of programmed death ligand-1 (PD-L1) in serous effusions of metastatic lung adenocarcinoma and metastatic lung squamous cell carcinoma. (a) Hematoxylin and eosin (HE) staining of non-small-cell lung cancer (NSCLC) (metastatic adenocarcinoma) in pleural effusion, ×20. (b) HE staining of NSCLC (metastatic adenocarcinoma) from pleural effusion cell block, ×20. (c) Immunochemical (IHC) staining for Epithelial cell adhesion molecule, displaying positive staining in the cell membrane or cytoplasm, ×20. (d) IHC staining for thyroid transcription factor 1, exhibiting positive staining in the nucleus, ×20. (e) IHC staining for Napsin A, revealing cytoplasmic granular positivity, ×20. (f) IHC staining demonstrating PD-L1 membrane positivity and high expression, ×40. (g) HE staining of NSCLC (metastatic squamous cell carcinoma) in pleural effusion, ×20. (h) HE staining of NSCLC cell blocks (metastatic squamous cell carcinoma) from pleural effusion, ×40. (i) IHC staining for Cytokeratin 5/6 (CK5/6), showing positive staining in the cell membrane or cytoplasm, ×20. (j) IHC staining for p63, displaying nuclear positivity, ×20. (k) IHC staining positive for p40 in the nucleus, ×20. (l) IHC staining of PD-L1, exhibiting cytoplasmic membrane positivity and low expression, ×20. Scale bars: 100 μm.
| Pathological type | n | Ep-CAM | CK7 | TTF1 | NapsinA | CK5/6 | P40 | Calretinin |
|---|---|---|---|---|---|---|---|---|
| Metastatic lung adenocarcinoma | 144 | + | + | + | + | - | - | - |
| Metastatic lung squamous cell carcinoma | 5 | + | - | - | - | + | + | - |
| Positive rate (%) | 100 | 96.6 | 96.6 | 96.6 | 3.4 | 3.4 | 0 |
TTF1: Thyroid transcription factor 1, Ep-CAM: Epithelial cell adhesion molecule
Positive expression of PD-L1 protein in malignant pleural fluid of NSCLC patients
Among the 149 cases of metastatic NSCLC in pleural effusion, PD-L1 staining was positive on the cell membrane. The positive expression rate was <1% in 17 cases [Figure 2a], between 1% and 49% in 29 cases [Figure 2b], and 50% or more in 17 cases [Figure 2c], resulting in an overall positive rate of 42.3%.
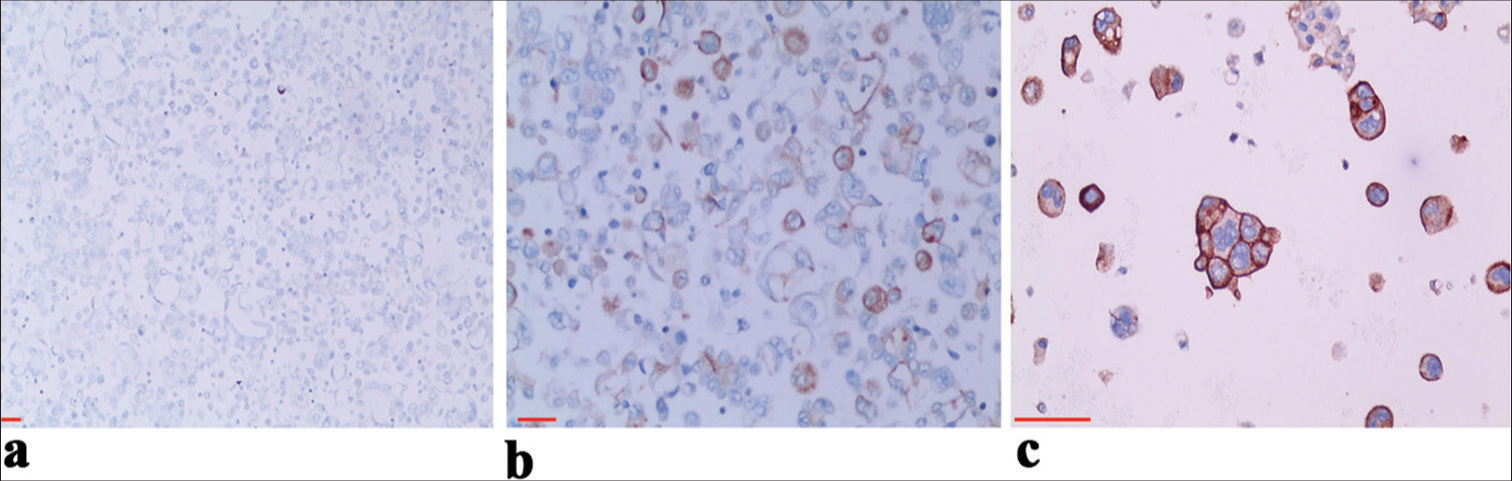
- Different levels of programmed death ligand-1 (PD-L1) expression in serous cavity effusion metastatic lung adenocarcinoma. (a) PD-L1 expression (<1%) on immunochemical (IHC) staining in metastatic non-small-cell lung cancer (NSCLC) from pleural effusion, ×10. (b) PD-L1 expression on IHC staining in metastatic NSCLC from pleural effusion (1–49%), ×20. (c) PD-L1 expression (≥50%) in pleural effusion metastatic NSCLC, ×40. Scale bars: 100 μm.
Relationship between PD-L1 expression in malignant pleural fluid of NSCLC and tumor clinicopathological features
In NSCLC patients with malignant pleural fluid, PD-L1-positive expression intervals were associated with tumor diameter and metastasis to other organs (P < 0.05). There was no significant relationship with gender, age, smoking status, tumor size, pleural involvement, degree of tumor differentiation, lymph node metastasis, or pathological stage (P > 0.05) [Table 2]. Specifically, PD-L1 expression was linked to tumor differentiation, lymph node metastasis, and metastasis to other organs (P < 0.05), but not to gender, age, smoking habits, mass size, tumor location, pleural involvement, or pathological stage (P > 0.05) [Table 2].
| Clinicopathological parameters | n | PD-L1 | χ2 | P | PD-L1 interval of positive rate | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 149 | + | - | ≥50% | 1–49% | <1% | |||||
| Sex | ||||||||||
| Male | 72 | 35 (48.6) | 37 (51.4) | 2.287 | 0.130 | 9 (12.5) | 15 (20.8) | 11 (15.3) | 0.796 | 0.85 |
| Female | 77 | 28 (36.4) | 49 (63.6) | 8 (10.4) | 14 (18.2) | 6 (7.8) | ||||
| Age (years) | ||||||||||
| ≤60 | 49 | 21 (42.9) | 28 (57.1) | 0.010 | 0.921 | 4 (8.2) | 13 (26.5) | 4 (8.2) | 3.195 | 0.363 |
| >60 | 100 | 42 (42.0) | 58 (58.0) | 13 (13.0) | 16 (16.0) | 13 (13.0) | ||||
| Diameter of mass (cm) | ||||||||||
| ≤3 | 93 | 41 (44.1) | 52 (55.9) | 0.330 | 0.566 | 3 (3.2) | 11 (11.8) | 8 (8.6) | 14.60 | 0.002 |
| >3 | 56 | 22 (39.3) | 34 (60.7) | 14 (25.0) | 18 (32.1) | 9 (16.1) | ||||
| Involvement of the pleura | ||||||||||
| Have | 74 | 34 (46.0) | 40 (54.0) | 0.809 | 0.308 | 8 (10.8) | 17 (23.0) | 9 (12.2) | 0.587 | 0.899 |
| Not have | 75 | 29 (38.7) | 46 (61.3) | 9 (12.0) | 12 (6.0) | 8 (10.7) | ||||
| Differentiation degree | ||||||||||
| Medium+High | 79 | 28 (35.4) | 51 (64.6) | 3.911 | 0.048 | 4 (5.1) | 17 (21.5) | 7 (8.8) | 5.446 | 0.142 |
| Low | 70 | 35 (50.0) | 35 (50.0) | 13 (18.6) | 12 (17.1) | 10 (14.3) | ||||
| Lymph node metastasis | ||||||||||
| Have | 76 | 52 (68.4) | 24 (31.6) | 43.430 | <0.001 | 12 (15.8) | 25 (32.9) | 15 (19.7) | 2.338 | 0.505 |
| Not have | 73 | 11 (15.1) | 62 (84.9) | 5 (6.9) | 4 (5.5) | 2 (2.7) | ||||
| Metastasis to other organs | ||||||||||
| Have | 61 | 48 (78.7) | 13 (21.3) | 56.096 | <0.001 | 9 (14.8) | 26 (42.6) | 13 (21.3) | 7.964 | 0.047 |
| Not have | 88 | 15 (17.0) | 73 (83.0) | 8 (9.1) | 3 (3.4) | 4 (4.5) | ||||
| Pathological type | ||||||||||
| Adenocarcinoma | 144 | 61 (42.4) | 83 (57.6) | 0.011 | 0.916 | 17 (11.8) | 29 (20.1) | 15 (10.4) | 5.589 | 0.133 |
| Squamous cell carcinoma | 5 | 2 (40.0) | 3 (60.0) | 0 (0) | 0 (0) | 2 (40.0) | ||||
PD-L1: Programmed death ligand-1
Correlation of PDL-1 expression in malignant pleural fluid with ALK expression and EGFR mutation in NSCLC
PD-L1 expression in the malignant pleural fluid of NSCLC patients was positively correlated with ALK expression (rs = 11.49, P < 0.05) and not correlated with EGFR mutation (rs = 0.004, P > 0.05) [Table 3].
| ALK | PD-L1 | rs | P | EGFR | PD-L1 | rs | P | ||
|---|---|---|---|---|---|---|---|---|---|
| + | - | + | - | ||||||
| + | 2 | 1 | + | 15 | 20 | ||||
| - | 40 | 54 | 11.49 | 0.001 | - | 27 | 35 | 0.004 | 0.95 |
PD-L1: Programmed death ligand-1, ALK: Anaplastic lymphoma kinase, EGFR: Epidermal growth factor receptor, rs: Spearman´s rank correlation coefficient
Relationship between PD-L1 expression in malignant pleural fluid of NSCLC and OS time of patients
Tracking the survival of 149 NSCLC patients with malignant pleural fluid revealed a significant disparity (P < 0.05) in median OS between those who were PD-L1 positive and those who were negative [Figure 3].
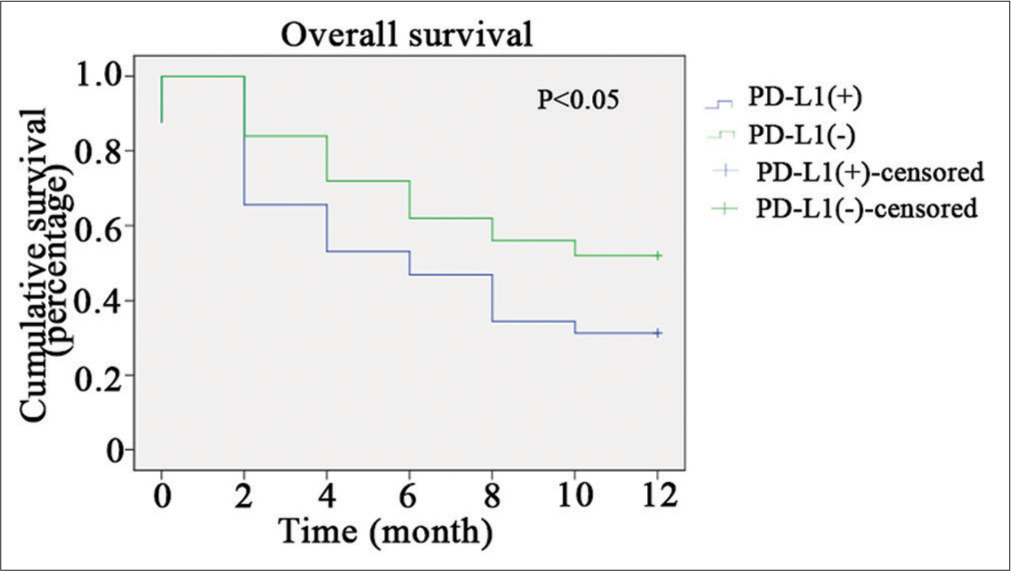
- Relationship between programmed death ligand-1 (PDL1) expression in malignant pleural fluid of non-small-cell lung cancer and overall survival time of patients.
DISCUSSION
The 2019 NSCLC Guidelines from the National Comprehensive Cancer Network moved PD-L1 testing from Class 2A to Class 1 recommendation.[15] PD-L1 is the most valuable predictor for NSCLC immune response to treatment. However, clinical studies and applications in patients with metastatic NSCLC with malignant pleural effusion are still limited. The novelty and advantage of this study is to detect the expression of PD-L1-22C3 in wax samples of malignant pleural effusion cells of NSCLC by IHC method and its relationship with clinicopathological features, so as to provide a theoretical basis for immunotherapy of patients with metastatic NSCLC complicated with pleural effusion.
At present, the four most commonly used PD-L1 antibodies in major international multi-center clinical trials are as follows: DAKO’s 22C3, 28–8, and Roche Diagnostics (Ventana)’s SP263 and SP142. Famous blue print plan phase 1 study of four antibodies (28–8, 22 c3, SP263, and SP142) consistency between the evaluated.[16] The results showed that the three antibodies (28–8, 22C3, and SP263) had highly consistent staining effects on the expression of PDL1 in tumor cells, while the positive rate of SP142 was low. The PD-L1 assay of DAKO 22C3 is the earliest approved companion diagnosis for immunotherapy of NSCLC and has high reliability. Although a number of studies have shown high consistency among 22C3, 28–8, and SP263, there is insufficient prospective clinical evidence to support the feasibility of interoperability of antibody test results. In a consistent study of the expression of PD-L1 22C3 and 28–8 in 420 Japanese NSCLC surgical specimens: The expression of PD-L1 in the 22C3 detection group, at 25% and 50% cutoff values, was higher than that in the 28–8 group, and the evaluation of PD-L1 expression with 28–8 instead of 22C3 might miss some positive patients. The consistency of results of 28–8, SP263, and 22C3 was analyzed by comparing real-world data, and both 28–8 and SP263 were likely to miss some patients who tested positive for 22C3. Therefore, in this study, PD-L1-22C3 was used to detect the wax block samples of NSCLC malignant pleural effusion cells, which has unique advantages, and its expression results are relatively reliable.
PD-L1 expression is detected across various tumor types, including NSCLC, breast, gastric, liver, kidney cancer, multiple myeloma, glioma, and melanoma, while PD-1 is primarily expressed in the lymphocytes of tumor patients. Zheng et al.[17] observed that PD-L1 was significantly upregulated in gastric cancer tissues compared to adjacent non-cancerous tissues, with its levels correlating with tumor size and differentiation, indicating a poorer prognosis. Lin et al.[18] discovered that positive expressions of PD-1 and PD-L1 in lung cancer tissues were associated with tumor size, lymph node metastasis, and histopathological grade. In our study involving 149 NSCLC patients with malignant pleural fluid, we found that PD-L1-positive expression was detected in <1% of 17 patients, 1–49% in 29 patients, and 50% or more in 17 patients, yielding an overall positivity rate of 42.3%. The distribution of PD-L1-positive expression was correlated with tumor size and the occurrence of metastases to other organs (P < 0.05). In addition, PD-L1 expression showed correlations with tumor differentiation, lymph node metastasis, and metastases to other organs (P < 0.05). These findings suggest that PD-L1 contributes to the progression and development of NSCLC, with its expression linked to tumor advancement and unfavorable outcomes. Hence, PDL1 emerges as a critical molecular biomarker for assessing malignancy progression and prognostication in NSCLC. In our study involving 149 patients with malignant pleural effusion in NSCLC, the positive rate of PD-L1 expression was 42.3%. This aligns with existing literature reporting PD-L1-positive rates in NSCLC ranging from 39.9% to 53.1%.[19,20] Yang et al[21] demonstrated a correlation between PD-L1 expression and lymph node metastasis and TNM Classification of Malignant Tumors staging, consistent with our findings analyzing the relationship between PD-L1 expression and clinicopathological features. However, contrasting results from Chen et al.[22] suggested no association between PD-L1 expression and gender, smoking status, lymph node metastasis, histological type, or differentiation. Therefore, our results warrant further validation through large-scale studies.
With the advancements in targeted therapy for NSCLC, molecular targeted treatments focusing on driver genes such as ALK and EGFR have reached clinical maturity. ALK is expressed not only in lung cancer but also in breast, cervical, and various other tumors.[23] Wang et al.[24] reported a positive expression rate of echinoderm microtubule-associated protein-like 4-ALK fusion protein of approximately 6.7% in lung adenocarcinoma, with a significant proportion of patients being under 60 years old. EGFR mutations are prevalent in female non-smoking NSCLC adenocarcinomas, and studies by Harrison et al[25] revealed higher mutation rates of the EGFR gene in Asian populations compared to white and black populations. For cases exhibiting ALK fusion protein positivity alongside EGFR gene mutations, pertinent studies have demonstrated clinical benefits with treatment involving EGFR-tyrosine kinase inhibitor and crizotinib.[26] In our investigation, we explored the correlation between PD-L1 expression and ALK expression as well as EGFR mutation. We found a positive correlation between PD-L1 and ALK expression (P < 0.05), while no significant correlation was observed between PD-L1 and EGFR mutation (P > 0.05). Understanding the interplay among genes can elucidate the mechanisms of drug resistance encountered during targeted therapy in clinical tumors. Furthermore, it can furnish a theoretical foundation for selecting targeted therapy drugs, determining their combinations, and implementing personalized treatment strategies. At present, multi-target synergistic therapeutic regimens and efficacy analyses for lung cancer patients are undergoing clinical studies for validation.
In recent years, immunotherapy has heralded a revolutionary breakthrough in cancer treatment. At present, a variety of PD-1/PD-L1 inhibitors, including nivolumab, atezolizumab, pembrolizumab, and durvalumab, among others, have emerged, with some obtaining approval for first-line treatment in NSCLC. The previous studies have demonstrated significant benefits for patients with high PD-L1 expression on tumor cells in terms of median OS, 1-year survival rate, and objective response rate following anti-PD-1/PD-L1 immunotherapy. The assessment of PD-L1 expression levels through IHC stands as the primary method to determine the suitability of NSCLC for immune checkpoint inhibitor treatment and the potential benefits derived from such therapy.[27] This underscores PDL1 as a pivotal target for immunotherapy in NSCLC. Trials such as CheckMate-227 and CheckMate-9LA, evaluating dual immunotherapy, have showcased a 6-year OS rate of 22% in patients with stage IV or recurrent NSCLC and PD-L1 ≥1% who received first-line dual immunotherapy. Notably, the complete response rate or partial response rate of 15% of patients exceeds 80%, while the 6-year OS rate can soar to 59%. Even patients with PD-L1 <1% exhibit improved OS benefits compared to traditional chemotherapy.[28] These results underscore the significance of different PD-L1 expression levels in influencing the efficacy of immunotherapy. In our study involving 149 NSCLC patients with malignant pleural effusion, the positive expression rate of PD-L1 was <1% in 17 cases, 1–49% in 29 cases, and more than 50% in 17 cases, yielding a total positive rate of 42.3%. Importantly, there was a significant difference in OS between the PD-L1 protein positive expression group and the PD-L1 protein negative expression group (P < 0.05). IHC assessment of PD-L1 antibody in malignant pleural effusion of advanced NSCLC patients offers a theoretical foundation for guiding clinical tumor immunotherapy.
The existing body of research underscores the pivotal role of PD-L1 in the development and progression of NSCLC, with its expression being associated with advanced disease stages, adverse prognoses, and impacts on patient OS. IHC analysis of PD-L1 in pleural fluid wax blocks presents as a straightforward, rapid, minimally invasive, and consistent method for evaluating patients with advanced NSCLC and malignant pleural fluid. Moreover, PD-L1 expression in patients with intermediate to advanced NSCLC and malignant pleural fluid holds substantial clinical significance for refining treatment strategies and monitoring prognosis.
It is important to note that this study is limited by its single-center design and the relatively small sample size of collected cases. Consequently, certain findings, such as the positive correlation between ALK expression and PD-L1 expression, may lack robustness due to the limited number of cases. Moving forward, we aim to conduct large-scale, high-quality studies to detect PD-L1, ALK, and delve further into the tumor microenvironment and related pathways. Such endeavors are anticipated to unveil the intricate interaction between gene expression and the tumor immune microenvironment, ultimately furnishing more effective treatment strategies for NSCLC patients and addressing their survival needs comprehensively.
SUMMARY
In our study, we found that the positive rate of PD-L1 expression (42.3%) was closely associated with tumor differentiation, lymph node metastasis, and distant organ metastasis. These factors significantly influence NSCLC invasion, metastasis, and OS. IHC evaluation of PD-L1 in malignant pleural effusion provides a theoretical basis for the application of immunotherapy in metastatic NSCLC patients with pleural effusion and aids in predicting patient prognosis. In the future, large-sample studies are expected to unravel the intricate interaction between these gene expressions and the tumor immune microenvironment, thereby providing more effective treatment strategies for NSCLC patients. The IHC assessment of PD-L1 in malignant pleural effusions provides a valuable foundation for implementing immunotherapy in the treatment of metastatic NSCLC patients with pleural effusion. This diagnostic approach is minimally invasive, straightforward, and rapid, making it particularly significant for individuals whose primary symptom is malignant pleural effusion.
AVAILABILITY OF DATA AND MATERIALS
Source data and reagents are available from the first author on reasonable request.
ABBREVIATIONS
ALK: Anaplastic Lymphoma Kinase
ARMS: Amplification refractory mutation system
CK5/6: Cytokeratin 5/6
CK7: Cytokeratin 7
DNA: Deoxyribonucleic acid
EGFR: Epidermal growth factor receptor
EpCAM: Epithelial cell adhesion molecule
HE: Hematoxylin and eosin
IFN-γ: Interferon-γ
NK: Natural killer cell
NSCLC: Non-small-cell lung cancer
ORR: Objective remission rate
OS: Overall survival
PD1: Programmed death receptor-1
PD-L1: Programmed death ligand-1
TTF1: Thyroid transcription factor 1
WHO: World
AUTHOR CONTRIBUTIONS
LCG and SH: Designed the study, conducted the research, and revised the final manuscript; DMG: Conceived the study and drafted the manuscript;DMG and LH: Developed the methodology and performed the data analysis; DMG: Also prepared the figures and tables. All authors have approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study complies with the Declaration of Helsinki. The study was approved by the Ethics Committee of the first affiliated hospital of Soochow University (Ethics File Approval letter of Review Board 2024-227). The Institutional Review Board has granted an exemption from obtaining informed consent from the patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409-36.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid advances in resectable non-small cell lung cancer: A narrative review. JAMA Oncol. 2024;10:249-55.
- [CrossRef] [PubMed] [Google Scholar]
- Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol Cancer. 2023;22:40.
- [CrossRef] [PubMed] [Google Scholar]
- Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-95.
- [CrossRef] [PubMed] [Google Scholar]
- Engineered bacteria in tumor immunotherapy. Cancer Lett. 2024;589:216817.
- [CrossRef] [PubMed] [Google Scholar]
- Better efficacy of intrapleural infusion of bevacizumab with pemetrexed for malignant pleural effusion mediated from nonsquamous non-small cell lung cancer. Onco Targets Ther. 2018;11:8421-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of physiotherapy interventions in pleural effusion patients: A comprehensive review. Cureus. 2024;16:e61195.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant pleural effusion: From diagnostics to therapeutics. Clin Chest Med. 2018;39:181-93.
- [CrossRef] [PubMed] [Google Scholar]
- Current status of and progress in the treatment of malignant pleural effusion of lung cancer. Front Oncol. 2023;12:961440.
- [CrossRef] [PubMed] [Google Scholar]
- Novel therapies for malignant pleural effusion: Antiangiogenic therapy and immunotherapy (review) Int J Oncol. 2021;58:359-70.
- [CrossRef] [PubMed] [Google Scholar]
- The 2015 World Health Organization classification of lung tumors: New entities since the 2004 classification. Pathologica. 2018;110:39-67.
- [Google Scholar]
- Immunohistochemistry with 3 different clones in anaplastic lymphoma kinase fluorescence in situ hybridization positive non-small-cell lung cancer with thymidylate synthase expression analysis: A multicentre, retrospective, Italian study. Pathologica. 2022;114:278-87.
- [CrossRef] [PubMed] [Google Scholar]
- Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. 2019;17:255-89.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208-22.
- [CrossRef] [PubMed] [Google Scholar]
- Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104-11.
- [Google Scholar]
- Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget. 2017;8:83986-994.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32.
- [CrossRef] [PubMed] [Google Scholar]
- Non-small cell lung cancer in China. Cancer Commun (Lond). 2022;42:937-70.
- [CrossRef] [PubMed] [Google Scholar]
- EGFR mutation status in non-small cell lung cancer receiving PD-1/PD-L1 inhibitors and its correlation with PD-L1 expression: A meta-analysis. Cancer Immunol Immunother. 2022;71:1001-16.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 expression is associated with advanced non-small cell lung cancer. Oncol Lett. 2016;12:921-7.
- [CrossRef] [PubMed] [Google Scholar]
- Combined EGFR/ALK expression analysis in laryngeal squamous cell carcinoma. In Vivo. 2019;33:815-9.
- [CrossRef] [PubMed] [Google Scholar]
- Value of serum tumor markers for predicting EGFR mutations and positive ALK expression in 1089 Chinese non-small-cell lung cancer patients: A retrospective analysis. Eur J Cancer. 2020;124:1-14.
- [CrossRef] [PubMed] [Google Scholar]
- Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167-79.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. 2020;13:58.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges of PD-L1 testing in non-small cell lung cancer and beyond. J Thorac Dis. 2020;12:4541-8.
- [CrossRef] [PubMed] [Google Scholar]
- Chemoimmunotherapy vs. immunotherapy for first line treatment of advanced non-small cell lung cancer with a PD-L1 expression >/=50% or >/=90. Clin Lung Cancer. 2023;24:235-43.
- [CrossRef] [PubMed] [Google Scholar]








