Translate this page into:
Fibronectin type III domain containing protein 5/irisin alleviated sepsis-induced acute kidney injury by abating ferroptosis through the adenosine 5'-monophosphate-activated protein kinase/nuclear factor erythroid-2-related factor 2 signaling pathway

*Corresponding author: Yunfeng Lu, Department of Emergency, The First People’s Hospital of Tongxiang, Tongxiang, Zhejiang, China. luyunfeng6662023@163.com
-
Received: ,
Accepted: ,
How to cite this article: Gui S, Zhu C, Lu Y. Fibronectin type III domain containing protein 5/irisin alleviated sepsis-induced acute kidney injury by abating ferroptosis through the adenosine 5'-monophosphate-activated protein kinase/nuclear factor erythroid-2-related factor 2 signaling pathway. CytoJournal. 2024;21:54. doi: 10.25259/Cytojournal_62_2024
Abstract
Objective:
Ferroptosis has been described in association with acute kidney injury (AKI)-induced sepsis. Fibronectin type III domain containing protein 5 (FNDC5)/irisin plays a crucial role in renal protection. The objective of this study was to investigate whether FNDC5/irisin is involved in AKI-induced sepsis by modulating ferroptosis, and the molecular mechanisms that may be involved.
Material and Methods:
A sepsis-induced AKI model was built in vivo and in vitro through lipopolysaccharide (LPS) intervention. FNDC5, adenosine 5'-monophosphate-activated protein kinase (AMPK), phospho-AMPK (p-AMPK), nuclear factor erythroid-2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), glutathione peroxidase 4 (GPX4), and acyl-CoA synthetase long-chain family member 4 (ACSL4) concentrations in cells and mouse kidney tissues were appraised by Western blot. Pro-inflammatory cytokines concentrations in cell supernatants and mouse kidney tissues were appraised by enzyme-linked immunosorbent assay. Fe2+ concentration in cells and mouse kidney tissue was appraised by kit. The apoptosis rate of cells and mouse kidney tissue was measured by flow cytometry. Automatic biochemical analyzer was to test serum creatinine (SCr) and blood urea nitrogen (BUN). The kidney tissue sections from each groups were observed by hematoxylin and eosin staining.
Results:
LPS abated FNDC5 concentration in human kidney-2 cells and mouse kidney tissue (P < 0.001). Overexpression of FNDC5 can abated proinflammatory cytokines concentrations in cells and mouse kidney tissue (P < 0.01). Meanwhile, overexpression of FNDC5 can boost GPX4 protein concentration, abate ACSL4 protein, and abate Fe2+ concentration in cells and mouse kidney tissues (P < 0.05). In addition, the overexpression of FNDC5 can reduce the rate of apoptosis (P < 0.01). In vivo experiments showed that FNDC5 overexpression reduced serum BUN and SCr concentrations and alleviated pathological damage in the mouse renal tissues (P < 0.05) and exhibited a certain renal protective effect. FNDC5 overexpression can boost p-AMPK/AMPK, Nrf2, and HO-1 protein concentrations (P < 0.01).
Conclusion:
FNDC5/irisin improves sepsis-induced acute renal injury by abating ferroptosis through the AMPK/Nrf2 signaling pathway.
Keywords
Fibronectin type III domain containing protein 5
Irisin
Acute kidney injury
Ferroptosis
Adenosine 5’-monophosphate-activated protein kinase
INTRODUCTION
Acute kidney injury (AKI) is a common complication of sepsis and remains a risk factor for chronic kidney disease progression. Its incidence and mortality rates are high. Most patients develop AKI before they start drug therapy.[1-3] The pathogenesis related to sepsis-induced AKI is complex.[4] Relevant mechanisms that have been reported to be associated with this process include renal hemodynamic changes, coagulation dysfunction, inflammatory response, microvascular endothelial dysfunction, and renal tubular epithelial cell injury.[5] Some studies have pointed out that different types of programmed cell death, such as cell apoptosis, pyrogenesis, necroptosis, autophagy, and ferroptosis, play a key role in sepsis-induced AKI.[6-10] Ferroptosis is defined as iron-dependent cell regulatory death caused by membrane damage mediated by a large degree of lipid peroxidation. It is a cell death from that depends on reactive oxygen species (ROS), which is related to iron accumulation and lipid peroxidation.[11] Moreover, ferroptosis are essential to AKI.[12,13] Liu et al.[12] discovered 4754 differentially expressed genes in the kidney tissues collected from sepsis-induced AKI model mice through bioinformatics analysis, and ferroptosis is the highest-scored enrichment pathway of the differentially expressed genes. However, the specific molecular mechanism of ferroptosis in sepsis-induced AKI has not been fully elaborated.
Irisin is a muscle factor discovered in 2012, derived from the protein hydrolysis and cleavage of fibronectin type III domain containing protein 5 (FNDC5) protein. It has the major influences in the modulation of oxidative stress, inflammation, cell apoptosis, and fibrosis.[14,15] Zhang et al.[16] discovered that irisin can abate ischemia or reperfusion-induced renal cell apoptosis and oxidative stress by upmodulating uncoupling protein 2 concentration, thereby improving renal injury. Formigari et al.[17] demonstrated that the protective effect of aerobic exercise on the kidney is related to the up-regulation of irisin level in the muscles and serum, and increase of renal adenosine 5'-monophosphate-activated protein kinase (AMPK) activity in the diabetes rat model. Blocking irisin receptor can eliminate the protective effect of exercise on diabetes kidney. This finding indicates that FNDC5/irisin plays a crucial role in renal protection.
Previous study has discovered that Irisin can abate ferroptosis.[18,19] Zhang et al.[20] showed that irisin can prevent renal ischemia/reperfusion damage by alleviating inflammatory response and reducing oxidative stress, its mechanism was related to the up-regulation of glutathione peroxidase 4 (GPX4). However, the effect of FNDC5/irisin on sepsis-induced AKI ferroptosis remains unclear. Therefore, we speculate that FNDC5/irisin reduces sepsis-induced AKI by downmodulating ferroptosis, offering a novel strategy for AKI treatment.
MATERIAL AND METHODS
Cell culture and treatments
Human renal tubular epithelial cells, human kidney-2 (HK-2) cell line, were sourced from the Institute of Cell Research, Chinese Academy of Sciences (SCSP-511, Shanghai, China). Cells were authenticated by short tandem repeat test, and passed the mycoplasma detection. The cells were placed in Dulbecco’s modified Eagle’s/Nutrient Mixture F-12 medium (11320033, Gibco, Carlsbad, CA, USA). Cell medium spiked with 10% fetal bovine serum (10099141C, Gibco, Carlsbad, CA, USA), and penicillin-streptomycin (15070063, Gibco, Carlsbad, CA, USA). The incubator was set at 5% CO2 and 37°C.
On reaching 80% cell fusion, the cells were randomly divided into the following subgroups: Control, lipopolysaccharide (LPS), LPS+negative control overexpressing lentivirus (oeNC), LPS+ FNDC5-overexpressing lentivirus (oe-FNDC5), LPS+oe-FNDC5+P5499, and LPS+oe-FNDC5+erastin subgroups. The cells were cultured normally in the control subgroup. LPS (100 μg/mL; L2630, Sigma-Aldrich, St Louis, MO, USA) was added for 24 h processing in LPS subgroup. The LPS+oe-NC subgroup cells were infected with a empty vector named oe-NC for 72 h of transfection before 24 h of LPS treatment. The LPS+oe-FNDC5 subgroup was infected with an oe-FNDC5 for 72 h of transfection before 24 h of LPS treatment. The LPS+oe-FNDC5+P5499 subgroup was infected with an oe-FNDC5 for 72 h before 24 h of LPS treatment and then treated with the AMPK inhibitor P5499 (10 μmol/L, 171260, Sigma-Aldrich, St Louis, MO, USA) for 1 h. The LPS+oe-FNDC5+erastin subgroup was infected with an oe-FNDC5 72 h before LPS treatment and then treated with the ferroptosis activator erastin (10 μmol/L, E7781, Sigma-Aldrich, St Louis, MO, USA) for 1 h.
Animal experiments
Specific pathogen-free grade male C57BL/6 mice (n = 60; 6–8 weeks old, 20–25 g) were provided by the Animal Experimental Center of Wuhan University (Wuhan, China). Animals were adaptive raised for 1 week. All mice were maintained the environment with 12 h light/dark cycles and 22 ± 2°C, and free to obtain food and water.
The mice were randomly divided into six subgroups with 10 mice each: Sham, LPS, LPS+oe-NC, LPS+oe-FNDC5, LPS+oeFNDC5+P5499, and LPS+oe-FNDC5+erastin subgroup. A mouse sepsis induced by AKI model was established through the intraperitoneal injection of 10 mg/kg LPS in LPS subgroup, whereas the sham subgroup was injected with equal volume of physiological saline.[21] The LPS+oe-NC and LPS+oeFNDC5 subgroups were injected with oe-NC and oe-FNDC5 through the tail vein (1 × 108 TU/mL, 100 μL) 30 min before LPS injection. The LPS+oe-FNDC5+P5499 and LPS+oeFNDC5+erastin subgroups were injected with an oe-FNDC5 through the tail vein (1 × 108 TU/mL, 100 μL) 30 min before LPS injection. After successful modeling, 20 mg/kg P5499 and 5 mg/kg erastin were injected intraperitoneally. After 24 h, blood was collected from the tail vein. The mice were sacrificed by injecting 100 mg/kg pentobarbital sodium intraperitoneally, the kidney tissues were rapidly separated and collected for subsequent experiments. All experimental protocols of this study were approved by Beijing Biocisco Biomedical Technology Co., Ltd animal ethics committee (No: MDL 2023–05–13–01).
Lentiviral vectors for FNDC5 overexpression
The full-length complementary DNA of FNDC5 was constructed by Shanghai GeneChem Co., Ltd (Shanghai, China). The prime sequences were: 5'-ATGCACCCCGGGCCGCCCCG-3' (forward) and 5'-GTCCCCTCTCTCCCTGAGC-3' (reverse). Transduced cells were packaged with a lentiviral vector. FNDC5 and negative control overexpression vectors were named oe-FNDC5 and oe-NC respectively. Lentivirus packaging and infection referenced previous reports.[22] A day before transfection, human embryonic kidney 293T (HEK 293T) cells (Cell bank of Shanghai Institutes for Life Science, Chinese Academy of Sciences, Shanghai, China) with 90–95% fusion were inoculated in a Petri dish. Lipofectamine 2000 (11668500, Thermo Fisher Scientific, MA, USA) was utilized to co-transfect HEK 293T cells with FNDC5 overexpression vector (20 μg), plasmid pHelper 1.0 (15 μg), and plasmid pHelper 2.0 (10 μg) for 48 h of transfection. The virus titer was determined, and 2 × 109 TU/mL lentivirus particles were utilized to infect 2 × 105 HK-2 cells at 37°C for 72 h. Stable infected cells were collected for follow-up experiments.
Western blot
A radioimmunoprecipitation assay lysis buffer (R0030, Solarbio, Beijing, China) was for the extraction of total protein, and the protein quantification was performed using a bicinchoninic acid kit (PC0020, Solarbio, Beijing, China). The extracted protein (20 μg) was separated and transferred to polyvinylidene fluoride membranes (IPVH00010, Millipore, Boston, MA, USA). The diluted primary antibodies with 5% skim milk powder (D8340, Solarbio, Beijing, China) were employed for overnight incubation of the membranes at 4°C. The primary antibodies were FNDC5 (1:1000, ab174833, Abcam, Cambridge, UK), phospho-AMPK (p-AMPK, 1:1000, 2537, CST, MA, USA), AMPK (1:1000, 2532, CST, MA, USA), nuclear factor erythroid-2-related factor 2 (Nrf2, 1:1000, A0674, ABclonal, Wuhan, China), heme oxygenase-1 (HO-1, 1:1000, 86806, CST, MA, USA), GPX4 (1:1000, 52455, CST, MA, USA), acyl-CoA synthetase long-chain family member 4 (ACSL4, 1:1000, A6826, ABclonal, Wuhan, China), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000, 5174, CST, MA, USA). Then, the membranes were incubated with secondary antibody (Solarbio, SE134, 1:5000, Beijing, China) for 1 h. Under the condition of avoiding light, after coloration was observed in the enhanced chemiluminescence solution (PE0010, Solarbio, Beijing, China), photographs were obtained with a gel imager. The gray values of the protein bands in each subgroup were measured with Image J gray analysis software (1.8.0, Media Cybernetics, Silver Spring, MD, USA), and GAPDH was set as the endogenous control.
Enzyme-linked immunosorbent assay (ELISA)
The cell culture medium was centrifuged to obtain the cell supernatant. Mouse kidney tissues were made into tissue homogenates after adding pre-cooled PBS. After the cell supernatant and tissue homogenate were diluted with PBS, and next the samples and reagents were added to the enzyme-linked immunosorbent assay (ELISA) plates according to the instructions of ELISA kits. The optical density value was measured at 450 nm. The interleukin (IL)-6, IL-qβ, and tumor necrosis factor (TNF)-α levels were calculated according to the standard curve. IL-6 (H007-1-2), IL-qβ (H002-1-2), and TNF-α (H052-1-2) ELISA kits were provided by Jiancheng Bioengineering Institute (Nanjing, China), the detection range of ELISA kits were 0–500 pg/mL.
Flow cytometry
Approximately 1 × 106 HK-2 cells were collected and centrifuged (400 g, 4°C, 5 min). After washing with PBS, the cells were suspended in 200 μL of PBS, 10 μL of annexin V-fluorescein isothiocyanate, and 10 μL of propidium iodide (BJ-10153, Bangjing, Shanghai, China). The suspension was mixed gently, and kept at 4°C for 30 min. The cells were measured through flow cytometry (Accuri C6, BD Bioscience, Franklin lakes, NJ, USA).
Iron assay
The experimental operation was performed following the instructions of the Fe2+ kit (I291, 0–50 μmol/L Dojindo, Shanghai, China). After PBS washing, HK-2 cells or mouse kidney tissue homogenates were added the iron assay buffer. After centrifugation, the supernatant was collected, mixed with an iron reducer solution, and incubated. Then, an iron probe solution was added and incubated for 1 h. The absorbance (593 nm) was determined with an enzyme-labeling instrument (AMR-100, Allsheng, Hangzhou, China).
Biochemical determination
The level of serum creatinine (SCr) and blood urea nitrogen (BUN) in mice was tested by an automatic biochemical analyzer (BS-420, Mindray, Shenzhen, China).
Hematoxylin and eosin staining
Renal tissue sections (4 μm) were made using the routine procedure, dewaxed to water routinely, and stained with hematoxylin and eosin (H&E; G1120, Solarbio, Beijing, China). Microscope (BX5, Olympus, Tokyo, Japan) was used to observe and photographthe pathological changes in the kidneys in accordance with previous reports.[23,24] The main observation indexes of renal tubular injury included tubulitis, atrophy, necrosis, tubular dilation, tubular cast formation, vacuolization, and congestion/hemorrhage. Examinations were performed with five fields of ×200 magnification, and the values were averaged. Renal injury was evaluated on the basis of the H&E results, and the percentage of damaged tubules were calculated: 0, no injury; 1, <25%; 2, 25%–50%; 3, 51%–75%; and 4, >75%.[23,24]
TdT-mediated dUTP nick end-labeling (TUNEL) staining
Cell apoptosis in vivo was evaluated through TUNEL staining in accordance with the instructions of a commercially available kit (C1089, Beyotime, Shanghai, China). 4',6-diamidino-2-phenylindole (DAPI, C1005, Beyotime, Shanghai, China) was for the staining of cell nuclei. Observation and photography were carried out using a microscope (DM1000, Leica, Wetzlar, Germany).
Statistical analysis
The Statistical Package for the Social Sciences 22.0 software (IBM, Armonk, NY, USA) was utilized for statistical analysis, and all data were presented as mean and standard deviation. Independent sample t-test was used in comparing differences between two subgroups, and differences among multiple subgroups were utilized through single-factor analysis of variance. The criteria for significant difference were set as P < 0.05.
RESULTS
FNDC5/irisin ameliorated LPS-induced inflammatory response and alleviates oxidative stress through activation of AMPK/Nrf2 signaling pathway
Compared with the control subgroup, the LPS subgroup showed dramatically abated FNDC5, p-AMPK/AMPK, Nrf2, and HO-1 concentrations (P < 0.001). The LPS+oeFNDC5 subgroup versus the LPS+oe-NC subgroup exhibited dramatically boosted FNDC5, p-AMPK/AMPK, Nrf2, and HO-1 concentrations (P < 0.01). The LPS+oe-FNDC5+P5499 subgroup versus the LPS+oe-FNDC5 subgroup showed dramatically abated FNDC5, p-AMPK/AMPK, Nrf2, and HO-1 concentrations (P < 0.01), [Figure 1a-e]. ELISA results suggested that the IL-6, IL-1b, and TNF-a concentrations in the HK-2 cells of the LPS subgroup were dramatically higher than those cytokine levels in the control subgroup (P < 0.001). FNCD5 overexpression diminished these cytokine levels in the LPS-treated HK-2 cells (P < 0.01). The IL-6, IL-1b, and TNF-a concentrations in the LPS+oe-FNDC5 +P5499 subgroup were dramatically higher than those of the LPS+oe-FNDC5 subgroup (P < 0.01), [Figure 1f-h]. LPS dramatically increased apoptosis rate relative to that in the control subgroup (P < 0.001), and FNDC5 overexpression reduced apoptosis rate in the LPS-treated cells (P < 0.01). The apoptosis rate in the LPS+oe-FNDC5+P5499 subgroup was dramatically higher than those of the LPS+oe-FNDC5 subgroup (P < 0.01), [Figure 1i and j]. In general, FNDC5/irisin ameliorated the inflammatory response caused by LPS and alleviated oxidative stress by activation of AMPK/Nrf2 signaling pathway.
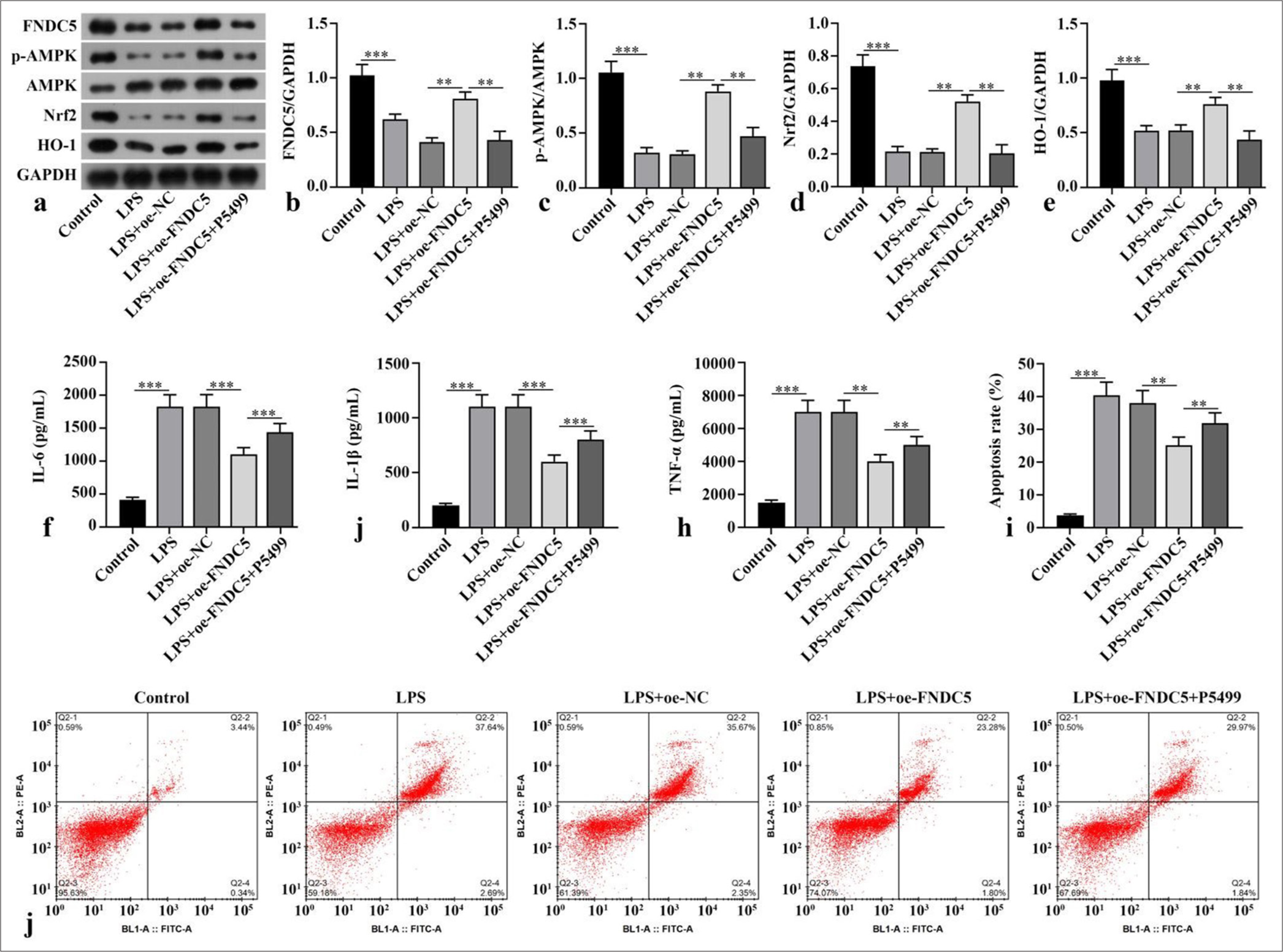
- FNDC5/irisin improved the HK-2 cell model by activating the AMPK/Nrf2 signaling pathway. LPS was used to stimulate the HK-2 cells for the establishment of a model of renal injury induced by sepsis in vitro. Lentivirus overexpression FNDC5 was constructed and transfected into cells. The cells were incubated with AMPK inhibitor (P5499) for 1 h. (a-e) FNDC5, p-AMPK, AMPK, Nrf2, and HO-1 expression were measured by Western blot. (f-h) IL-6, IL-1b, and TNF-a concentrations were measured with ELISA. (i and j) Cell apoptosis was measured through flow cytometry. n = 3, **P < 0.01, ***P < 0.001. (FNDC5: Fibronectin type III domain containing protein 5, HK-2: Human kidney-2, AMPK: Adenosine 5'-monophosphate-activated protein kinase, p-AMPK: phospho- adenosine 5'-monophosphate-activated protein kinase, Nrf2: Nuclear factor erythroid-2-related factor 2, HO-1: Heme oxygenase-1, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, LPS: Lipopolysaccharide, oe-NC: Negative control overexpressing lentivirus, oe-FNDC5: Fibronectin type III domain containing protein 5 overexpressing lentivirus, IL-6: Interleukin-6, IL-1b: Interleukin-1b, TNF-a: tumor necrosis factor-a, ELISA: Enzyme-linked immunosorbent assay).
FNDC5/irisin suppressed ferroptosis and apoptosis in the LPS-induced HK-2 cells
FNDC5, p-AMPK/AMPK, Nrf2, HO-1, and GPX4 concentrations of the LPS subgroup were dramatically abated versus those of the control subgroup (P < 0.001), whereas ACSL4 protein expression was dramatically boosted (P < 0.001). Compared with the LPS+oe-NC subgroup, the LPS+oeFNDC5 subgroup showed dramatically boosted FNDC5, p-AMPK/AMPK, Nrf2, HO-1, and GPX4 concentrations (P < 0.05), whereas ACSL4 protein concentration was dramatically abated (P < 0.01). Compared with the LPS+oeFNDC5 subgroup, the LPS+oe-FNDC5+erastin subgroup showed dramatically abated FNDC5, p-AMPK/AMPK, Nrf2, HO-1, and GPX4 concentrations (P < 0.05), but ACSL4 concentration was dramatically boosted (P < 0.01), [Figure 2a-g]. The Fe2+ concentration in the LPS subgroup was dramatically higher than with that of the control subgroup (P < 0.001). The Fe2+ concentration in the LPS+oeFNDC5 subgroup was dramatically lower versus that of the LPS+oe-NC subgroup (P < 0.05). The Fe2+ concentration in the LPS+oe-FNDC5+erastin subgroup was dramatically higher than that in the LPS+oe-FNDC5 subgroup (P < 0.05), [Figure 2h]. The apoptosis rate in the LPS+oe-FNDC5+erastin subgroup was dramatically higher than that in the LPS+oeFNDC5 subgroup, as further confirmed by flow cytometry (P < 0.001), [Figure 2i and j]. These results showed that FNDC5/irisin suppressed ferroptosis and apoptosis in the LPS-treated HK-2 cells, and this effect was correlated with the AMPK/Nrf2 pathway.
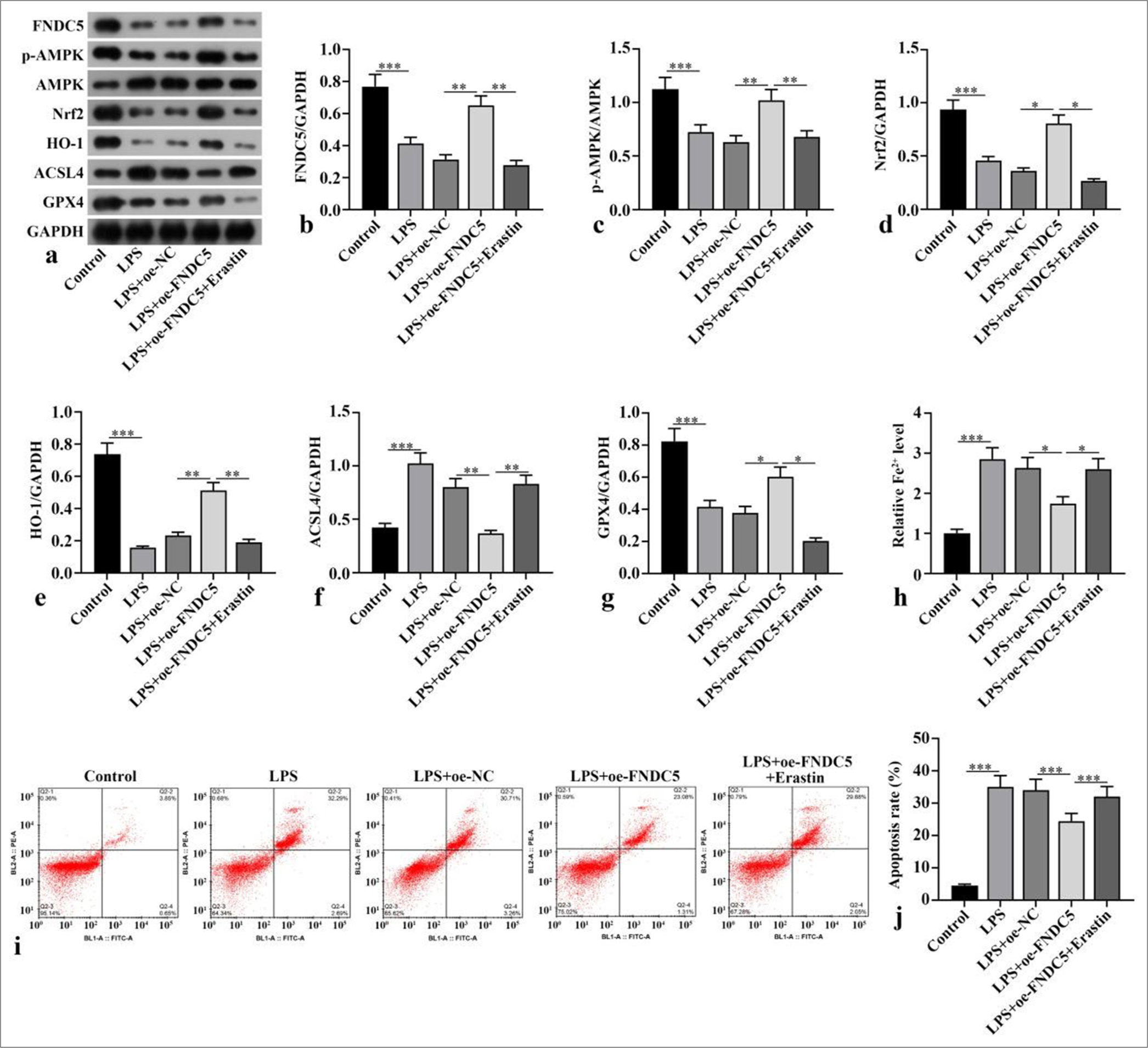
- FNDC5/irisin suppressed ferroptosis and apoptosis in LPS-induced HK-2 cells. A oe-FNDC5 was constructed and transfected into the cells. The cells were treated with a ferroptosis activator (erastin) for 1 h. (a-g) FNDC5, AMPK, p-AMPK, Nrf2, HO-1, ACSL4, and GPX4 expression levels were measured with Western blot. (h) Fe2+ concentration was measured in accordance with the kit instructions. (i and j) Cell apoptosis was measured through flow cytometry. n = 3, *P < 0.05, **P < 0.01, ***P < 0.001. (FNDC5: Fibronectin type III domain containing protein 5, HK-2: Human kidney-2, AMPK: Adenosine 5'-monophosphate-activated protein kinase, p-AMPK: Phospho- Adenosine 5'-monophosphate-activated protein kinase, Nrf2: Nuclear factor erythroid-2-related factor 2, HO-1: Heme oxygenase-1, GPX4: Glutathione peroxidase 4, ACSL4: Acyl-CoA synthetase long-chain family member 4, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, LPS: Lipopolysaccharide, oe-NC: Negative control overexpressing lentivirus, oe-FNDC5: Fibronectin type III domain containing protein 5 overexpressing lentivirus).
FNDC5/irisin abated ferroptosis and alleviated renal pathological damage induced by LPS by activating the AMPK/Nrf2 signaling pathway
Compared with the sham subgroup, serum BUN and Scr concentrations in LPS subgroup boosted dramatically (P < 0.001). Compared with LPS+oe-NC subgroup, serum BUN and Scr concentrations in LPS+oe-FNDC5 subgroup abated dramatically (P < 0.05). Compared with the LPS+oeFNDC5 subgroup, the LPS+oe-FNDC5+P5499 and LPS+oeFNDC5+erastin subgroups showed dramatically boosted serum BUN and Scr concentrations (P < 0.05), [Figure 3a and b]. H&E staining observed that in sham subgroup, there was no inflammatory cell infiltration in renal interstitium and cortex, and no pathological changes of renal tubular epithelial cells. In the LPS and LPS+oe-NC subgroups, renal cortex and interstitium was found inflammatory cell infiltration, and the swelling and vacuolar degeneration of most renal tubular epithelial cells were observed. Renal injury score was dramatically boosted in the LPS and LPS+oe-NC subgroups versus the sham group (P < 0.001). The renal injury in the LPS+oe-FNDC5 subgroup was dramatically improved, and the renal injury score in LPS+oe-FNDC5 subgroup was dramatically lower than that of the LPS+oe-NC subgroup (P < 0.001). Compared with LPS+oe-FNDC5 subgroup, renal interstitial edema and inflammatory cell infiltration were dramatically aggravated. The renal injury score was dramatically boosted in the LPS+oe-FNDC5+P5499 and LPS+oe-FNDC5+erastin subgroup compared with LPS+oe-FNDC5 subgroup (P < 0.01) [Figure 3c and d]. FNDC5, p-AMPK/AMPK, Nrf2, HO-1, and GPX4 protein concentrations in the kidney tissues of the LPS subgroup versus the sham subgroup were dramatically abated (P < 0.001), whereas the ACSL4 protein levels was dramatically boosted (P < 0.001). The FNDC5, p-AMPK/AMPK, Nrf2, HO-1, and GPX4 protein concentrations in the kidney tissues of the LPS+oe-FNDC5 subgroup versus the LPS+oe-NC subgroup was dramatically boosted (P < 0.01), whereas the ACSL4 protein concentration was dramatically abated (P < 0.01). Versus LPS+oe-FNDC5 subgroup, FNDC5, p-AMPK/AMPK, Nrf2, HO-1, and GPX4 protein concentrations in kidney tissue of LPS+oe-FNDC5+P5499 subgroup and LPS+oe-FNDC5+Erastin subgroup abated dramatically (P < 0.01), while ACSL4 protein concentration boosted dramatically, as confirmed by the Western blot results (P < 0.01), [Figure 3ek]. Fe2+ concentration in kidney tissue of LPS subgroup was dramatically higher than that in sham subgroup (P < 0.001). Fe2+ concentration of kidney tissue in LPS+oe-FNDC5 subgroup lower dramatically that that in LPS+oe-NC subgroup (P < 0.01). Compared with LPS+oe-FNDC5 subgroup, Fe2+ concentrations of the kidney tissue in LPS+oe-FNDC5+P5499 subgroup and LPS+oe-FNDC5+erastin subgroup boosted dramatically (P < 0.01) [Figure 3l]. These results showed that FNDC5/irisin abates LPS-induced ferroptosis and alleviates renal pathological damage through activating the AMPK/Nrf2 pathway.
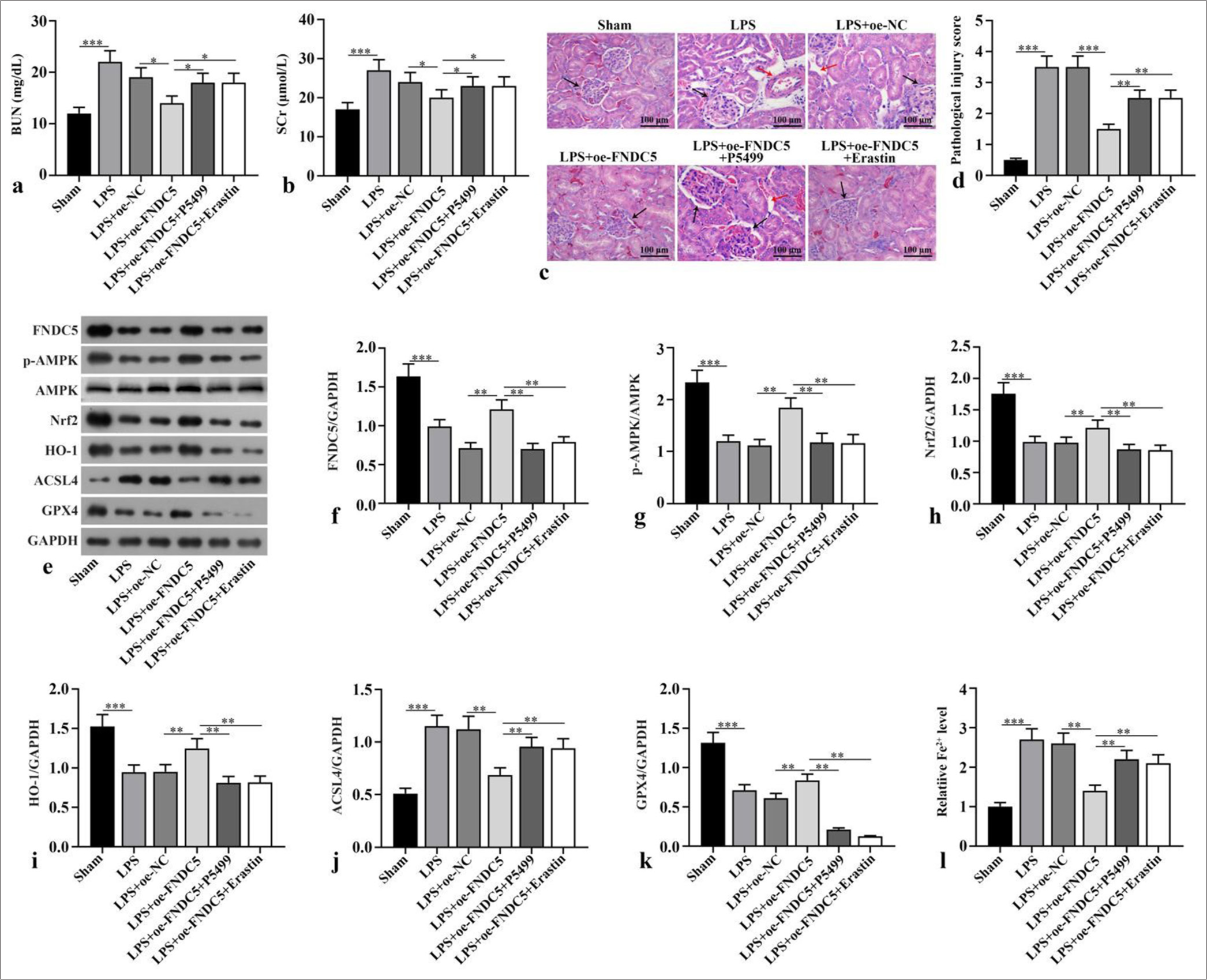
- FNDC5/irisin inhibited ferroptosis and alleviated renal pathological damage induced by LPS by activating AMPK/Nrf2 signaling pathway. The mice were sacrificed after the intraperitoneal injection of LPS or equal volume of normal saline, and blood and kidney samples were collected. After successful modeling, oe-FNDC5 was constructed, and the mice received AMPK inhibitor (P5499) and ferroptosis activator (erastin). (a and b) BUN and SCr were measured. (c and d) Hematoxylin and eosin (H&E) staining was applied for the evaluation of renal tubule injury. The black arrows represent the glomerulus, and the red arrows indicate the vacuoles in the proximal tubules. Magnification: ×200. (e-k) FNDC5, p-AMPK, AMPK, Nrf2, HO-1, ACSL4, and GPX4 expression were measured by western blot. (l) Fe2+ concentration was measured according to the kit instructions. n = 10, *P < 0.05, **P < 0.01, ***P < 0.001. (BUN: Blood urea nitrogen, SCr: Serum creatinine, LPS: Lipopolysaccharide, oe-NC: Negative control overexpressing lentivirus, oe-FNDC5: Fibronectin type III domain containing protein 5 overexpressing lentivirus, FNDC5: Fibronectin type III domain containing protein 5, AMPK: Adenosine 5’-monophosphate-activated protein kinase, p-AMPK: Phospho- Adenosine 5’-monophosphate-activated protein kinase, Nrf2: Nuclear factor erythroid-2-related factor 2, HO-1: Heme oxygenase-1, GPX4: Glutathione peroxidase 4, ACSL4: Acyl-CoA synthetase long-chain family member 4, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, H&E: Hematoxylin and eosin).
FNDC5/Irisin activated AMPK/Nrf2 signaling pathway to abate alleviate renal cell apoptosis
IL-6, IL-1b, and TNF-a concentrations in the kidney tissues of the LPS subgroup versus the sham subgroup were dramatically boosted (P < 0.001). Versus LPS+oe-NC subgroup, IL-6, IL-1b, and TNF-a concentrations in kidney tissues of LPS+oe-FNDC5 subgroup abated dramatically (P < 0.01). IL-6, IL-1b, and TNF-a concentrations in the kidney tissues of the mice in the LPS+oe-FNDC5+P5499 and LPS+oe-FNDC5+erastin subgroups versus the LPS+oeFNDC5 subgroup were dramatically boosted (P < 0.01), as demonstrated by the ELISA results [Figure 4a-c]. The apoptosis rate in the kidney tissues of the LPS subgroup was dramatically boosted, in contrast to that in the sham subgroup (P < 0.001). In contrast with LPS+oe-NC subgroup, the apoptosis rate of kidney tissue in LPS+oe-FNDC5 subgroup abated dramatically (P < 0.01). Compared with the apoptosis rate in LPS+oe-FNDC5 subgroup, the apoptosis rates of kidney tissue in LPS+oe-FNDC5+P5499 subgroup and LPS+oe-FNDC5+Erastin subgroup were boosted dramatically (P < 0.01), [Figure 4d and e]. These results showed that FNDC5/irisin abate ferroptosis and alleviate renal cell apoptosis by AMPK/Nrf2 pathway.
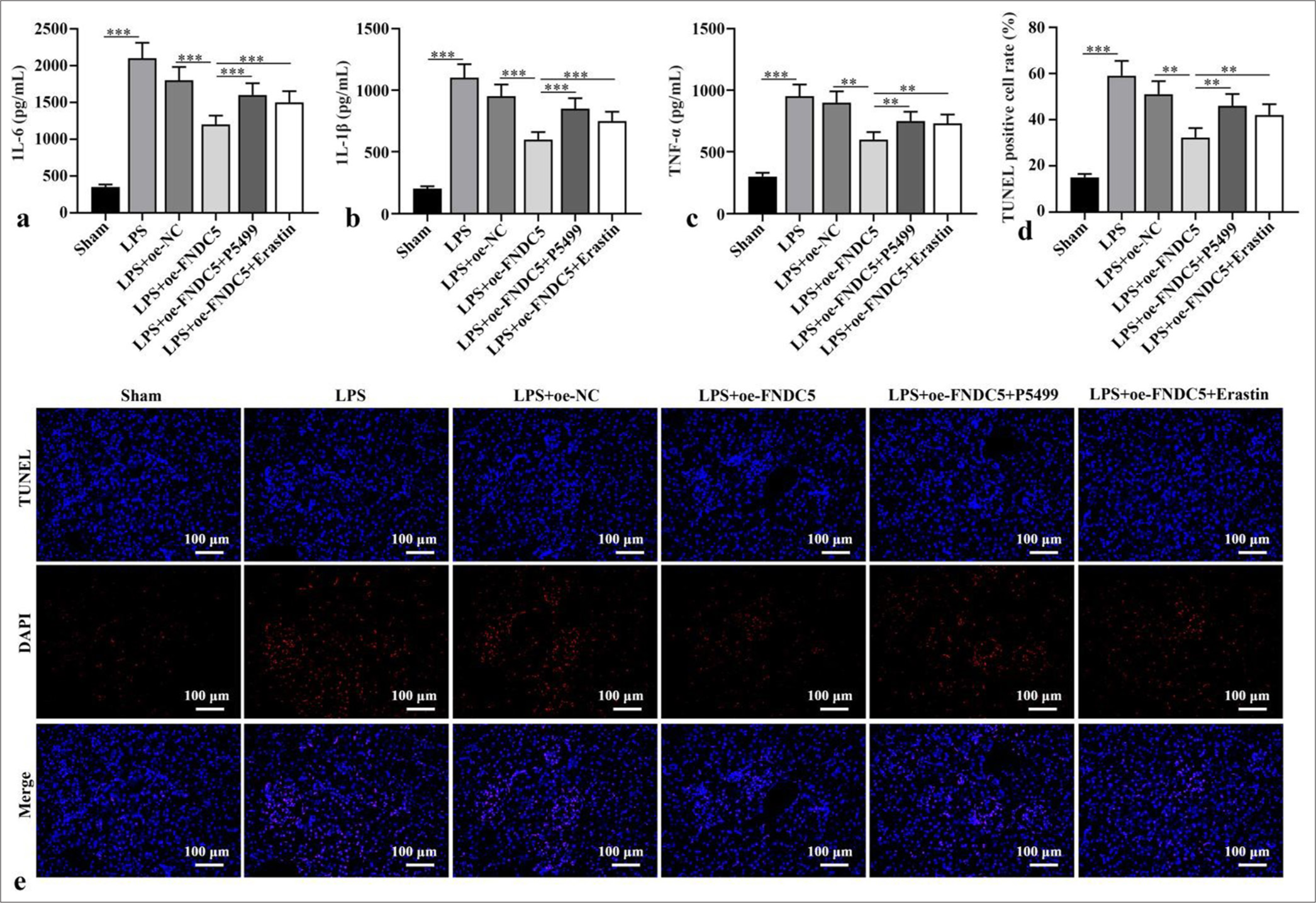
- FNDC5/Irisin activated AMPK/Nrf2 pathway to abate alleviate renal cell apoptosis. (a-c) ELISA was to test IL-6, IL-1b, and TNF-a levels. (d and e) Apoptosis in renal tissues was measured through TUNEL staining. Magnification: ×200. n = 10, **P < 0.01, ***P < 0.001. (IL-6: Interleukin-6, IL-1b: Interleukin-1b, TNF-a: Tumor necrosis factor-a, LPS: Lipopolysaccharide, oe-NC: Negative control overexpressing lentivirus, oe-FNDC5: Fibronectin type III domain containing protein 5 overexpressing lentivirus, FNDC5: Fibronectin type III domain containing protein 5, TUNEL: TdT-mediated dUTP nick end-labeling, DAPI: 4',6-diamidino-2-phenylindole, AMPK: Phospho- Adenosine 5’-monophosphate-activated protein kinase, Nrf2: Nuclear factor erythroid-2-related factor 2, ELISA: Enzyme-linked immunosorbent assay).
DISCUSSION
Sepsis can lead to multiple-organ damage, mainly affecting the kidneys.[25] AKI is a serious complications of sepsis and a type of renal dysfunction linked to inflammation and oxidative stress.[26] LPS is commonly utilized in the construction of experimental sepsis-related AKI models.[27] LPS could activate the inflammatory pathway, boost the release of proinflammatory cytokines, including IL-1b, IL-6, TNF-a, and chemokine, and activate the complement system by binding to toll-like receptor 4. Systemic inflammation is the main pathophysiological factor of sepsis-induced AKI, in which excessive inflammation accompanied by a large increase in ROS level causes the damages in the mitochondrial structure and function.[5] These effects could aggravate kidney damage. In addition, oxidative stress is an important mechanisms related to sepsis-induced AKI.[28] Chen et al.[29] demonstrated that forsythiaside A can alleviate sepsis-induced AKI through alleviating inflammation and apoptosis, and exert a protective efficacy in the kidney. In this study, we established an cell model using LPS-treated HK-2 cells. We discovered that LPS stimulation abated FNDC5 protein concentration. The overexpression of FNDC5 abated cytokines (IL-6, IL-1b, and TNF-a) concentrations and apoptosis rate, suggesting alleviating LPS-induced inflammatory response and inhibiting cell apoptosis. Ferroptosis is characterized by iron-dependent accumulation of ROS that exceeds the redox content maintained by glutathione and phospholipid hydroperoxidase.[30] The disorder of iron ion and decreased activity of the cystine/glutamate antiporter system (cystine/glutamate antiporter system, Xc-) and GPX4 are the main causes of ferroptosis.[31] GPX4 participates in the processes of ferroptosis, it converts glutathione into oxidized glutathione, and reduces cytotoxic lipid peroxides into corresponding alcohols. The decreased GPX4 activity can result in the accumulation of lipid peroxide, which is a sign of ferroptosis.[31] Zhou et al.[32] demonstrated that the down-regulation of microRNA-214-3p expression boosted GPX4 concentration in the kidney tissues of cisplatin-induced AKI mice and thus abated renal tubular ferroptosis in cisplatin-induced AKI mice. ACSL4 can catalyze the synthesis of acyl coenzyme A from fatty acids; thus, it is considered the key enzyme in fatty acid catabolism.[33] Wang et al.[34] discovered that the knockout of ACSL4 substantially abated ferroptosis and renal function, mitigated pathological damage, and substantially abated renal inflammation and macrophage infiltration in AKI mice. Our study observed that the overexpression of FNDC5 boosted GPX4 protein concentration and abated ACSL4 protein level in the HK-2 cells induced by LPS. Fe2+ concentration was measured in the cells, and the results showed that overexpression of FNDC5 abated Fe2+ concentration in HK-2 cells, demonstrating that FNDC5/irisin plays a role in sepsis-induced AKI by modulating ferroptosis.
Then, in the animal study, a septic-induced AKI mouse model was established through the intraperitoneal injection of LPS. Serum BUN and Scr concentrations were measured for the evaluation of renal function. Serum BUN and Scr concentrations were substantially boosted after LPS injection, and H&E staining results showed the inflammatory cell infiltration in the renal cortex and interstitium, and the swelling and vacuolar degeneration of most renal tubular epithelial cells, indicating that the mouse model was established successfully. We also observed that FNDC5 concentration in the kidney tissues of mice treated with LPS was substantially abated, and the overexpression of FNDC5 boosted serum BUN and Scr concentrations. H&E staining results showed that pathological damage in the kidney tissues was substantially improved, indicating that overexpression of FNDC5 exerted a certain renal protective effect. Then, ferroptosis-related indexes in the mouse kidney were measured. The overexpression of FNDC5 boosted GPX4 protein expression, abated ACSL4 protein expression, and abated Fe2+ concentration. The major proinflammatory cytokines and related indexes of oxidative stress concentrations were measured. The overexpression of FNDC5 abated IL-6, IL-1b, TNF-a, and apoptosis rate. These results indicated that FNDC5/irisin can abate ferroptosis and alleviate renal pathological damage induced by LPS, inflammatory reaction, and oxidative stress.
AMPK is an important energy sensor in cell metabolism and can respond to various metabolic stress, including oxidative stress, inflammation, and hypoxia.[35] Nrf2 is the main transcription factor that regulating the expression of antioxidant enzymes, has a major role in cell defense against oxidative stress and inflammation by activating antioxidant cascades.[36] Sulforaphane can abate ferroptosis in the cardiomyocytes of mice with diabetic cardiomyopathy through AMPK-mediated Nrf2 activation.[37] Saleh et al.[38] discovered that the up-regulation of AMPK/phosphatidylinositol-3-kinase/AKT pathway can stimulate Nrf2-regulated antioxidant enzymes and become a potential therapeutic target for AKI induced by diabetic renal ischemia/reperfusion injury. In this study, the overexpression of FNDC5 can boost p-AMPK/AMPK, Nrf2, and HO-1 concentrations in LPS-induced HK-2 cells or mouse kidney, indicating that the role of FNDC5/irisin in sepsis-induced AKI is related to AMPK/Nrf2 pathway.
SUMMARY
FNDC5/irisin abates ferroptosis through the AMPK/Nrf2 signal pathway and reduces renal pathological damage, inflammatory response, and oxidative stress in sepsis-induced. The results showed that targeting FNDC5/irisin may be a potential method for the sepsis-induced AKI.
AVAILABILITY OF DATA AND MATERIALS
The dataset analyzed during the present study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
AKI – Acute kidney injury
FNDC5 – Fibronectin type III domain containing protein 5
AMPK – Adenosine 5'-monophosphate-activated protein kinase
Nrf2 – Nuclear factor erythroid-2-related factor 2
LPS – Lipopolysaccharide
p-AMPK – phospho-AMPK
HO-1 – Heme oxygenase-1
GPX4 – Glutathione peroxidase 4
ACSL4 – Acyl-CoA synthetase long-chain family member 4
IL-6 – Interleukin-6
IL-1β – Interleukin-1β
TNF-α – Tumor necrosis factor -α
ELISA – Enzyme-linked immunosorbent assay
TUNEL – TdT-mediated dUTP nick end-labeling
SCr – Serum creatinine
BUN – Blood urea nitrogen
H&E – Hematoxylin and eosin
ROS – Reactive oxygen species
HK-2 – Human kidney-2
oe-NC – negative control overexpressing lentivirus
oe-FNDC5 – FNDC5 overexpressing
AUTHOR CONTRIBUTIONS
SHG, CCZ and YFL: Contributed to the study conception and design. Material preparation, data collection, and analysis; SHG: Wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experimental protocols of this study were approved by Beijing Biocisco Biomedical Technology Co., Ltd animal ethics committee (No: MDL 2023-05-13-01). As all animal experiments were conducted at the Beijing Biocisco Biomedical Technology Co., Ltd, the animal ethics committee of this institution also approved the animal experiments of our study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Inhibition of aerobic glycolysis alleviates sepsisinduced acute kidney injury by promoting lactate/sirtuin 3/AMPKregulated autophagy. Int J Mol Med. 2021;47:19.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083-99.
- [CrossRef] [PubMed] [Google Scholar]
- Sepsis-associated acute kidney injury. Crit Care Clin. 2021;37:279-301.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoregulatory mechanism of acute kidney injury in sepsis: A narrative review. Biomed Pharmacother. 2023;159:114202.
- [CrossRef] [PubMed] [Google Scholar]
- Sepsis-induced AKI: From pathogenesis to therapeutic approaches. Front Pharmacol. 2022;13:981578.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed cell death in sepsis associated acute kidney injury. Front Med (Lausanne). 2022;9:883028.
- [CrossRef] [PubMed] [Google Scholar]
- Lyn attenuates sepsis-associated acute kidney injury by inhibition of phosphoSTAT3 and apoptosis. Biochem Pharmacol. 2023;211:115523.
- [CrossRef] [PubMed] [Google Scholar]
- Necroptosis in renal ischemia/reperfusion injury: A major mode of cell death? Arch Biochem Biophys. 2020;689:108433.
- [CrossRef] [PubMed] [Google Scholar]
- LncRNA GAS5 inhibits miR-579-3p to activate SIRT1/PGC-1a/Nrf2 signaling pathway to reduce cell pyroptosis in sepsis-associated renal injury. Am J Physiol Cell Physiol. 2021;321:C117-33.
- [CrossRef] [PubMed] [Google Scholar]
- The pathophysiology of sepsis-associated AKI. Clin J Am Soc Nephrol. 2022;17:1050-69.
- [CrossRef] [PubMed] [Google Scholar]
- Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021;31:107-25.
- [CrossRef] [PubMed] [Google Scholar]
- Transcriptomic analysis and laboratory experiments reveal potential critical genes and regulatory mechanisms in sepsis-associated acute kidney injury. Ann Transl Med. 2022;10:737.
- [CrossRef] [PubMed] [Google Scholar]
- Ferroptosis: A new insight for treatment of acute kidney injury. Front Pharmacol. 2022;13:1065867.
- [CrossRef] [PubMed] [Google Scholar]
- A PGC1-a-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463-8.
- [CrossRef] [PubMed] [Google Scholar]
- FNDC5/Irisin: Physiology and pathophysiology. Molecules. 2022;27:1118.
- [CrossRef] [PubMed] [Google Scholar]
- Irisin pretreatment protects kidneys against acute kidney injury induced by ischemia/reperfusion via upregulating the expression of uncoupling protein 2. Biomed Res Int. 2020;2020:6537371.
- [CrossRef] [PubMed] [Google Scholar]
- Renal protection induced by physical exercise may be mediated by the irisin/AMPK axis in diabetic nephropathy. Sci Rep. 2022;12:9062.
- [CrossRef] [PubMed] [Google Scholar]
- Post-treatment with irisin attenuates acute kidney injury in sepsis mice through anti-ferroptosis via the SIRT1/Nrf2 pathway. Front Pharmacol. 2022;13:857067.
- [CrossRef] [PubMed] [Google Scholar]
- Postconditioning with irisin attenuates lung ischemia/reperfusion injury by suppressing ferroptosis via induction of the Nrf2/HO-1 signal axis. Oxid Med Cell Longev. 2022;2022:9911167.
- [CrossRef] [PubMed] [Google Scholar]
- Involvement of GPX4 in irisin's protection against ischemia reperfusion-induced acute kidney injury. J Cell Physiol. 2021;236:931-45.
- [CrossRef] [PubMed] [Google Scholar]
- Maresin 1 attenuates lipopolysaccharide-induced acute kidney injury via inhibiting NOX4/ROS/NF-kB pathway. Front Pharmacol. 2021;12:782660.
- [CrossRef] [PubMed] [Google Scholar]
- Overexpression of activating transcription factor 3 exerts suppressive effects in HepG2 cells. Mol Med Rep. 2019;19:869-76.
- [CrossRef] [Google Scholar]
- Irisin is induced in renal ischemia-reperfusion to protect against tubular cell injury via suppressing p53. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165792.
- [CrossRef] [PubMed] [Google Scholar]
- Nrf2 signaling attenuates epithelial-to-mesenchymal transition and renal interstitial fibrosis via PI3K/Akt signaling pathways. Exp Mol Pathol. 2019;111:104296.
- [CrossRef] [PubMed] [Google Scholar]
- Dexmedetomidine alleviates lipopolysaccharide-induced acute kidney injury by inhibiting p75NTR-mediated oxidative stress and apoptosis. Oxid Med Cell Longev. 2020;2020:5454210.
- [CrossRef] [PubMed] [Google Scholar]
- Sirtuin 6 overexpression relieves sepsis-induced acute kidney injury by promoting autophagy. Cell Cycle. 2019;18:425-36.
- [CrossRef] [PubMed] [Google Scholar]
- ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10:2342-57.
- [CrossRef] [PubMed] [Google Scholar]
- Forsythiaside A ameliorates sepsis-induced acute kidney injury via anti-inflammation and antiapoptotic effects by regulating endoplasmic reticulum stress. BMC Complement Med Ther. 2023;23:35.
- [CrossRef] [PubMed] [Google Scholar]
- FNDC5/irisin reduces ferroptosis and improves mitochondrial dysfunction in hypoxic cardiomyocytes by Nrf2/HO-1 axis. Cell Biol Int. 2022;46:723-36.
- [CrossRef] [PubMed] [Google Scholar]
- Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-214-3p aggravates ferroptosis by targeting GPX4 in cisplatin-induced acute kidney injury. Cell Stress Chaperones. 2022;27:325-36.
- [CrossRef] [PubMed] [Google Scholar]
- AcylCoA synthetase long chain family member 4 plays detrimental role in early brain injury after subarachnoid hemorrhage in rats by inducing ferroptosis. CNS Neurosci Ther. 2021;27:449-63.
- [CrossRef] [PubMed] [Google Scholar]
- ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022;51:102262.
- [CrossRef] [PubMed] [Google Scholar]
- AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251-62.
- [CrossRef] [PubMed] [Google Scholar]
- Eat your broccoli: Oxidative stress, NRF2, and sulforaphane in chronic kidney disease. Nutrients. 2021;13:266.
- [CrossRef] [PubMed] [Google Scholar]
- Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12:708-22.
- [CrossRef] [PubMed] [Google Scholar]
- Polyethylene glycol capped gold nanoparticles ameliorate renal ischemia-reperfusion injury in diabetic mice through AMPK-Nrf2 signaling pathway. Environ Sci Pollut Res Int. 2022;29:77884-907.
- [CrossRef] [PubMed] [Google Scholar]








