Translate this page into:
Fine-needle aspiration of basaloid scalp lesion: Potential diagnostic pitfall

*Corresponding author: Casem Ballouk, Department of Pathology, Wayne State University, Detroit, United States. hj5236@wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Ballouk C, Alazawi S, Khan M, Shidham VB. Fine-needle aspiration of basaloid scalp lesion: Potential diagnostic pitfall. CytoJournal. 2024;21:35. doi: 10.25259/Cytojournal_81_2023
QUIZ DESCRIPTION
Gradually, enlarging 4 × 4 cm, well-circumscribed, mobile scalp nodule over the left parietal area in a 38-year-old man, noted after hitting a towel rack 7 months ago. Papanicolaou stained fine-needle aspiration smears stained showed groups of basaloid cells and eosinophilic shadows (ghost cells) with focal foreign body giant cells [Figure 1].
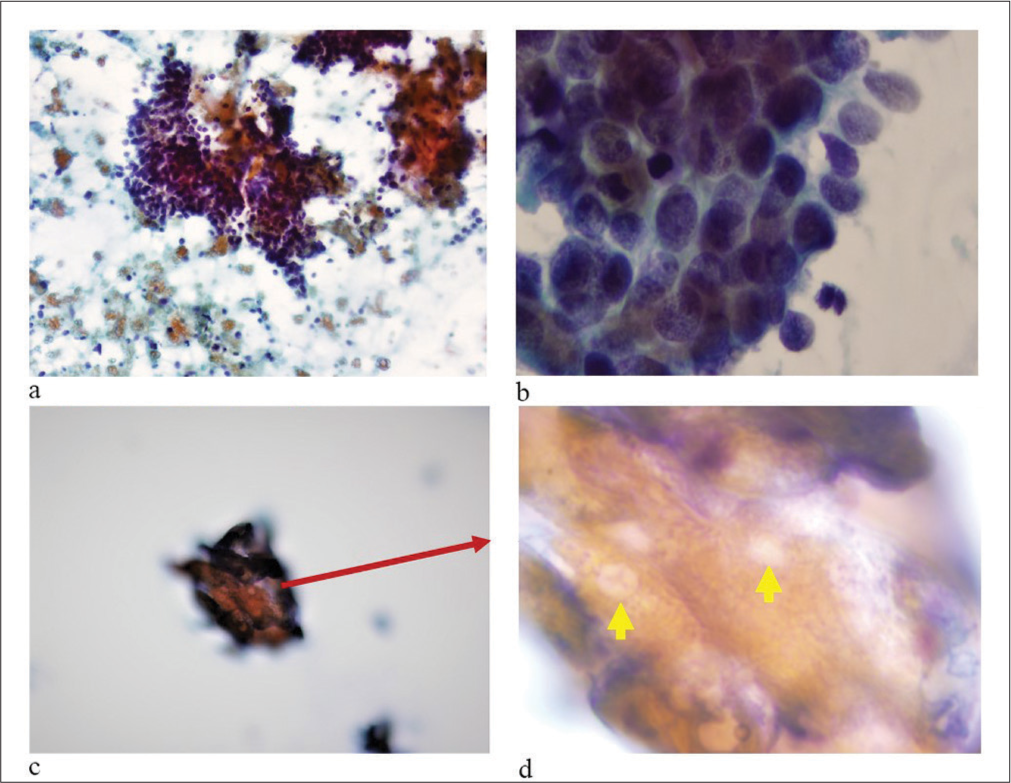
- (a) The aspirate showed scattered groups of basaloi d cells with cohesive clumps of keratin debris. (b) ×60 the basaloid cells had scant cytoplasm with indistinct cell borders and hyperchromatic nuclei. (c) ×20 Tight clumps of keratinized debris with red arrow indicatingthe area undergoing zooming Tight clumps of keratinized debris with some identifiable ghost cells (yellow arrows in d). (d) 100×: ghost cells identified by yellow arrows. The tightly cohesive groups of anucleate keratinized cells did not spread well as compared to other squamous epithelial lesions and so the details of individual cells as ghost cells in cytology smears were difficult to evaluate and photograph (Pap stained direct smear. a: ×10; b: ×60; c: ×20; d: ×100 Oil with light increased).
MORPHOLOGY QUIZ
1. What is the most likely diagnosis?
Pilomatricoma
Melanoma
Merkle cell carcinoma
Squamous cell carcinoma
Proliferating trichilemmal tumor
Answer: a
EXPLANATION
Option a: Pilomatricoma showed cellular aspirates comprised of micro-fragments containing groups of basaloid cells without peripheral palisading. The basaloid cells showed high nuclear: cytoplasmic ratio, evenly dispersed chromatin with a few cells showing prominent nucleoli. Clumps of refractile keratin and multinucleated giant cells were also seen in the background. These tumors can have mitosis and, hence, could be misinterpreted as carcinomas. Even thought, it may be difficult to evaluate in fine-needle aspiration (FNA) aspirate, ghost cells (a.k.a. eosinophilic shadow cells), which have no nuclear staining, are clues to correct diagnosis.
Option b: Melanoma may present as asymmetrical and gradually enlarging lesion with irregular borders. However, this lesion is symmetrical with well-defined borders and uniform tan color. Desmoplastic melanoma is usually present on the head-and-neck region and can appear as erythematous, pink or pale nodules or plaque.[1] Histologically, melanoma can mimic features of other tumors such as neuroendocrine tumors, poorly differentiated carcinoma, and lymphoma.[2] A typical case of melanoma shows high nuclear to cytoplasmic ratio with a prominent cherry red nucleoli and hyperchromatic nuclei with irregular nuclear membrane.[2] Immunohistochemistry is widely used in melanoma to differentiate it from other tumors. S-100 is commonly used and has good sensitivity.[2] Other markers have good specificity such as Melan-A, tyrosinase, or HMB45.[2]
Option c: Merkel cell carcinoma (MCC) often appears as asymptomatic red, pink, or blue papules or nodules and lacks distinctive clinical features. Histopathologically, it is marked by round nuclei with finely granular chromatin, inconspicuous nucleoli, scant cytoplasm, and nuclear crush artifacts, along with multiple mitotic figures and apoptotic bodies resembling small cell carcinoma.[3] MCC shows high sensitivity to cytokeratin 20 and neurofilaments, whereas negative staining for thyroid transcription factor 1 and cytokeratin 7 helps distinguish it from small cell carcinoma.[3]
Option d: Squamous cell carcinoma (SCC) usually presents as a red scaly plaque. SCC can be classified with various degrees of differentiation from well to poorly differentiated. SCC can be classified into many subtypes and histopathologic features include atypical squamous cells, keratin pearls, and sometimes hyperkeratosis.[4] Well-differentiated tumors exhibit interconnecting follicular infundibular type squamous epithelium.[4] SCC is characterized by atypical squamous cells, whereas pilomatricoma consists of basaloid cells and shadow cells. In addition, immunohistochemistry is not typically used except in the cases of poorly differentiated SCC.[4] Cutaneous SCC stains positive with p63, p40, MUC1 (epithelial membrane antigen), CK5/6, MNF116, and high-molecular weight 34 E12. BerEp4 should be negative in SCC.[5]
Option e: Proliferating trichilemmal tumor is a tumor originating from the outer root sheath of a hair follicle.[6] The fine-needle aspirates are usually cellular and show syncitial sheets of squamous epithelial cells, amorphous material, keratin, and clusters of small cells with hyperchromatic nuclei mostly basaloid cells.[6] This lesion does not show ghost cells, calcification, cholesterol clefts, and foreign body giant cells.[6]
FOLLOW-UP OF THE CASE
The lesion showed [Figure 2a-d] two types of cells: Basaloid cells and shadow cells. Basaloid cells are present along the periphery in variable proportion. They are small, monotonous with scant cytoplasm with indistinct cellular borders and evenly distributed chromatin. Basaloid cells merge gradually or abruptly with so-called ghost or shadow cells. Ghost cells have abundant pink cytoplasm and open space at their center. Focally, the periphery of the lesion showed foreign body giant cell reaction.
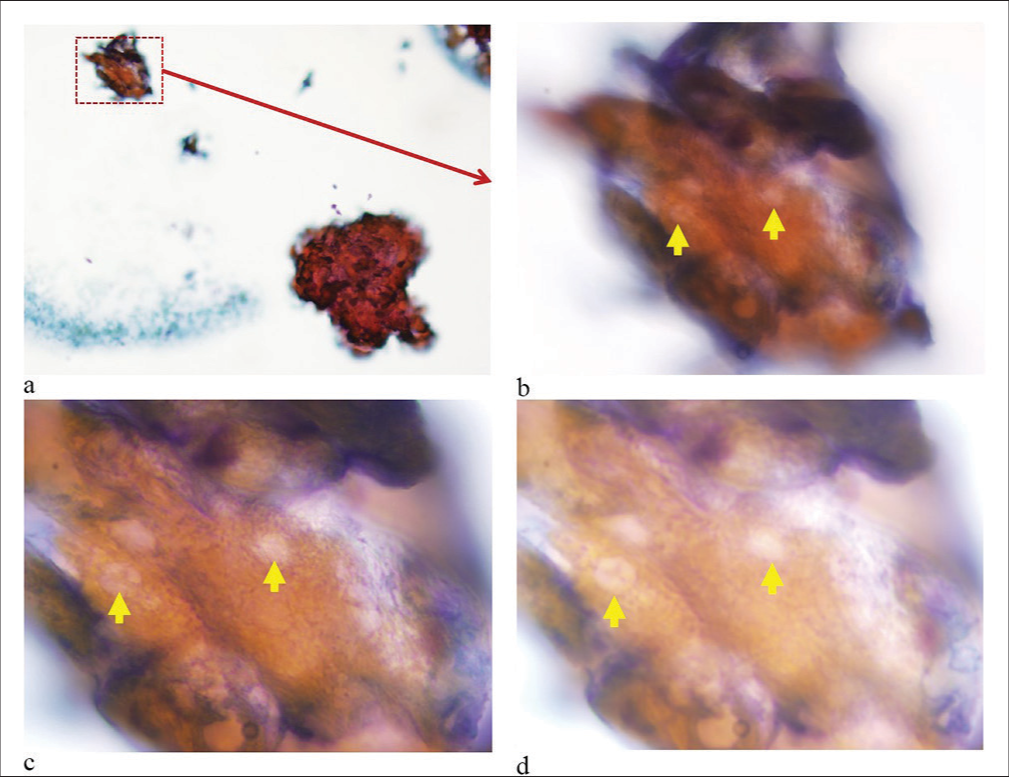
- (a-d) Clumps of keratinized debris with some identifiable ghost cells (yellow arrows). The tightly cohesive groups of keratinized cells did not spread well as compared to other squamous epithelial lesions, making the details of individual cells as ghost cells in cytology smears difficult to appreciate and photograph. (Pap stained direct smear. a: ×10; b: ×60; c: ×100 Oil, Focus 1 to highlight the overall structure; d: ×100 Oil, Focus 2 to provide enhanced detail with increased light.
The excisional biopsy showed an intradermal, fairly well-circumscribed lesion measuring 4 × 4 × 4 cm. Microscopic images are shown in Figure 3.
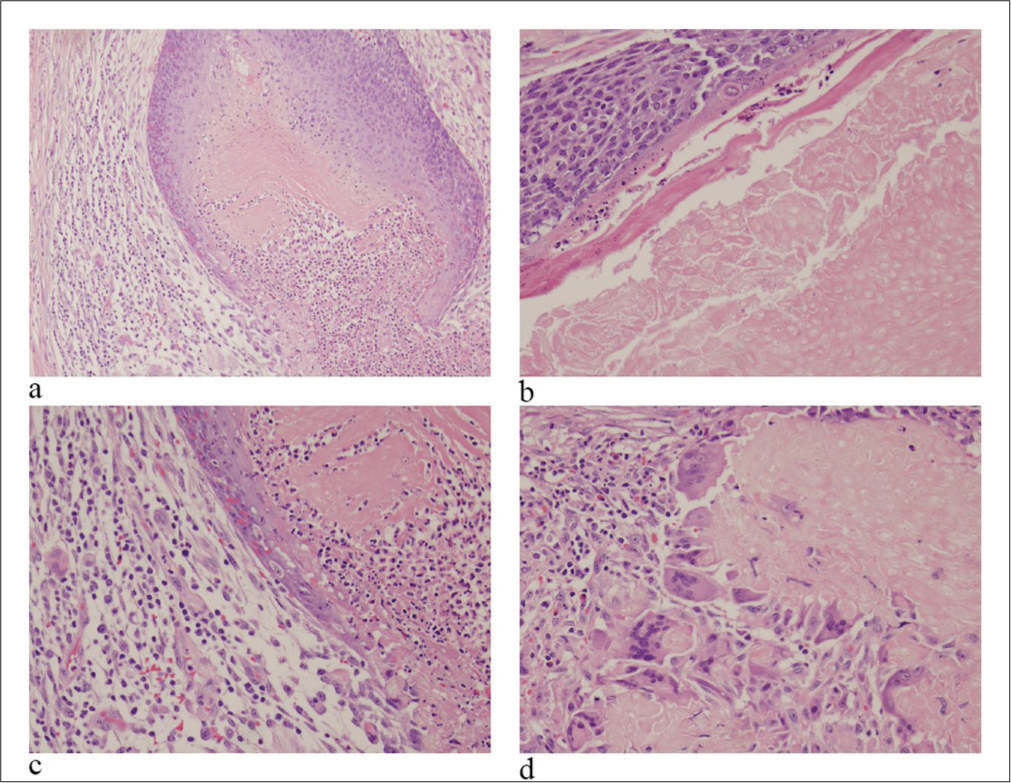
- Hematoxylin and Eosin (HE) stained sections of resection (a: ×4; b-d: ×20 ). (a) Islands of basaloid cells at periphery with center showing shadow cells. (b) Basaloid cells with shadow cells. (c and d) Giant cells surrounding an area with keratinized ghost cells.
ADDITIONAL QUIZ QUESTIONS
2. How does the expression of beta-catenin and p63 in pilomatricoma differ in intensity and distribution from other hair matrix tumors?
Pilomatricoma exhibits nuclear beta-catenin and nuclear p63
Pilomatricoma exhibits membranous beta-catenin and cytoplasmic p63
Pilomatricoma exhibits nuclear beta-catenin and absent p63
Pilomatricoma exhibits absent both beta-catenin and p63
Answer: a
EXPLANATION
a) Pilomatricoma exhibits nuclear beta-catenin and nuclear p63 Explanation: The expression and distribution of certain proteins, such as beta-catenin and p63, can be used to differentiate pilomatricomas from other types of hair matrix tumors. Beta-catenin was positive in the basaloid cell population, showing both cytoplasmic and nuclear staining.[7,8] Nuclear localization is particularly characteristic of pilomatricoma and can help distinguish it from other skin tumors, where beta-catenin is usually restricted to the membrane.[8] p63 is a marker that is often used to identify squamous epithelium and squamous differentiation.[8] In pilomatricoma, p63 typically shows strong positivity in the basaloid cells surrounding the ghost cells, reflecting the squamous nature of the tumor.[7]
3. What is the recommended treatment approach for pilomatricoma?
Surgical excision with clear margins
Observation as the pilomatricoma will spontaneously regress
Steroid injection
Radiation therapy
Topical chemotherapy
Answer: a
a) Surgical excision with clear margins. Because it is a benign tumor, complete removal with clear margins is usually curative and prevents recurrence.[9]
BRIEF REVIEW OF TOPIC
Pilomatricoma (also known as pilomatrixoma and calcifying epithelioma of Malherbe) is a benign tumor of hair follicle matrix that most frequently develops with bimodal age distribution of first and sixth decade.[9] Pilomatricoma usually presents as a firm nodule with the overlying skin appearing normal but can present with blue-red discoloration.[9] The tumor can occasionally have keratotic appearance, imitating squamous cell carcinoma, or can present with telangiectasia resembling basal cell carcinoma (BCC).[9] Although malignancy is rare, pilomatricoma can progress into pilomatrix carcinoma.[10] Pilomatricoma is commonly seen in the head-and-neck area.[10] Involvement of palms and soles or genitalia has not been reported.[10] Surgical excision with clear margins is the recommended treatment.[10] Following total resection, recurrence is uncommon.[10]
The histological appearance of a pilomatricoma is solid and nests of basaloid cells. This is different from other types of hair matrix tumors which typically have peripheral palisading. The presence of ghost cells, which are tiny, pale cells that seem “ghostly” under the microscope, is a crucial diagnostic clue of pilomatricomas. They arise when the cytoplasm of maturing cells is replaced by keratin, a protein found in the epidermis, or outermost layer of the skin.[11] Beta-catenin in pilomatricoma often shows both nuclear and cytoplasmic accumulation and p63 typically demonstrates strong nuclear positivity in the basaloid cells surrounding the ghost cells.[7,8] The nuclear distribution of both beta-catenin and p63 is a distinguishing feature from other hair matrix tumors, where they may show a predominantly membranous pattern of beta-catenin and variable P63.
Pilomatricomas are commonly misdiagnosed and are rarely considered as part of the differential diagnosis due to multiple overlapping cytologic features as shown in Figure 4. Clinical differential diagnosis includes but is not limited to BCC which lacks shadow cells and frequently has a mucinous stroma. BCC has many subtypes and are characterized as high-risk and low-risk histologic subtypes. Nodular BCC is the most common and falls under low-risk subtypes.[12] Nodular BCC can appear as a pearly, translucent papule or nodule with telangiectasia and histopathology usually show basaloid keratinocytes with peripheral palisading and clefting.[12,13] Micronodular, infiltrative, sclerosing, and morpheaform are considered higher-risk subtypes and more likely to recur than low-risk histologic subtypes which include superficial and nodular BCC.[13]
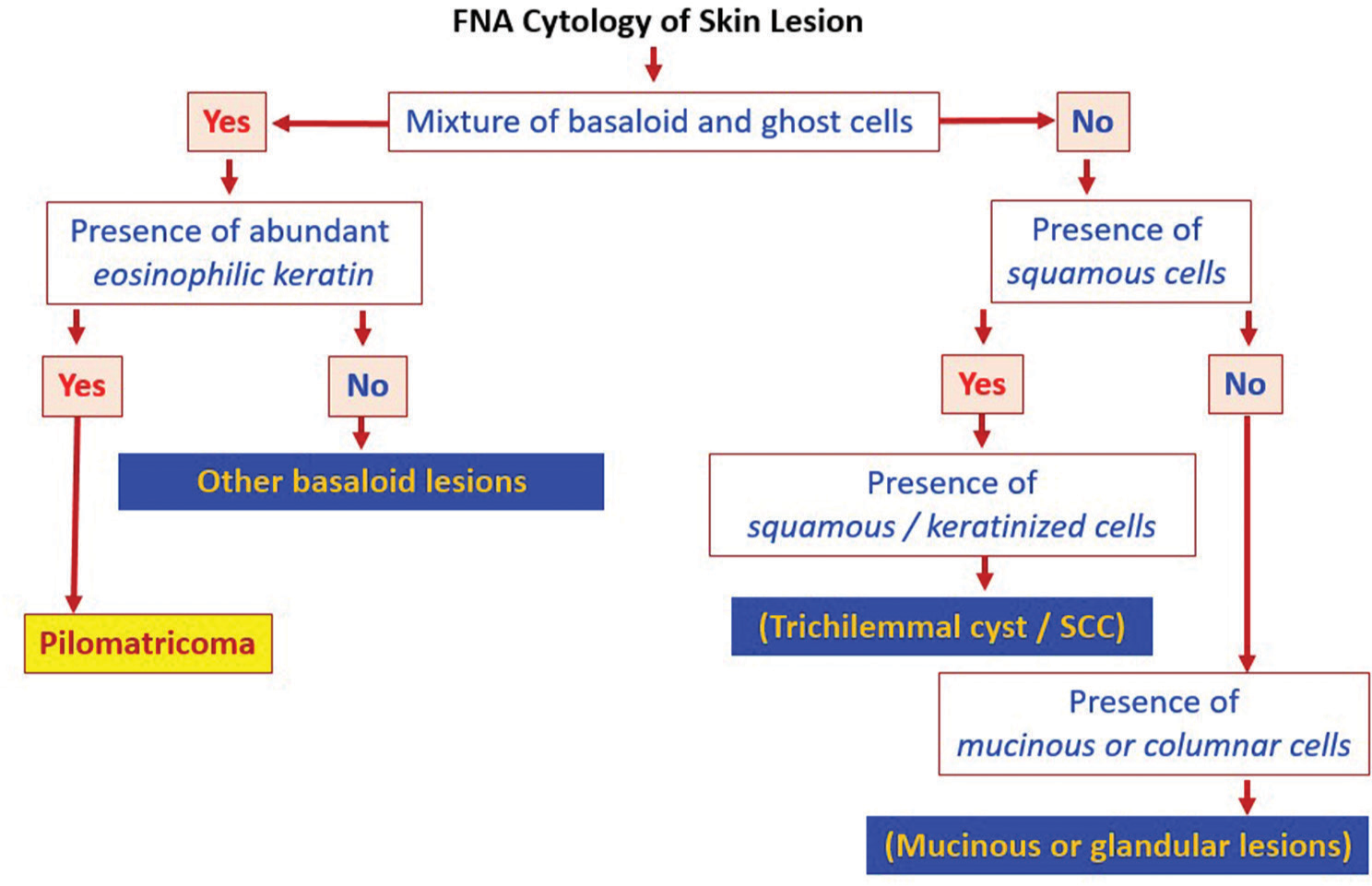
- Pilomatricoma fine-needle aspiration alg orithm. SCC: Squamos cell carcinoma The figure was made using the software PowerPoint for Microsoft 365 (2024), Microsoft, USA.
Trichilemmal cysts (TCs) are benign cystic lesions originating from follicular isthmus epithelium and can present similar to pilomatricoma with slow-growing, asymptomatic, and firm nodules in the scalp.[14] However, histologically TC found to have squamoid cells with abundant eosinophilic cytoplasm, compared to basaloid small cells in pilomatricoma.
Epidermal inclusion cyst was considered on the differential and usually occurs on the scalp, neck, back, and extremities.[15] However, it is less likely given absence of central punctum on examination. Lipoma is a benign adipose tumors and can present similar to pilomatricoma but less likely since FNA did not visualize adipose tissue cells.[16]
MCCs do not have distinctive clinical features and can present as asymptomatic red or pink or blue papules or nodules.[3] Histopathologic features include round nuclei with finely granular chromatin, inconspicuous nucleoli, scant cytoplasm, showing molding, and crush artifact.[3] The high nucleus: cytoplasm ratio gives it a blue or basaloid appearance (3). In addition, immunostains have good sensitivity and can further confirm the diagnosis of MCC which includes cytokeratin 20, low-molecular-weight cytokeratins (e.g., CAM5.2), and neurofilaments.[3] Electron microscopy shows paranuclear and/or cytoplasmic bundles of tonofilaments (keratin intermediate filaments), cytoplasmic dense-core granules, and desmosomal attachments.[3] FNA of MCC typicall show small, round, or oval cells with round-to-oval nuclei and scant cytoplasm. Nuclei have fine chromatin and small, inconspicuous nucleoli. Occasional nuclear moldingcan be observed.[17]MCC is less lik ely because histopathology lacks shadow cells. Table 1[3,4,13-18] shows the differential diagnosis for pilomatricoma.
| Diagnosis | Description | Physical examination | Histology | Fine-needle aspiration |
|---|---|---|---|---|
| TC | Slow-growing, asymptomatic or mildly painful. Usually present in the scalp. | Freely mobile, and firm, skin-colored nodules. TC Can be erythematous after trauma. | Cyst lumen contains homogeneous eosinophilic and keratinous materials. Cystic structure lined by stratified squamous epithelium without a granular cell layer.[14] | Anucleate and nucleated squamous cells without atypia, basaloid cells without peripheral palisading but with abrupt keratinization and keratinous debris.[14] |
| EIC | Asymptomatic, slowly enlarging, usually occur on the scalp, skin of head and neck, back and extremities.[15] | Firm, round, and subcutaneous nodule with central punctum.[15] | EIC is lined by a stratified squamous epithelium with a granular layer and contains lamellated keratin flakes.[18] | numerous anucleate squames alongside nucleated benign squamous cells. With secondary infection, aspirates turn turbid, showing inflammatory cells like neutrophils and histiocytes.[15] |
| Lipoma | Asymptomatic. | Skin-colored subcutaneous nodule. | Lobules of mature adipocytes separated by thin fibrous septa. | Mature adipocytes, which appear as large, uniform, and empty-appearing cells with eccentric nuclei.[16] |
| BCC | Slow-growing and commonly found in sun-exposed areas. | Nodular BCC can appear as a pearly, translucent papule or nodule with telangiectasia. | Nodular subtype-basaloid keratinocytes with peripheral palisading and clefting.[13] | Clusters of basaloid cells, possibly with peripheral palisading and mucinous stroma. |
| SCC | Typically found on sun-exposed areas. | Red, scaly plaques or nodules, often with ulceration or crusting. | Atypical squamous cells with pleomorphism and mitotic figures, forming nests and infiltrating the dermis. Keratin pearls may be present.[4] | Dysplastic squamous cells, singly dispersed or in clusters, with high nucleus-to-cytoplasm ratio and keratin pearls. |
| MCC | Varying presentation. | Red or pink or blue papules or nodules | Round nuclei with finely granular chromatin, inconspicuous nucleoli, scant cytoplasm, showing molding, and crush artifact.[3] | Small, round, or oval cells with round-to-oval nuclei and scant cytoplasm. Nuclei have fine chromatin and small, inconspicuous nucleoli. Occasional nuclear molding can be observed.[17] |
TC: Trichilemmal cyst, EIC: Epidermal inclusion cyst, BCC: Basal cell carcinoma, SCC: Squamous cell carcinoma, MCC: Merkel cell carcinoma
SUMMARY
Rare pilomatrixoma can be elusive if not kept in the differential of basaloid neoplasms in fine needle aspiration of subcutaneous head-and-neck lesions. FNA from pilomatrixoma can be cellular with cells showing high nuclear to cytoplasmic ratio, prominent nuclei, and mitotic figures. Clumps of refractile keratin and “ghost cells” are major clues to appropriate diagnosis. Atypical basaloid cells and mitotic figures may lead to a diagnostic pitfall to report this entity as a malignancy. Ghost cells are important for the cytological diagnosis of pilomatricoma.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this published article. No additional datasets were generated or analyzed during the current study. Therefore, data sharing is not applicable to this article as all necessary information is provided within.
ABBREVIATIONS
FNAC – Fine-needle aspiration cytology.
AUTHOR CONTRIBUTIONS
CB, SA, MYAK and VBS: Made substantial contributions to the conception and design of the work, led the initial drafting of the manuscript, and was primarily responsible for its overall structure and content; CB and SA: Significantly contributed to the study design and data analysis; MYAK: Provided critical insights into the methodology and interpretation of results; VBS: Offered expert guidance on the clinical implications and supervised the project.
All authors critically reviewed and revised the manuscript to ensure its intellectual content and integrity, and have given final approval of the version to be published. They all agree to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Wayne State University School of Medicine University, Detroit, Michigan, USA IRB waives required approval and patient consent since the patient has been anonymized and fully de-identified. This case report has been prepared and submitted in strict compliance with all relevant guidelines and regulations, ensuring the complete anonymity of the patient and the absence of any identifying information.
CONFLICT OF INTEREST
Given his role as Editor in Chief Emeritus, Vinod B. Shidham had no involvement in the peer-review of this article and has no access to information regarding its peer review. The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28:1005-11.
- [Google Scholar]
- Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-44.
- [CrossRef] [Google Scholar]
- Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12.
- [CrossRef] [Google Scholar]
- Keratinocytic/epidermal tumours In: Elder D, Massi D, Scolyer R, Willemze R, eds. World Health Organization (WHO) Classification of skin tumours Vol 11. (4th ed). Argonay, France: International Agency for Research on Cancer (IARC); 2018. p. :31-43. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9913635/ [Last accessed on 2023 Jan 04]
- [Google Scholar]
- Cytologic findings of proliferating trichilemmal tumor (PTT) of scalp. Diagn Cytopathol. 2020;48(1):86-89.
- [CrossRef] [Google Scholar]
- Ki67/MART1 and p63/SOX10 dual immunohistochemistry allows a correct interpretation of the melanocytic component in the diagnosis of pigmented pilomatricoma. Indian J Dermatol. 2021;66:525-9.
- [CrossRef] [Google Scholar]
- Does endometrial morular metaplasia represent odontogenic differentiation? Virchows Arch. 2021;479:607-16.
- [CrossRef] [Google Scholar]
- A clinical review of 209 pilomatricomas. J Am Acad Dermatol. 1998;39:191-5.
- [CrossRef] [Google Scholar]
- Pilomatricoma: An unusual dermatologic neoplasm. Hawaii J Med Public Health. 2012;71:282-6.
- [Google Scholar]
- Pilomatrixoma and pilomatrical carcinoma. ExpertPath. Available from: https://app.expertpath.com/document/pilomatrixoma-and-pilomatrical-car-/a75411a1-6792-437f-877d-2ff415c7f97e?searchTerm=pilomatricoma [Last accessed on 2023 Jan 04]
- [Google Scholar]
- Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- [CrossRef] [Google Scholar]
- Basal cell carcinoma: Pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-79.
- [Google Scholar]
- Trichilemmal cyst: Clinical and sonographic features. J Ultrasound Med. 2019;38:91-6.
- [CrossRef] [Google Scholar]
- Cytological diagnosis of epidermal inclusion cyst of breast: A rare benign lesion. J Nat Sci Biol Med. 2014;5:460-2.
- [CrossRef] [Google Scholar]
- Atypical spindle cell/pleomorphic lipomatous tumour (ASPLT): A report of three FNA cases and comparison with spindle cell/pleomorphic lipoma cytopathology. Cytopathology. 2023;34:346-52.
- [CrossRef] [Google Scholar]
- Fine-needle aspiration cytology of recurrent Merkel cell carcinoma of eye-brow. J Cytol. 2014;31:179-80.
- [CrossRef] [Google Scholar]
- Libre Pathology. https://librepathology.org/wiki/Epidermal_inclusion_cyst [Last accessed on 2023 May 06]
- [Google Scholar]







