Translate this page into:
Gastrointestinal stromal tumor mimicking perineurioma: A case report

*Corresponding author: Guohua Yu, Department of Pathology, Affiliated Yantai Yuhuangding Hospital, Qingdao University, Yantai, China. guohuayu@qdu.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Pan Y, Gao Y, Yang P, Yu G. Gastrointestinal stromal tumor mimicking perineurioma: A case report. CytoJournal. 2024;21:51. doi: 10.25259/Cytojournal_104_2024
Abstract
Although gastrointestinal stromal tumor (GIST) can present with various histological characteristics, GIST mimicking perineurioma has not been previously reported. We present the case of a 47-year-old woman diagnosed with GIST after laparoscopic resection of a stomach tumor near the lesser curvature of the gastric body close to the cardia. Morphological features resembled a perineurioma. c.1504_1509 (p.A502_Y503) duplication was found in exon 9 of kinase insert domain receptor (c-KIT). This specific mutation is associated with constitutive activation of the c-KIT, which is crucial in the pathogenesis of GIST. Such unique histological characteristics broaden our understanding of the morphological diversity within GISTs and underscore the importance of considering an extended differential diagnosis when encountering atypical gastrointestinal tumors. This rare presentation may challenge conventional diagnostic criteria and could influence therapeutic strategies, emphasizing the need for comprehensive histological and molecular assessments in patient management.
Keywords
Gastrointestinal stromal tumor
Immunohistochemistry
Molecular pathology
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) originate from the interstitial cells of Cajal and predominantly occur in adults aged 50–70 years.[1] Most occur in the stomach (56.4%) and small intestine (32.7%); only 5.9% are found in the colon and rectum.[2] Clinically, GIST typically presents with abdominal pain, nausea, vomiting, and a palpable mass; however, the symptoms largely depend on the tumor’s location and size. Many patients manifest with gastrointestinal bleeding while others may experience dysphagia. Histopathologically, GISTs typically appear as well-demarcated masses with variable size. Grossly, they are nodular and grayish-white with solid consistency; however, parts of the tumor may be soft. Additional findings may include hemorrhage, necrosis, and cystic degeneration. In some instances, the tumor may invade the surrounding tissue. Morphologically, GISTs exhibit various cellular patterns, including spindle cell, epithelioid cell, and mixed cell types as well as other less common variants. The degree of cellular atypia and mitotic activity can vary significantly.[3] Tumor cells are frequently spindle-shaped or short spindle-shaped and arranged in fascicles, interlacing patterns, or whorls.[4] Kinase insert domain receptor (c-Kit) is a receptor tyrosine kinase that plays a pivotal role in cell signaling, regulating processes such as proliferation, differentiation, and survival. Mutations in c-Kit have been linked to various malignancies, including GIST, mastocytosis, melanoma, and acute myeloid leukemia, where they drive abnormal cell growth and tumor progression.[1] Investigating the c-Kit mutation in our study is essential, as it not only underscores its involvement in the specific pathology under examination but also points to broader therapeutic implications across multiple disease contexts.[5] We present a case of GIST mimicking perineurioma, which has not been previously reported.
CASE REPORT
A 47-year-old woman was admitted with a 10-day history of persistent abdominal pain. She denied diarrhea and constipation. Physical examination revealed no abdominal tenderness or distension. She had no medical history other than acute appendicitis 4 years previously for which she had undergone laparoscopic appendectomy. She denied any family history of similar conditions or genetic disorders. Abdominal computed tomography showed a mass near the lesser curvature of the gastric body close to the cardia [Figure 1a], which strongly suggested GIST. The patient subsequently underwent a laparoscopic partial gastrectomy for tumor removal.
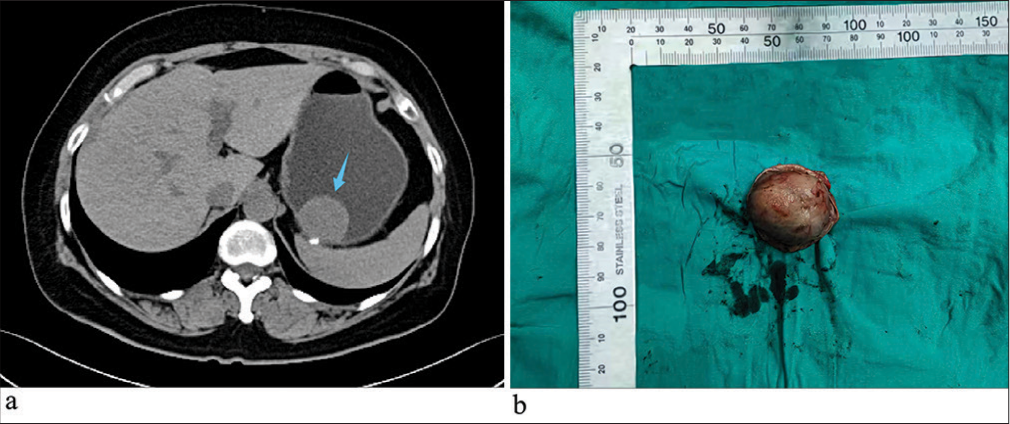
- (a) Abdominal computed tomography with contrast enhancement showed a mass near the cardia on the lesser curvature side of the gastric body (blue arrow). (b) The tumor was nodular and measured 3 × 2.3 × 1.7 cm.
Pathological examination showed that the tumor exhibited an exophytic growth pattern and appeared grayish-yellow [Figure 1b]. After fixation with 10% formalin neutral buffer solution (brand name: Tissue Fixative Solution, manufacturer name: Shandong Jianze Medical Technology Co., Ltd., approval number: Lu Yan Medical Device Preparation 20210050, and company address: 169 Airport Road, Zhifu District, Yantai City, Shandong Province, China) and dehydration, specimens were embedded in paraffin, sectioned (4 μm thickness), and stained with hematoxylin and eosin. Microscopically, the tumor cells were arranged as onion skins or whirlpools. The stroma exhibited hyalinization and mucinous degeneration under low power. The tumor cells were spindle or short spindle shaped with abundant eosinophilic cytoplasm, mild nuclear atypia, and unclear nucleoli. No mitosis was observed at high magnification [Figure 2a-c].
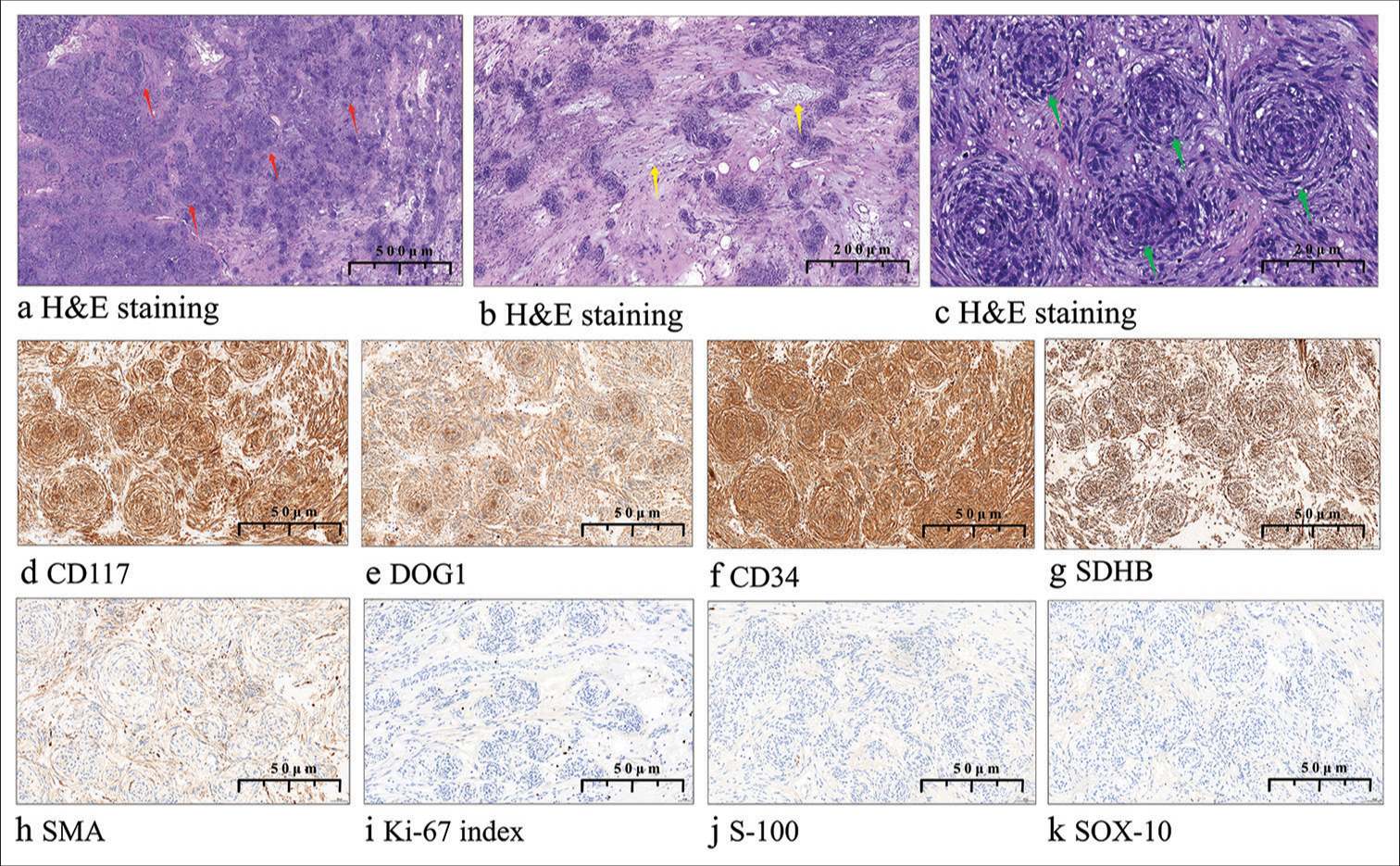
- (a) Histopathologically, the tumor was composed of nodules of different size in a stromal background (red arrow, ×10). (b) Tumor cells were distributed at an uneven density and the stroma was hyalinized with mucinous degeneration (yellow arrow, ×15). (c) Tumor cells were spindle arranged in a perineurium-like pattern (green arrow, ×30). (d-h)Tumor tissue was immunohistochemically positive (×20) for cluster of differentiation 117, discovered on gastrointestinal stromal tumor-1, cluster of differentiation 34, succinate dehydrogenase iron-sulfur subunit B, and smooth muscle actin. (i)The Ki-67 antigen index was ≤1%. (j-k)Staining for S-100 calcium-binding protein and SRY-box 10 protein was negative. (H&E: Hematoxylin and eosin, CD117: Cluster of differentiation 117, DOG1: Discovered on gastrointestinal stromal tumor-1, CD34: Cluster of differentiation 34, SDHB: Succinate dehydrogenase iron-sulfur subunit B, SMA: Smooth muscle actin, Ki-67 index: Ki-67 antigen index, S-100: S-100 calcium-binding protein, SOX-10: SRY-box 10 protein).
Immunohistochemical staining (IHC staining) was performed following standard protocols using the EnVision method[6] to assess Cluster of differentiation 117 (CD117; catalog number: ZA-0523, clone EP10, 1:100, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China), discovered on GIST-1 (DOG1; catalog number: ZM-0371, clone OTI1C6, 1:25, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China), Smooth muscle actin (SMA; catalog number: ZM-0003, clone UMAB237, 1:200, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China), Cluster of differentiation 34 (CD34; catalog number: ZM-0046, clone 10C9, 1:200, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China), S-100 calcium-binding protein(S100; catalog number: ZM-0224, clone 15E2E2+4C4.9, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China), SRY-box 10 protein (SOX10; catalog number: ZA-0624, clone EP268, 1:100, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China), Succinate dehydrogenase iron-sulfur subunit B (SDHB; catalog number: ZM-0162, clone OTI1H6, 1:400, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China), and Ki-67 antigen (Ki-67; catalog number: ZM-0166, clone UMAB107, 1:50, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China). The primary purpose of the IHC staining was to assist in the differential diagnosis and confirm the diagnosis of GIST. By detecting specific markers, we were able to distinguish GIST from other potential mimicking neoplasms. This approach was crucial in establishing the definitive diagnosis, as these markers are well recognized in the literature for their diagnostic value in identifying GIST. Immunohistochemically, the tumor cells were positive for CD117, DOG1, CD34, SDHB, and SMA. The Ki-67 index was ≤1%. Staining was negative for S-100 and SOX10 [Figure 2d-k]. The detection of these proteins was undertaken to confirm the diagnosis of GIST, assess the tumor’s proliferative potential, and exclude other differential diagnoses such as perineurioma, which was part of the histological mimicry observed in this case. Using Sanger sequencing, c.1504_1509 (p.A502_Y503) duplication was found in exon 9 of c-KIT. This mutation is linked to constitutive KIT receptor activation, (the KIT receptor, which belongs to the receptor tyrosine kinase family, normally plays a crucial role in cell signaling pathways), driving uncontrolled cell proliferation and survival, which are key in GIST pathogenesis. It indicates a more aggressive tumor phenotype and may affect prognosis and treatment, as exon 9 mutations can alter response to tyrosine kinase inhibitors. No mutation was detected in exons 12 or 18 of platelet-derived growth factor receptor alpha (PDGFRA) [Figure 3a-d]. A diagnosis of GIST mimicking a perineurioma (low risk) was made based on all the above. Two months after surgery, the patient had no sign of recurrence.
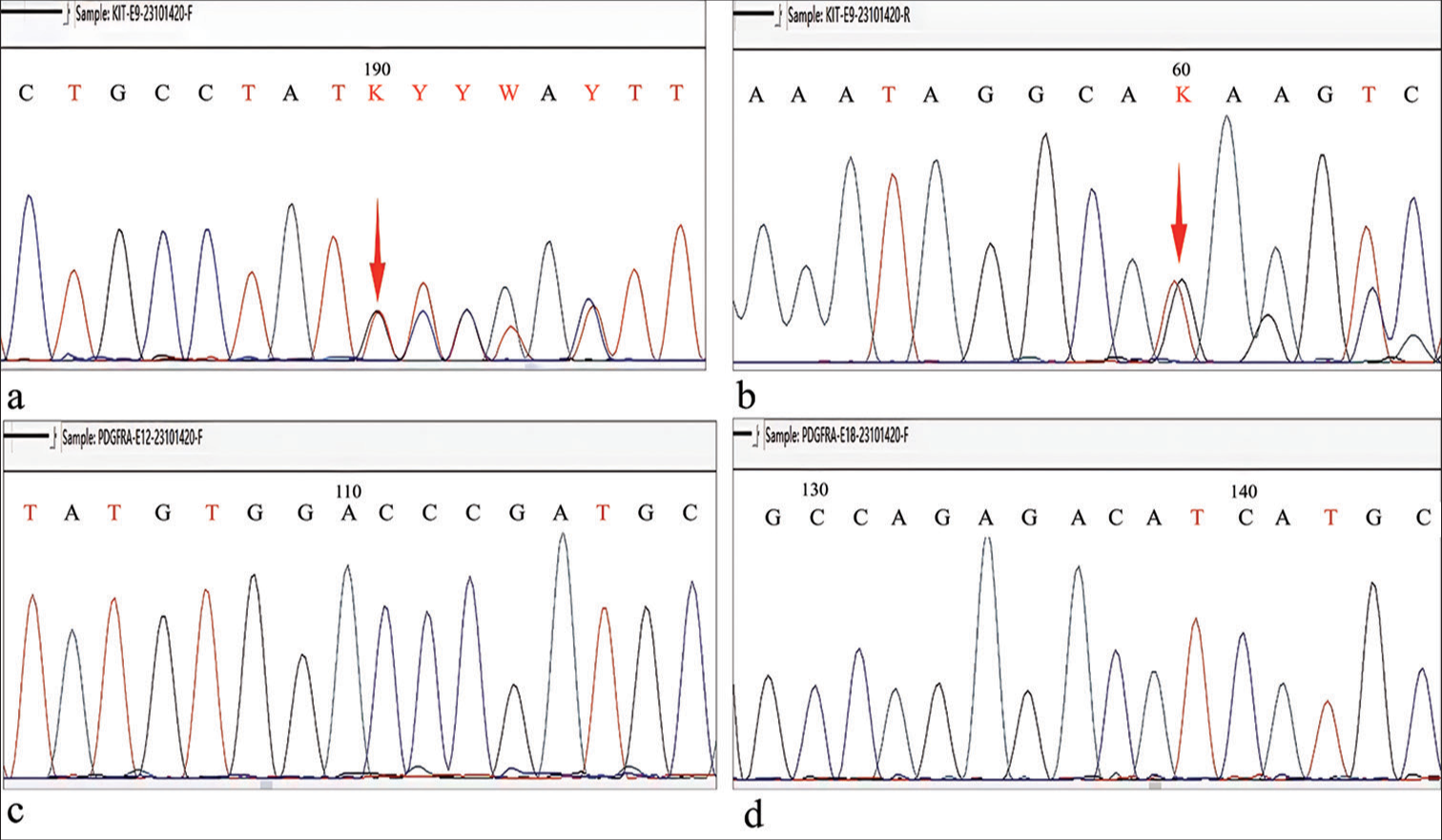
- (a-b) Forward and reverse Sanger sequencing demonstrated c.1504_1509 (p.A502_Y503) duplication in exon 9 of Kinase Insert Domain Receptor (red arrow). (c-d)Forward sequencing of exon 12 and exon 18 of platelet-derived growth factor receptor alpha showed no mutation. (A: Adenine, C: Cytosine, T: Thymine, G: Guanine, K: G or T (keto bases), Y: C or T (pyrimidine bases).
DISCUSSION
GIST is the most prevalent mesenchymal cell neoplasm which occurs within the gastrointestinal tract.[7] Histologically, GISTs are diverse and exhibit unpredictable morphology.[2] Examples include pleomorphic rhabdomyosarcomatous GIST, rhabdoid signet-ring GIST, myxoid epithelioid GIST, organoid nesting pattern GIST, hemangiopericytoma-like histological pattern GIST, and others [Table 1].[7-10] In our case, the tumor cells were arranged like perineurium without an axon-like structure in the center of the nodule. c.1504_1509 duplication was observed in exon 9 of KIT despite the p.A502_Y503 duplication caused by this mutation is more common. This mutation form is usually associated with a good patient outcome.[1,5]
| S. No. | First author/year | Age (year)/gender | Histopathological type | Molecular mutations |
|---|---|---|---|---|
| 1. | Takahashi et al., 2009[7] | 57/F | Hemangiopericytoma-like histological pattern | A 42 bp deletion was found from codons 560 to 573 of exon 11 of the KIT gene |
| 2. | Zámecník et al., 2005[8] | 93/F | Biphasic adenosarcoma-like structure | NA |
| 3. | Cai et al. 2015[9] | 63/M | Foam cell-like structure | Mutation in exon 13 of the c-KIT gene |
| 4. | Shi et al. 2023[10] | NA | Rhabdoid and signet-ring-like cells | KIT gene mutation |
NA: Not available, M: Man, F: Female
The low Ki-67 index detected in this case signifies a minimal proliferative rate, aligning with the tumor’s non-aggressive behavior and the absence of recurrence in the patient 2 months following surgical intervention. Nonetheless, the identification of a mutation in exon 9 implies that tumors exhibiting low proliferative rates may still possess aggressive genetic characteristics.[3] Aggressive clinical behavior is present in 59.3% of GISTs with KIT exon 9 mutations, indicating a high or intermediate-risk scenario.[8,11] Consequently, exon 9 mutations are not directly linked to probability of recurrence.[10] However, their presence is associated with histological morphology phenotype.[2] The histological morphology in our patient’s tumor did not resemble perineurium; however, exon 9 was mutated and a spindle-shaped pattern was present. Mutations in exon 9 are linked to cell membrane localization and continued susceptibility to activation by stem cell factor. Moreover, the GIST mutation in exon 9 indicates substantial activation of the non-dependent carcinogenic pathway of KIT.[1] Exon 9 mutations are thought to change the overall structure of the protein, which mimics the ligand binding manner and causes secondary aggregation and receptor activation.[11] Point mutations, deletions, insertions/duplications, and compound deletion mutations are just a few of the many uncommon mutation types.[12] These characteristics may explain the limited activity of the tyrosine kinase inhibitor imatinib in the adjuvant setting in patients with exon 9-mutant GISTs. They may also explain the lower sensitivity of advanced exon 9-mutant tumors to standard dose imatinib. Sunitinib, another tyrosine kinase inhibitor, has shown better activity in GIST patients with exon 9 mutations.[2,5]
The differential diagnosis of GIST includes schwannoma, solitary fibrous tumor, and leiomyoma. Schwannoma is usually located in the muscularis propria layer of the stomach and exhibits a prominent lymphocytic mantle and lymphoplasmacytic infiltration. IHC staining shows S-100 positivity and CD117 negativity. Molecular testing does not reveal any KIT mutations. Solitary fibrous tumor is a distinct soft-tissue neoplasm associated with NGFI-A Binding Protein 2 - Signal Transducer and Activator of Transcription 6 gene fusion. It comprises spindle cells and varying proportions of collagenous stroma and is frequently accompanied by hemangiopericytoma-like structures. Tumor cells are positive for CD34 and B-cell lymphoma 2 protein and show no KIT mutation. Leiomyoma is associated with the muscular layer of the mucosa and is characterized by eosinophilic cells with round, blunt nuclei. Tumor cells are positive for Iintermediate filament protein desmin(Desmin), Actin filament(Actin) and SMA but negative for CD117.
Several factors influence clinical outcome in patients with GIST including risk classification, tumor size, mitotic index, and the completeness of surgical resection.[12] Mutations in c-KIT or PDGFRA are found in 85% to 90% of GISTs and studies suggest that postoperative targeted therapy can improve patient outcomes to some extent. Efficacy of imatinib-targeted therapy and patient outcome are both associated with the type of c-KIT or PDGFRA mutation. Exon 9 c-KIT mutations are typically stable and associated with a high degree of malignancy, aggressive behavior, and poor outcome.[13] Whether histological features can predict outcome in such GISTs is unknown and should be investigated.
The GIST in our patient shows their diversity of histological features, indicating that pathologists should consider that GISTs may have non-conventional manifestations and characteristics.[7] Understanding the molecular and histological characteristics of these atypical GIST cases might help to inform future treatment strategies, especially targeted therapies based on genetic mutations. Studying such cases may also help physicians better predict disease course and prognosis. Identifying specific gene mutation type in GIST patients appears to be essential for selecting precise individualized treatment and predicting outcome.[1,13]
SUMMARY
In summary, the histological characteristics of the case described in this case report have not been thoroughly documented in the literature. A homozygous variation c.1504_1509 (p.A502_Y503) duplication in exon 9 of c-KIT was identified, which is considered to be significantly linked to a favorable prognosis. This unusual feature could enrich the histological spectrum of GIST and possess certain significance in differential diagnosis.
AVAILABILITY OF DATA AND MATERIALS
All the data and materials supporting this work are available upon reasonable request to the corresponding author.
ABBREVIATIONS
GIST – Gastrointestinal stromal tumor
SFT – Solitary fibrous tumor
CT – Computed tomography
H&E – Hematoxylin and eosin
IHC staining – Immunohistochemical staining
CD117 – Cluster of Differentiation 117
DOG1 – Discovered on GIST-1
SMA – Smooth Muscle Actin
CD34 – Cluster of Differentiation 34
SOX10 – SRY-Box 10 protein
SDHB – Succinate Dehydrogenase Iron-Sulfur Subunit, B
BCL-2 – B-cell lymphoma 2 Protein
Desmin – Iintermediate filament protein desmin
Actin – Actin filament
c-KIT – Kinase Insert Domain Receptor
PDGFRA – Platelet-derived Growth Factor Receptor Alpha
NAB2-STAT6 NGFI – A Binding Protein 2 - Signal
Transducer and Activator of Transcription 6
AUTHOR CONTRIBUTIONS
YP and GH Y: Were responsible for the design of the study; YP and YG: Contributed to data collection, analysis, the drafting, and revision of the manuscript; PY: Provided help and advice on IHC staining and molecular sequencing; GH Y: Contributed to review and editing and conceptualization. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENT
We would like to thank individuals (such as our research partners, editors, and anonymous reviewers) who have contributed substantially to the completion of this study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board of the Yantai Yuhuangding Hospital (No. 2021-040). Written informed consent was obtained from the patient or her families. The study was conducted in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
Given his role as editorial member, Guohua Yu had no involvement in the peer-review of this article and has no access to information regarding its peer-review. The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Clinicopathologic profile of gastrointestinal stromal tumors (GISTs) with primary KIT exon 13 or exon 17 mutations: A multicenter study on 54 cases. Mod Pathol. 2008;21:476-84.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal stromal tumors presenting as omental masses--a clinicopathologic analysis of 95 cases. Am J Surg Pathol. 2009;33:1267-75.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: A report from the national institutes of health gastrointestinal stromal tumor Clinic. JAMA Oncol. 2016;2:922-8.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment I. Histopathology. 2006;49:406-10.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal stromal tumor in the duodenum exhibiting hemangiopericytoma-like histological pattern. Pathol Int. 2009;59:98-101.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal stromal tumor (GIST) with glandular component. A report of an unusual tumor resembling adenosarcoma. Cesk Patol. 2005;41:150-6.
- [Google Scholar]
- Gastrointestinal stromal tumor with foam cell-like and micro-capsule structures: Report of a case. Zhonghua Bing Li Xue Za Zhi. 2015;44:920-1.
- [Google Scholar]
- Morphological, immunohistochemical, and genetic analyses of epithelioid gastrointestinal stromal tumors. Ann Diagn Pathol. 2023;67:152208.
- [CrossRef] [PubMed] [Google Scholar]
- A subset of gastrointestinal stromal tumors previously regarded as wild-type tumors carries somatic activating mutations in KIT exon 8 (p.D419del) Mod Pathol. 2013;26:1004-12.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a prognostic nomogram to predict recurrence in high-risk gastrointestinal stromal tumour: A retrospective analysis of two independent cohorts. EBioMedicine. 2020;60:103016.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib. Ann Oncol. 2023;34:615-25.
- [CrossRef] [PubMed] [Google Scholar]








