Translate this page into:
Granulomatous inflammation and organizing pneumonia: Role of computed tomography-guided lung fine needle aspirations, touch preparations and core biopsies in the evaluation of common non-neoplastic diagnoses
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Fine-needle aspirations (FNAs) and core biopsies (CBs), with or without touch preparations (TPs), are performed to characterize pulmonary lesions. Although a positive (P) or suspicious report is sufficient for further management, the significance of unsatisfactory (U), negative (N) and atypical (A) cytological diagnoses remains uncertain. The aims of the study were to correlate U, N and A cytological diagnoses with histological and/or clinical/radiological follow-up and evaluate the utility of FNAs, TPs and CBs.
Materials and Methods:
We performed a retrospective search and examined 30 consecutive computed tomography-guided transthoracic U, N and A lung FNAs (n = 23) and TPs (n = 7) with surgical pathology (SP) (n = 17) and/or clinical/radiological follow-up (n = 13) and compared them to 10 SP-confirmed P FNAs, which served as controls.
Results:
The 30 FNAs and TPs were from 29 patients. All 6 U specimens were scantly cellular. Granulomas, the most common specific benign cytological diagnosis, were evident in 8 (of 13) and 7 (of 11) N and A cytology cases, respectively. Histology corroborated the presence of granulomas identified on cytology. Organizing pneumonia was the second leading benign specific diagnosis (5/17), but it was rendered on histology (n = 5) and not FNAs or TPs. Evaluation of the A cases revealed that type II pneumocytes were the source of “atypical”, diagnoses often associated with granulomas or organizing pneumonia and lacked 3-D clusters evident in all P cases.
Discussion:
U, N and A FNAs and TPs lacked 3-D clusters seen in carcinomas and were negative on follow-up. Granulomas and organizing pneumonia were the most common specific benign diagnoses, but the latter was recognized on histology only. In the absence of a definitive FNA result at the time of on-site assessment, a CB with a TP containing type II pneumocytes increases the likelihood of a specific benign diagnosis.
Keywords
Benign
core biopsy
fine-needle aspiration
lung
pulmonary
touch preparations and imprints
INTRODUCTION
Greater numbers of pulmonary nodules are being encountered, many times incidentally, due to the increased use of imaging. Most nodules are typically sampled by fine-needle aspiration (FNA) or core biopsy (CB), with or without touch preparations (TPs). Though some nodules are malignant, many nodules represent benign processes.[1] Therefore, distinction between the two is significant to prevent unnecessary intervention and morbidity[2] in benign cases and delayed diagnosis and treatment[2] in malignant ones.
High-grade malignancies with abundant cellularity are relatively straightforward to diagnose. Interpretation is not always easy or definitive, though. For instance, reactive changes can be misinterpreted and lead to false positive[3] or equivocal “atypical” results. Furthermore, samples diagnosed as “negative” sometimes fail to describe a specific pathological entity to explain the underlying lesion identified by imaging. Management of patients with non-specific diagnoses poses challenges, including whether or not the lesion is adequately sampled; if so, is the lesion truly negative; how best to proceed without “specific” results; which modality, FNA or CB, offers greater yield.
The aims of the present study were to correlate cytology preparations, FNAs and TPs, diagnosed as unsatisfactory (U), negative (N) and atypical (A) with histological and/or clinical/radiological follow-up and evaluate the outcomes of the FNAs, TPs and CBs.
MATERIALS AND METHODS
We performed a retrospective computerized search and examined 30 consecutive computed tomography (CT)-guided transthoracic U, N and A pulmonary FNAs (n = 23) and TPs (n = 7) with surgical pathology (SP) (n = 17) and/or clinical/radiological follow-up (n = 13). These cases were selected from a total of 93 consecutive CT-guided FNAs performed during a 75-week period; the remaining 63 cases (non-U, N or A) were diagnosed as either suspicious (n = 6) or positive for neoplasm/malignancy. The U, N and A cases were compared to 10 consecutive SP-confirmed P FNAs, which served as controls.
At our institution, pulmonary lesions are evaluated by FNAs and/or TPs (of CBs) and all undergo on-site assessment by a cytopathologist and/or cytotechnologist. The method (s), FNA and/or TP, used to obtain the specimen varies with the radiologist performing the procedure and/or impression at the time of on-site immediate assessment.
All cytological and histological specimens were reviewed simultaneously by two study pathologists. Cytomorphological findings evaluated for all FNAs and TPs included: Epithelial cellularity, epithelial arrangement, type II pneumocytes, nuclear features, macrophages, multinucleated giant cells, inflammation, granulomas and necrosis. All concurrent and subsequent histological specimens were reviewed to confirm the diagnoses and to evaluate for the presence of type II pneumocytes.
Clinical findings and radiological studies, including chest X-rays, CT scans and positron emission tomography (PET) scans, documented both at the time of presentation and during subsequent follow-up, were documented for each cytological case diagnosed as U, N, or A.
This study was approved by the Institutional Review Board.
RESULTS
The U (n = 6), N (n = 13) and A (n = 11) FNAs and TPs were from 29 patients (19 females; 10 males; age range 16-82; average age 61) [Figure 1]. 17 cases (3 U, 7 N, 7 A) had either concurrent or subsequent SP correlation as well as clinical/radiological follow-up. Seven of 17 cytological specimens with surgical correlation were comprised of only TPs of the CBs. Ten of 17 cytological specimens were comprised of FNAs with either concurrent CB (n = 5; TPs performed on 4/5), follow-up wedge resection (n = 4; 3-15 weeks post FNA), or follow-up transbronchial biopsy (n = 1; 3 weeks post FNA). Thirteen cases from 12 patients (3 U, 6 N, 4 A) had only clinical/radiological follow-up.
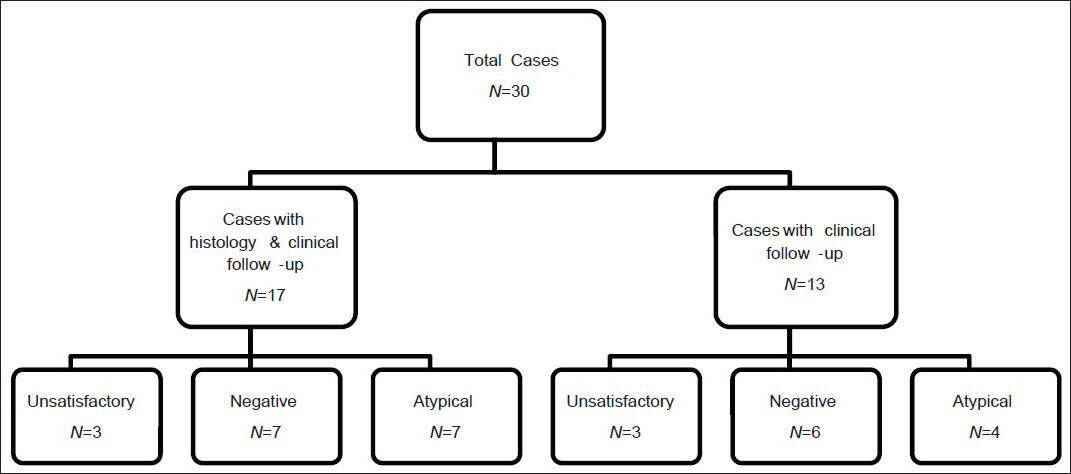
- Summary of unsatisfactory, negative and atypical cases. The third tier shows the cytologic diagnoses rendered
For patients without histology, radiological follow-up ranged from 17 to 158 weeks (median 82 weeks; mean 91 weeks). The nodules with the shortest follow-up intervals (17 weeks and 24 weeks) decreased or remained unchanged, respectively. In the other cases, cultures tested positive for Nocardia (n = 1) and the nodules resolved, diminished in size or remained stable [Tables 1 and 2]. Clinical/radiological follow-up ranged from 0 to 78 weeks for cases with histology (median 28 weeks; mean 28 weeks; no clinical follow-up for 1 case with concurrent biopsy showing granulomas) [Table 1].
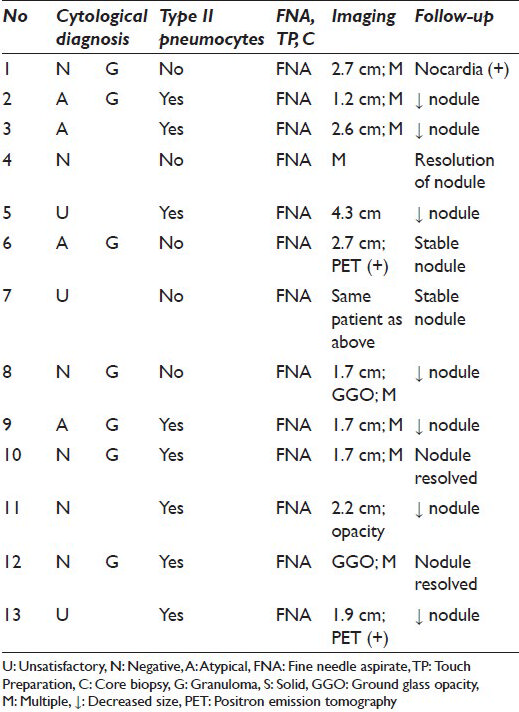
All (30) U, N and A cases were negative on follow-up (histological and/or clinical/radiological). A specific cytological diagnosis (i.e. granuloma) was rendered on 15 cases [Figure 2]. Eight of these had concurrent/follow-up histology, 7 of which also had granulomas; 1 biopsy was non-diagnostic. Ten of 12 cases with CBs had specific diagnoses of granuloma (n = 6), organizing pneumonia (n = 3), or infarct (n = 1).
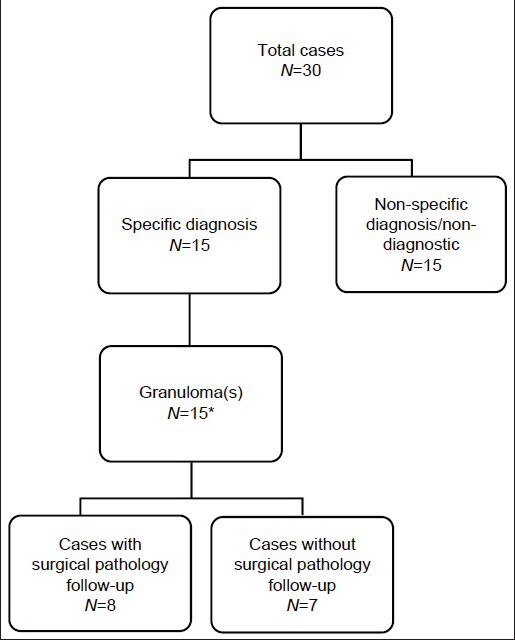
- Specific versus non-specific cytopathological diagnoses of unsatisfactory, negative and atypical cases. *One case had extensive necrosis and inflammation with acid-fast bacilli
Unsatisfactory (U) cases
All 6 U specimens were scantly cellular with too few cells for a diagnosis. Three of the U specimens had concurrent or follow-up SP diagnosed as having organizing pneumonia (n = 1), granuloma (n = 1) and non-diagnostic (n = 1). Four of 6 U cases showed CT/PET findings suspicious for neoplasia/malignancy at the time of presentation.
Negative (N) cases
Out of the 13 N cases, 8 showed granulomatous inflammation on cytology and/or histology. Other diagnoses included acute inflammation, reactive epithelioid cells, bland epithelial/epithelioid cells, macrophages and inflammation and necrosis associated with hemosiderin-laden macrophages. Seven of 13 cases had histology showing granulomas (n = 3: Same diagnosis on cytology), organizing pneumonia (n = 2), infarct (n = 1) and non-diagnostic (n = 1). Three of 13 N cases showed CT/PET findings that were suspicious for neoplasm.
Atypical (A) cases
Out of the 11 A cases, 7 were diagnosed as granulomatous inflammation on cytology and/or histology. Diagnoses on the remaining cytology specimens included “cannot exclude a neoplasm” and “rare atypical cells insufficient to render a specific diagnosis.” Seven of 11 A cases had histology, which demonstrated granulomas (n = 4: Same diagnosis on cytology), organizing pneumonia (n = 2) and type II pneumocyte hyperplasia (n = 1). CT/PET at time of presentation favored neoplasia in 5 cases and the differential diagnosis included neoplasia for the remaining.
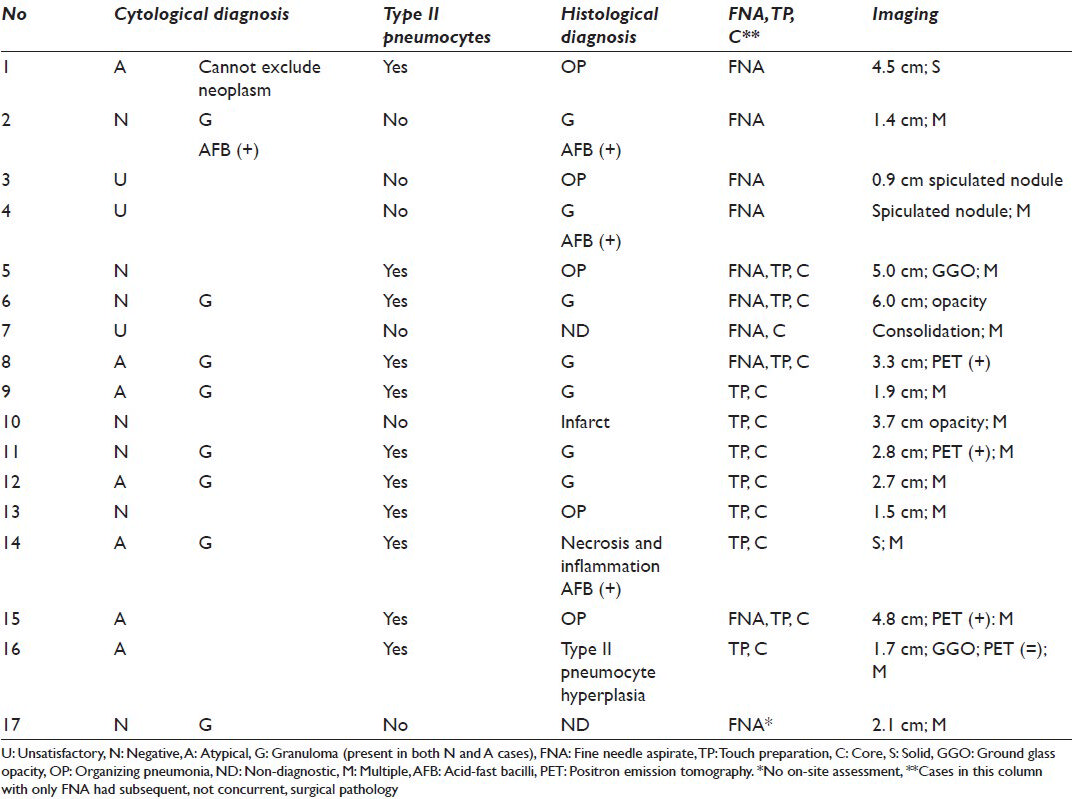
Type II pneumocytes were identified in 11 of 17 cases with histology [Figures 3–5]. Seven of 11 were diagnosed as atypical on cytology and the reactive type II pneumocytes appeared to be the source of the atypical interpretation, as they represented the only epithelioid cells. Cases without type II pneumocytes were never interpreted as atypical. Four cases were diagnosed as negative and had type II pneumocytes on cytology and histology. The histology of the 11 cases was organizing pneumonia (n = 4), granulomas (n = 6), type II pneumocyte hyperplasia (n = 1) [Figures 4 and 5].
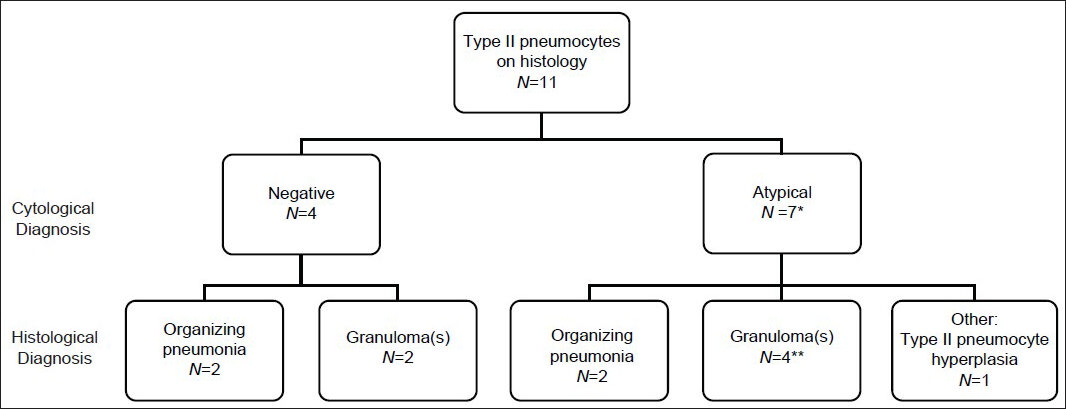
- Cases with type II pneumocytes and histological follow-up. Second tier is cytological diagnosis. Third tier is histological diagnosis. *One case diagnosed as “atypical cannot exclude neoplasm.” **One case diagnosed as having extensive necrosis and inflammation associated with acid-fast bacilli. All cases were comprised of fine needle aspiration (FNA) with concurrent core biopsy and touch preparation (TP) or only core biopsy with TP, except the one case diagnosed as “atypical cannot exclude neoplasm,” which had a FNA followed by a wedge resection 3 weeks later that showed organizing pneumonia
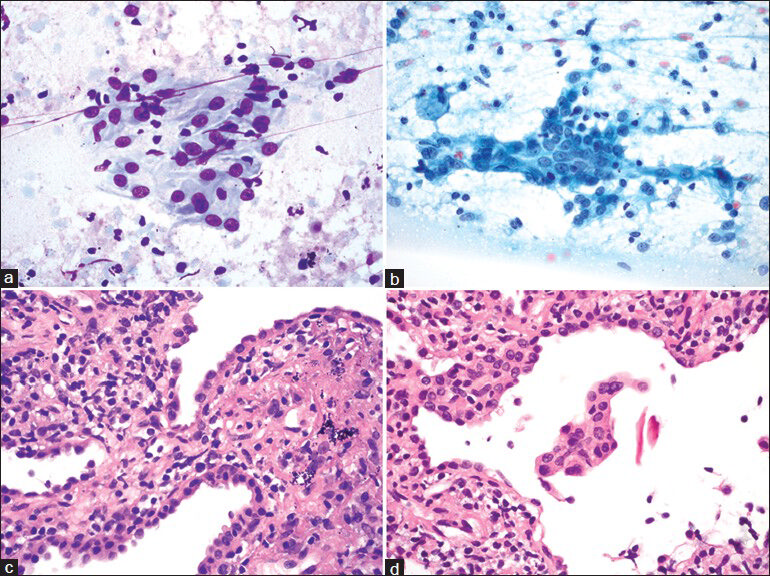
- Type II pneumocytes. (a and b) Touch preparations show type II pneumocytes arranged in flat sheets (a and b). These epithelial cells have small but conspicuous nucleoli, bland chromatin and a moderate amount of rather dense cytoplasm. (c and d) Corresponding core biopsy showing type II pneumocyte hyperplasia. ((a) Diff Quik, ×600; (b) Papanicolaou, ×600; (c and d) H and E, ×600)
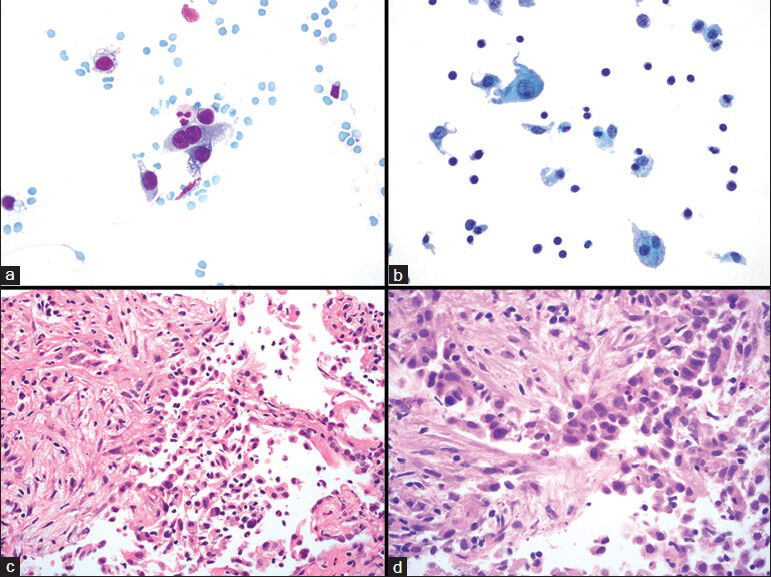
- Type II pneumocytes and organizing pneumonia. (a and b) Fine needle aspiration showing type II pneumocytes arranged as single cells, including one that is binucleated (a). (b) Type II pneumocyte present in the left upper portion of the image; other cells include histiocytes, which have a lower nuclear-to-cytoplasmic ratio and small lymphocytes. (c and d) Histological sections of corresponding core biopsy show organizing pneumonia with associated type II pneumocyte hyperplasia. (a) Diff Quik, ×600; (b) Papanicolaou stain on ThinPrep, ×600; (c) H and E, ×400. (d) H and E, ×600)
Follow-up of cytology
We had no false negative cases by cytology. We had specific cytological diagnoses in 15/30 (50%) cases, all showing granulomas; seven cases were histologically confirmed [Figure 6]. On CBs, specific diagnoses were rendered in 10/12 (83%) cases and included granulomas (n = 6), organizing pneumonia (n = 3) and infarct (n = 1). When evaluating only the 17 patients with concurrent/follow-up histology, 9 had granulomas; 8 were diagnosed on cytology (4 N; 4 A) and 8 on histology.
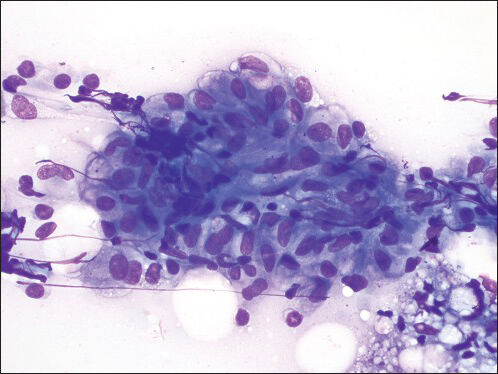
- Granuloma fine needle aspiration direct smear shows a cluster of epithelioid histiocytes consistent with a granuloma (Diff Quik, ×600)
Special stains for fungi, Pneumocystis and acid-fast bacilli (AFB) were performed on cases with granulomas, inflammation and/or necrosis; AFB were identified in 3 cases [Table 1].
Organizing pneumonia
Though cytology and histology are equivalent for diagnosing granulomas, the same is not true for organizing pneumonia. Of 17 patients with a histological specimen, 5 had organizing pneumonia diagnosed on concurrent CBs or subsequent resections; none were diagnosed on cytology (FNAs or TPs).
Positive cases
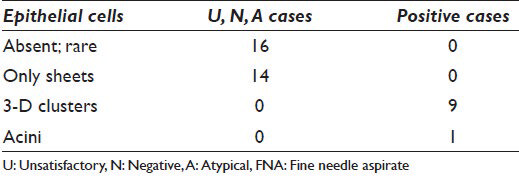
The P cases were from 10 patients (7 females; 3 males; age range 55-86; average age: 68). Final SP diagnoses were adenocarcinoma (9/10) and adenosquamous carcinoma (1/10). On cytology, the P cases had moderate to numerous epithelial cells, whereas U, N, A lacked epithelial cells or were scantly cellular. When present, the epithelial cells in U, N, A had sheets comprised of approximately 6-20 cells and no 3-D clusters [Figures 4 and 5; Table 3]. In contrast, the epithelial cells of the carcinomas formed 3-D clusters in 9/10 cases and acini in one [Table 3]. Inflammatory cells were evident in both groups – U, N, A and P – but were more often present in the former.
DISCUSSION
FNAs are useful in diagnosing malignancies because they offer high sensitivity (80-95%) and specificity (98-100%).[456] When compared with small biopsies, FNAs are equivalent at definitively and specifically classifying non-small cell carcinomas,[7] and optimal results according to some, are obtained when the two modalities are performed concurrently.[7] Nonetheless, CBs are associated with greater incidence of pneumothorax and hemorrhage relative to FNAs.[1] Specific benign cytological diagnoses, in contrast to malignant ones, have been reported in only up to 37[8] -50% of cases[456] and 60% of solitary pulmonary nodules are unnecessarily resected for benign disease.[9] Given these data, we sought to evaluate U, N and A results of cytological preparations (FNAs and TPs) by comparing them with their histological and/or clinical/radiological follow-up.
Though correlation with clinical information and imaging can help avoid interpretive errors,[2] the radiological impression does not always correlate with histology.[10] Studies have demonstrated that PET scans have a sensitivity of 96%, but a specificity of 77%.[11] In our study, the radiological findings (e.g. CT and/or PET scans) for 11 lesions (of 29 patients) were “favored to be” or “consistent with” neoplasia; 7 were considered to be non-neoplastic (e.g. infectious); and for the remaining lesions, no specific impression was provided by radiology.
Studies have demonstrated that growth and volume doubling time of nodules are features of malignancy,[12] whereas slow (or no) growth favors a benign process.[1314] In one study, a doubling time of 20 weeks correlated with carcinomas.[15] None of the cases in our study demonstrated growth with 17-158 weeks radiological follow-up, suggesting that the nodules represent underlying benign processes. Although this study is arguably limited by a lack of histological follow-up for 13 cases, clinical and radiological follow-up did not warrant additional procedures.
Follow-up of cytology
Whereas we had no false negative cases in our study, others have shown that 9[16]-36.8% of patients with a negative diagnosis were subsequently found to have a malignancy.[810] False negative diagnoses usually result from sampling errors – either owing to poor technique or inability to access the lesion due to its small size or location.[817]
Our results differ from those of others for a few possible reasons: (1) Based on the radiology, the needle was in the lesion in all but one U case in our series, (2) we typically have on-site assessment that allows us to request additional tissue, (3) the radiologist and/or cytologist correlate the on-site findings with the imaging and (4) in cases of FNAs, CB (s) are performed, if feasible, when the preliminary cytology diagnosis is not specific.
The rate of a specific benign cytological diagnoses reported in the literature ranges from 7%[8] to 50%[45619] and includes Candida, organizing pneumonia and granulomatous inflammation.[8] On CBs, the reported specific benign diagnoses is between 20.0%[20] and 81.7% respectively.[19] Our results are similar to the higher end of these spectra. Again, this may be attributable to the use of rapid on-site assessment, a multidisciplinary approach and request for CB when feasible.
Granuloma
Granulomas represent one of the most common inflammatory pulmonary lesions sampled by FNA.[18] Our findings demonstrate that cytology and histology are comparable in diagnosing granulomas, since equal numbers of granulomas were diagnosed by each method.
Type II pneumocytes
Reactive type II pneumocytes can be a source of atypical and malignant diagnoses.[2122] Cases without type II pneumocytes on histology were never diagnosed as A on cytology. We noted type II pneumocytes in cases of organizing pneumonia (n = 4) and granulomas (n = 6). Cytological features of cells that corresponded to type II pneumocytes on histology included: Sheets (comprised of approximately 6-20 cells) without 3-D arrangements and few single cells, including binucleated cells; cytoplasm with a granular appearance and sometimes fine vacuoles; nuclei without hyperchromasia; nucleoli that tended to be inconspicuous; nuclear irregularity, when present, was mild and best seen on a Pap stain [Table 3 Figures 4 and 5]. Relative to macrophages, type II pneumocytes had higher nuclear: cytoplasmic ratios and unlike mesothelial cells, they had no “windows.”
In contrast to type II pneumocytes, the epithelial cells in the control group of malignant cytology cases consistently had 3-D and/or acinar formation and showed nuclear irregularity and hyperchromasia. Features described by others that favor a reactive process include sparsely cellular specimens,[21] cohesive cell groups with scalloped borders[22] representing the hobnail appearance of reactive pneumocytes clinging to the alveolar walls,[23] and intercellular windows or gaps.[2] Distinguishing lung adenocarcinomas with a predominantly lepidic growth pattern from type II pneumocytes can be challenging, because both have low-grade cytology. Features in favor of the former include the presence of tissue fragments, intercytoplasmic connections, intranuclear inclusions and sparse multinucleation.[21]
Organizing pneumonia
Our findings suggest that a histological sample is more likely to yield a diagnosis of organizing pneumonia than a cytology preparation. The loose fibroblastic tissue associated with organizing pneumonia may not be easily recognized on FNAs or TPs, but presence of type II pneumocytes appears to be suggestive of lesional tissue.
While the number of cases is our series could be greater, the findings support that in the absence of a neoplastic diagnosis or granuloma (s), cytology and CB combination with on-site assessment is more likely to yield a specific benign answer. Similar findings have been reported[24] and advocated by some.[25]
CONCLUSION
Based on the above study our results indicate that FNAs provide a specific benign, non-neoplastic diagnosis when the underlying lesion represents a granulomatous process. Organizing pneumonia, a source of mass lesions on imaging studies, however, is more likely to be diagnosed on histology through a CB rather than FNA. Recognizing features of type II pneumocytes in cytological preparations is important, because they are often the source of atypical diagnoses and suggestive of lesional tissue. In conclusion, there is a role for the selective performance of a CB in the presence of a mass lesion and in the absence of a specific benign or neoplastic diagnosis by FNA – a concurrent CB with on-site TP assessment is likely to improve the yield of a specific benign diagnosis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. AS analyzed/interpreted the data and drafted the study.
SMC collected the data, contributed to the design of the study and revised it for intellectual content. JPC analyzed/interpreted the data and drafted the study. All authors read and approved the manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. No funding was provided, and no support in the form of grants, gifts, equipment, and/or drugs was received. There are no relevant financial disclosures for the authors. This study was conducted with approval from Institutional Review Board (IRB) of the institution associated with this study. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model. (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol. 2012;46:19-22.
- [Google Scholar]
- Respiratory cytology: Differential diagnosis and pitfalls. Diagn Cytopathol. 2010;38:297-307.
- [Google Scholar]
- Respiratory cytology: A review of non-neoplastic mimics of malignancy. Diagn Cytopathol. 1993;9:89-97.
- [Google Scholar]
- Percutaneous transthoracic aspiration needle biopsy. Ann Thorac Surg. 1978;26:399-405.
- [Google Scholar]
- Subtyping of non-small cell lung carcinoma: A comparison of small biopsy and cytology specimens. J Thorac Oncol. 2011;6:1849-56.
- [Google Scholar]
- Negative predictive value of pulmonary fine needle aspiration cytology. Acta Cytol. 1992;36:283-6.
- [Google Scholar]
- Repeat needle biopsies combined with clinical observation are safe and accurate in the management of a solitary pulmonary nodule. Cancer. 2005;103:599-607.
- [Google Scholar]
- The clinical outcome of needle aspirations of the lung when cancer is not diagnosed. Ann Thorac Surg. 1986;41:592-6.
- [Google Scholar]
- PET-FDG imaging and transthoracic needle lung aspiration biopsy in evaluation of pulmonary lesions. A comparative risk-benefit analysis. Chest. 1995;108:441-6.
- [Google Scholar]
- Combined use of positron emission tomography and volume doubling time in lung cancer screening with low-dose CT scanning. Thorax. 2011;66:315-9.
- [Google Scholar]
- Distribution of stage I lung cancer growth rates determined with serial volumetric CT measurements. Radiology. 2006;241:554-63.
- [Google Scholar]
- Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252-9.
- [Google Scholar]
- Natural growth and disease progression of non-small cell lung cancer evaluated with 18F-fluorodeoxyglucose PET/CT. Lung Cancer. 2012;78:51-6.
- [Google Scholar]
- Diagnosis of pulmonary masses by fine-needle aspiration. Am J Surg. 1988;156:441-5.
- [Google Scholar]
- Causes of false results in transthoracic fine needle lung aspirates. Acta Cytol. 1993;37:16-20.
- [Google Scholar]
- Inflammatory and neoplastic processes of the lung: Differential diagnosis and pitfalls in FNA biopsies. Diagn Cytopathol. 1995;13:448-62.
- [Google Scholar]
- Percutaneous core needle biopsy vs. fine needle aspiration in diagnosing benign lung lesions. Acta Cytol. 1999;43:756-60.
- [Google Scholar]
- CT-guided thoracic core biopsies: Value of a negative result. Cancer Imaging. 2006;6:163-7.
- [Google Scholar]
- Distinction between bronchioloalveolar carcinoma and hyperplastic pulmonary proliferations: A cytologic and morphometric analysis. Diagn Cytopathol. 1997;16:396-401.
- [Google Scholar]
- Transthoracic fine needle aspiration and core biopsy of pulmonary lesions. A study of 296 patients. Acta Cytol. 2002;46:1061-8.
- [Google Scholar]
- Cytologic features of benign solitary pulmonary nodules with radiologic correlation and diagnostic pitfalls: A report of six cases. Acta Cytol. 2009;53:201-10.
- [Google Scholar]








