Translate this page into:
Gray zone Bethesda category III – Atypia of undetermined significance lesions of the thyroid: Potential diagnostic issues and image morphometry as a useful adjunct to cytomorphology

*Corresponding author: Pranab Dey, Department of Cytology and Gynecological Pathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. deypranab@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saini T, Kundu R, Rohilla M, Gupta P, Gupta N, Srinivasan R, et al. Gray zone Bethesda category III – Atypia of undetermined significance lesions of the thyroid: Potential diagnostic issues and image morphometry as a useful adjunct to cytomorphology. CytoJournal. 2024;21:38. doi: 10.25259/Cytojournal_86_2023
Abstract
Objective:
Atypia of undetermined significance (AUS) is an indeterminate category which presents a significant challenge for pathologists and clinicians. The management options are dependent on the rate of malignancy for a given populace.
Material and Methods:
This is a retrospective analysis of 61 cases of the AUS Bethesda category III with grouping into neoplastic and non-neoplastic according to the histopathology data and clinical follow-up. Detailed cytomorphological features were analyzed and image morphometry was done using image J software. Student’s t-test was used.
Results:
Out of the total 61 cases, 35 were neoplastic cases of AUS (histopathology proven), and 26 were non-neoplastic (on follow-up) cases. The risk of neoplasia and risk of malignancy observed were 57.4% and 47.5%, respectively. Neoplastic cases displayed prominent intranuclear inclusions (54%) and pseudopapillary clusters (20%). Majority of non-neoplastic cases revealed fine chromatin (96%) and pale chromatin (4%) while among neoplastic cases, 14% showed pale chromatin. Neoplastic cases showed moderate to marked nuclear pleomorphism (20%) compared to non-neoplastic cases which were monomorphic to mildly pleomorphic. None of the non-neoplastic cases exhibited frequent nuclear overlapping, nuclear grooving, or nucleoli which emphasizes the need for scrutiny of smears for these features. On image morphometry, cases with malignant outcome had larger nuclear area, perimeter, diameter, and nuclear density which were statistically significant.
Conclusion:
The study illustrates the importance of identifying subtle cytomorphological features and usefulness of image morphometry as an adjunctive objective tool in AUS cases. This helps in making an accurate cytological diagnosis which guides the treating clinician regarding surgical management or need for clinical follow-up.
Keywords
Atypia of undetermined significance
Cytomorphology
Fine-needle aspiration cytology
Image morphometry
Neoplastic
Thyroid
INTRODUCTION
The fine-needle aspiration (FNA) technique is a proficient, time-saving, and simple technique that can be used as a first-hand screening tool for thyroid lesions. Reporting of thyroid nodules is done by the universal 6-tiered Bethesda system. Atypia of undetermined significance (AUS) has compromised morphology and is reserved for cases with cytopathological features that are insufficient to be classified under higher Bethesda category but showing more atypia to put under benign category.[1] The diagnosis of follicular-patterned lesions is challenging for the cytopathologist.
The final histopathology outcome of AUS cases ranges from colloid goiter to carcinoma. Most importantly, there is substantial evidence that the actual risk of malignancy (ROM) of AUS is significantly higher than the estimated incidence. Therefore, it is crucial to identify the peculiar cytopathological features to determine the likelihood of malignancy and guide clinical decision-making.[2] Nonetheless, cytomorphological assessment is subjective with interobserver variations when reporting thyroid cytopathology especially in AUS cases. Computerized nuclear morphometry is an objective, economical, and consistent tool to detect changes that are too small to be visually perceived and could be used constructively to classify these gray zone lesions of the thyroid. Fine-needle aspiration cytology (FNAC) when corroborated with morphometry can prove beneficial in AUS category and, thus, can aid a cytopathologist in settling cases with diagnostic dilemma.[3] Literature on this is scarce.[4,5]
A timely informed and correct cytological diagnosis is fundamental to triaging patients with thyroid nodules and minimizes non-essential surgeries. In the present study, we retrospectively compared the cytomorphological features of histopathology-proven neoplastic and non-neoplastic AUS cases (histopathology proven/clinically stable) and assessed the cytomorphological features contributing to diagnostic pitfalls. Further, nuclear morphometry was done on FNAC smears to assess the role of image morphometry in delineating benign from malignant outcomes in this category.
MATERIAL AND METHODS
The present study is a retrospective study carried out in accordance with the guidelines laid down in the Declaration of Helsinki. The research was carried out according to the Institutional Ethics Guidelines after obtaining due approval of the Departmental Research Ethics Committee. The inclusion and exclusion criteria were outlined as follows: -
Inclusion criteria
Sixty-one cases with thyroid nodules were retrieved from the pathology database, which were diagnosed as AUS or follicular lesion of undetermined significance (FLUS) on FNA from 2017 to 2022. The enrolled patients had only primary thyroid disease.
Exclusion criteria
Cases with slides unavailable for analysis and follow-up information being unavailable were not included in the study. Further, cases with metastasis were excluded from the study.
Thyroid nodules that did not progress to malignancy in the past 2 years were taken to be benign on follow-up. Cytological characteristics were analyzed by two independent observers Tarunpreet Saini and Pranab Dey (TS and PD), who were blinded to the outcome, and histopathological slides were reviewed by an independent observer (UNS). Informed consent was taken at the time of FNA in all the cases and before surgical procedures wherever required.
The second edition of the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was used for making the diagnoses in this study. As per the third edition,[6] AUS is used and not AUS/FLUS. Hence, we use the latest AUS terminology in this paper.
Image morphometry
Intact and well-separated cells were selected and overlapping cells and three-dimensional clusters or papillae were excluded while assessing, and on an average 30 non-overlapping nuclei per case were assessed by two cytopathologists (TS and PD). The steps in image morphometry include image capturing, preprocessing, segmentation, feature extraction, and classification. Images were captured at ×40 magnification using an Olympus DP74 microscope. Morphometric analysis was done using Image J software (version 1.8.0). The colored images were converted to 8–bit gray images, and the gray threshold was adjusted to detect the nuclei. The automatic measurements of the nuclear morphometric features were done by the Image J software. The nuclear area, nuclear diameter, nuclear perimeter, the standard deviation of nuclear areas (SDNAs), and nuclear-integrated density were measured. These parameters were compared between the benign and malignant groups. Statistical analysis was performed using Student’s t-test and Levene’s Test for Equality of Variances. The cutoff P value was considered as 0.05.
RESULTS
A total of 61 patients were included in the study [Figure 1]. There were 47 women and 14 men, with age varying from 20 years to 71 years. This study had 35 neoplastic cases (histopathology proven), risk of neoplasia (RON) being 57.4% and 26 nonneoplastic (on follow-up) cases of category III. These 35 neoplastic cases were distributed among papillary thyroid carcinoma (PTC) (23), follicular adenoma (FA) (6), follicular carcinoma (FC) (3), non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) (2), and poorly differentiated thyroid carcinoma (1). The ROM was 47.5%. FNAC smears of AUS with neoplastic and non-neoplastic outcomes revealed a similar trend in cellularity, microfollicle formation [Figure 2], oncocytic change, and fire flares. There was significant conflicting variation for other cytomorphological features. Neoplastic cases displayed prominent intranuclear inclusions (54%) and pseudopapillary clusters (20%) [Figure 3] in signification proportion as compared to non-neoplastic cases that showed these features in a minority of cases constituting 8% and 7%, respectively. Regarding nuclear features, neoplastic cases showed moderate to marked nuclear pleomorphism (20%) compared to non-neoplastic cases, which were monomorphic to mildly pleomorphic. Majority of non-neoplastic cases revealed fine chromatin (96%) and pale chromatin (4%), while in neoplastic cases, 14% showed pale chromatin. None of the non-neoplastic cases exhibited frequent nuclear overlapping, frequent nuclear grooving, or nucleoli, pronouncing that cytopathologist should take utmost care of these features while ascertaining the diagnosis. Non-neoplastic cases exhibited more colloid than neoplastic cases, as shown in Table 1.
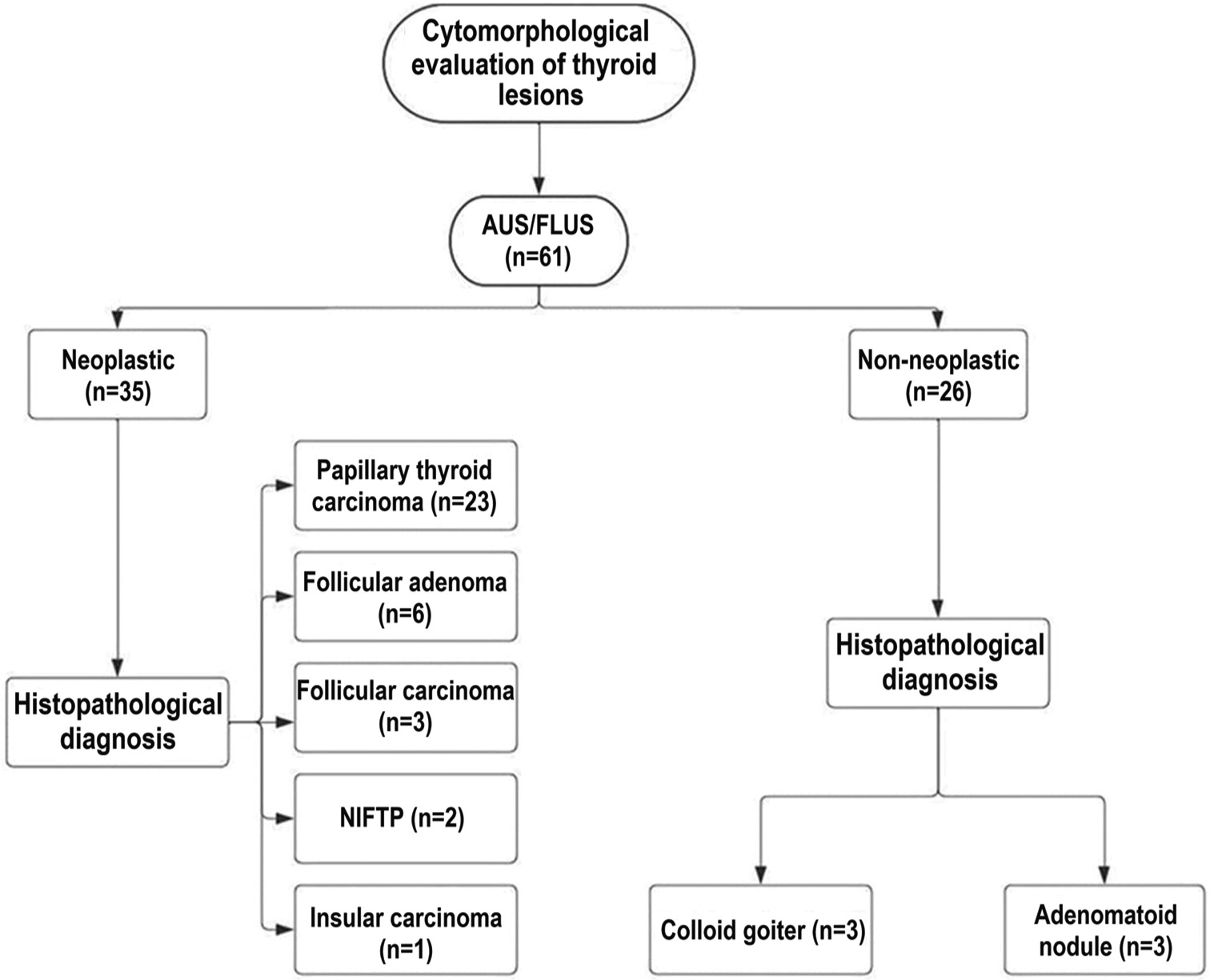
- Work flow of current study with histopathological outcome. (AUS: Atypia of undetermined significance, FLUS: Follicular lesion of undetermined significance, NIFTP: Non-invasive follicular thyroid neoplasm with papillary-like nuclear features). This flowchart was created using the Intelligent Diagramming Lucidchart, accessible at: https://www.lucidchart.com (Accessed July 19, 2024).
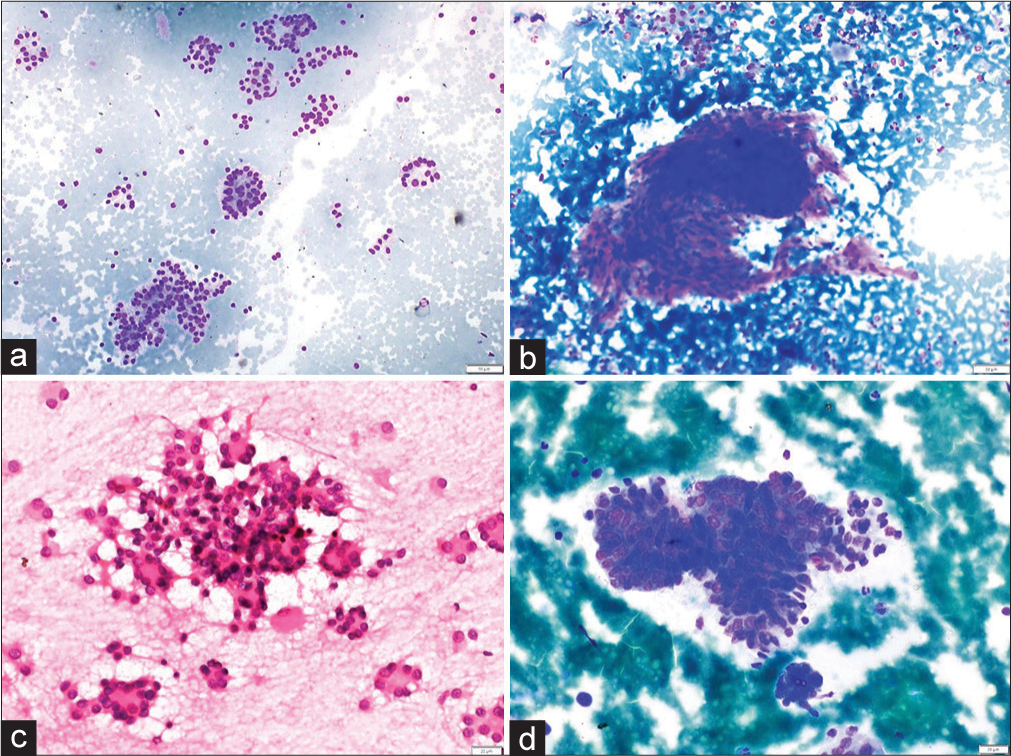
- (a and b) Non-neoplastic. (a) Smear showing microfollicles in a case of adenomatous nodule (MGG, ×100). (b) Aspirate smear showing a pseudo papillary cluster of follicular epithelial cells in colloid goiter (MGG, ×100). (c and d) Neoplastic. (c) Plentiful microfollicles in follicular adenoma (H&E, ×400). (d) Cohesive cluster of cells in papillary thyroid carcinoma. The cluster is avascular and the peripheral cells are irregularly arranged giving rise to vague papillae like arrangement. (MGG, ×400). (MGG: May Grunwald Giemsa stain, H and E: Hematoxylin and Eosin).
- (a and b) Non-neoplastic. (a). Smear showing cluster and few scattered follicular epithelial cells with occasional intranuclear inclusion and an occasional nuclear groove in lymphocytic thyroiditis (MGG, ×400). (b) Aspirate smear showing follicular epithelial cells with mild pleomorphism (MGG, ×400). (c and d) Neoplastic. (c) Intranuclear inclusion in papillary thyroid carcinoma (MGG, ×400). (d) An occa sional sheet and few scattered follicular epithelial cells with mild pleomorphism in papillary thyroid carcinoma (MGG, ×400). (MGG: May Grunwald Giemsa stain, H and E: Hematoxylin and Eosin).
| Cytological features | Neoplastic (35)-Histologically proven (%) | Non-neoplastic (26) Histologically proven/clinically stable (%) |
|---|---|---|
| Cellularity | ||
| Scant to low | 19 (54) | 14 (54) |
| Moderate to high | 16 (46) | 12 (46) |
| Colloid | ||
| Scant to low | 23 (66) | 15 (58) |
| Moderate to high | 12 (34) | 11 (42) |
| Microfollicles | ||
| Present | 15 (43) | 12 (46) |
| Absent | 20 (57) | 14 (54) |
| Pseudopapillary clusters | ||
| Present | 7 (20) | 2 (8) |
| Absent | 28 (80) | 24 (92) |
| Intranuclear inclusions | ||
| Present | 19 (54) | 2 (8) |
| Absent | 16 (46) | 24 (92) |
| Nuclear pleomorphism | ||
| Monomorphic to mild | 28 (80) | 26 (100) |
| Moderate to marked | 7 (20) | 0 (0) |
| Nuclear chromatin | ||
| Fine | 30 (86) | 25 (96) |
| Pale/Granular | 5 (14) | 1 (4) |
| Nuclear overlapping | ||
| Occasional to minimal | 24 (68) | 26 (100) |
| Frequent | 11 (32) | 0 (0) |
| Nuclear grooving | ||
| Absent to occasional | 32 (92) | 26 (100) |
| Frequently seen | 3 ( 8) | 0 (0) |
| Nucleoli | ||
| Present | 16 (46) | 0 (0) |
| Absent | 19 (54) | 26 (100) |
| Oncocytic change | ||
| Present | 4 (11) | 4 (15) |
| Absent | 31 (89) | 22 (85) |
AUS: Atypia of undetermined significance
Causes for discrepancies
Microfollicular arrangement
Obvious microfollicular arrangement was seen in the adenomatous nodule, now called follicular nodular disease (FND) as per the recent World Health Organization (WHO) 5th edition[7] (4), FA (6), and FC (3). These entities, by the presence of a capsule or by capsular/vascular invasion, are distinguishable on histopathological assessment. Follicular variant of PTC, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), and FND are other diagnostic considerations. This was one of the common causes of discordance in the current study.
FND
Hyperplastic nodules (FND) (4) may show focal nuclear atypia. Based on focal atypia, less cellular smears, and lack of colloid, few lesions were placed in category III in the present study. Attention should be paid to pseudo-inclusions and characteristic nuclear features generally indicative of malignancy.
NIFTP
This entity presents a diagnostic challenge on pre-operative FNAC. Its diagnosis is still debatable. Furthermore, it is not uncommon for NIFTP to be associated with coexisting conventional PTC. In a large meta-analytic study,[8] it was shown that the prevalence of NIFTP among the conventional PTC is near about 4.4%. In an index study, two cases finally turned out to be NIFTP, which was considered as a malignant outcome.
Malignancies coexisting with FND
It is a well-known fact that, in a significant number of cases, papillary thyroid carcinoma (PTC) arises in the niche of FND previously called as multinodular goiter. Hence, aspirate if accidently taken from goiter component can lead to diagnostic error. In such cases, a cytopathologist should always rely on ultrasound-guided FNAC.
Interpretation error
Finally, interpretation error can also be a cause of misdiagnosis. Hence, emphasis should be given to nuclear features as ascribed in our study. Proper history, time duration of disease, and biochemical and radiological reports should always be considered while giving the final diagnosis. Cells with vacuolated cytoplasm deserve scrutiny of nuclear characteristics so that they are not mistaken for macrophages. Nuclei of these cells can be matched with actual macrophages elsewhere in the smear. Furthermore, features such as papillary fragments, pleomorphism, elongation of nuclei, rare grooves, and occasional nuclear inclusions, which denote PTC may be observed.
Sampling error
This is an important diagnostic pitfall. As thyroid is a vascular organ, more precision is needed during the procedure.
Image morphometry
The average time from image acquisition to final output was about 8–10 min for each case with additional 2–3 min required for putting the data into Excel sheets for analysis. A representative case of AUS with benign outcome is shown in Figure 4. Figure 5 demonstrates the image morphometry of an AUS with malignant outcome (PTC). All the nuclear morphometric features were highly significant between the malignant and benign groups of AUS cases. The mean nuclear area, standard deviation of nuclear area (SDNA), nuclear perimeter, diameter, and integrated density in the malignant group were 26.205 ± 8.195 µm2 (mean ± standard deviation), 8.642 ± 3.059 µm, 23.988 ± 3.901, 7.308 ± 1.037, and 3460.521 ± 1136.319, respectively. The statistical analysis showed that these values were significantly higher than those observed in the benign group, as depicted in Table 2. P values observed were 0.004, 0.058, 0.293, 0.124, and 0.001 for mean nuclear area, SDNA, nuclear perimeter, diameter, and integrated density using Levene’s test for equality of variances and were < 0.001 using t-test for equality of means.
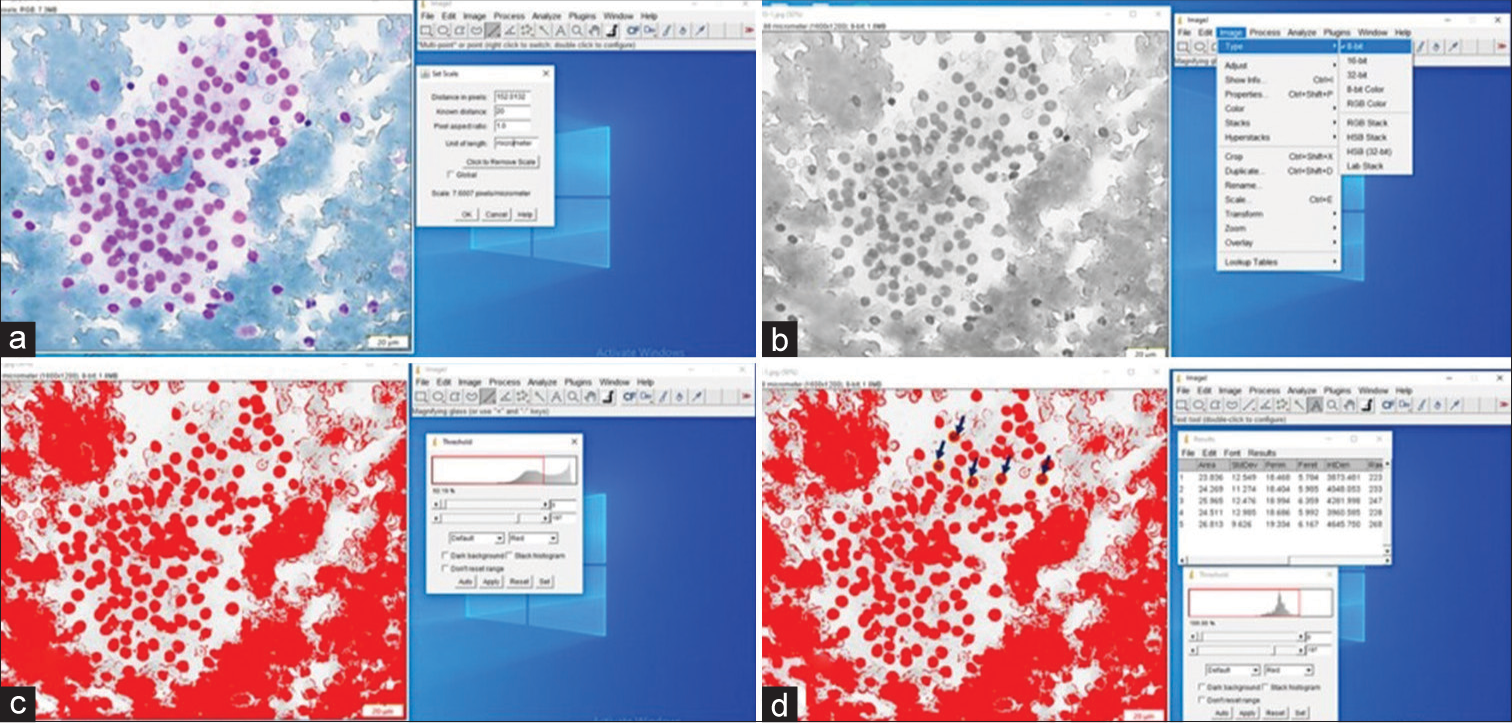
- Image morphometry in atypia of undetermined significance lesion of thyroid with the outcome of follicular nodular disease (colloid goiter). (a) Captured image is displayed and the calibration is set up. (b) The colored image is converted into a 8-bit gray image. (c) The nuclei are detected by adjusting the gray threshold. (d) The individual nuclei are detected (arrows) and morphometric parameters are measured.
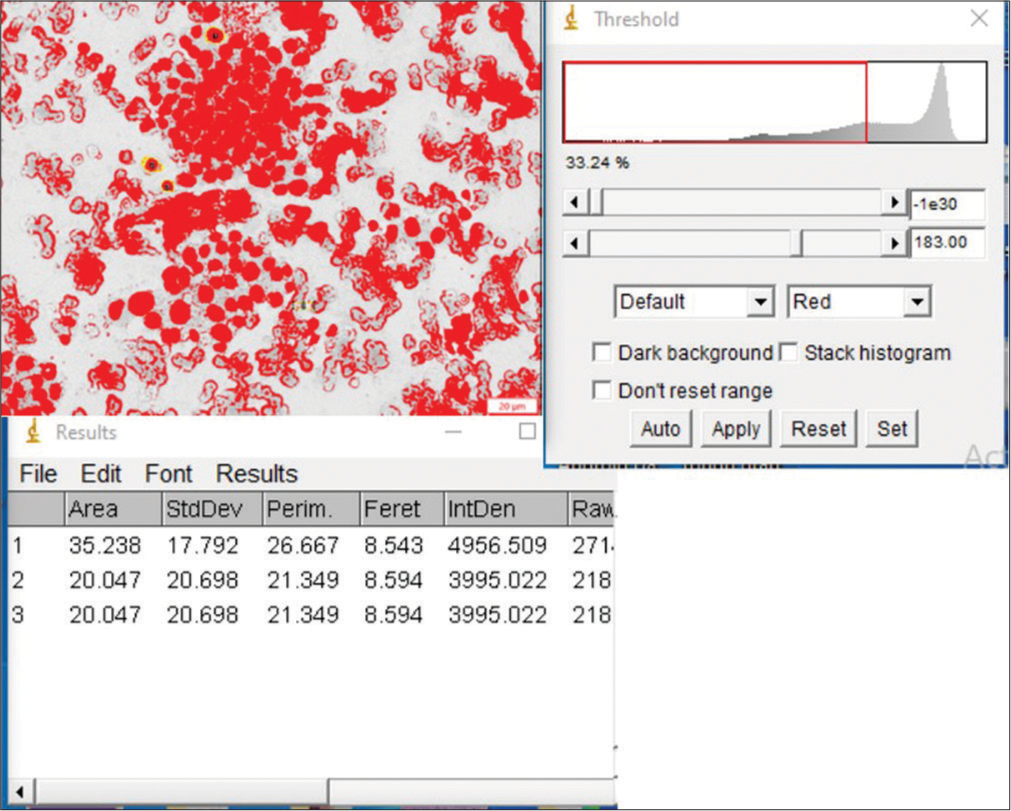
- Image morphometry in a case of atypia of undetermined significance lesion with the outcome of papillary thyroid carcinoma.
| Parameter | Benign outcome | Malignant outcome | Levene’s test for equality of variances (P-value) |
|---|---|---|---|
| Mean area (µm2), nucleus (±Standard deviation) |
16.222 4.533 | 26.205 8.195 | 0.004 |
| Mean standard deviation of nuclear area (µm2) (±Standard deviation) |
5.441 2.258 | 8.642 3.059 | 0.058 |
| Mean perimeter (µm), nucleus (±Standard deviation) |
17.965 3.386 | 23.988 3.901 | 0.293 |
| Mean nuclear diameter (µm) (±Standard deviation) |
5.670 0.818 | 7.308 1.037 | 0.124 |
| Mean integrated density, nucleus (±Standard deviation) |
2188.860 735.687 |
3460.521 1136.319 |
0.001 |
AUS: Atypia of undetermined significance, µm: Micrometer
DISCUSSION
This paper outlines the specific cytomorphological characteristics of the Bethesda category III, AUS lesions of the thyroid and places emphasis on common diagnostic errors. According to our analysis, significant attention should be paid to substantial intranuclear inclusions, pseudopapillary clusters, and nuclear traits like pale chromatin and nuclear pleomorphism since they connect to a higher category lesion. Although there is some subjectivity in applying the Bethesda system, the AUS instances are the most perplexing. They should not be treated as a waste basket, and before reaching a final diagnosis, weightage should be given to cytomorphological features. Further, the subjectivity inherent to cytomorphological evaluation is non-existent for image morphometry, which was assessed as an additional objective tool in our current work.
Many authors have tried to analyze the risk of malignancy (ROM) in AUS and subclassify the category.[9-14] The variability in results across different set-ups may be attributed to an element of subjectivity. Results from different centers may differ due to differences in FNA procedures, needle size, ultrasound guidance use, and the cytopathologist’s competence. At many centers, FNAC is done by the radiologist or clinician rather than the pathologist. Differences in smear preparatory methodology are yet another cause. A repeat FNA is a recommendation for an initial diagnosis of category III, and molecular testing should be introduced according to the latest Bethesda. A repetitive diagnosis of category III is seen in 20–28% of thyroid lesions.[15] Nonetheless, a repeat FNA is yielding with a definitive diagnosis invariably.
Our tertiary care facility holds multidisciplinary meetings fortnightly where pathologists and radiologists participate, and cases are discussed to determine the best further course of action wherever required. Our study emphasizes better cytomorphological criteria that support malignancy or benignity and avoiding diagnostic pitfalls that can lead to under or overdiagnosis and plan patient management accordingly. In our study, 23 out of 61 cases turned out to be PTC (37.7%), followed by FA (6) and FC (3), NIFTP (2), and one case of poorly differentiated thyroid carcinoma. In our study, most neoplastic cases displayed intranuclear pseudo-inclusions (54%), prominent nucleoli (46%), and pseudopapillary clusters (papillaroid clusters of follicular epithelial cells lacking fibrovascular cores) and moderate to marked pleomorphism in 20% of cases. Majority of nonneoplastic cases revealed fine chromatin (96%) and pale chromatin (4%), while in neoplastic cases, 14% showed pale chromatin. None of the non-neoplastic cases exhibited frequent nuclear overlapping, frequent nuclear grooving or nucleoli pronouncing that cytopathologist should take utmost care of these features while ascertaining the diagnosis. While smears show pleomorphism, intranuclear pseudo-inclusions, pale chromatin, and nuclear grooves, the diagnosis may be TBSRTC category IV or TBSRTC category V based on cell quantity. Probably, one should focus both on cell quantity and degree of atypia to label a case TBSRTC category V.
Non-neoplastic cases exhibited more colloids as compared to neoplastic cases. FNAC smears of AUS with neoplastic and non-neoplastic outcomes revealed a similar trend in microfollicle formation, oncocytic change, and fire flares.
Image morphometry can be used as an adjunctive modality for making pre-operative assessments and its importance in gray zone areas of AUS cases in thyroid cytopathology which need additional ancillary techniques cannot be overemphasized. It is an inexpensive objective tool.[16] It is precise and objective in comparison to the manual assessment of nuclear features which have subjectivity. In our study, image morphometry was able to delineate the malignant and benign outcomes of AUS cases. The RON and ROM observed were 57.4% and 47.5%, respectively. The average time from image acquisition to final output was about 8–10 min for each case with additional 2–3 min required for putting the data into Excel sheets. Compared to molecular testing, it is cheaper, less time-consuming, and not much demanding technically. Doing it on the cytology smears which show intact and well-preserved cells yields superior results than when done on histopathology sections.[17]
Our observations on image morphometry in Category III AUS thyroid lesions are in line with Kang et al.,[4] who performed morphometry in 84 cases of AUS which had histologic follow-up and noted a significant difference between the benign and malignant groups. Moreover, the nuclear irregularities in AUS/FLUS lesions may be helpful in differentiating benign from malignant thyroid lesions. Artacho-Pérula et al.[5] studied 55 patients with FA, FC, and PTC by image analysis. An increase in nuclear size was noted for carcinoma as compared to adenoma. This increase achieved greater significance when volume-weighted mean nuclear volume was evaluated.
The most updated 2023 Bethesda System divides AUS into AUS with Nuclear Atypia and AUS-Other.[6] The results of our study and the inferences drawn thereby are not affected by utilizing the new Bethesda III terminology. The recent 2022 WHO classification of thyroid neoplasms has many new/alternative terminologies, most characteristic one being thyroid FND, thereby avoiding terms such as multinodular goiter, hyperplastic nodule, neoplastic, or adenomatous hyperplasia.[7] It is pertinent to note that the updated terminology of the latest TBSRTC is in line with the recent 2022 WHO classification of thyroid neoplasms.
The potential shortcomings or limitations of the present study include a relatively small sample size and a short duration of clinical follow-up which was 2 years. Nevertheless, the study is reproducible and paves way for building up of diagnostic algorithmic models especially in resource-limited set-ups based on robust morphometric findings observed in the study, as depicted in Table 2.
SUMMARY
The present study emphasizes on better cytomorphological criteria to classify the lesions of the thyroid either benign or malignant and prioritize cases that should be put in the Bethesda category III AUS when it is utmost needed for better management of patients. To reiterate, this category should not to be treated as a waste basket. Objective analysis using image morphometry and its utility as an adjunct to cytomorphological assessment in differentiating benign versus malignant outcomes in AUS lesions of the thyroid are demonstrated.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
AUS: Atypia of undetermined significance
FA: Follicular adenoma
FC: Follicular carcinoma
FLUS: Follicular lesion of undetermined significance
FNA: Fine-needle aspiration
FNAC: Fine-needle aspiration cytology
FND: Follicular nodular disease
NIFTP: Non-invasive follicular thyroid neoplasm with papillary-like nuclear features,
PTC: Papillary thyroid carcinoma
ROM: Risk of malignancy
RON: Risk of neoplasia
SDNAs: Standard deviation of nuclear areas,
TBSRTC: The Bethesda system for reporting thyroid cytopathology
WHO: World health organization
AUTHOR CONTRIBUTIONS
TS: Data collection, analysis and drafting of the manuscript; RK: Literature review, manuscript preparation and cytology reporting; MR, PG, NG, RS: Cytology reporting; UNS: Histopathology reporting; PD: Conception of the idea for the study, reporting, analysis and drafting and overall execution. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was approved by Postgraduate Institute of Medical Education and Research, Chandigarh Institutional Ethics Committee (Intramural), and the approval number is: IEC-INT/2024/Study-2102.
Informed consent was obtained from all the patients before fine-needle aspiration cytology and surgical resection wherever applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid. 2023;33:1039-44.
- [CrossRef] [Google Scholar]
- Approach to cytological indeterminate thyroid nodules. Gland Surg. 2019;8:S98-104.
- [CrossRef] [Google Scholar]
- Computerized nuclear morphometry in the diagnosis of thyroid lesions with predominant follicular pattern. Ecancermedicalscience. 2009;3:146.
- [CrossRef] [Google Scholar]
- Morphometric analysis of thyroid follicular cells with atypia of undetermined significance. J Pathol Transl Med. 2016;50:287-93.
- [CrossRef] [Google Scholar]
- Objective differential classification of thyroid lesions by nuclear quantitative assessment. Histol Histopathol. 1997;12:425-31.
- [Google Scholar]
- The 2023 Bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol. 2023;12:319-25.
- [CrossRef] [Google Scholar]
- Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33:27-63.
- [CrossRef] [Google Scholar]
- Systematic review and meta-analysis of the impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on cytological diagnosis and thyroid cancer prevalence. Endocr Pathol. 2019;30:189-200.
- [CrossRef] [Google Scholar]
- Cytologic subclassification of atypia of undetermined significance may predict thyroid nodules more likely to be malignant at surgery. Diagn Cytopathol. 2016;44:492-8.
- [CrossRef] [Google Scholar]
- Cytological sub-classification of atypia of undetermined significance may predict malignancy risk in thyroid nodules. Acta Cytol. 2021;65:205-12.
- [CrossRef] [Google Scholar]
- Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology. 2013;24:385-90.
- [CrossRef] [Google Scholar]
- Thyroid atypia of undetermined significance or follicular lesion of undetermined significance: An indispensable Bethesda 2010 diagnostic category or waste garbage? Acta Cytol. 2014;58:319-29.
- [CrossRef] [Google Scholar]
- Risk of malignancy according to sub-classification of the atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) category in the Bethesda system for reporting thyroid cytopathology. Cytopathology. 2017;28:65-73.
- [CrossRef] [Google Scholar]
- Malignancy rates for Bethesda III and IV thyroid nodules: A retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC Endocr Disord. 2020;20:48.
- [CrossRef] [Google Scholar]
- Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol. 2003;29:203-6.
- [CrossRef] [Google Scholar]
- Morphologic and planimetric diagnosis of follicular thyroid lesions on fine needle aspiration cytology. Anal Quant Cytol Histol. 1995;17:247-56.
- [Google Scholar]









