Translate this page into:
High-grade transformation in adenoid cystic carcinoma: Can it be diagnosed on cytology? A cytohistological correlation

*Corresponding author: Shruti Gupta, Department of Pathology, AIIMS, Raebareli, Uttar Pradesh, India. drshrutimlb@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Maurya S, Gupta S, Soni A, Kumari N, Rajwanshi A. High-grade transformation in adenoid cystic carcinoma: Can it be diagnosed on cytology? A cytohistological correlation. CytoJournal 2024;21:10. doi: 10.25259/Cytojournal_38_2023
Abstract
Adenoid cystic carcinomas (ADCC) are distinctive salivary gland neoplasms with characteristic histomorphology. The diagnosis of dedifferentiation/high-grade transformation (HGT) indicates poor prognosis and is most often made on histopathology. We present a case of ADCC arising from a minor salivary gland tumor exhibiting HGT, reaching up to the submandibular gland and having lymph node metastases, suspected on fine-needle aspiration cytology. The index case highlights the awareness of the entity of the HGT of salivary gland tumors and raises suspicion for cytological diagnosis.
Keywords
Adenoid cystic carcinoma
High-grade transformation
Cytopathology
Histopathology
Dedifferentiation
INTRODUCTION
Adenoid cystic carcinoma (ADCC) is a slowly growing but highly invasive tumor with a high recurrence rate and a propensity toward perineural spread. ADCC accounts for 14.5% of all salivary gland tumors and 41% of malignant tumors of the large and small salivary glands.[1] Fine-needle aspiration has been utilized for the evaluation of salivary gland lesions with high sensitivity and specificity.[2] This report highlights cytohistomorphological correlation in a case of metastatic high-grade transformation (HGT) of ADCC in a 52-year-old male patient presenting with cervical lymphadenopathy and an intraoral mass.
CASE REPORT
A 52-year-old male presented to the otorhinolaryngology outpatient department with a slow-growing, painless left submandibular swelling noticed 2 weeks back. On local examination, the swelling was 1 × 1 cm deep, firm, mobile non tender, and fixed to the underlying skin. The patient was referred for fine-needle aspiration cytology (FNAC) of the submandibular mass. After due informed consent and under all aseptic precautions, FNAC was done using a 23-gauge needle and a 20-mL syringe attached to Cameco’s handle. The smears were cellular and showed tumor cells in groups, clusters, and attempted glandular formation as well as singly scattered. The individual tumor cells had round-to-oval nuclei, coarse chromatin, and prominent nucleoli with a moderate amount of cytoplasm. Few of these cells had vacuolated cytoplasm [Figure 1a-c]. Based on the cytomorphology, a diagnosis of metastatic adenocarcinoma in submandibular swelling was given.
![(a-c) Microphotographs from fine-needle aspiration smears from the submandibular lymph node showing tumor cells in loose cohesive clusters having round-to-oval nuclei, coarse chromatin, and prominent nucleoli with a moderate amount of cytoplasm, (×40, ×200, ×400, May Grunwald Giemsa [MGG]). (d-f) Microphotograph from fine-needle aspiration smears from the sublingual swelling showing basaloid cells with oval to angulated hyperchromatic nuclei, indistinct nucleoli, and scant cytoplasm, (×40, ×100, ×200, MGG).](/content/105/2024/21/1/img/Cytojournal-21-10-g001.png)
- (a-c) Microphotographs from fine-needle aspiration smears from the submandibular lymph node showing tumor cells in loose cohesive clusters having round-to-oval nuclei, coarse chromatin, and prominent nucleoli with a moderate amount of cytoplasm, (×40, ×200, ×400, May Grunwald Giemsa [MGG]). (d-f) Microphotograph from fine-needle aspiration smears from the sublingual swelling showing basaloid cells with oval to angulated hyperchromatic nuclei, indistinct nucleoli, and scant cytoplasm, (×40, ×100, ×200, MGG).
Subsequently, the patient was examined, and an intraoral hard, fixed swelling in the floor of the mouth in the left sublingual region measuring approximately 1.5 × 1 cm was found. The patient was again sent for FNAC for the intraoral swelling. The smears were cellular and showed tumor cells with hyaline globules. These tumor cells had uniform basaloid cells with scant cytoplasm, oval to angulated hyperchromatic nuclei, and indistinct nucleoli. The findings were characteristics of ADCC arising from the minor salivary gland [Figure 1d-f]. Dual malignancy was suspected, and smears from the submandibular swelling were reviewed; however, no features of metastatic ADCC could be confirmed on the smears from the swelling. The possibility of a high-grade transformation in ADCC was considered.
The patient underwent excision of the left submandibular gland and left sublingual mass with left-side cervical lymph node dissection. On examination, the resected sublingual gland specimen measured 2 × 1 × 1 cm with a gray-white firm cut surface. Microscopy showed ADCC with the tumor disposed in cords, tubules, and cribriform pattern having hyperchromatic, angulated nuclei with scant cytoplasm, and the lumen showed the presence of pale mucoid secretions [Figure 2a and b]. However, the tumor showed a gradual transition to high grade and extended into the adjoining soft tissue arranged in cords, solid sheets, and focal glands present in the hyalinized stroma [Figure 2c and d]. The tumor breached to the capsule of the sublingual gland; however, the inked margins were free of tumor.
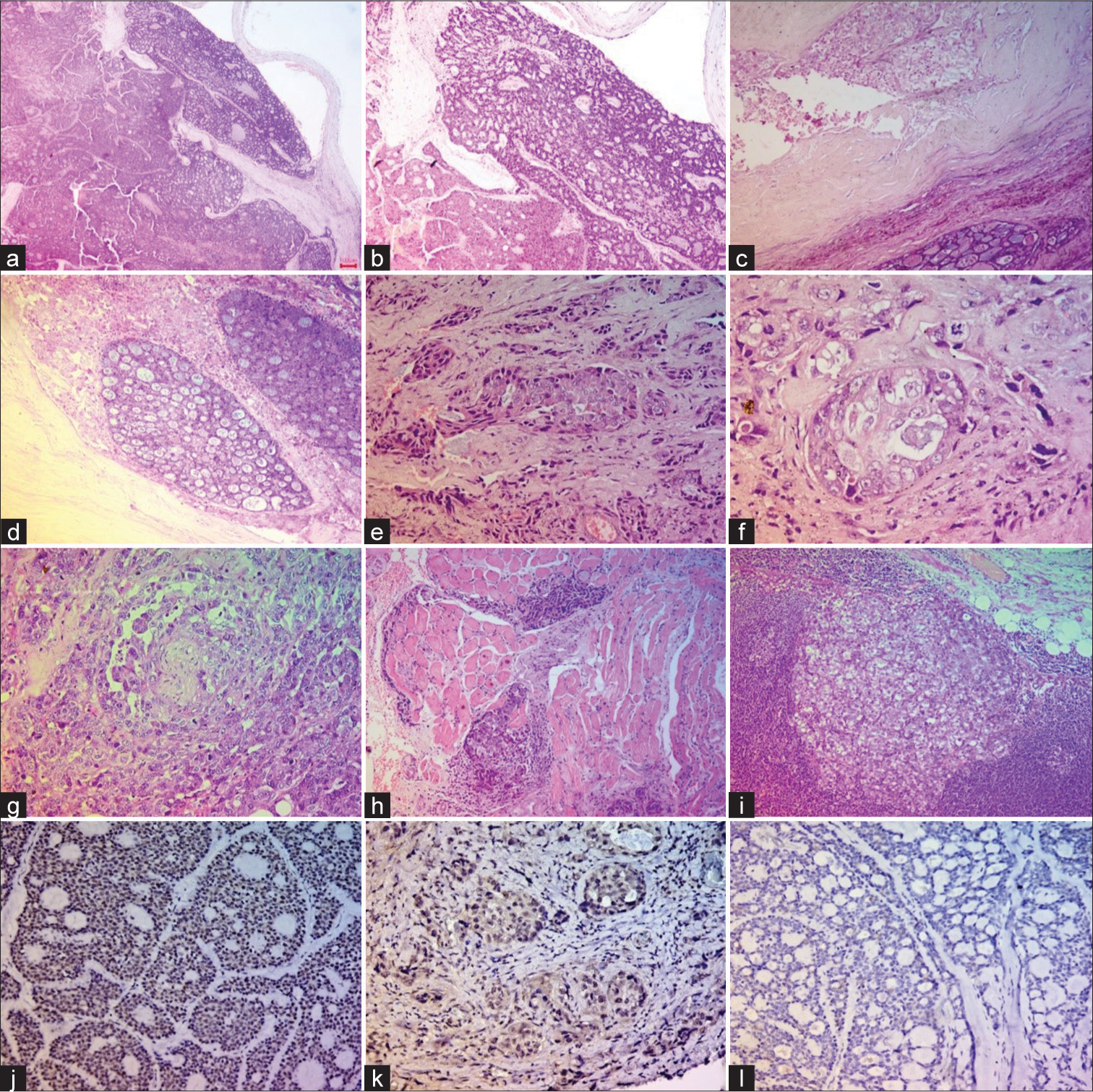
- Microphotograph panel showing features of high-grade transformation (HGT) of adenoid cystic carcinoma (ADCC). (a and b) Sections from sublingual tumors showing classical cribriform pattern of ADCC. (c and d) Section showing areas of high-grade transformation; both basaloid cells disposed in the cribriform pattern with bands of hyalinization, and sheets of pleomorphic tumor cells with vesicular nuclei and prominent nucleoli are noted. (e and f) Section showing invasive sheets of poorly differentiated tumor cells. (g) Section showing perineural invasion by poorly differentiated tumor cells. (h) Section showing poorly differentiated tumor cells invading surrounding muscle tissue. (i) Lymph node metastases. (j and k) Immunohistochemical expression of tumor protein p53 (p53) in both basaloid tumor cells and poorly differentiated areas. (l) Tumor cells are immunonegative for human epidermal growth factor receptor 2. ((a-i): hematoxylin and eosin, (j-k): p53, l: Human epidermal growth factor receptor 2 (HER2), immunoperoxidase (IPOX), (a-c): 40x, d: 100x, e: 200x, (f-g): 400x, (h-i): 100x, (j-l): 100x).
The resected submandibular specimen measured 2 × 2 × 2 cm comprised a firm gray-white mass with fibrofatty tissue. Sections from the submandibular gland showed tumors arranged in cords, acini, and solid sheets. The individual tumor cells were pleomorphic with vesicular nuclei, prominent nucleoli, and scant cytoplasm [Figure 2e and f]. Perineural invasion and large areas of tumor necrosis were seen, and dedifferentiated areas were present [Figure 2g and h].
The radical neck dissection of levels II, III, IV, and V lymph nodes yielded 43 lymph nodes; 37 of which showed tumor metastasis and extranodal extension. The nodal metastases showed tumor cells with high-grade morphology [Figure 2i].
A final diagnosis of ADCC with HGT was offered. Immunohistochemistry (IHC), done on both well-differentiated and high-grade areas, revealed tumor cell immunopositivity for p53 [Figure 2j and k].
DISCUSSION
The classical adenoid cystic carcinomas more commonly arise from the minor salivary glands. The cytological diagnosis of ADCC is straightforward in most cases with occasional incidences of overlaps with other neoplasms such as pleomorphic adenoma, basal cell adenoma, basal cell adenocarcinoma, and myoepithelioma.[3] A diagnosis of high-grade transformation of ADCC is usually not made on FNAC. However, in the index case, a suspicion was made, based on the poorly differentiated morphology in the submandibular swelling.
HGT was initially described as dedifferentiated carcinoma by Cheuk et al.[4] and is defined as the transformation of a well-differentiated tumor into a high-grade malignancy that lacks the distinct histologic characteristics of the original neoplasm.[4]
In ADCC-HGT, the high-grade carcinoma is usually either poorly differentiated adenocarcinoma or less commonly “undifferentiated” carcinoma.[5] Nagao[5,6] discussed six cases, wherein the high-grade carcinoma cells exhibited large pleomorphic nuclei with a moderate amount of cytoplasm and high mitotic activity. These tumor nuclei had vesicular chromatin with conspicuous nucleoli.[6] Similar findings were found in the index case as well. The high-grade areas had solid patterns and showed perineural invasion and lymph node metastases.
On immunohistochemical (IHC) analysis, TP53 gene mutation, human epidermal growth factor receptor 2 overexpression, cyclin D1 overexpression, and loss of retinoblastoma expression have been variably demonstrated in the high-grade component in contrast to the parent ADCC.[7,8] In the present case, p53 immunopositivity was demonstrated in both basaloid tumor cells (classical ADCC) and high-grade areas [Figure 2j-l]. HGT in salivary gland carcinomas is associated with aggressive clinical behavior and a poor prognosis. Studies have revealed that ADCC-HGT has an increased tendency for lymph node metastasis, as high as 43–57%.[9]
The current guidelines advocate surgical excision, followed by irradiation for ADCC of the salivary gland.[10]
In the index case, the patient developed large lymph nodes within 2 months after radical surgical excision and neck dissection and expired within 4 months of diagnosis despite radiotherapy. Seethala et al.,[10,11] in their series of 11 cases of ADCC-HGT, showed cervical lymph node metastases in 4 cases.[11] In another study, the same author has postulated the role of c-myc amplification in HGT of adenoid cystic carcinomas.[12]
Further insights regarding the pathogenesis of high transformation of ADCC need to be evaluated.
In practice, the potential for HGT/dedifferentiation in most of the salivary gland carcinomas needs a thorough sampling of all salivary gland tumors to avoid missing a high-grade component. The possibility of HGT of a specific entity should be considered while classifying any salivary gland tumor as undifferentiated or unclassifiable high-grade carcinomas on biopsies with limited tumor tissue.
SUMMARY
HGT has been described in limited reports in the literature. Most reports describe the HGT identified on histological examination. Owing to its rarity and the lack of specific features, cytologic specimens from metastatic ADCC with high grade/de-differentiation may be easily confused with a variety of poorly differentiated primary or secondary carcinomas that may be more commonly encountered at the site of distant metastasis. However, in such cases, the possibility of HGT can be suggested in cytology.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
ADCC - Adenoid cystic carcinoma
HGT - High grade transformation
FNAC - Fine needle aspiration cytology.
AUTHOR CONTRIBUTIONS
SM: Involved in diagnosis and data collection SG: Involved in diagnosis, conceptualization, drafting and editing the manuscript, AS:Involved in patient management, NK: Involved in diagnosis and reviewing the manuscript, AR: Involved in diagnosis and reviewing the manuscript. All authors are responsible for the manuscript’s content and have participated in the study concept and design, analysis and interpretation of the data, drafting or revising of the manuscript, and approved the manuscript as submitted.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Ethics Committee AIIMS, Raebareli (approval number: 2024-6- OTH-EXP- 6, Date: 24.02.24).
The authors certify that they have obtained all appropriate patient consent.
CONFLICT OF INTEREST
Given his role in the International Editorial Panel, Arvind Rajwanshi had no involvement in the peer-review of this article and has no access to information regarding its peer review. The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
SUPPLEMENTARY MATERIAL
Supplementary material associated with this article can be found doi: 10.25259/Cytojournal_38_2023
FUNDING
Not applicable.
References
- Value of cytopathology in the diagnosis of adenoid cystic carcinoma and an analysis of misdiagnoses. BMC Surg. 2023;23:52.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology in diagnosis of salivary gland lesions: A study with histologic comparison. Cytojournal. 2013;10:5.
- [Google Scholar]
- Adenoid cystic carcinoma cytology: Salivary gland and nonsalivary gland. Diagn Cytopathol. 2020;48:1282-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dedifferentiation in adenoid cystic carcinoma of salivary gland: An uncommon complication associated with an accelerated clinical course. Am J Surg Pathol. 1999;23:465-72.
- [CrossRef] [PubMed] [Google Scholar]
- "Dedifferentiation" and high-grade transformation in salivary gland carcinomas. Head Neck Pathol. 2013;7:S37-47.
- [CrossRef] [PubMed] [Google Scholar]
- Dedifferentiated adenoid cystic carcinoma: A clinicopathologic study of 6 cases. Mod Pathol. 2003;16:1265-72.
- [CrossRef] [PubMed] [Google Scholar]
- Dedifferentiation of adenoid cystic carcinoma: Report of a case implicating p53 gene mutation. Hum Pathol. 2001;32:1403-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical lymph node metastasis in high-grade transformation of head and neck adenoid cystic carcinoma: A collective international review. Adv Ther. 2016;33:357-68.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of extra-salivary adenoid cystic carcinoma. Cytopathology. 2020;31:215-22.
- [CrossRef] [Google Scholar]
- Adenoid cystic carcinoma with high-grade transformation: A report of 11 cases and a review of the literature. Am J Surg Pathol. 2007;31:1683-94.
- [CrossRef] [PubMed] [Google Scholar]
- Progressive genetic alterations of adenoid cystic carcinoma with high-grade transformation. Arch Pathol Lab Med. 2011;135:123-30.
- [CrossRef] [PubMed] [Google Scholar]
- Undifferentiated and dedifferentiated head and neck carcinomas. Semin Diagn Pathol. 2021;38:127-36.
- [CrossRef] [PubMed] [Google Scholar]








