Translate this page into:
Histopathological findings of 687 thyroid nodules, suspicious for malignancy on ultrasound, with an indeterminate cytopathological diagnosis after the combination of the Bethesda System and BRAF mutation status

Jiawei Li

Kai Zhang
*Corresponding authors: Jiawei Li, PhD Department of Oncology, Shanghai Medical College, Fudan University Department of Medical Ultrasound, Fudan University Shanghai Cancer Center, Shanghai, China. jiaweili2006@163.com
Kai Zhang, MD Department of Oncology, Shanghai Medical College, Fudan University Department of Medical Ultrasound, Fudan University Shanghai Cancer Center, Shanghai, China zhangk86@foxmail.com
-
Received: ,
Accepted: ,
How to cite this article: Meng X, Hou R, Zhang M, Chen J, Zhang K, Li J. Histopathological findings of 687 thyroid nodules, suspicious for malignancy on ultrasound, with an indeterminate cytopathological diagnosis after the combination of the Bethesda system and BRAF mutation status. CytoJournal. 2025;22:1. doi: 10.25259/Cytojournal_97_2024
Abstract
Objective:
The conflicting results of the Bethesda system for reporting thyroid cytopathology (BSRTC) and B-Raf proto-oncogene (BRAF) mutation status during pre-operative fine-needle aspiration cytology (FNAC) of thyroid nodules create a dilemma for clinicians in devising appropriate treatment strategies for patients. This study provides a report on the histopathological findings of 687 thyroid nodules with an indeterminate cytological diagnosis after the combination of the BSRTC and BRAF mutation status.
Material and Methods:
The clinical data of patients with thyroid nodules, suspicious of malignancy at ultrasound (US), who underwent US-guided FNAC between December 2020 and March 2023 at our cancer center were reviewed. Patients with an indeterminate diagnosis, that is, conflicting results of the BSRTC and BRAF mutation status after FNAC, were enrolled. The following four combinations of BSRTC and BRAF mutation status were considered indeterminate: (1) Group 1, BSRTC I and positive for a BRAF mutation; (2) Group 2, BSRTC II and positive for a BRAF mutation; (3) Group 3, BSRTC III and positive for a BRAF mutation; and (4) Group 4, BSRTC V and negative for a BRAF mutation. Finally, only patients who underwent surgical treatment at our center were included in the data analysis.
Results:
Among the 1,044 eligible patients, 687 underwent surgical treatment. Of the 687 patients, 117 were in Group 1, 14 in Group 2, 394 in Group 3, and 162 in Group 4. Histopathological examination showed that 677 (98.5%) patients had papillary thyroid cancer, including 585 with papillary thyroid microcarcinoma, whereas only 10 (1.5%) had benign nodules. The malignancy rates were 98.3%, 100%, 98.7%, and 98.1% for Groups 1 to 4, respectively. Among the 387 patients in category 4A by the thyroid imaging reporting and data system (TI-RADS 4A) through the US, the malignancy rate was 98.4%, and for the 116 nodules <5 mm in diameter in the US, the malignancy rate was 99.1%. When combining TI-RADS 4A and a nodule diameter <5 mm, the malignancy rate was 98.9% (88/89). A total of 179 patients (26.1%) had histopathologically confirmed central cervical lymph node metastasis, and 46 (6.8%) had lateral cervical lymph node metastasis. Two nodules in Group 1, five nodules in Group 3, and three nodules in Group 4 were determined to be benign post-surgery. The benign thyroid nodules included seven dysplastic, one adenomatous, one fibrotic, and one hyperplastic.
Conclusion:
Thyroid nodules, suspicious of malignancy on US, after the combined interpretation of BSRTC and BRAF mutation status following pre-operative FNAC had a high risk of malignancy. Repeat US-guided FNAC for indeterminate thyroid nodules is highly recommended in clinical practice.
Keywords
B-Raf protein
Thyroid neoplasms
Pathological conditions
Fine-needle
Ultrasonography
INTRODUCTION
Thyroid cancer is the most common endocrine cancer and ranks 5th among malignant tumors in females, according to a recent report.[1] The global incidence of thyroid cancer is rapidly increasing, largely due to the availability of enhanced imaging screening. This rapid increase is also due to the increased incidence of papillary thyroid cancer (PTC), particularly nodules with a maximum diameter of 1 cm or less, named papillary thyroid microcarcinoma (PTMC).[2]
Ultrasonography is the primary imaging modality for thyroid nodules. The evaluation is mainly based on the thyroid imaging reporting and data system (TI-RADS) guidelines[3] which formulate the risk of malignancy in thyroid nodules after considering sonographic features, such as composition, echogenicity, shape, margin, echogenic foci, and blood flow. The TI-RADS guidelines were first proposed by Horvath et al. in 2009[4,5] and subsequently, several modified national TIRADS classification systems have been established to assist in stratifying the risk of malignancy of thyroid nodules.[6] It has been acknowledged that the TI-RADS guidelines provide clinicians with an easy-to-apply guide toward the accurate diagnosis and tailored treatment of thyroid nodules. US-guided pre-operative fine-needle aspiration cytology (FNAC) has been widely used for thyroid nodules, suspicious of malignancy because it is a rapid, simple, and less invasive diagnostic method.[7]
The interpretation of FNAC is mainly based on the Bethesda system for reporting thyroid cytopathology (BSRTC) guidelines, which has six diagnostic categories: BSRTC I - nondiagnostic (ND) or unsatisfactory (UNS), BSRTC II - benign, BSRTC III - atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS), BSRTC IV - follicular neoplasm (FN)/suspicious for an FN (SFN), BSRTC V - suspicious for malignancy (SM), and BSRTC VI – malignant.[8] BSRTC is a valuable scoring system for cytopathologists to quantitatively interpret the results of FNAC and assist surgeons in making clinical decisions. Although FNAC has been acknowledged to have good sensitivity, its poor specificity may limit its clinical value.[9] In a recent meta-analysis, the sensitivity and specificity of the BSTRC system were 97.0% and 50.7%, respectively.[10] This is because FNAC cannot provide a precise diagnosis for BSRTC categories I, III, IV, and V.[11] It was reported that 10–60% of thyroid nodules with indeterminate cytology are malignant.[12] Management of indeterminate nodules varies widely between follow-up, repeat FNAC, and surgical resection. Therefore, the uncertain diagnosis of thyroid nodules after FNAC poses a dilemma for clinicians.
Molecular testing provides additional information to the BSRTC system to facilitate the diagnosis of indeterminate thyroid nodules.[13,14] The B-Raf proto-oncogene (BRAF) mutation is the most common genetic alteration in thyroid nodules and plays an important role in tumorigenesis.[15] The incidence of a BRAF mutation among PTCs has been reported to be 30–80%,[16-18] while it is rarely present in benign thyroid nodules. Therefore, BRAF mutation analysis is an important complement to cytology and can significantly improve the diagnostic accuracy of FNAC.[16,19,20] However, in clinical practice, we found that there are conflicts between cytological and molecular results after FNAC, which have raised dilemmas for surgeons in determining appropriate surgical planning: (1) BSRTC I and positive for a BRAF mutation (I and BRAF+), (2) BSRTC II and positive for a BRAF mutation (II and BRAF+), (3) BSRTC III and positive for a BRAF mutation (III and BRAF+), and (4) BSRTC V and negative for a BRAF mutation (V and BRAF-).
In this study, with the primary purpose of studying the malignancy rate of the indeterminate thyroid nodules, we retrospectively analyzed the clinical data of 687 patients who underwent thyroid surgery at our hospital with one of the above-mentioned four conflicting cytological and molecular results during pre-operative FNAC. This is the first report of the four conflicting cytological and molecular results after FNAC.
MATERIAL AND METHODS
Patients
With the ethical approval of the Cancer Center (No. 050432-4-2018), the clinical and imaging data of patients who underwent US-guided FNAC between December 2020 and March 2023 at our hospital were retrieved from the archives. This study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients. The inclusion criteria were (1) pre-operative US examination of the thyroid gland at our hospital; (2) thyroid nodules, suspicious of malignancy on US; (3) maximum diameter of thyroid nodules on US <2 cm; and (4) FNAC with conflicting BSRTC and BRAF mutation status (I and BRAF+, II and BRAF+, III and BRAF+, and V and BRAF-). Patients were excluded if there were missing or incomplete data from US images, FNAC, and/or BRAF mutation status. A total of 1,044 patients with 1,044 thyroid nodules were enrolled in this study. Among them, 687 patients underwent surgical treatment at the cancer center, and histopathological examination of sections from paraffin-embedded specimens was performed. A flowchart of the patient selection process is shown in Figure 1. This study reports the histopathological results of 687 thyroid nodules, suspicious of malignancy on US, with an indeterminate cytological diagnosis after combining the BSTRC system and BRAF mutation status.
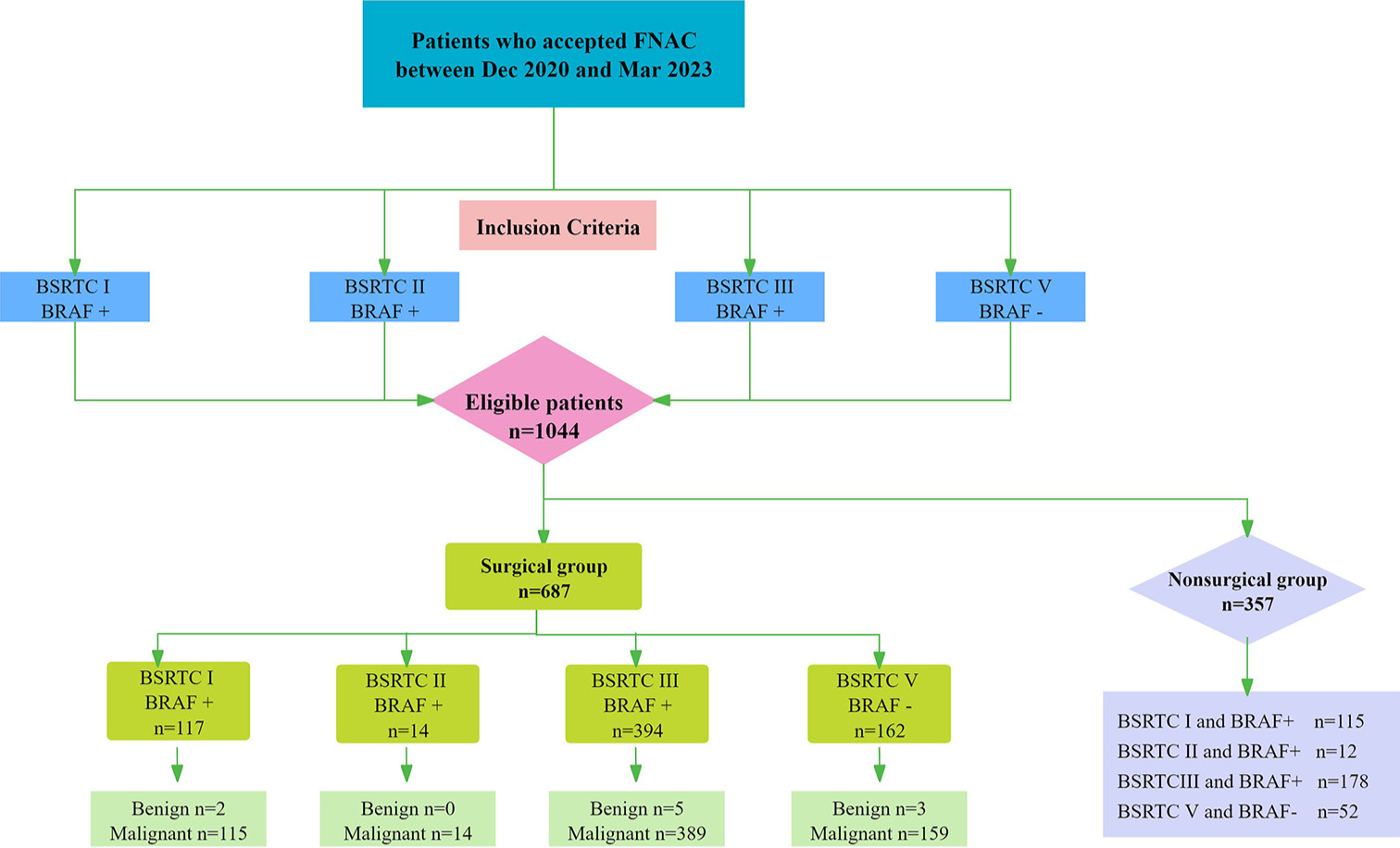
- Flow chart of patient selection. FNAC: Fine needle aspiration cytology, BSRTC: Bethesda system for reporting thyroid cytopathology, BRAF: B-Raf proto-oncogene, serine/threonine kinase.
US examination of the thyroid
US examination of the thyroid was conducted using a Logic E9 (GE Healthcare, Kretz, Zipf, Austria), EPIQ 7 (Philips Medical Systems, Bothell, WA, USA), Aixplorer (Supersonic Imaging, Aix-en-Provence, France), Aplio 500 (Toshiba Medical Systems, Japan), and Mylab XVISION (Esaote, Genoa, Italy) equipped with a 5-14 MHz linear-array transducer. Gray-scale images of the thyroid nodules were obtained after a thorough assessment of the thyroid gland. Thyroid nodules, suspicious of malignancy, were carefully assessed for the following characteristics: (1) maximum diameter on US, (2) ratio of tall to wide (equal/more than 1), (3) calcification (yes), and (4) multifocality on US (yes). Based on these sonographic features, the malignancy risk of thyroid nodules was categorized using the TI-RADS guidelines. At our cancer center, the TI-RADS category developed by Kwak et al. was adopted: [5] TI-RADS 3, no suspicious US features (malignancy: 1.7%); TI-RADS 4A, one suspicious US feature (malignancy: 3.3%); TI-RADS 4B, two suspicious US features (malignancy: 9.2%); TI-RADS 4C, three or four suspicious US features (malignancy: 44–72.4%); and TI-RADS 5, five suspicious US features (malignancy: 87.5%). Multifocality on US was defined as more than one solitary thyroid nodule, suspicious of malignancy, detected by a US physician.
US-guided FNAC
Head-and-neck surgeons recommend US-guided FNAC for thyroid nodules suspicious of malignancy on US. All patients provided written informed consent before undergoing FNAC. Standard 23-gauge injection needles were used to perform three to four passes on the target lesion; two passes for cytological diagnosis and one or two passes for molecular diagnosis. For multiple thyroid nodules, the most suspicious nodule was selected as the target lesion for FNAC. Specimens for cytological diagnosis were assessed and reported by a cytopathologist according to the BSTRC system.
The BSRTC categories
The BSRTC classifies the cytological features of thyroid nodules into six categories: (1) BSRTC I: ND/UNS, (2) BSRTC II: benign, (3) BSRTC III: AUS/FLUS, (4) BSRTC IV: FN/SFN, (5) BSRTC V: SM, and (6) BSRTC VI: Malignant.[8]
BRAF mutation analysis
BRAF mutations were assessed at the Department of Molecular Pathology using cellular samples from US-guided FNAC. Briefly, DNA from FNAC specimens was extracted, and real-time polymerase chain reaction (PCR) was performed on the BioRad- CFX96 real-time PCR system (Compact Fluorescence eXpression, Bio-Rad, Hercules, CA, USA, NO.20180701). BRAF mutation amplification and analysis were performed using specific primers (F: GCTAGCTCTGATAGGAAAATCAG and R: GTATCTCAGGAGCATCTCAGG, JN0060) and the human BRAF gene V600E mutation fluorescence PCR diagnostic kit (Amoy Diagnostics, Xiamen, China, NO.4485694). The carboxyfluorescein fluorescence signal (FAM) of the detection system indicated the BRAF mutation status. When the sample FAM Ct value was ≥27, the sample was determined to be negative; otherwise, the sample was considered mutation-positive.
The NGS analysis
A thyroid cancer multigene panel (RigenBio, Shanghai, China) was used to prepare sequencing libraries. The thyroid cancer NGS panel is a multiplex PCR-based test for genetic alterations of 26 genes related to thyroid cancer (Guanine nucleotide-binding protein, alpha stimulating [GNAS], Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha [PIK3CA], Enhancer of zeste homolog 1 [ EZH1], V-akt murine thymoma viral oncogene homolog 1 [AKT1], BRAF, KIT proto-oncogene [ KIT], [CTNNB1, Eukaryotic translation initiation factor 1A, X-linked [EIF1AX], Tumor protein 53 [TP53], 6-Neuro trophin receptor kinase [NTRK] 1, Fms-like tyrosine kinase [ FLT3], Harvey Rat Sarcoma Viral Oncogene Homolog [HRAS], Fibroblast Growth Factor Receptor [FGFR], Speckle-type POZ protein [SPOP], Kirsten ratsarcoma viral oncogene homolog [ KRAS], Neuroblastoma RAS viral oncogene homolog [NRAS], Gene of phosphate and tension homology deleted on chromosome ten [ PTEN], Rearranged during transformation proto-oncogene [RET], Checkpoint kinase 2 [CHEK2], Telomerase reverse transcriptase [TERT] promoter, Zinc finger protein 148 [ZNF148], Thyroid stimulating hormone receptor Gene [TSHR], Anaplastic lymphoma kinase [ALK], rearranged during transformation [ RET], NTRK3, and peroxisome proliferator activated receptor gamma [ PPARG]). Briefly, genomic DNA and total RNA extracted from sufficient fine-needle aspiration (FNA) samples were tested using the AllPrep DNA/RNA Kit (Qiagen, Germany, NO. 80244,) and quantified using a NanoDrop 2000 unit (Thermo Fisher Scientific, Wilmington, DE, USA). Total RNA was reverse-transcribed into complementary DNA. Target regions were amplified using multiplex PCR, and a unique index and universal adapter were added to each amplified library. The purified indexed libraries were quantified and size-checked using a Qubit fluorometer (Thermo Fisher Scientific, Wilmington, DE, USA, NO. Q33040) and an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). Subsequently, qualified libraries were sequenced on a NovaSeq 6000 platform (Illumina, CA, USA).[21]
Surgical treatment and histopathological reporting of thyroid nodules
All patients who participated in this study underwent a total thyroidectomy or lobectomy performed by experienced thyroid surgeons. Therapeutic central cervical lymph node (CCLN) dissection (Level VI) was routinely performed, and lateral cervical lymph node (LCLN) dissection (Levels II to V) was performed in cases of histopathologically diagnosed lymph node metastasis or suspicious lymph nodes detected on pre-operative imaging. All resected specimens were subjected to paraffin section-based histopathological examination and the diagnoses were confirmed by two board-certified histopathologists. Multifocality on histopathology was defined as more than one confirmed malignant nodule on post-surgical histopathology.
Statistical analysis
Data were classified as categorical or continuous. Categorical data are presented as frequency and percentage (%), and continuous numerical data are presented as mean and standard deviation or median and interquartile range (IQR), depending on data normality. The Chi-square (χ2) test or Fisher’s exact test was applied to compare categorical data. Statistical significance was set at P < 0.05. All statistical analyses were performed using the statistical package for the social sciences (SPSS) 23.0 (SPSS Inc., Chicago, IL, USA). The figure 1 was performed on WPS 365 software (Beijing, China).
RESULTS
Clinicopathological characteristics and sonographic features of the patients
A total of 1,044 patients, including 822 (78.7%) females and 222 (21.3%) males with conflicting cytological and BRAF mutation status results, were eligible. The median diameter of the thyroid nodules was 6 mm (IQR, 5–8 mm), and the median age of the patients was 46 years (IQR, 37–54 years). The clinicopathological characteristics and sonographic features of all patients are summarized in Table 1.
| Variables | Total (n=1044) | Non-surgical group (n=357) | Surgical group (n=687) |
|---|---|---|---|
| Gender | |||
| Female | 822 (78.7) | 285 (79.8) | 537 (78.2) |
| Age (years old) | |||
| <50 | 637 (61.0) | 220 (61.6) | 419 (60.8) |
| Size at US | |||
| <5 mm | 193 (18.5) | 77 (21.6) | 116 (16.9) |
| 5–10 mm | 720 (69.0) | 244 (68.3) | 476 (69.3) |
| 10–20 mm | 131 (12.5) | 36 (10.1) | 95 (13.8) |
| Ratio of tall to wide | |||
| ≥1 | 249 (23.9) | 91 (25.5) | 158 (23.0) |
| Calcification | |||
| Yes | 544 (52.1) | 170 (47.6) | 374 (54.4) |
| Multifocality at US | |||
| Yes | 278 (26.6) | 105 (29.4) | 173 (25.2) |
| TI-RADS | |||
| 4A | 591 (56.6) | 204 (57.1) | 387 (56.3) |
| 4B | 299 (28.6) | 95 (26.6) | 204 (29.7) |
| 4C | 141 (13.5) | 57 (16.0) | 84 (12.2) |
| 5 | 13 (13.2) | 1 (0.3) | 12 (1.7) |
| The Bethesda system and BRAF status | |||
| I and BRAF+ | 232 (22.2) | 115 (32.2) | 117 (17.0) |
| II and BRAF+ | 26 (2.5) | 12 (3.4) | 14 (2.0) |
| III and BRAF+ | 572 (54.8) | 178 (49.9) | 394 (57.4) |
| V and BRAF- | 214 (20.5) | 52 (14.5) | 162 (3.4) |
TI-RADS: Thyroid imaging reporting and data system, US: Ultrasound, BSRTC: Bethesda system for reporting thyroid cytopathology, I and BRAF+: BSRTC I and positive BRAF mutation, II and BRAF+: BSRTC II and positive BRAF mutation, III and BRAF+: BSRTC III and positive BRAF mutation, V and BRAF-: BSRTC V and negative BRAF mutation
Clinicopathological characteristics and sonographic features for patients in the surgical group
A total of 687 patients, including 537 females (78.2%) and 150 males (21.8%), underwent surgical treatment for thyroid nodules suspicious of malignancy. Table 2 shows the clinicopathological characteristics and sonographic features of these patients, categorized by the conflicting results for BSRTC and BRAF mutation status. The median diameter of the thyroid nodules was 6 mm (IQR, 5–8 mm), and the median age of the patients was 46 years (IQR, 37–53 years). Among them, 677 (98.5%) patients had histopathologically confirmed PTCs, including 585 PTMCs (86.4%). Only 10 (1.5%) were confirmed to be benign nodules, including two nodules in Group 1 [Figure 2a and b], five nodules in Group 3 [Figure 3a-e], and three nodules in Group 4 [Figure 4a-c]. The benign thyroid nodules included seven dysplastic, one fibrotic [ Figure 2b], one adenomatous [ Figure 3d] , and one hyperplastic [Figure 4c]. A total of 173 patients (25.2%) presented with multiple nodules, suspicious of malignancy on US, and post-surgery, and 170 patients (24.7%) had histopathologically confirmed multiple malignant tumors. CCLN metastasis was histopathologically confirmed in 179 patients (26.1%), and LCLN metastasis was confirmed in 46 patients (6.8%).
| Variables | Total (n=687) | I and BRAF+(n=117) | II and BRAF+(n=14) | III and BRAF+(n=394) | V and BRAF-(n=162) |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 537 (78.2) | 99 (84.6) | 10 (71.4) | 304 (77.2) | 124 (76.5) |
| Age (years old) | |||||
| < 50 | 417 (60.7) | 61(52.1) | 6 (42.9) | 251 (63.7) | 99 (61.1) |
| Size at US | |||||
| < 5 mm | 116 (16.9) | 21 (17.9) | 1 (7.1) | 80 (20.3) | 14 (8.6) |
| 5–10 mm | 476 (69.3) | 83 (71) | 13 (92.9) | 274 (69.5) | 106 (65.4) |
| 10–20 mm | 95 (13.8) | 13(11.1) | / | 40 (10.2) | 42 (26.0) |
| Ratio of tall to wide | |||||
| ≥1 | 158 (23) | 24 (20.5) | 2 (14.3) | 99 (25.1) | 33 (20.4) |
| Calcification | |||||
| Yes | 374 (54.4) | 60 (51.3) | 9 (64.3) | 213 (54.1) | 92 (56.8) |
| Multifocality at US | |||||
| Yes | 173 (25.2) | 36 (30.8) | 3 (21.4) | 108 (27.4) | 26 (16.0) |
| TI-RADS | |||||
| 4A | 387 (56.3) | 75 (64.1) | 11 (78.6) | 219 (55.6) | 82 (51.0) |
| 4B | 204 (29.7) | 31 (26.5) | 1 (7.1) | 125 (31.7) | 47 (29.0) |
| 4C | 84 (12.2) | 10 (8.5) | 2 (14.3) | 46 (11.7) | 26 (16.0) |
| 5 | 12 (1.8) | 1 (0.9) | / | 4 (1.0) | 7 (4) |
| Multifocality at pathology | |||||
| Yes | 170 (24.7) | 41 (35) | 3 (21.4) | 98 (24.9) | 28 (17.3) |
| Lymph node metastasis at the central neck | |||||
| Yes | 179 (26.1) | 29 (24.8) | 5 (35.7) | 102 (25.9) | 43 (26.5) |
TI-RADS: Thyroid imaging reporting and data system, US: Ultrasound, BSRTC: Bethesda system for reporting thyroid cytopathology, I and BRAF+: BSRTC I and positive BRAF mutation, II and BRAF+: BSRTC II and positive BRAF mutation, III and BRAF+: BSRTC III and positive BRAF mutation, V and BRAF-: BSRTC V and negative BRAF mutation
![Illustration of benign thyroid nodules with Bethesda system for reporting thyroid cytopathology I and positive BRAF mutation. Ultrasound of the neck reveals that: (a) A dysplasia nodule in the left lobe with the size of 10 × 7 × 8 mm (thyroid imaging reporting and data system [TI-RADS] 4B). (b) A fibrotic nodule in the right lobe with the size of 6 × 5 × 5 mm (TI-RADS 4A).](/content/105/2025/22/1/img/Cytojournal-22-1-g002.png)
- Illustration of benign thyroid nodules with Bethesda system for reporting thyroid cytopathology I and positive BRAF mutation. Ultrasound of the neck reveals that: (a) A dysplasia nodule in the left lobe with the size of 10 × 7 × 8 mm (thyroid imaging reporting and data system [TI-RADS] 4B). (b) A fibrotic nodule in the right lobe with the size of 6 × 5 × 5 mm (TI-RADS 4A).
![Illustration of benign thyroid nodules with Bethesda system for reporting thyroid cytopathology III and positive BRAF mutation. Ultrasound of the neck reveals that: (a) A dysplasia nodule in the left lobe with the size of 7 × 4 × 3 mm (thyroid imaging reporting and data system [TI-RADS] 4A). (b) A dysplasia nodule in the right lobe with the size of 15 × 10 × 10 mm (TI-RADS 4A). (c) A dysplasia nodule in the right lobe with the size of 4 × 4 × 5 mm (TI-RADS 4B). (d) An adenomatous nodule in the left lobe with the size of 19 × 9 × 14 mm (TI-RADS 4A). (e) A dysplasia nodule in the right thyroid with the size of 5 × 4 × 4 mm (TI-RADS 4A).](/content/105/2025/22/1/img/Cytojournal-22-1-g003.png)
- Illustration of benign thyroid nodules with Bethesda system for reporting thyroid cytopathology III and positive BRAF mutation. Ultrasound of the neck reveals that: (a) A dysplasia nodule in the left lobe with the size of 7 × 4 × 3 mm (thyroid imaging reporting and data system [TI-RADS] 4A). (b) A dysplasia nodule in the right lobe with the size of 15 × 10 × 10 mm (TI-RADS 4A). (c) A dysplasia nodule in the right lobe with the size of 4 × 4 × 5 mm (TI-RADS 4B). (d) An adenomatous nodule in the left lobe with the size of 19 × 9 × 14 mm (TI-RADS 4A). (e) A dysplasia nodule in the right thyroid with the size of 5 × 4 × 4 mm (TI-RADS 4A).
![Illustration of benign thyroid nodules with Bethesda system for reporting thyroid cytopathology V and negative BRAF mutation. Ultrasound of the neck reveals that: (a) A dysplasia nodule in the left lobe with the size of 4 × 5 × 5 mm (thyroid imaging reporting and data system [TIRADS] 4A). (b) A dysplasia nodule in the left thyroid with the size of 5 × 5 × 3 mm (TI-RADS 4A). (c) A hyperplastic nodule in the right thyroid with the size of 4 × 5 × 4 mm (TI-RADS 4C).](/content/105/2025/22/1/img/Cytojournal-22-1-g004.png)
- Illustration of benign thyroid nodules with Bethesda system for reporting thyroid cytopathology V and negative BRAF mutation. Ultrasound of the neck reveals that: (a) A dysplasia nodule in the left lobe with the size of 4 × 5 × 5 mm (thyroid imaging reporting and data system [TIRADS] 4A). (b) A dysplasia nodule in the left thyroid with the size of 5 × 5 × 3 mm (TI-RADS 4A). (c) A hyperplastic nodule in the right thyroid with the size of 4 × 5 × 4 mm (TI-RADS 4C).
The malignancy rate in the surgical group categorized by the conflicting results of BSRTC and BRAF status
The malignancy rates in Groups 1–4 were 98.3%, 100%, 98.9%, and 98.1% (P = 0.789), respectively. The malignancy rates for TI-RADS 4A, 4 B, 4C, and 5 were 98.4%, 98.5%, 98.8%, and 100%, respectively (P = 1.000). The malignancy rates according to the diameter of the nodule on US were 99.1%, 98.7%, and 96.8% (P = 0.278) for nodules <5 mm, 5–10 mm, and 10–20 mm, respectively. When combining TI-RADS 4A and nodule diameter <5 mm, there was a malignancy rate of 98.9% (88/89) [Table 3].[6]
| All patients | I and BRAF+ | II and BRAF+ | III and BRAF+ | V and BRAF- | |
|---|---|---|---|---|---|
| Overall (%) | 98.5 (677/687) | 98.3 (115/117) | 100 (14/14) | 98.7 (389/394) | 98.1 (159/162) |
| TI-RADS | |||||
| TI-RADS 4A (%) | 98.4 (381/387) | 98.7 (74/75) | 100 (11/11) | 98.6 (216/219) | 97.6 (80/82) |
| TI-RADS 4B (%) | 98.5 (201/204) | 96.8 (30/31) | 100 (1/1) | 98.4 (123/125) | 100 (47/47) |
| TI-RADS 4C (%) | 98.8 (83/84) | 100 (10/10) | 100 (2/2) | 100 (46/46) | 96.2 (25/26) |
| TI-RADS 5 (%) | 100 (12/12) | 100 (1/1) | / | 100 (4/4) | 100 (7/7) |
| P-value | 0.837 | 0.591 | / | 1.000 | 0.377 |
| Size at US | |||||
| <5 mm (%) | 99.1 (115/116) | 100 (21/21) | 100 (1/1) | 98.8 (79/80) | 100 (14/14) |
| 5–10 mm (%) | 98.7 (470/476) | 98.8 (82/83) | 100 (13/13) | 99.3 (272/274) | 97.2 (103/106) |
| 10–20 mm (%) | 96.8 (92/95) | 92.3 (12/13) | / | 95 (38/40) | 100 (42/42) |
| P-value | 0.278 | 0.242 | / | 0.067 | 0.664 |
| TI-RADS 4A and <5 mm (%) | 98.9 (88/89) | 100 (18/18) | 100 (1/1) | 98.3 (57/58) | 100 (12/12) |
TI-RADS: Thyroid imaging reporting and data system, US: Ultrasound, BSRTC: Bethesda system for reporting thyroid cytopathology, I and BRAF+: BSRTC I and positive BRAF mutation, II and BRAF+: BSRTC II and positive BRAF mutation, III and BRAF+: BSRTC III and positive BRAF mutation, V and BRAF-: BSRTC V and negative BRAF mutation
The rate of CCLN metastasis in the surgical group categorized by the conflicting results of BSRTC and BRAF status
The overall CCLN metastasis rate was 26.4% (179/677). The rates of CCLN metastasis in BSRTC Groups 1–4 were 25.2%, 35.7%, 26.2%, and 27.0%, respectively. There were no statistically significant differences between the four groups (P = 0.839). When categorizing patients according to TIRADS, the rates of CCLN metastasis for TI-RADS 4A, 4B, 4C, and 5 were 20.1%, 27.4%, 41%, and 83.3%, respectively, with significant differences (P < 0.001). According to the nodule diameter on US, the rate of CCLN metastasis was significantly different (P < 0.001) for nodules <5 mm (12.2%), 5–10 mm (26%), and 10–20 mm (46.7%), respectively. The rate of CCLN metastasis was 13.6% (12/88) when TIRADS 4A was combined with a nodule diameter of <5 mm [Table 4].[29]
| All patients | I and BRAF+ | II and BRAF+ | III and BRAF+ | V and BRAF- | |
|---|---|---|---|---|---|
| Overall (%) | 26.4 (179/677) | 25.2 (29/115) | 35.7 (5/14) | 26.2 (102/389) | 27.0 (43/159) |
| TI-RADS | |||||
| TI-RADS 4A (%) | 20.1 (80/381) | 36.4 (27/74) | 27.3 (3/11) | 20.8 (45/216)& | 18.8 (15/80) |
| TI-RADS 4B (%) | 27.4 (55/201) | 26.7 (8/30) | 100 (1/1) | 28.5 (35/123) | 23.4 (11/47) |
| TI-RADS 4C (%) | 41 (34/83)‡ | 30 (3/10) | 50 (1/20) | 41.3 (19/46) | 44 (11/25) |
| TI-RADS 5 (%) | 83.3 (10/12)# | 100 (1/1) | / | 75 (3/4) | 85.7 (6/7)# |
| P-value | <0.001 | 0.397 | / | <0.003 | <0.001 |
| Size at US | |||||
| <5 mm (%) | 12.2 (14/115)✶ | 14.3 (3/21) | 0 (0/1) | 10.1 (8/79)✶ | 21.4 (3/14) |
| 5–10 mm (%) | 26 (122/470)† | 26.8 (22/82) | 38.5 (5/13) | 26.1 (71/272)† | 23.3 (24/103) |
| 10–20 mm (%) | 46.7 (43/92) | 33.3 (4/12) | / | 60.5 (23/38) | 38.1 (16/42) |
| P-value | <0.001 | 0.451 | / | <0.001 | 0.195 |
| TI-RADS 4A and<5 mm (%) | 13.6 (12/88) | 11.1 (2/18) | 0 (0/1) | 12.3 (7/57) | 25 (3/12) |
Analysis of NGS testing
In the NGS-tested cohort, 12 other molecular alterations were detected in 20 thyroid nodules [Table 5]. Most tumors contained either a single mutation or no mutation. In addition to BRAF mutations, CCDC6-RET was the most commonly expressed mutation. No mutations were detected by NGS in the benign group.[34]
| Gene mutation | Total | PTCs | PTMCs |
|---|---|---|---|
| CCDC6-RET | 7 | 4 | 3 |
| ETV6-NTRK3 | 2 | 2 | 0 |
| TP53 | 3 | 1 | 2 |
| NRAS | 3 | 0 | 3 |
| GNAS | 1 | 0 | 1 |
| TERT | 1 | 0 | 1 |
| CD74-NRG1 | 1 | 0 | 1 |
| PIK3CA | 1 | 0 | 1 |
| CHEK2 | 1 | 0 | 1 |
| EGFR | 1 | 0 | 1 |
| SPOP | 1 | 0 | 1 |
| EIF1AX | 1 | 0 | 1 |
| Total | 23 | 7 | 16 |
NGS: Next-generation sequencing, CCDC6-RET: Coiled-coil domain containing 6-retproto-oncogene, ETV6-NTRK3: Ets variant gene 6-Neurotrophin receptor kinase 3, TP53: Tumor protein 53, NRAS: Neuroblastoma RAS viral oncogene homolog, GNAS: Guanine nucleotide-binding protein, alpha stimulating, TERT: Telomerase reverse transcriptase, CD74-NRG1: CD74-neuregulin 1, PIK3CA: Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, CHEK2: Checkpoint kinase 2, EGFR: Epidermal growth factor receptor, SPOP: Speckle-type POZ protein, EIF1AX: Eukaryotic translation initiation factor 1A, X-linked, PTCs: Papillary thyroid carcinomas, PTMCs: Papillary thyroid microcarcinomas
DISCUSSION
PTC is the most common type of malignant thyroid tumor. Pre-operative diagnosis mainly depends on the combined interpretation of cytopathology (BSRTC) and BRAF mutation status. However, the conflicting cytopathological results and BRAF mutation status (i.e., indeterminate cases) pose a significant challenge for surgical clinicians in making decisions for appropriate treatment of thyroid nodules, suspicious of malignancy, on US.[22] In this study, we summarized the clinical data of 1044 indeterminate thyroid nodules with the following four conditions in pre-operative US-guided FNAC: (1) I and BRAF+, (2) II and BRAF+, (3) III and BRAF+, and (4) V and BRAF-. A total of 687 patients underwent surgical treatment, with a malignancy rate of 98.5%, and the metastasis rate of the CCLNs was 26.1%. Even for patients categorized as TI-RADS 4A and with nodules <5 mm in diameter, which are deemed to be less likely to be malignant on US, the malignancy rate was 98.9%, and the metastasis rate of CCLNs was 13.6%. The malignancy rates in the four conflicting groups ranged from 98.1% to 100%. Nodule diameter and TI-RADS scores did not correlate with malignancy rates in the four conflicting groups. The rates of CCLN metastasis ranged from 25.2% to 35.7% in the four groups, but there were no statistical differences.
For I & BRAF+, II & BRAF+, and III & BRAF+, which indicate benign or inconclusive cytological findings but positive for a BRAF mutation, the malignancy rates ranged from 98.3% to 100%. The high malignancy rates of these three combinations of cytology and BRAF mutation status demonstrate that BRAF mutation analysis decreases the false-negative rate of FNAC and plays a vital role in identifying thyroid nodules with sonographic features that are suspicious of malignancy but with benign or inconclusive cytological findings. Numerous studies have evaluated the diagnostic utility of BRAF mutations in thyroid nodules with BSRTC I or BSRTC III and positive for a BRAF mutation.[23-25] These studies concluded that for such thyroid nodules, repeat FNAC with BRAF mutation testing is highly suggested. However, to the best of our knowledge, there is a lack of studies on thyroid nodules with BSRTC II and positive for a BRAF mutation, probably because BSRTC II is considered to be benign. Surprisingly, in this study, 14 thyroid nodules with BSRTC II and positive for BRAF mutation that were surgically resected were all diagnosed as malignant. Consistent with previous studies, Kim et al. revealed that 15 of 17 thyroid nodules with benign cytology results and positive for a BRAF mutation were confirmed to be malignant.[26] Chen et al. also found that among 36 thyroid nodules positive for a BRAF mutation and benign by cytology, 31 were confirmed to be malignant.[19] This further highlights that repeat FNAC is recommended for thyroid nodules with negative or benign FNAC findings and positive for a BRAF mutation.
Although a BRAF mutation is an important diagnostic molecular marker for PTC, the absence of a mutation of the gene cannot exclude malignancy. In this study, we found that 98.1% (159/162) of thyroid nodules that were negative for a BRAF mutation and with suspicious cytological findings were malignant. This is higher than the malignancy rate of 85.7% (42/49) reported in the literature.[20] Perhaps this difference may be because our hospital is a cancer center with a high referral rate, which may increase the proportion of nodules that are malignant. Another important implication of these results is that the three benign thyroid nodules from the 162 nodules deserve attention. For thyroid nodules with BSRTC V cytology and negative for a BRAF mutation, repeat FNAC and BRAF mutation testing are also recommended to avoid unnecessary surgery for benign cases.
The malignancy rate of these indeterminate thyroid nodules was further analyzed based on the diameter of the thyroid nodule and TI-RADS on US. Unexpectedly, the TI-RADS category had no effect on the malignancy rates of these indeterminate nodules. US is a sensitive and reliable imaging modality for the differentiation of thyroid nodules. All nodules suspected of being malignant on US, with conflicting cytological and genetic results, should be recommended for repeat FNAC. The malignancy rate in thyroid nodules 10– 20 mm in diameter appeared to be lower than that in nodules <5 mm and 5–10 mm in diameter, but the difference was not statistically significant (P > 0.05). In recent years, numerous studies have explored the association between thyroid nodule diameter and the malignancy rate in thyroid cancer, but the association remains controversial.[27,28]
The metastasis rate of CCLNs was 26.4%, with no statistically significant differences among the four indeterminate groups. However, the rate of CCLN metastasis increases with increased thyroid nodule diameter and higher TI-RADS category. This is consistent with previous studies showing that a larger tumor diameter and higher TI-RADS category are associated with CCLN metastasis, which may indicate a heavier tumor burden and greater invasiveness.[29-31] Therefore, it is important to determine the pathological nature of thyroid nodules, suspicious of malignancy on US, as they may carry the risk of CCLN metastasis, which is not visible on US examination.
In recent years, NGS, which analyzes polygene expression, has shown clinical efficacy in the diagnosis of malignant thyroid tumors.[32,33] In 2014, the Cancer Genome Atlas project published the genomic datasets of PTC, describing the most comprehensive gene alterations, including point mutation, fusion genes, and somatic copy alterations.[34] In this study, 12 other genetic mutations were identified in 20 thyroid nodules. According to previous studies, alterations in genes including CHEK2, TERT, GNAS, NRAS, PIK3CA, RET, NTRK1, EIF1AX, TP53, and epidermal growth factor receptor were also associated with malignant thyroid nodules.[21,35-37] However, the SPOP mutation was usually detected in benign thyroid nodules.[38] The number of patients who underwent NGS was insufficient for statistical analysis, thus, a related study is expected to be performed in the future.
The limitations of this study should be considered when interpreting the results. First, the malignancy rate for indeterminate cases was expected to be overestimated, considering the high incidence of malignancy at the cancer center. Second, the results were considered to be biased because of the low number of cases in the BSRTC II and BRAF+ categories. Third, the results were obtained from a single center; therefore, a multicenter study is warranted. Finally, there were no data for the repeat FNAC. A prospective study in cooperation with surgeons is planned where repeat FNAC for indeterminate cases will be suggested.
SUMMARY
For thyroid nodules suspicious of malignancy on US among indeterminate cases with I and BRAF+, II and BRAF+, III and BRAF+, and V and BRAF-, there was a high probability of malignancy even for thyroid nodules with TI-RADS 4A and nodules <5 mm in diameter on US. These indeterminate thyroid nodules, suspicious of malignancy on US may carry the risk of CCLN metastasis, which is not visible on US. Repeated FNAC is recommended for conflicting cytology results and BRAF mutation status.
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
BSRTC: Bethesda system for reporting thyroid cytopathology
BRAF: B-Raf proto-oncogene, serine/threonine kinase
FNAC: Fine-needle aspiration cytology
US: Ultrasound
TI-RADS: Thyroid imaging reporting and data system
PTC: Thyroid cancer
PTMC: Papillary thyroid microcarcinoma
CCLN: Central cervical lymph node
LCLN: Lateral cervical lymph node
PCR: Polymerase chain reaction
NGS: Next generation sequencing
IQR: Interquartile range
CFX96: Compact Fluorescence eXpression
GNAS: Guanine nucleotide-binding protein, alpha stimulating
PIK3CA: Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
EZH1: Enhancer of zeste homolog 1
AKT1: V-akt murine thymoma viral oncogene homolog 1
KIT: KIT proto-oncogene
EIF1AX: Eukaryotic translation initiation factor 1A, X-linked
TP53: Tumor protein 53
NTRK: Neuro trophin receptor kinase
FLT3: Fms-like tyrosine kinase
HRAS: Harvey Rat Sarcoma Viral Oncogene Homolog
FGFR: Fibroblast Growth Factor Receptor
SPOP: Speckle-type POZ protein
KRAS: Kirsten ratsarcoma viral oncogene homolog
NRAS: Neuroblastoma RAS viral oncogene homolog
PTEN: Gene of phosphate and tension homology deleted on chromosome ten
RET: Rearranged during transformation proto-oncogene
CHEK2: Checkpoint kinase 2
TERT: Telomerase reverse transcriptase promoter
ZNF148: Zinc finger protein 148
TSHR: Thyroid stimulating hormone receptor Gene
ALK: Anaplastic lymphoma kinase
PPARG: Peroxisome proliferator activated receptor gamma
AUTHOR CONTRIBUTIONS
XQM, RQH, and MDZ: Drafted the concept; XQM and RQH: Wrote major parts; JYC, KZ, and JWL: Provided critical feedback and revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENT
All clinicians involved in ultrasound image collection, fine needle aspiration, cytological interpretation, genetic test, thyroid surgery, and histological report are appreciated.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study involving human participants was reviewed and approved by Ethics Committee of Fudan University Shanghai Cancer Center (Approval No. 050432-4-2018), dated Jan 12, 2024. The informed consent was obtained from all patients before fine-needle aspiration cytology and surgical resection. The study was conducted in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63.
- [CrossRef] [PubMed] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid imaging reporting and data system (TI-RADS): A user's guide. Radiology. 2018;287:1082.
- [CrossRef] [PubMed] [Google Scholar]
- An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748-51.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology. 2011;260:892-9.
- [CrossRef] [PubMed] [Google Scholar]
- ACR Thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587-95.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration in the investigation of thyroid nodules. Dtsch Arztebl Int. 2016;113:353-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159-65.
- [CrossRef] [PubMed] [Google Scholar]
- Implementation of the Bethesda system for reporting the thyroid cytopathology: Study on 5729 cases from a cancer center. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55:258-62.
- [Google Scholar]
- The Bethesda system for reporting thyroid cytopathology: A meta-analysis. Acta Cytol. 2012;56:333-9.
- [CrossRef] [PubMed] [Google Scholar]
- Approach to cytological indeterminate thyroid nodules. Gland Surg. 2019;8:S98-104.
- [CrossRef] [PubMed] [Google Scholar]
- The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27:1341-6.
- [CrossRef] [PubMed] [Google Scholar]
- High diagnostic accuracy of epigenetic imprinting biomarkers in thyroid nodules. J Clin Oncol. 2023;41:1296-306.
- [CrossRef] [PubMed] [Google Scholar]
- Decision making in indeterminate thyroid nodules and the role of molecular testing. Surg Clin North Am. 2019;99:587-98.
- [CrossRef] [PubMed] [Google Scholar]
- The Raf/MEK/ERK pathway: New concepts of activation. Biol Cell. 2001;93:53-62.
- [CrossRef] [PubMed] [Google Scholar]
- BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-62.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: A study of 1,587 patients. Cancer Biol Med. 2019;16:121-30.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of molecular testing in fine-needle aspiration biopsy samples: An experience in a Chinese population. Exp Mol Pathol. 2014;97:292-7.
- [CrossRef] [Google Scholar]
- Value of BRAF V600E in high-risk thyroid nodules with benign cytology results. AJNR Am J Neuroradiol. 2018;39:2360-5.
- [CrossRef] [PubMed] [Google Scholar]
- The role of BRAFV600E mutation and ultrasonography for the surgical management of a thyroid nodule suspicious for papillary thyroid carcinoma on cytology. Ann Surg Oncol. 2009;16:3125-31.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic performance of next-generation sequencing and genetic profiling in thyroid nodules from a single center in China. Eur Thyroid J. 2022;11:e210124.
- [CrossRef] [PubMed] [Google Scholar]
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- BRAF (V600E) vs. TIRADS in predicting papillary thyroid cancers in Bethesda system I, III, and V nodules. Cancer Biol Med. 2019;16:131-8.
- [CrossRef] [PubMed] [Google Scholar]
- The value of Korean, American, and Chinese ultrasound risk stratification systems combined with BRAF (V600E) mutation for detecting papillary thyroid carcinoma in cytologically indeterminate thyroid nodules. Endocrine. 2024;84:549-59.
- [CrossRef] [PubMed] [Google Scholar]
- BRAF(V600E) mutation test on fine-needle aspiration specimens of thyroid nodules: Clinical correlations for 4600 patients. Cancer Med. 2022;11:40-9.
- [CrossRef] [PubMed] [Google Scholar]
- What to do with thyroid nodules showing benign cytology and BRAF(V600E) mutation? A study based on clinical and radiologic features using a highly sensitive analytic method. Surgery. 2015;157:354-61.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid. 2011;21:1191-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association between thyroid nodule size and malignancy rate. Ann R Coll Surg Engl. 2020;102:43-8.
- [CrossRef] [PubMed] [Google Scholar]
- Using ultrasonographic features to predict the outcomes of patients with small papillary thyroid carcinomas: A retrospective study implementing the 2015 ATA patterns and ACR TI-RADS categories. Ultrasonography. 2022;41:298-306.
- [CrossRef] [PubMed] [Google Scholar]
- A nomogram for predicting lateral lymph node metastasis in cN0 unifocal papillary thyroid microcarcinoma. BMC Cancer. 2023;23:718.
- [CrossRef] [PubMed] [Google Scholar]
- Lateral lymph node metastasis in papillary thyroid microcarcinoma: A study of 5241 follow-up patients. Endocrine. 2024;83:414-21.
- [CrossRef] [PubMed] [Google Scholar]
- Next-generation sequencing in thyroid cancer. J Transl Med. 2016;14:322.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: A randomized clinical trial. JAMA Oncol. 2021;7:70-7.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676-90.
- [CrossRef] [PubMed] [Google Scholar]
- TERT accelerates BRAF mutant-induced thyroid cancer dedifferentiation and progression by regulating ribosome biogenesis. Sci Adv. 2023;9:eadg7125.
- [CrossRef] [PubMed] [Google Scholar]
- Differences in cancer phenotypes among frequent CHEK2 variants and implications for clinical care-checking CHEK2. JAMA Oncol. 2022;8:1598-606.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermal growth factor receptor overexpression is a marker for adverse pathologic features in papillary thyroid carcinoma. J Surg Res. 2013;185:217-24.
- [CrossRef] [PubMed] [Google Scholar]
- The genetic landscape of benign thyroid nodules revealed by whole exome and transcriptome sequencing. Nat Commun. 2017;8:15533.
- [CrossRef] [PubMed] [Google Scholar]








