Translate this page into:
Hypoxia-induced S-phase kinase-interacting protein 2 knockdown repressed the progression of melanoma through extracellular signal-regulated kinase 1/2 pathway

*Corresponding author: Yong Hu, Department of Dermatology and Venereal Diseases, Yihe Women’s and Children’s Hospital, No.111 Jingshun Road, Chaoyang District, Beijing, China. ayong.yong@163.com
-
Received: ,
Accepted: ,
How to cite this article: Hu Y. Hypoxia-induced S-phase kinase-interacting protein 2 knockdown repressed the progression of melanoma through extracellular signal-regulated kinase 1/2 pathway. CytoJournal. 2025;22:9. doi: 10.25259/Cytojournal_117_2024
Abstract
Objective:
Hypoxia intensely drives the development of malignant tumors, including skin cutaneous melanoma (SKCM). S-phase kinase-interacting protein 2 (SKP2) is known to participate in the progression of human tumors. The purpose of this study is to explore whether SKP2 acts as a hypoxic response gene during SKCM progression.
Material and Methods:
SKP2 expression in SKCM tissues was analyzed using The Cancer Genome Atlas database. Anoxic experiments were conducted to simulate an anoxic environment. 5-Ethynyl-2'-deoxyuridine and colony formation assays were used to evaluate SKCM cell growth. Scratch healing and Transwell assays were applied to measure the migration and invasion abilities of SKCM cells. An immunoblotting assay was used to detect the levels of extracellular signal-regulated kinase (ERK)1/2 pathway proteins. In addition, the ERK-specific agonist LM22B-10 was added to confirm whether the ERK1/2 signaling pathway is required for SKP2-mediated SKCM progression under hypoxic conditions.
Results:
SKP2 was significantly upregulated in SKCM tissues and closely related to adverse outcomes in patients. Moreover, SKP2 levels increased in SKCM cells under normoxic conditions and further elevated under hypoxic conditions. SKP2 deficiency led to the reduced proliferation, migration, and invasion potential of cells under hypoxic conditions. Mechanically, SKP2 silencing blocked the ERK1/2 pathway in hypoxic cells, and the activation of the ERK1/2 pathway rescued the suppression effect of SKP2 on the hypoxia-induced progression of SKCM.
Conclusion:
SKP2 deficiency repressed the hypoxic-induced progression of SKCM through the ERK1/2 pathway. This novel discovery regarding the SKP2/ERK1/2 axis might provide new insights into the pathogenesis of SKCM.
Keywords
Cutaneous melanoma
S-phase kinase-interacting protein 2
Hypoxia
Extracellular signal-regulated kinase 1/2
INTRODUCTION
Skin cutaneous melanoma (SKCM) is a type of malignant tumor that originates from epidermal normal melanocytes; it features high malignancy, rapid development, and poor prognosis.[1] Over the past decade, the incidence of SKCM has been on the rise, with SKCM once becoming the malignancy with the fastest-growing incidence worldwide.[2,3] Although China has a relatively low incidence of SKCM, 8,000–10,000 new cases of SKCM are reported in China annually, and the annual growth rate of SKCM cases in the country is 3–5%.[4] In contrast to other neoplastic disorders, SKCM still lacks efficacious treatment. Although the early diagnosis of SKCM enhances patient outcomes, the 5-year survival rate for advanced SKCM remains low.[5] Therefore, further exploring the pathogenesis of SKCM and searching for new potential biomarkers and targets must be regarded as an urgent matter that will provide a basis for improving the prognosis of SKCM.
Hypoxia is one of the most important and crucial microenvironmental characteristics in the majority of solid tumor tissues.[6,7] Previous studies have shown that the degree of hypoxia is associated with the malignancy degree of tumors.[8,9] Furthermore, hypoxia is an independent prognostic factor for human cancers, resulting in a poor prognosis for patients with tumors.[10,11] Other studies have revealed that hypoxia is predominantly involved in biological processes, such as cell proliferation and metabolism, glycolysis, and immune response, as well as tumorigenesis and metastasis.[12] These conclusions imply that hypoxia may play an important role in the mechanisms of SKCM tumorigenesis. Therefore, the further exploration of the role of hypoxia in SKCM and its molecular mechanism may offer novel approaches and targets for the diagnosis and treatment of SKCM.
S-phase kinase-interacting protein (SKP2), also referred to as p45 or F-box with leucine-rich amino acid repeats 1, is a member of the F-box family.[13] F-box family proteins, which contain at least one F-box domain and were initially identified in cyclin F, are a component of the SKP1–cullin–Fbox ubiquitin-protein ligase complex, within which SKP2 acts as a substrate recognition factor to regulate the cell cycle, cell proliferation, and transcription by degrading cyclin.[14,15] Since the discovery of SKP2 in 1995, its functional role in tumor formation and development has been gradually elucidated.[16] SKP2 has been demonstrated to be upregulated in multiple tumors, such as lymphoma, prostate cancer, breast cancer, and lung cancer, and positively modulates the progression of tumors.[17-20] A previous study discovered that SKP2 was elevated in SKCM cells and its knockdown blocked the viability and invasion of SKCM cells.[21] However, the molecular details of the role of SKP2 in cell growth and metastasis remain unclear. Notably, a previous study demonstrated that hypoxia-inducible factor 1-alpha modulated SKP2 expression in liver cancer,[22] suggesting that a hypoxic microenvironment may play a critical role in SKP2-regulated SKCM development. This finding provides a basis for exploring the role of SKP2 in SKCM and its relationship with hypoxia.
The present study investigated the effects of SKP2 on cell biological function and the relationship between SKP2 and the extracellular signal-regulated kinase (ERK)1/2 pathway in hypoxia-treated melanoma cells. Its overall objective is to gain a deepened understanding of the regulatory role of SKP2 in SKCM cell proliferation, migration, and invasion under hypoxic conditions.
MATERIAL AND METHODS
Online database analysis
SKP2 levels in SKCM tissues were downloaded for Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/detail.php?clicktag=degenes). GEPIA and PrognoScan database were applied to quantify the relationship of SKP2 with the prognosis of patients with SKCM.
Cell culture
Human epidermal melanocytes (HEM; cat. no. CP-H108) and the human SKCM cell lines A-375 (cat. no. CL-0014), A-875 (cat. no. CL-0255), SK-MEL-28 (cat. no. CL-0717), and SK-MEL-1 (cat. no. CL-0440) were all acquired from Priscilla (Wuhan, China) and subjected to mycoplasma testing. Short tandem repeat showed that the cells lacked cross-contamination.
HEM cells were cultured in Ham’s F10 medium (cat. no. PM151110A; Prisilla, Wuhan, China) containing 20% fetal bovine serum (FBS; cat. no. 164210; Prisilla, Wuhan, China) and 1% penicillin/streptomycin, and SK-MEL-28 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (cat. no. PM150312B; Prisilla, Wuhan, China) supplemented with 20% FBS and 1% penicillin/streptomycin. The cells were cultured in a cell incubator with 95% humidity and 5% CO2 at a temperature of 37℃. In addition, anoxic experiments were conducted to simulate an anoxic environment. The cells were inoculated into a six-well plate at a density of 1 × 105 cells/well and cultured under hypoxic conditions (5% CO2 and 1% O2) (cat. no. 3307E; Thermo Fisher Scientific, Waltham, MA, the USA) with nitrogen equilibrium at 37℃ for 24 h.
Lentiviral transfection
In accordance with the procedure of a previous study, HIV-based packaging mix (GenePharma, Shanghai, China) was used to package SKP2 lentiviral small interfering RNA (si-RNA) to infect SKCM cells for knockdown, thereby establishing stable SKP2 knockdown cell lines. The specific primer sequences were as follows: si-SKP2-1, 5'-GCCTAAGCTAATCGAGAGA-3'; siSKP2-2, 5'-CCATTGTCAATACTCGCAA-3'; and si-NC, 5'-UUCUCCGAACGUGUCACGUTT-3'. Immunoblotting and quantitative real-time polymerase chain reaction (qRTPCR) were applied to detect SKP2 expression, and cells with stable SKP2 expression were screened.
qRT-PCR
After digestion by pancreatic enzymes (cat. no. PYG0107; BOSTER, Wuhan, China), cells with good growth were washed with phosphate-buffered solution (PBS) then centrifuged at 3000 rpm for 15 min at 4℃. TRIzol® reagent (cat. no. 12183555; Thermo Fisher Scientific, Waltham, MA, the USA) was used to extract total RNA. Subsequently, 20 μL of diethylpyrocarbonate water was added to prevent RNA degradation. RNA reverse transcription was performed by following the instructions included with the RNA reverse transcription kit (cat.no. 11141ES60; Yeasen Biotechnology, Shanghai, China). The quantitative polymerase chain reaction (qPCR) kit Hieff® qPCR SYBR Green Master Mix (cat. no. 11204ES08; Yeasen Biotechnology, Shanghai, China) was then used for qPCR. The specific primer sequences were as follows: SKP2 forward, 5'-ATCTTAGCGGCTACAGAAAG-3', SKP2 reverse, 5'-GAGGTAGTTGAGCTGGAAAA-3' and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5'-TGCACCACCAACTGCTTAGC-3', GAPDH reverse, 5'-GGCATTGACTGTGGTCATGAG-3'. GAPDH was used as an endogenous control for normalization. Data were analyzed using the 2ΔΔCq method.
Immunoblotting assay
SKCM cells were lysed with radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime, Beijing, China) containing protease inhibitors to extract total protein and protein quantification was performed using the bicinchoninic acid method (cat. no. ST2222-1g; Beyotime). Next, 10% sodium dodecyl sulfate–polyacrylamide gel was prepared and placed in a 4℃ refrigerator. On the next day, 20 μg of protein samples was added to gel for electrophoresis then transferred to polyvinylidene fluoride (PVDF) membranes (cat. no. G6015; Servicebio, Wuhan, China). Afterward, the membranes were blocked with 5% milk for 1 h and placed in the corresponding dilutions of the primary antibodies of ERK1/2 (1:1000; cat. no. ab184699; Abcam, Cambridge, the UK) and p-ERK1/2 (1:1000; cat. no. ab126445; Abcam) and incubated at 4℃ overnight. The membranes were then placed in secondary antibodies (1:3000; cat. no. ab6728; Abcam) at room temperature for 2 h. Finally, the PVDF membranes were uniformly covered with enhanced chemiluminescence liquid (cat. no. P0018HS; Beyotime) in the darkroom for exposure. Proteins were quantitatively analyzed by ImageJ software (version 6.0; Media Cybernetics, Shanghai, China).
5-Ethynyl-2'-deoxyuridine (EdU) assay
The cells were inoculated into 96-well plates at a density of 1 × 104 cells per well for 48 h. After 24 h of transfection, the cells were added with an appropriate amount of EdU for incubation for 2 h. EdU and 4',6-diamidino-2-phenylindole staining methods were performed in accordance with the instructions of the EDU-555 cell proliferation detection kit (cat. no. C0071S; Beyotime). Images of each group were acquired using a fluorescence microscope (Olympus Corporation, Tokyo, Japan), and the number of EdU-positive cells was quantized using ImageJ software.
Colony formation assay
The cells were inoculated into six-well plates at a density of 50 cells/well. After 24 h of transfection, the medium containing transfection reagents was discarded and the cells were incubated with Iscove’s modified Dulbecco’s medium (cat. no. PM150510B; Prisilla, Wuhan, China) mixed with 10% extra FBS for 3 weeks, during which the medium was changed every 2 days. Subsequently, the medium was discarded. After rinsing, fixation, staining with crystal violet (cat. no. C0121; Beyotime, Shanghai, China), and rinsing again, the colonies of each group were observed under an optical microscope (Olympus Corporation, Tokyo, Japan) and photographed to calculate the number of colonies formed.
Transwell assay
Transwell chambers were precoated with Matrigel. The cells were digested using trypsin. Subsequently, serum-free medium was added to suspend the cells, and the cell concentration was adjusted to 5 × 105 cells/mL. A total of 200 μL of the cell suspension were added to the upper chamber of each Transwell plate, and Roswell Park Memorial Institute-1640 medium (cat. no. PM150110B; Priscilla, Wuhan, China) containing serum was added to the lower chamber. The cells in the Transwells were incubated in an incubator for 24 h. After being washed with PBS, the cells were removed from the Transwells and fixed in paraformaldehyde for 10 min. After the cells were stained with 0.1% crystal violet solution for 10 min, five visual fields were selected to observe the number of invading cells using an optical microscope (Olympus Corporation, Tokyo, Japan) and analyzed with ImageJ software (version 1.46, National Institutes of Health).
Scratch healing assay
A marker was employed to draw lines evenly on the back of a 24-well plate at an interval of 0.5–1.0 cm with at least five lines across each well. The transfected cells were inoculated into 24-well plates, each containing 1 × 105 cells, ensuring they were incubated overnight. On the 2nd day, the tip of a pipette was used to create a scratch. The scratched cells were added with serum-free medium then cultured in a 5% CO2 incubator at 37℃. Scratch healing was observed with an optical microscope (Olympus Corporation, Tokyo, Japan), and the cells were analyzed using Image J software. Cell scratch healing rate = (0 h scratch area – 24 h scratch area)/0 h scratch area × 100%.
Statistical analysis
The Statistical Package for the Social Sciences 21.0 statistical software (IBM, Armonk, NY, the USA) was utilized for statistical analysis, and the results were presented as mean ± standard deviation. Student’s t-test was applied to compare differences between two groups. Significant differences among multiple groups were determined through oneway analysis of variance followed by Tukey’s post hoc test. P < 0.05 was regarded as a significant difference. All experiments were conducted in triplicate in at least three independent experiments.
RESULTS
High expression of SKP2 was observed in SKCM tissues and cells
We first evaluated SKP2 expression in SKCM tissues from The Cancer Genome Atlas using the GEPIA website to assess the effect of SKP2 on SKCM progression. The results in Figure 1a revealed a remarkable increase in SKP2 expression in SKCM tissues relative to that in normal tissues. A Kaplan–Meier curve was established to analyze the correlation of SKP2 expression with prognosis. The results of GEPIA and the PrognoScan database revealed that overall survival was better in patients with SKCM and low SKP2 expression than in those with high SKP2 expression [Figure 1b and c]. Subsequently, the expression pattern in SKCM cells was validated using qRT-PCR. As presented in Figure 1d, SKP2 expression in SKCM cells was significantly upregulated compared with that in HEM cells. These data indicate that the high expression of SKP2 was closely related to SKCM development.
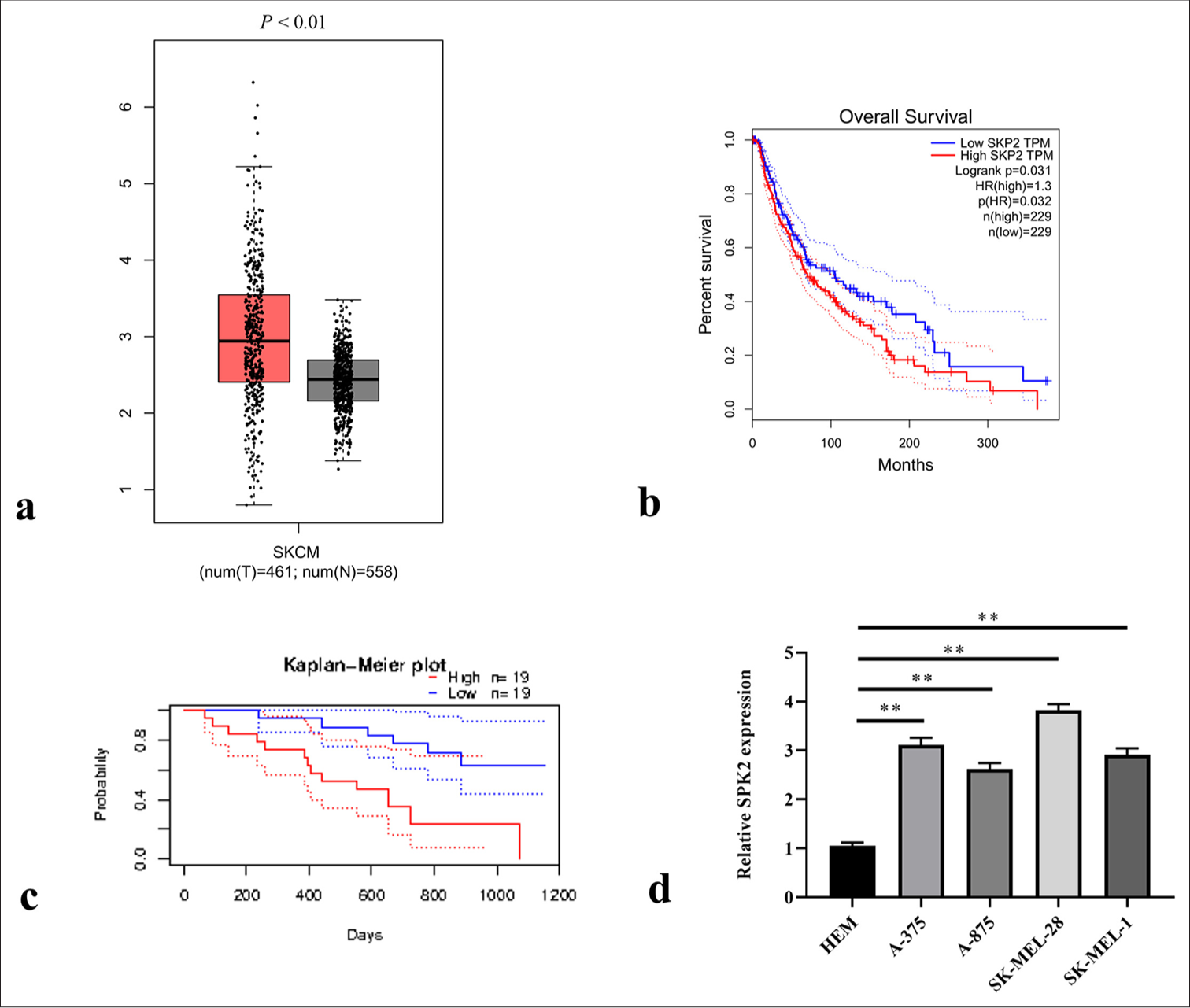
- SKP2 was elevated in skin cutaneous melanoma SKCM tissues and cells. (a) SKP2 expression in SKCM tissues from The Cancer Genome Atlas was analyzed using GEPIA. (b) Overall survival rates of patients with SKCM were compared between the high and low SKP2 groups using GEPIA and (c) the PrognoScan database. (d) SKP2 expression pattern in SKCM cells. ✶✶P < 0.01. SKCM: Skin cutaneous melanoma, SKP2: S-phase kinase-interacting protein 2, GEPIA: Gene expression profiling interactive analysis.
SKP2 knockdown inhibited the hypoxia-induced proliferation, migration, and invasion of SKCM cells
Hypoxia is an important feature of most solid tumors and promotes the malignant progression of tumors by activating signaling pathways in tumor cells. Therefore, we investigated the regulatory role of SKP2 in SKCM development under hypoxic conditions. We applied qRT-PCR to detect SKP2 expression in SKCM cell lines, and the results showed that SKP2 was elevated in SKCM cells under hypoxia treatment [Figure 2a], suggesting that hypoxia treatment caused an increase in SKP2 levels in SKCM cells. SK-MEL-28 cells with relatively high levels of SKP2 were selected for further studies. The cells were transfected with si-SKP2-1 or siSKP2-2 before hypoxia treatment. si-SKP2-1 was selected for subsequent experiments due to its relatively high silencing efficiency [Figure 2b]. Immunoblotting assay discovered the low expression of SKP2 in si-SKP2 SK-MEL-28 cells under normoxic and hypoxic conditions [Figure 2c]. We next evaluated the effects of SKP2 on the proliferation of SKCM cells. As exhibited in Figure 2d and e, the cells treated with hypoxia had stronger proliferation potentials than those treated with normoxia. However, SKP2 knockdown markedly repressed cell formation under normoxic and hypoxic conditions. The EdU assay demonstrated that the number of EdU-positive cells dramatically increased under hypoxic conditions, and the knockdown of SKP2 markedly blocked EdU-positive cells under normoxic and hypoxic conditions [Figure 2f and g]. Furthermore, the scratch healing assay revealed a significant increase in the wound healing rate of SK-MEL-28 cells under hypoxic conditions, whereas the silencing of SKP2 remarkably decreased wound healing rates [Figure 2h and i]. Similarly, the ability of SK-MEL-28 cells to invade enhanced under hypoxic conditions, and SKP2 knockdown mitigated these events [Figure 2j and k]. These results show that SKP2 knockdown attenuated the proliferation and metastasis of SKCM cells under hypoxia treatment.
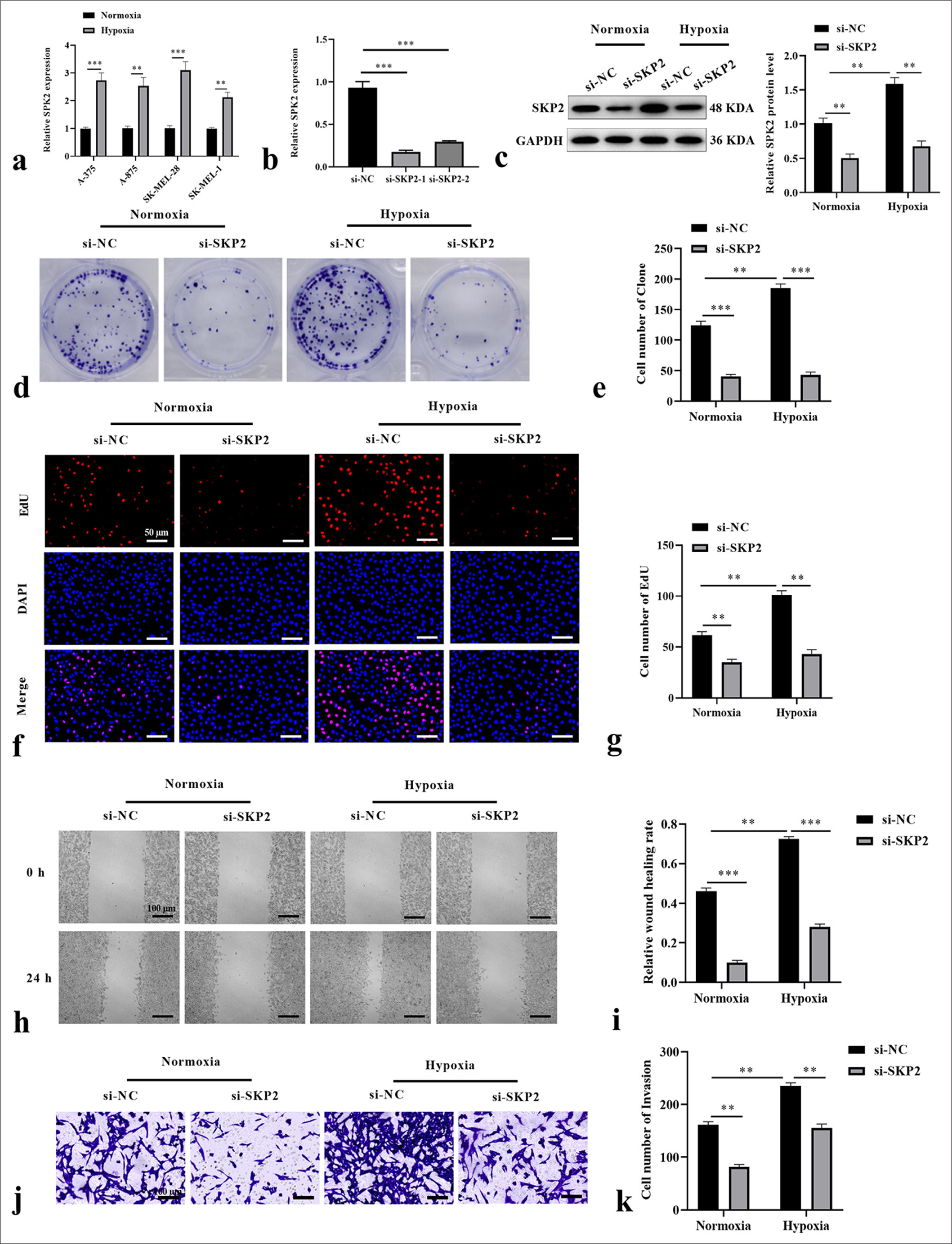
- SKP2 knockdown inhibited the hypoxia-induced proliferation, migration, and invasion of SKCM cells. SK-MEL-28 cells were treated with si-SKP2 before hypoxia treatment. (a) SKP2 expression in SKCM cell lines under hypoxia treatment. (b) SKP2 expression after transfection with si-SKP2-1 or si-SKP2-2. SKP2 level, cell formation number, EdU-positive cells, wound healing rate, and cell invasion were measured by (c) immunoblotting assay, (d and e) colony formation assay, (f and g) EdU assay (scale = 50 μm), (h and i) wound healing assay (scale = 100 μm), and (j and k) Transwell assay (scale = 100 μm), respectively. ✶✶P < 0.01; ✶✶✶P < 0.001. SKCM: Skin cutaneous melanoma, SKP2: S-phase kinase-interacting protein 2, si-SKP2: Small interfering S-phase kinase-interacting protein 2, EdU: 5-Ethynyl-2’-deoxyuridine.
SKP2 knockdown blocked the activation of the ERK1/2 pathway in hypoxia-treated SKCM cells
Considering that the inhibition of SPK2 has been shown to block VEGF-induced ERK1/2 phosphorylation and that the ERK1/2 pathway participated in SKCM progression, we investigated the effects of SKP2 on the ERK1/2 pathway in SKCM cells under hypoxic conditions. Immunoblotting assay was applied to detect the levels of p-ERK1/2 and ERK1/2 in hypoxia-treated cells. p-ERK1/2 was elevated in SK-MEL-28 cells under hypoxic conditions. Importantly, SKP2 knockdown dramatically suppressed the level of p-ERK1/2 in normoxia- and hypoxia-treated SKCM cells [Figure 3a and b]. To confirm whether the ERK1/2 signaling pathway was required for SKP2-mediated SKCM progression under hypoxic conditions, we activated this signaling pathway using the ERK-specific agonist LM22B-10. Cells were transfected with si-RNA for 48 h, cultured under hypoxic conditions, then treated with the ERK-specific agonist LM22B-10 (1000 ng/mL) for 24 h. As presented in Figure 3c and d, in the hypoxia-treated SKCM cells, the inhibition of p-ERK1/2 by si-SKP2 was partly rescued by LM22B-10. Together, the data suggest that SKP2 knockdown blocked the ERK1/2 pathway in hypoxia-treated SKCM cells.
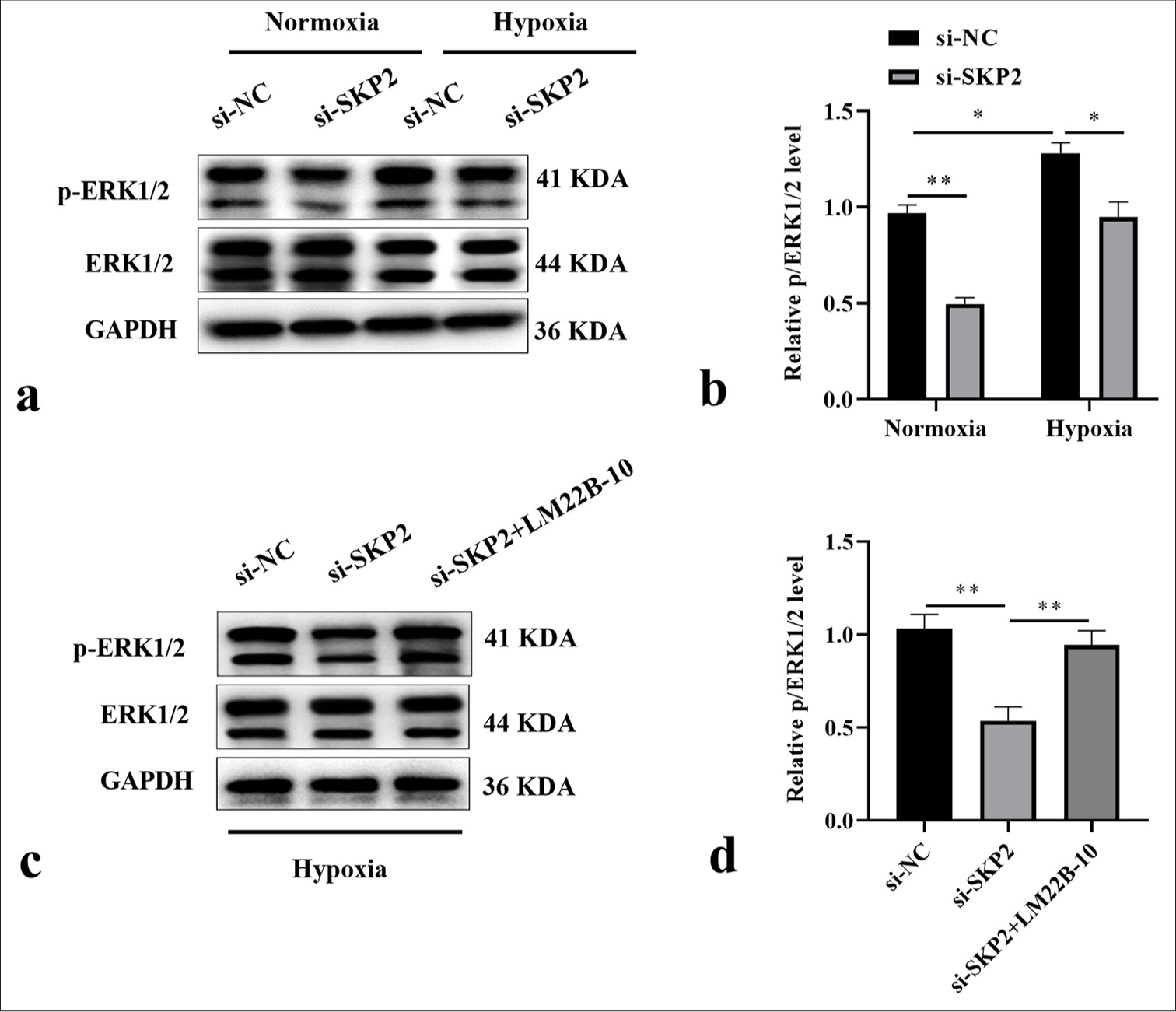
- SKP2 knockdown blocked the ERK1/2 pathway in hypoxia-treated SKCM cells. (a and b) p-ERK1/2 and ERK1/2 levels were examined in hypoxia-treated SKCM cells and (c and d) in SKCM cells cotransfected with si-SKP2 and LM22B-10 before hypoxia treatment. ✶P < 0.05; ✶✶P < 0.01. SKCM: Skin cutaneous melanoma, SKP2: S-phase kinase-interacting protein 2, ERK: Extracellular signal-regulated kinase, si-SKP2: Small interfering S-phase kinase-interacting protein 2.
SKP2 knockdown inhibited the hypoxia-induced proliferation, migration, and invasion of SKCM cells through the ERK1/2 pathway
Subsequently, we explored the effects of LM22B-10 on SKCM cell proliferation, migration, and invasion modulated by si-SKP2. Colony formation assays showed that in SK-MEL-28 cells under hypoxic conditions, the suppressive effect of SKP2 knockdown on cell formation was rescued by LM22B-10 [Figure 4a and b]. EdU assay results revealed that the number of EdU-positive cells among hypoxia-treated SK-MEL-28 cells dramatically increased under treatment with LM22B-10 [Figure 4c and d]. Furthermore, LM22B-10 remarkably increased wound healing rates; this effect was attenuated by si-SKP2 in SK-MEL-28 cells under hypoxic conditions [Figures 4e and f]. Similarly, the ability of SK-MEL-28 cells to invade was mitigated by si-SKP2, and the activation of ERK1/2 enhanced these events [Figure 4g and h]. Collectively, these findings demonstrate that the ERK1/2 pathway plays a key role in SKP2-regulated SKCM proliferation, migration, and invasion.
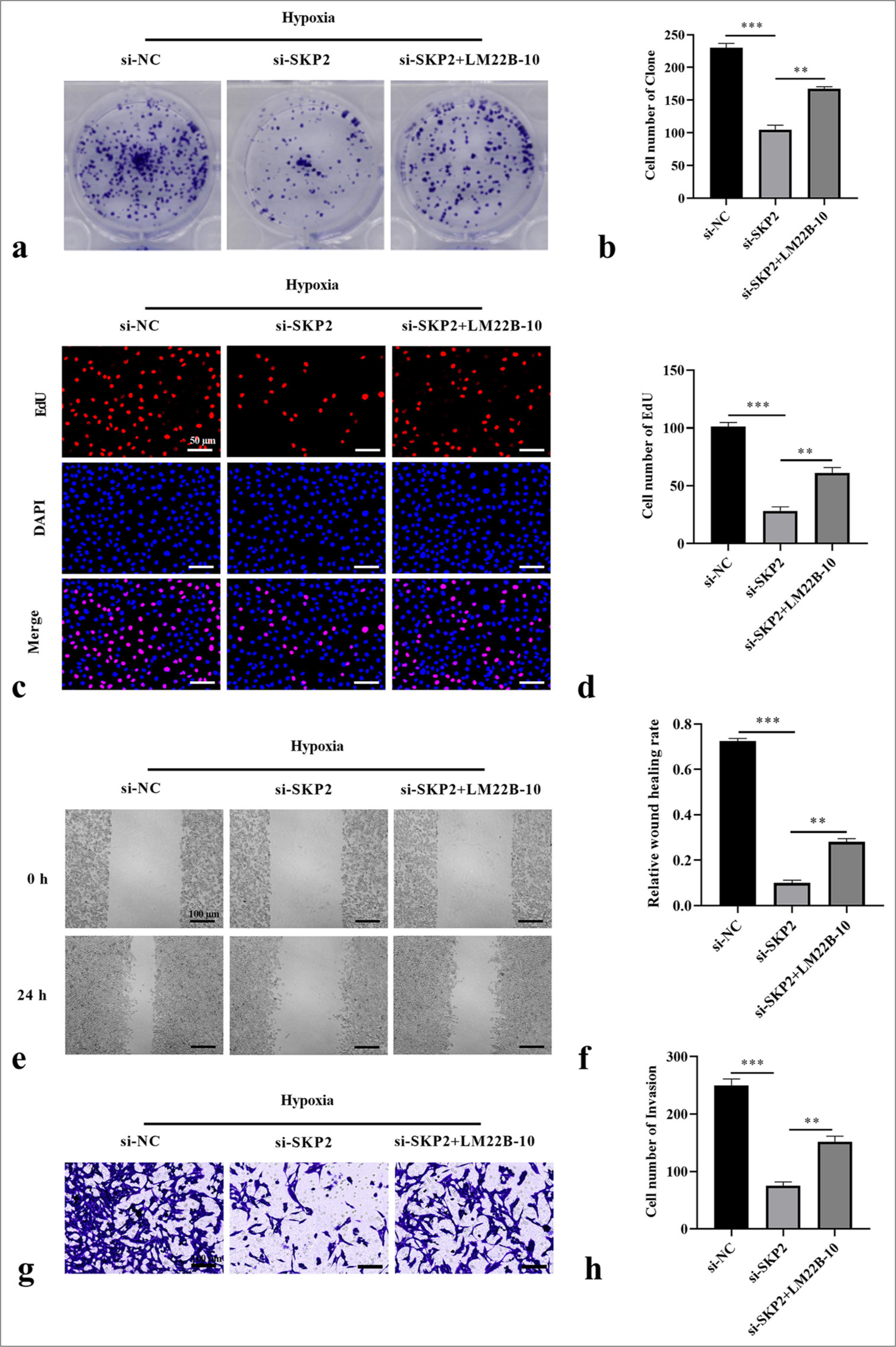
- SKP2 knockdown inhibited the hypoxia-induced proliferation, migration, and invasion of SKCM cells through the ERK1/2 pathway. (a and b) Cell formation number, (c and d) EdU-positive cells, (e and f) wound healing rate, and (g and h) cell invasion were measured by colony formation, EdU staining assay (scale = 50 μm), wound healing assay (scale = 100 μm), and Transwell assay (scale = 100 μm), respectively. ✶✶P < 0.01; ✶✶✶P < 0.001. SKCM: Skin cutaneous melanoma, SKP2: S-phase kinase-interacting protein 2, ERK: Extracellular signal-regulated kinase, EdU: 5-Ethynyl-2’-deoxyuridine.
DISCUSSION
SKCM, which accounts for 4% of all skin cancers, is highly aggressive and easily metastatic, making it the deadliest form of skin cancer.[23] The malignant progression of solid tumors is generally triggered by hypoxia.[24] In SKCM, hypoxia does not constrain the survival and proliferation of tumor cells; conversely, it activates the signaling pathways of various cells within the cancer cell or tumor microenvironment and promotes the malignant progression of tumors.[25] A multitude of recent studies related to melanoma have focused on the efficacy of immunotherapy and targeted therapies.[26,27] However, the molecular mechanism of SKCM development is still not widely discussed. Therefore, an improved comprehension of the mechanisms underlying the development of SKCM is required to create precise medical treatments for patients. However, the molecular mechanisms through which hypoxia induces the growth and metastasis of SKCM cells remain unknown. Here, we discovered that SKP2 was highly expressed in SKCM tissues and cells, and patients with SKCM and low SKP2 expression had good prognosis. Interestingly, SKP2 was considerably elevated in hypoxia-treated SKCM cell lines relative to normoxic cells. The role of SKP2 in the progression of hypoxic-treated SKCM cells was further investigated. We contend that SKP2 can be utilized as a target for SKCM.
As an important E3 ligase, SKP2 can promote tumor development through inducing the degradation of the substrate polyubiquitin or regulating protein interactions.[28] SKP2 serves as a carcinogenic modulator in transformation detection; it is highly expressed in human tumors and associated with the abnormal biological manifestations of tumors.[29] Numerous studies have revealed that SKP2 expression is positively correlated with the malignancy of tumors.[18,30,31] Importantly, SKP2 is elevated in human melanoma and related to poor prognosis.[32] Moreover, a recent study reported that SKP2 knockdown suppressed melanoma cell growth.[33] Another study demonstrated that the depletion of SKP2 led to cell cycle arrest in melanoma cells.[34] Zhu et al. revealed that SKP2 increased in hypoxia-treated medial vestibular nucleus cells and was involved in apoptosis.[35] Arnold et al. indicated that high levels of SKP2 participated in liver cancer progression under hypoxic treatment.[23] In line with the above works, our study illustrated that SKP2 was elevated in SKCM tissues and cells and that its high expression predicted the short survival time of patients. These results implied that SKP2 may act as an oncogene during the development of SKCM. Moreover, we found that SKP2 was elevated in SKCM cells under hypoxia treatment. We therefore believe that SKP2 could respond to hypoxic conditions and affect SKCM progression under hypoxic conditions. Our findings showing that SKP2 depletion blocked the proliferation of SKCM cells under hypoxic conditions, as well as migration, proved this hypothesis.
Hypoxia is an important pathological factor during tumor development, and the ERK1/2 pathway is a well-known oncogenic proliferation signaling pathway in various tumors, including SKCM. For example, a previous study confirmed that the ERK1/2 pathway can be activated in hepatocellular carcinoma cells under hypoxic conditions.[36] Another study revealed that in breast cancer, exposure to hypoxia led to the phosphorylation of ERK.[37] Importantly, a study confirmed the considerable elevation of p-ERK expression in melanoma cells after hypoxia exposure.[38] This result was in line with our current finding that the phosphorylation of ERK was upregulated in SKCM cells under hypoxic conditions. In particular, a previous study found that SKP2 positively regulated cellular-myelocytomatosis viral oncogene, thereby affecting the p-ERK pathway in breast cancer.[39] However, another study reported that SKP2 deficiency led to an increase, rather than a decrease, in p-ERK1/2 levels in myeloma cells.[40] Given the expression and role of SKP2 in SKCM cells under hypoxic conditions, we therefore speculated that some interactions may exist between SKP2 and the ERK1/2 pathway. As expected, our findings, which were obtained through Western blot analysis, showed for the first time that the absence of SKP2 repressed the expression of p-ERK1/2 in SKCM cells under hypoxic conditions, which suggested that the silencing of SKP2 can inactivate the ERK1/2 pathway. The ERK-specific agonist LM22B-10 (1000 ng/mL) was added to further ascertain the effect of the SKP2/ERK1/2 axis on cellular functions in SKCM cells. Our results revealed that the activation of the ERK1/2 pathway reversed the effects of SKP2 on the hypoxia-induced proliferation and metastasis of SKCM cells, implying that hypoxia-induced SKP2 knockdown repressed the progression of melanoma in vitro through the ERK1/2 pathway.
However, our study has some limitations. First, additional clinical samples and prospective data should be included to explore SKP2 expression and its relationship with clinical features. Second, animal experiments must be conducted to explore the potential role and mechanism of SKP2 in vivo. Third, further biological function experiments are needed to elucidate the detailed mechanism of SKP2 in the development of SKCM.
SUMMARY
Our results demonstrated for the first time that SKP2 was significantly elevated in SKCM cells under hypoxic conditions and SKP2 knockdown inhibited SKCM cell proliferation and migration through the ERK1/2 pathway under hypoxic conditions. These findings provide a new mechanism for comprehending hypoxia-induced SKCM progression.
ACKNOWLEDGMENT
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
DAPI: 4’,6-diamidino-2-phenylindole
DEPC: Diethypyrocarbonate
ECL: Enhanced chemiluminescence
EdU: 5-Ethynyl-2’-deoxyuridine
ERK: Extracellular signal-regulated kinase
FBS: Fetal bovine serum
FBXL1: F-box with leucine-rich amino acid repeats 1
GEPIA: Gene expression profiling interactive analysis
HEM: Human epidermal melanocytes
PBS: Phosphate balanced solution
PVDF: Polyvinylidene fluoride
qRT-PCR: Quantitative real-time polymerase chain reaction
SCF: SKP1-cullin-F-box
SDS-PAGE: Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis
SKCM: Skin cutaneous melanoma
SKP2: S-phase kinase-interacting protein 2
AUTHOR CONTRIBUTIONS
YH: Substantial contributions to the conception and design of the study. Substantial contributions to the acquisition, analysis, and interpretation of data, as well as the drafting and revision of the manuscript. Confirmed the authenticity of all the raw data, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval and consent to participate is not required as this study does not involve animal or human experiments, and the patient data came from a database.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Biology of melanoma. Hematol Oncol Clin North Am. 2021;35:29-56.
- [CrossRef] [PubMed] [Google Scholar]
- Chinese expert consensus on the surgical treatment of cutaneous/acral melanoma V1.0. Zhonghua Zhong Liu Za Zhi. 2020;42:81-93.
- [Google Scholar]
- Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol. 2011;31:357-77.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-driven adenosine accumulation: A crucial microenvironmental factor promoting tumor progression. Adv Exp Med Biol. 2016;876:177-83.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor hypoxia as a barrier in cancer therapy: Why levels matter. Cancers (Basel). 2021;13:499.
- [CrossRef] [PubMed] [Google Scholar]
- Role of hypoxia in the hallmarks of human cancer. J Cell Biochem. 2009;107:1053-62.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8:70.
- [CrossRef] [PubMed] [Google Scholar]
- Immunity, hypoxia, and metabolism-the menage a trois of cancer: Implications for immunotherapy. Physiol Rev. 2020;100:1-102.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct Target Ther. 2022;7:218.
- [CrossRef] [PubMed] [Google Scholar]
- Skp2: A critical molecule for ubiquitination and its role in cancer. Life Sci. 2024;338:122409.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10-7.
- [CrossRef] [Google Scholar]
- PD-L1 intrinsically promotes the proliferation of breast cancer cells through the SKP2-p27/p21 axis. Cancer Cell Int. 2024;24:161.
- [CrossRef] [PubMed] [Google Scholar]
- Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci U S A. 2001;98:2515-20.
- [CrossRef] [PubMed] [Google Scholar]
- The role of Skp2 in extranodal NK/T-cell lymphoma. Chin J Cancer. 2010;29:567-71.
- [CrossRef] [PubMed] [Google Scholar]
- High Skp2 expression is associated with a mesenchymal phenotype and increased tumorigenic potential of prostate cancer cells. Sci Rep. 2019;9:5695.
- [CrossRef] [PubMed] [Google Scholar]
- SKP2 promotes breast cancer tumorigenesis and radiation tolerance through PDCD4 ubiquitination. J Exp Clin Cancer Res. 2019;38:76.
- [CrossRef] [PubMed] [Google Scholar]
- Skp2 regulates non-small cell lung cancer cell growth by Meg3 and miR-3163. Tumour Biol. 2016;37:3925-31.
- [CrossRef] [PubMed] [Google Scholar]
- miR-590-5p Targets Skp2 to inhibit the growth and invasion of malignant melanoma cells. Dis Markers. 2022;2022:8723725.
- [CrossRef] [PubMed] [Google Scholar]
- Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis. J Hepatol. 2013;59:830-41.
- [CrossRef] [PubMed] [Google Scholar]
- Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- [CrossRef] [PubMed] [Google Scholar]
- Oxygen regulates molecular mechanisms of cancer progression and metastasis. Cancer Metastasis Rev. 2014;33:183-215.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-dependent drivers of melanoma progression. J Exp Clin Cancer Res. 2021;40:159.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted therapy and immunotherapy in melanoma. Dermatol Clin. 2023;41:65-77.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in targeted therapy and immunotherapy for melanoma (Review) Exp Ther Med. 2023;26:416.
- [CrossRef] [PubMed] [Google Scholar]
- Skp2: A critical molecule for ubiquitination and its role in cancer. Life Sci. 2024;338:122409.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of Skp2 expression and activity and its role in cancer progression. ScientificWorldJournal. 2010;10:1001-15.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibiting the role of Skp2 suppresses cell proliferation and tumorigenesis of human gastric cancer cells via the upregulation of p27kip1. Mol Med Rep. 2016;14:3917-24.
- [CrossRef] [PubMed] [Google Scholar]
- Amplification and overexpression of SKP2 are associated with metastasis of non-small-cell lung cancers to lymph nodes. Am J Pathol. 2004;165:175-80.
- [CrossRef] [PubMed] [Google Scholar]
- Cytoplasmic Skp2 expression is increased in human melanoma and correlated with patient survival. PLoS One. 2011;6:e17578.
- [CrossRef] [PubMed] [Google Scholar]
- Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J Dermatol Sci. 2006;42:215-24.
- [CrossRef] [PubMed] [Google Scholar]
- Skp2 regulates G2/M progression in a p53-dependent manner. Mol Biol Cell. 2008;19:4602-10.
- [CrossRef] [PubMed] [Google Scholar]
- Tanshinone IIA suppresses hypoxia-induced apoptosis in medial vestibular nucleus cells via a Skp2/BKCa axis. Curr Pharm Des. 2020;26:4185-94.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-inducible long noncoding RNA NPSR1-AS1 promotes the proliferation and glycolysis of hepatocellular carcinoma cells by regulating the MAPK/ERK pathway. Biochem Biophys Res Commun. 2020;533:886-92.
- [CrossRef] [PubMed] [Google Scholar]
- Sanguinarine combats hypoxia-induced activation of EphB4 and HIF-1α pathways in breast cancer. Phytomedicine. 2021;84:153503.
- [CrossRef] [PubMed] [Google Scholar]
- LRIG1 acts as a critical regulator of melanoma cell invasion, migration, and vasculogenic mimicry upon hypoxia by regulating EGFR/ERK-triggered epithelialmesenchymal transition. Biosci Rep. 2019;39:BSR20181165.
- [CrossRef] [PubMed] [Google Scholar]
- INSM1 promotes breast carcinogenesis by regulating C-MYC. Am J Cancer Res. 2023;13:3500-16.
- [Google Scholar]
- Over-expression of CKS1B activates both MEK/ERK and JAK/STAT3 signaling pathways and promotes myeloma cell drug-resistance. Oncotarget. 2010;1:22-33.
- [CrossRef] [PubMed] [Google Scholar]








