Translate this page into:
Inhibitory effect on endometrial cancer: Collagen type XII α1 chain

*Corresponding author: Qiong Jin, Department of Gynecology, Fuzhou First General Hospital Affiliated with Fujian Medical University, Fuzhou, China. jinqiong2024@163.com
-
Received: ,
Accepted: ,
How to cite this article: Shen Z, Huang M, Lin J, Wu S, Jin Q. Inhibitory effect on endometrial cancer: Collagen type XII α1 chain. CytoJournal. 2025;22:31. doi: 10.25259/Cytojournal_236_2024
Abstract
Objective
Endometrial cancer (EC) is one of the most common gynecological malignancies, and it poses a considerable threat to women’s lives. Therefore, searching for EC inhibitors and exploring the potential mechanism of action is particularly important. This article aims to investigate the potential effect of collagen type XII α1 chain (COL12A1) on macrophage polarization and its subsequent influence on the biological behavior of EC cells to further elucidate the underlying mechanisms of EC development.
Material and Methods
Quantitative real-time polymerase chain reaction and Western blot were used to detect the expression levels of COL12A1 messenger RNA and protein in EC cells. A subcutaneous tumor formation assay was performed in nude mice to evaluate the effect of COL12A1 on EC cell growth in vivo. Flow cytometry was utilized to assess the expression levels of macrophage surface markers under different treatments. Cell counting kit-8, Transwell assay, and Western blot experiments were conducted to investigate the effects of COL12A1 knockdown and various macrophage treatments on the biological behavior of EC cells.
Results
The expression of COL12A1 was upregulated in EC cells. Knockdown of COL12A1 significantly inhibited the viability, invasion, migration, and extracellular matrix abilities of EC cells and tumor growth in vivo. Overexpression of COL12A1 significantly promoted M2-type macrophage polarization, which enhanced the invasion, migration, and epithelial-mesenchymal transition abilities of EC cells.
Conclusion
The expression of COL12A1 is upregulated in EC, and COL12A1 promotes EC cell invasion and migration by activating macrophage M2 polarization.
Keywords
Collagen type XII alpha 1 chainCOL12A1
Endometrial cancer
Invasion
Migration
Tumor-associated macrophage polarization
INTRODUCTION
Endometrial cancer (EC) is a gynecological malignant tumor that arises in the glandular epithelium of the endometrium. The incidence rate of EC has been increasing annually, significantly affecting women’s quality of life.[1] A study has identified postmenopausal women as the primary population at risk, with an increasingly younger age of onset.[2] While the overall prognosis for this disease is favorable, patients with infiltration and metastasis exhibit poor outcomes. Recent research has confirmed that tumor development is not solely attributed to cellular changes within tissues but also results from interactions between tissue cells and the tumor microenvironment.[3] Notably, infiltration by tumor-associated macrophages (TAMs) has been linked to myxoid infiltration and lymphatic metastasis in EC, showing a negative correlation with patient prognosis.[4-6] Therefore, understanding its underlying mechanisms holds great significance in providing a biological foundation for treatment.
TAMs are a crucial cell subpopulation within the tumor microenvironment.[7] On the basis of differences in macrophage surface markers, activation status, and function, TAMs can be classified into M1 and M2 types. The classically activated M1 type is predominantly found in inflammatory environments, expressing epitope molecules such as cluster of differentiation (CD86/68) and major histocompatibility complex II. They secrete pro-inflammatory factors like interleukin (IL-6, IL-12, and IL-23) to mediate inflammatory responses. Meanwhile, alternatively, activated M2 type primarily exists in the tumor microenvironment and secretes anti-inflammatory factors IL-4 and IL-10. They participate in regulating tumorigenesis and development.[8] Monocytes present in peripheral circulation are recruited to tumor tissues by chemokines secreted by stromal cells and tumors. Under stimulation from the local microenvironment, these monocytes polarize into TAMs with an M2 phenotype. These differentiated TAMs express phenotypic molecules, including Arg1, CD68, CD163, and CD206, while producing various growth factors and cytokines that facilitate tumor cell proliferation, infiltration metastasis, and neovascularization.[9] Numerous studies have demonstrated that infiltration of M2-type macrophages, including EC, is linked to worsened prognosis,[10,11] although the underlying mechanisms remain unclear.
Epithelial-mesenchymal transformation (EMT) allows otherwise quiescent epithelial cells to become more mobile and may detach from the primary tumor site, enter the circulatory system, and ultimately colonize distant organs to form metastatic foci.[12] Cytoskeletal changes occur during EMT, with weakened adhesion between cells and increased expression levels of mesenchymal markers such as N-cadherin and Vimentin.[13] Tanaka’s study identified N-cadherin as an independent predictor of survival in patients with EC.[14] Extracellular matrix (ECM) alterations are important during EMT. The ECM represents a major component of the tumor microenvironment, and abnormal collagen expression within the ECM has been observed in various cancers.[15] This association is believed to modulate the interaction between collagen I fibrils and the surrounding matrix, thereby playing a pivotal role in regulating the tumor microenvironment.[16,17]
Collagen type XII alpha 1 chain (COL12A1), a gene encoding XII type collagen genes of alpha chain, is a member of the protofiber-associated collagen family, whereas XII type collagens are important cellular scaffolding and support proteins in the ECM.[18] Previous studies have found that dysregulation of COL12A1 expression is involved in various tumorigenesis, progression, and metastasis.[19,20] COL12A1 expression is increased in pancreatic cancer and associated with poor prognosis, and it can be used as an immune marker for pancreatic cancer.[21] By performing bioinformatics analysis, Hauptman found that genes involved in ECM receptor interactions, such as COL12A1, COL1A2, and COL3A1, were upregulated in cancers compared with adenomas, suggesting that ECM organizing processes are involved in the progression of adenomas to carcinomas.[22] The underlying mechanisms of COL12A1 in EC are still not well understood. This study seeks to explore how COL12A1 contributes to the progression of EC and uncover its potential mechanisms, thereby laying new theoretical groundwork for the treatment of EC.
MATERIAL AND METHODS
Cell culture and transfection
Human monocyte THP-1 cells (cat. SCSP-567) were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (cat. 11875093, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, cat. A5256701, Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin (cat. C0222, Beyotime Biotech, Shanghai, China). Human endometrial mesenchymal stromal cells (HESCs) and EC cells Ishikawa (cat. SC0202), AN3CA (cat. SC0199), HEC-1A (cat.SC0204), and RL-95 (cat.SC0200) were cultured in high-glucose Dulbecco’s Modified Eagle Medium (DMEM, cat. C11960500BT, Gibco, Grand Island, NY, USA). All EC cells were purchased from Yuchi Biological Technology Co., Ltd. (Shanghai, China), with correct STR identification and negative mycoplasma detection. All cell lines were incubated at 37°C with 5% carbon dioxide (CO2). The EC cells in the logarithmic growth phase were enzymatically dissociated and seeded into six-well plates.
Construction and transfection of small interfering RNA (siRNA) and overexpressed plasmid
Si-NC and si-COL12A1 were designed using the online GenScript siRNA Target Finder tool (GenScript, Piscatawy, NJ, USA) and synthesized by Ribo Bio (Guangzhou, China). The sequence is si-NC: 5'-UUC UCC GAACGU GUC ACG UTT-3', si-COL12A1: 5'-GAT CGG CAA TAC TCT CAC AGG CAT GGC TCG AGC CAT GCC TGT GAG AGT ATT GCT TTT TTG GAA TTC-3'. The overexpressed plasmid pcDNA-COL12A1 was completed by Feng Hui Biological Company (Hunan, China). An empty vector was used as a negative control. When the cell density reached 60–80%, the culture medium was replaced with serum-free DMEM. LipofectamineTM2000 (Invitrogen, Carlsbad, CA, USA) was used to transfect si-NC and si-COL12A1 into EC cells and transfect pc DNA-COL12A1 and Vetor into macrophages according to the manufacturer’s instructions. After 48 h, the transfected cells were collected for subsequent studies.
Induced differentiation of THP-1 cells
The THP-1 cell suspension was collected, and THP-1 monocytes were stimulated with Phorbol 12-myristate 13-acetate (PMA, cat. HY-18739, 100 ng/mL, Med Chem Express, New Jersey, USA) for 24 h to induce their differentiation into M0-type (inactivated state) macrophage.[23] Subsequently, lipopolysaccharides (LPS, cat. HY-D1056, 10 ng/mL, Med Chem Express, New Jersey, USA) and interferon-gamma (IFN-γ, cat. HY-P7025, 20 ng/mL, Med Chem Express, New Jersey, USA) were added and treated for 48 h to promote their polarization toward M1-type macrophages (M1 group).[24] IL-4 and IL-13 at a concentration of 20 ng/mL were applied for 48 h to stimulate the cells’ differentiation into M2-type macrophages (M2 group).[25] Then, Vector or pcDNA-COL12A1 plasmids were transfected into THP-1 differentiated M2-type macrophages using Lipofectamine 2000 according to the manufacturer’s instructions. After incubation for an additional 48 h, the cells were collected for subsequent experiments.
Macrophages co-cultured with EC cells
The transfected THP-1 cells were seeded at a density of 1 × 105 cells/well in the upper chamber (0.4 μm diameter, Corning, NY, USA) of the co-culture system, with 200 μL per well. EC cells were then resuspended in a DMEM medium containing 10% FBS at a concentration of 2500 cells/mL, and 800 μL of cell suspension was added to the lower chamber of the co-culture system. Care should be taken during this process to prevent the formation of air bubbles between the upper and lower chambers. Following this, the co-culture system was incubated in a cell culture incubator at 37℃ with 5% CO2 for a duration of 48 h. Finally, the cells were harvested for subsequent analysis.
RNA extraction and quantitative real-time polymerase chain reaction (RTqPCR)
The cells were subjected to TRIzol-based (cat. 15596026CN, Invitrogen, Carlsbad, CA, USA) total RNA extraction followed by an assessment of its purity. Reverse transcription was performed in accordance with the instructions provided with the reverse transcription kit (cat. N8080234, Invitrogen, Carlsbad, CA, USA). The resulting cDNA was diluted using RNase-free water (cat. R0021, Beyotime Biotech, Shanghai, China) and used as a template for PCR amplification on a fluorescent quantitative PCR instrument, employing the SYBR Green quantitative PCR kit (Qiagen, Germantown, MD, USA). The relative expression levels of the target genes were determined using the 2−ΔΔCt method. The primer sequence is as follows: COL12A1, forward, 5'-CCA CAG GTT CAA GAG GTC CC-3' and reverse, 5'-TGT GTT AGC CGG AAC CTG GA-3', glyceraldehyde-3-phosphate dehydrogenase (GAPDH); forward, 5'-ACA ACT TTG GTA TCG TGG AAG G-3' and reverse, 5'-GCC ATC ACG CCA CAG TTT C-3'.
Western blot
The cells were lysed, and the total cellular proteins (cat. 89900, Thermo Scientific, Waltham, MA, USA) were subsequently extracted and quantified spectrophotometrically. A denatured protein sample of 35 μg was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a polyvinylidene fluoride membrane (cat. 1620177, Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5% bovine serum albumin at 37°C for 2 h. Specific primary antibodies COL12A1 (cat. ab121304, Abcam), E-cadherin (cat. 3195, Cell signaling Technology), N-cadherin (cat.13116, Cell signaling Technology), Vimentin (cat. ab92547, Abcam, all diluted at 1:1000), and GAPDH (cat. ab181602, 1:5000, Abcam) were incubated with the membrane overnight at 4°C. Subsequently, an HRP-labeled secondary antibody (cat. ab205718, 1:5000, Abcam) was added and incubated at 37°C for 1 h. Finally, an enhanced chemiluminescence luminescent solution (cat. WBKLS0500, Millipore, Billerica, MA, USA) and an imaging system (iBright FL1000, Thermo Fisher Scientific, USA) were used for imaging. The gray value of the bands was determined using ImageJ (version 1.53k, NIH, Bethesda, MD, USA).
CCK-8 assay
The EC cells were routinely digested, centrifuged, and then resuspended. The cell concentration was 3 × 104 cells/mL in complete medium, and 100 μL per well was seeded in 96-well plates. After the cells were incubated for 0, 24, 48, and 72 h, each well was added with 10 μL CCK-8 solution (cat. 96992, Sigma–Aldrich, St. Louis, MO, USA) and further incubated for an additional 2 h. The absorbance at a wavelength of 450 nm was measured using a microplate reader (Synergy HT, BioTek, Winooski, VT, USA) to record the optical density value for plotting the cell growth curve.
Transwell assay
The cell invasion assay involved the even distribution of the diluted Matrigel gel solution onto the basement membrane of the upper chamber of the Transwell, followed by overnight incubation in a cell incubator to allow for semi-solidification of the matrix gel. On the next day, the EC cells were prepared as a single-cell suspension with a cell density of 4 × 104 cells/mL. Subsequently, 300 μL of the treated cell suspension from each group was added to the upper layer of the chambers, and 600 μL of medium containing 10% FBS was added to the lower layer. After 24 h, the cells in the lower layer were fixed using a solution consisting of 4% paraformaldehyde. Following fixation, crystal violet staining (0.1%, cat. C0121, Beyotime Biotech, Shanghai, China) was performed for 5 min on these cells. Five randomly selected non-overlapping fields were photographed under an inverted microscope (Olympus IX71, Tokyo, Japan) for counting and taking the average value. The cell migration assay did not necessitate the preparation of Matrigel matrix gel, and the remaining experimental procedures remained consistent with those employed in the invasion assay.
Subcutaneous tumor formation in nude mice
All 12 of 4-week-old BALB/c nude mice were purchased from Animal Laboratory (Shanghai, China). The mice were maintained at a temperature of 20–27 °C, a relative humidity of 40–60%, and a light/dark cycle of approximately 12 h and fed and watered ad libitum. After 1 week of adaptive feeding, they were randomly divided into two groups: si-NC and si-COL12A1 groups. The EC cells in the logarithmic phase were selected, trypsinized, and collected by centrifugation. Subsequently, the cells were resuspended in serum-free DMEM at a cell density of 1 × 106 cells/mL. Afterward, EC cell suspensions stably transfected with short hairpin shNC, and sh-COL12A1 were subcutaneously injected into the back of the nude mice at a volume of 200 μL each. The nude mice were euthanized through cervical dislocation at the end of a 5-week period, and subsequently, tumor tissues were excised. The length (a, mm) and width (b, mm) were measured with calipers to calculate the tumor volume (mm3) using the following formula: a × b2 × 0.5. Tumor volume growth curves and tumor weights were then measured for each group of nude mice. The animals used in this study have been approved by the Fuzhou First General Hospital Affiliated with Fujian Medical University Ethics Committee (No. 202210015).
Immunohistochemical Ki-67 staining
Tumor tissues were collected from nude mice, fixed, and embedded in paraffin. The paraffin sections were then deparaffinized. Subsequently, they were repaired using sodium citrate buffer and incubated with the primary antibody Ki-67 (1:100, cat. GB111141, Servicebio, Wuhan, China) at 37°C for 1 h. Afterward, they were washed 3 times with phosphate-buffered saline (PBS) for 3 min each time. Then, the secondary antibody (1:200, cat. GB23303, Servicebio, Wuhan, China) was added and incubated for 30 min before being washed again with PBS three times. Finally, a light-absorbing diaminobenzidine chromatography solution (cat. ZLI 9017, Zsgb-bio, Beijing, China) was used to develop color, followed by restaining with hematoxylin for 3 min. The tissues were then observed under a microscope (Eclipse 50i, Nikon fluorescence microscope, Tokyo, Japan), and photographs were taken. The Ki-67-positive cell rate was quantitatively analyzed by ImageJ, and the positive cell rate was calculated as follows: number of positive tumor cells/total number of tumor cells × 100.
Flow cytometry
The single cell suspension was obtained by washing the macrophages treated with different conditions 2 times with PBS, followed by resuspending the cells in 100 μL of PBS. By following the instructions provided for the antibodies, fluorescein 5-isothiocyanate-anti-CD68 (cat. 333805, BioLegend; San Diego, CA), PE-anti-CD206 (cat. 321105, BioLegend; San Diego, CA), APC-anti-CD86 antibodies (cat. 374207, BioLegend; San Diego, CA), and 5 μL of the corresponding fluorescent-labeled antibody were added to each tube, gently mixed, and incubated at 4°C for 30 min in darkness. After two washes with PBS, the cells were resuspended in 300 μL of PBS. The Beckman Coulter Gallios flow cytometer (Danaher, Washington, USA) was used to detect the expression of surface differentiation antigens in macrophages.
Statistics and analysis
The results of each experiment were statistically analyzed using the Statistical Package for the Social Sciences (version 21.0) software (IBM, Chicago, IL, USA). All experiments were independently repeated 3 times. Data are presented as mean ± standard deviation; differences between two groups were compared using an independent samples t-test; and comparisons among multiple groups were analyzed using analysis of variance, followed by the least significant difference test for post hoc. P < 0.05 was considered statistically significant.
RESULTS
COL12A1 was upregulated in EC cells
The expression of COL12A1 in HESCs and EC cells Ishikawa, AN3CA, HEC-1A, and RL-95 was assessed using RT-qPCR. A significant upregulation in COL12A1 messenger RNA (mRNA) expression was found in EC cell lines compared with HESCs (P < 0.001, Figure 1a). Western blot experiments confirmed a substantial increase in COL12A1 protein levels in EC cells (P < 0.01, Figure 1b and c). The two EC cell types exhibiting the highest COL12A1 expression were selected for subsequent investigations. The results collectively indicated a pronounced upregulation of COL12A1 across all EC cells, suggesting its potential involvement in the pathogenesis and progression of EC.
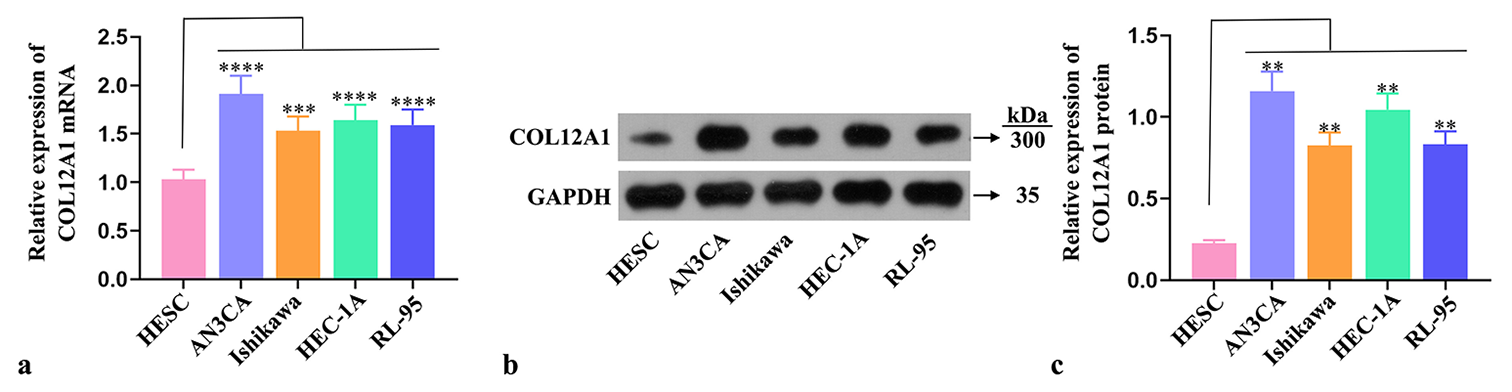
- COL12A1 upregulation in EC cells. (a) RT-qPCR detection of the expression of COL12A1 mRNA in EC cells. (b and c) Western blot analysis of the protein expression of COL12A1 in EC cells. ✶✶P < 0.01, ✶✶✶P < 0.001, and ✶✶✶✶P < 0.0001 compared with HESC group. Statistical analysis of significance using one-way ANOVA. COL12A1: Collagen type XII alpha 1 chain, EC: Endometrial cancer, mRNA: Messenger RNA, ANOVA: Analysis of variance, RT-qPCR: Quantitative real-time polymerase chain reaction.
Knockdown of COL12A1 suppressed the effects of invasion, migration, and EMT in EC cells
Si-COL12A1 was transfected into two types of EC cells to investigate the effect of COL12A1 on EC progression, and the successful transfection efficiency of COL12A1 was confirmed (P < 0.05, Figure 2a-c). CCK-8 assay was employed to assess the effect of COL12A1 on the proliferative capacity of both types of EC cells, revealing a significant inhibition in their proliferation on knockdown of COL12A1 (P < 0.05, Figure 2d and e). Cell migration assay results demonstrated a notable reduction in migration capabilities following COL12A1 knockdown in both cell types (P < 0.05, Figure 2f-h). Transwell assay showed that the invasive ability of both cell types was significantly reduced after COL12A1 knockdown (P < 0.05, Figure 2i-k). Western blot analysis detected alterations in the expression levels of EMT-related proteins N-cadherin, Vimentin, and E-cadherin in both types of EC cells. Notably, downregulation of COL12A1 significantly upregulated E-cadherin protein expression while downregulating N-cadherin and Vimentin protein expression levels (P < 0.05, Figure 2l-n). Collectively, these findings suggested that suppressing COL12A1 inhibited in vitro proliferation, migration, invasion, and EMT abilities in EC cells.
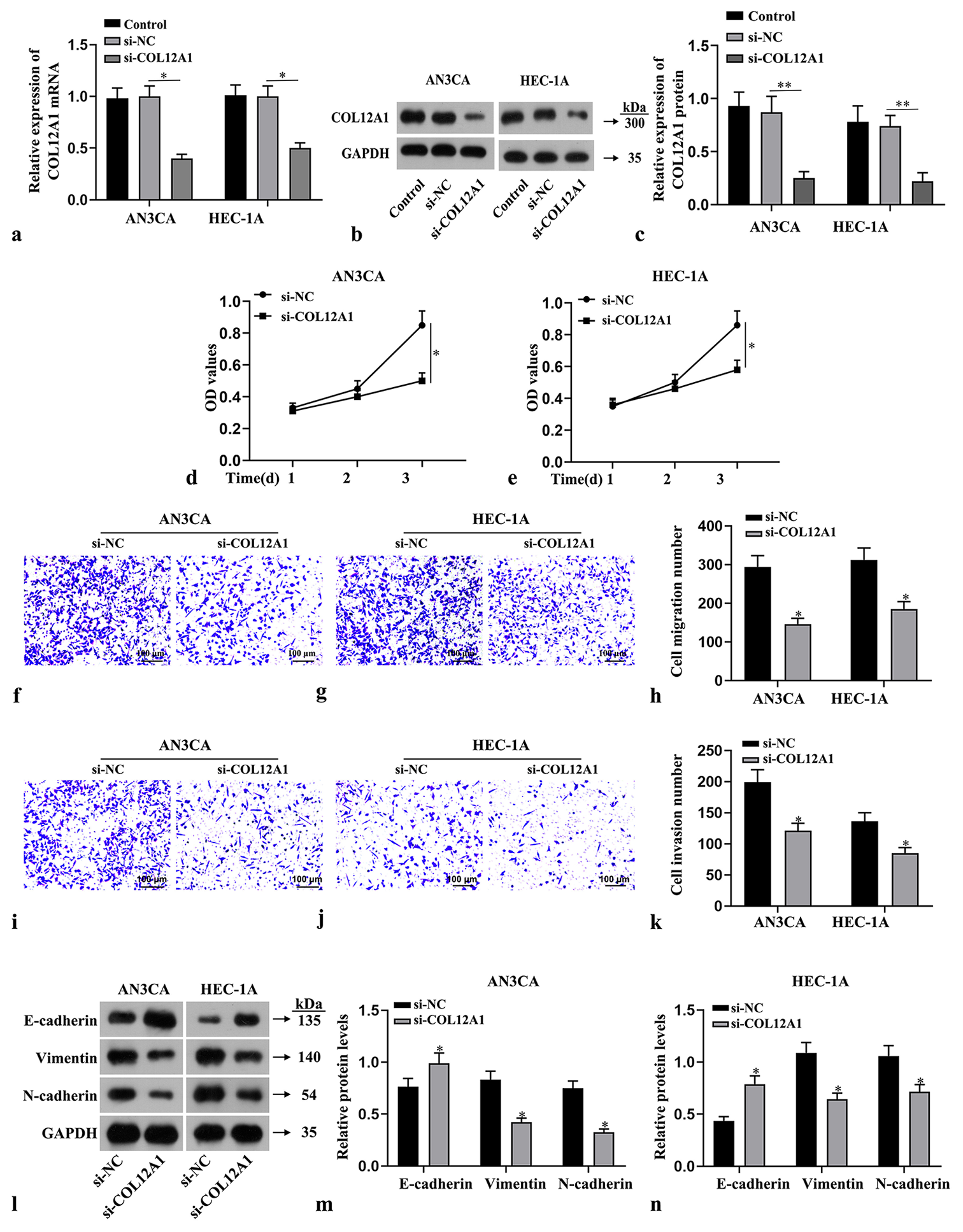
- Suppression of the effects of invasion, migration, and EMT in EC cells by knockdown of COL12A1. (a-c) Assessment of the transfection efficiency of COL12A1 by RT-qPCR and Western blot. (d and e) CCK-8 assay evaluation of the viability of EC cells. (f-h) Cell migration assay detection of the migratory capacity of EC cells (Magnification 40×). (i-k) Transwell assay measurement of the invasion capabilities of EC cells (Magnification 40×). (l-n) Western blot analysis of the protein expression levels of N-cadherin, Vimentin, and E-cadherin in EC cells. ✶P < 0.05 compared with si-NC group. Scale bar = 100 μm. Statistical analysis of significance using one-way ANOVA or Student’s t-test. COL12A1: Collagen type XII alpha 1 chain, EMT: Epithelial-mesenchymal transformation, EC: Endometrial cancer, ANOVA: Analysis of variance, RT-qPCR: Quantitative real-time polymerase chain reaction.
Knockdown of COL12A1 inhibited EC tumor growth in vivo
EC cell suspensions that were stably transfected with siNC and si-COL12A1 were subcutaneously injected into the nude mice to investigate the effect of COL12A1 on the in vivo growth of EC tumors. After a period of 5 weeks, the mice were euthanized, and a significant reduction in tumor volume and weight was observed following the knockdown of COL12A1 (P < 0.05, Figure 3a-c). Immunohistochemical analysis revealed a notable decrease in Ki-67 positive expression within the tumor tissues on COL12A1 suppression (P < 0.05, Figure 3d and e). These findings provided compelling evidence that silencing COL12A1 effectively inhibited the growth ability of EC tumors in an in vivo setting.
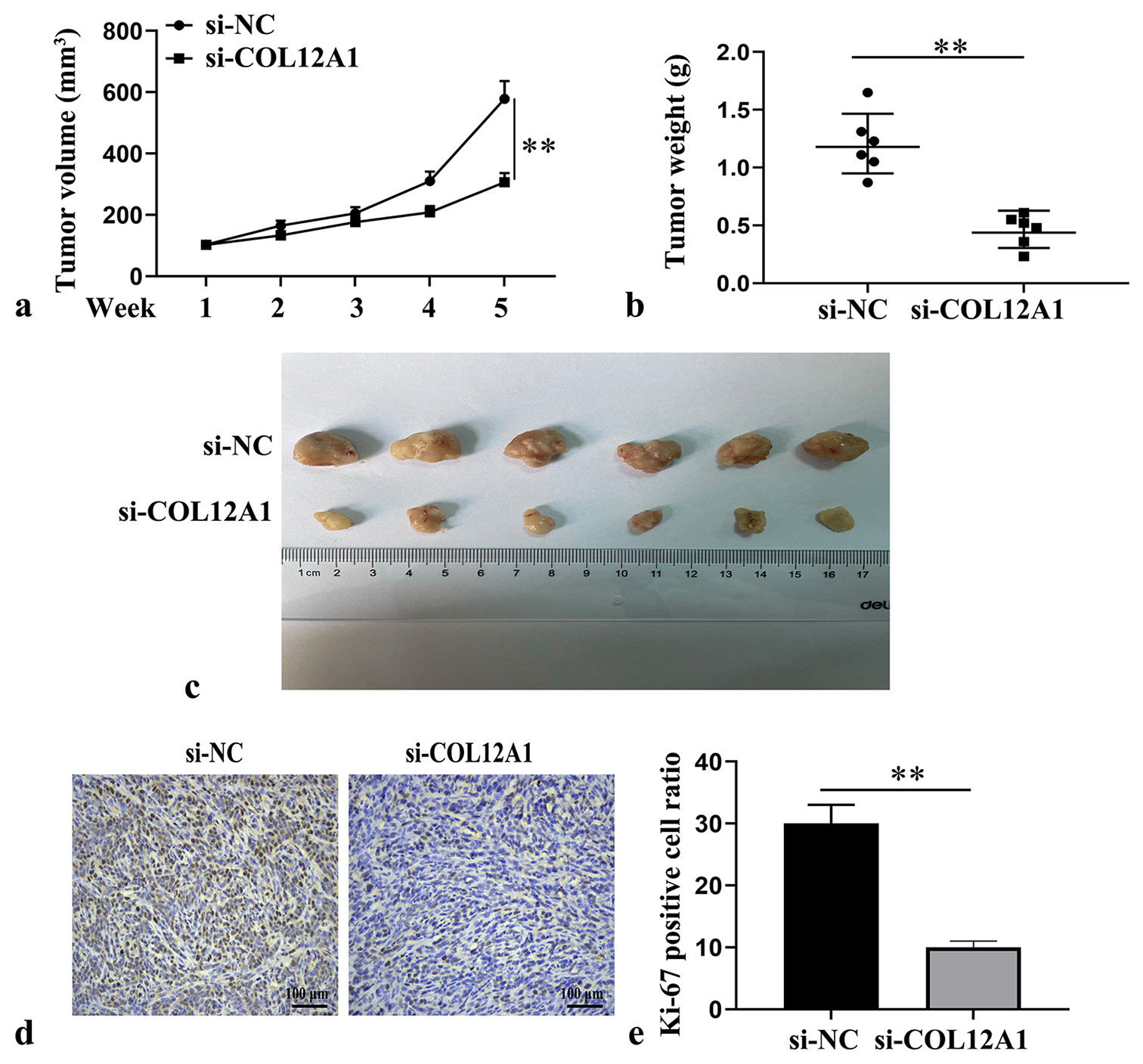
- Inhibition of EC tumor growth in vivo by knockdown of COL12A1. (a) Assessment of tumor growth kinetics of nude mice in each group (n = 6). (b) Quantification of tumor mass of nude mice in each group (n = 6). (c) Representative images of transplanted tumor in nude mice at 5 weeks. (d and e) Immunohistochemical analysis of Ki-67 expression in tumor tissue (Magnification 40×). ✶P < 0.05. Scale bar of Ki μm. Statistical analysis of significance using one-way ANOVA or Student’s t-test. EC: Endometrial cancer, COL12A1: Collagen type XII alpha 1 chain, ANOVA: Analysis of variance.
Overexpression of COL12A1 promoted macrophage M2 polarization
Light microscopy was employed to observe the morphological characteristics of macrophages. The THP-1 cells underwent a transition from suspension agglomerative growth to adherent growth with protruding pseudopods following PMA treatment [Figure 4a]. Flow cytometry experiments revealed that LPS + IFN-γ treatment significantly augmented the proportion of CD86+ cells, a marker for M1 macrophages, and IL-4 + IL-13 treatment notably increased the proportion of CD206+ cells, a marker for M2 macrophages (P < 0.05, Figure 4b-d), thereby confirming successful induction of macrophage polarization. COL12A1 mRNA expression was significantly higher in M2-type macrophages than in M1-type macrophages (P < 0.05, Figure 4e). The overexpressed COL12A1 was transfected into THP-1-derived macrophages treated with IL-4 and IL-13 (P < 0.05, Figure 4f-h), and flow cytometry was performed to assess the proportion of M2-type macrophages. The results demonstrate that the IL-4 + IL-13 + pcDNA-COL12A1 group had a significant increase in the percentage of CD206+ cells compared with the IL-4 + IL-13 + vector group (P < 0.01, Figure 4i and j), indicating that overexpression of COL12A1 effectively promoted M2 macrophage polarization.
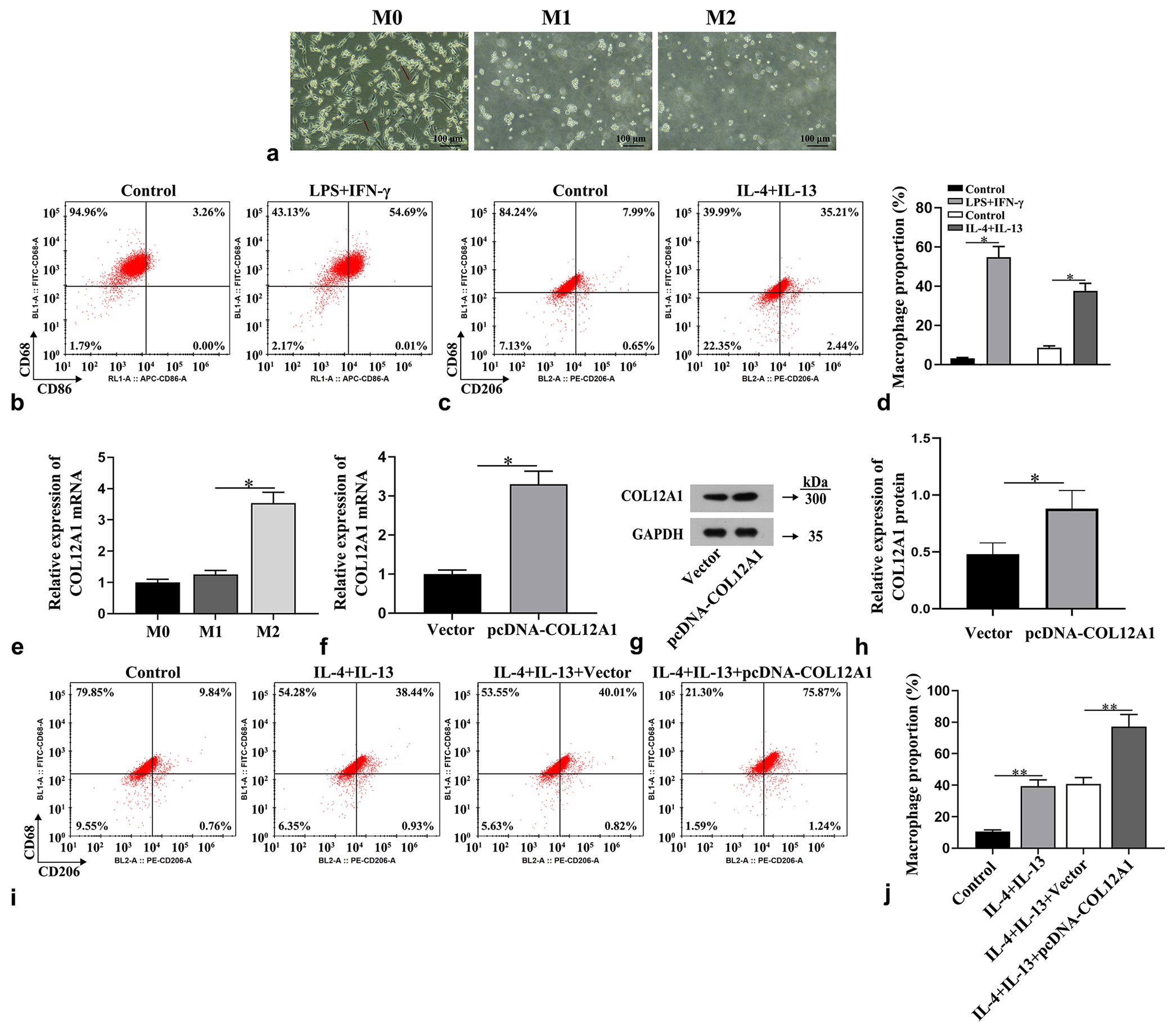
- Promotion of macrophage M2 polarization by overexpression of COL12A1. (a) Examination of the morphology of macrophages using optical microscopy (Magnification 40×). (b-d) Flow cytometry used to determine the proportion of M1 and M2 macrophages. (e) Detection of COL12A1 mRNA expression by RT-qPCR. (f-h) Detection of COL12A1 overexpression efficiency. (i and j) Flow cytometry used to determine the proportion of M2 macrophages. ✶P < 0.05 and ✶✶P < 0.01. Scale bar = 100 μm. CD: Cluster of differentiation, IL: Interleukin. Statistical analysis of significance using one-way ANOVA or Student’s t-test. COL12A1: Collagen type XII alpha 1 chain, mRNA: Messenger RNA, ANOVA: Analysis of variance, RT-qPCR: Quantitative real-time polymerase chain reaction.
Effect of COL12A1 on EC cell invasion, migration, and EMT by inducing macrophage M2 polarization
To investigate the impact of differently treated macrophage supernatants on the proliferation, invasion, migration, and EMT abilities of EC cells, we observed through CCK-8 assay, Transwell assay, and Western blot experiments that co-cultivation of IL-4- and IL-13-treated macrophages with
EC cells significantly enhanced their proliferation (P < 0.01, Figure 5a), invasion, migration (P < 0.01, Figure 5b-d), and EMT abilities (P < 0.01, Figure 5e-h). These results suggested that a higher proportion of M2-type macrophages induced stronger proliferation, invasion, migration, and EMT abilities in co-culture conditions with EC cells. Therefore, COL12A1 promoted EC cell invasion, migration, and EMT ability by facilitating M2 polarization in macrophages.
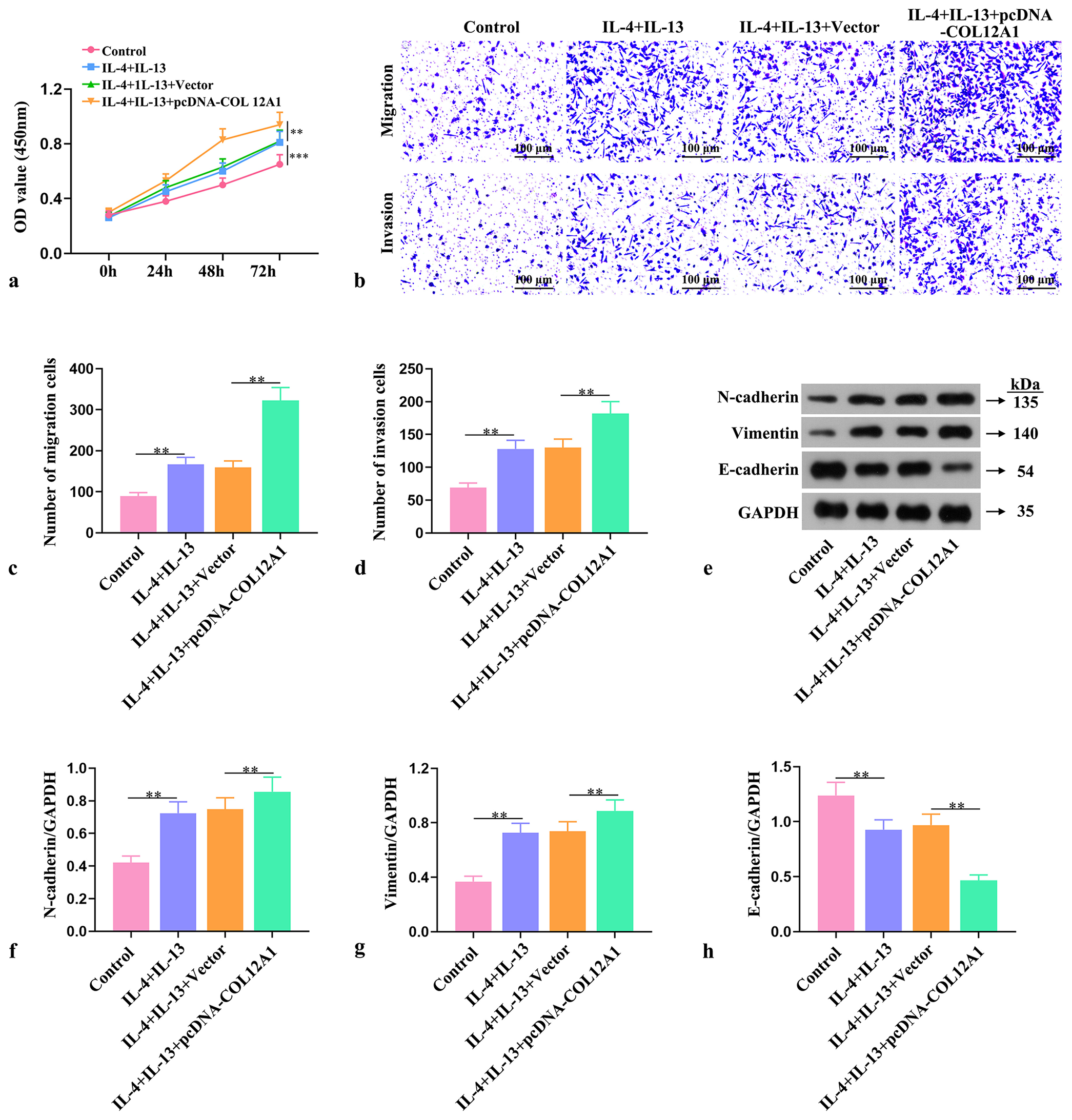
- Effect of COL12A1 on EC cell invasion, cell migration, and EMT by inducing macrophage M2 polarization. (a) Assessment of EC cell viability through CCK-8 assay. (b-d) Evaluation of the invasive and migratory abilities of EC cells by Transwell assay (Magnification 40×). (e-h) Western blot analysis of the protein expression levels of N-cadherin, Vimentin, and E-cadherin in EC cells. ✶✶P < 0.01 and ✶✶✶P < 0.001. Scale bar = 100 μm. OD: Optical density. Statistical analysis of significance using one-way ANOVA. COL12A1: Collagen type XII alpha 1 chain, EMT: Epithelial-mesenchymal transformation, EC: Endometrial cancer, ANOVA: Analysis of variance.
DISCUSSION
COL12A1 is highly expressed in several types of tumors, including gastric,[26] colorectal,[27] and pancreatic adenocarcinomas.[28] Knockdown experiments targeting COL12A1 demonstrated its ability to inhibit malignant phenotypes of cancer cells. Jiang et al.[ 20] found that COL12A1 expression in gastric cancer tissue increased significantly, proving that COL12A1 is a prognostic factor in patients with gastric cancer and a potential therapeutic target. In the present study, knockdown experiments were conducted on COL12A1 to investigate its biological function in EC cells. Specifically, its effect on EC cell invasion and migration and EMT were assessed. The results revealed that knocking down COL12A1 significantly suppressed the invasion, migration, and EMT abilities of EC cells in vitro and in vivo. Collectively, the findings suggested that COL12A1 exerted pro-cancer effects in EC.
The tumor microenvironment of EC comprises immune cells, non-immune stromal cells, and ECM proteins, which encompass immunosuppressive cell populations such as regulatory T-cells, M2-type macrophages, and myeloid-derived suppressor cells. This environment fosters the progression of EC.[29] Among these components, TAMs exert the most significant influence on tumor progression and play a crucial role in evading immune surveillance mechanisms. Their presence often indicates a worsened prognosis.[30-32] Macrophages can be categorized into M1-type macrophages (classically activated), which possess antitumor effects, and M2-type macrophages (alternatively activated), which exhibit pro-tumor effects.[33] Under in vitro conditions, THP-1 cells can be polarized to M1-type macrophages by INF-γ combined with LPS or to M2-type macrophages by IL-4 combined with IL-13 after induction with PMA.[34] In the tumor microenvironment, TAMs predominantly display an M2 phenotype.[35] Studies have demonstrated that TAMs can enhance the malignant biological behavior of tumor cells by secreting a diverse array of mediators capable of remodeling the tumor microenvironment.[36] The protumorigenic properties exhibited by TAMs during tumor progression render them an important therapeutic target in cancer treatment.[37] The current therapeutic strategies for TAMs aim to either directly reduce their abundance or polarize them toward an antitumor phenotype.[38] Therefore, reducing the proportion of M2-type macrophages within the tumor microenvironment remains a valuable direction in the field of tumor therapy. A recent study reported that the knockdown of COL12A1 suppressed the infiltration of M2-type macrophages in breast cancer.[39] Similarly, the present study showed a high expression of COL12A1 in M2-type macrophages. The overexpression of COL12A1 significantly enhanced the proportion of IL-4 combined with IL-13-induced production of M2-type macrophages, indicating that COL12A1 promotes monocyte-macrophage polarization toward the M2 phenotype. Therefore, the pro-carcinogenic effect of COL12A1 on EC may involve its regulation of macrophage M2 polarization. CCK-8, Transwell migration and invasion, and EMT assays were conducted to assess the effects of COL12A1-treated macrophages on proliferation, migration, invasion, and EMT in EC cells. The results revealed that overexpression of COL12A1 further augmented the pro-growth and invasive properties of macrophages toward tumors. COL12A1 not only exerted its promotional effect on EC cells but also regulated the polarization of macrophages within the tumor microenvironment. Liu’s study determined that the genes COL1A1, COL4A1, and COL12A1 were significantly associated with macrophage M2 infiltration in gastric cancer.[26] These experiments collectively illustrated that COL12A1 enhances growth and metastasis in EC cells by promoting M2-type polarization in macrophages.
This study has some limitations. It verified the cell performance characteristics of COL12A1 on macrophage polarization only, and a detailed study of the mechanism was not conducted. In addition, only specific EC cell lines were studied, which may not be representative of the characteristics of all types and subtypes of EC cells, so sample bias may be present. In future studies, multi-omics and bioinformatics analyses regarding EC and COL12A1 could contribute to a deeper understanding of the underlying mechanisms.
CONCLUSION
This study revealed that the expression of COL12A1 was upregulated in EC cells. Knockdown of COL12A1 significantly inhibited EC cell proliferation, migration, invasion, and EMT abilities. Overexpression of COL12A1 may enhance the proliferation and metastatic potential of EC cells by modulating M2-type macrophage polarization. These findings provide experimental and theoretical evidence supporting the utilization of COL12A1 as a biological marker and therapeutic target for EC.
AVAILABILITY OF DATA AND MATERIALS
The data analyzed are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
ZS and QJ: Designed the research study; MH and JL: Performed the research; SW: Collected the data and made statistical analysis; QJ: Help and advice on the experiments; ZS and QJ: Analyzed the data and drafted the manuscript. All authors contributed to important editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ABBREVIATIONS
CCK-8: Cell counting kit-8
CD86/68: Cluster of differentiation 86/68
COL12A1: Collagen type XII α1 chain
DMEM: High-glucose Dulbecco’s modified eagle medium
EC: Endometrial cancer
ECM: Extracellular matrix
EMT: Epithelial-mesenchymal transformation
GAPDH: Glyceraldehyde- 3-phosphate dehydrogenase
HESCs: Human endometrial mesenchymal stromal cells
IFN-γ: Interferon-gamma
IL: Interleukin
LPS: Lipopolysaccharides
PMA: Phorbol 12-myristate 13-acetate
TAMs: Tumor-associated macrophages
ACKNOWLEDGMENT
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The animals used in this study have been approved by the Fuzhou First General Hospital Affiliated with Fujian Medical University Ethics Committe e (No.202210015) .
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: This study is supported by the Fuzhou Health Science and Technology Plan Project (2022-S-wq11).
References
- Association of endometrial cancer risk with postmenopausal bleeding in women: A systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-22.
- [CrossRef] [PubMed] [Google Scholar]
- Cuproptosis patterns and tumor microenvironment in endometrial cancer. Front Genet. 2022;13:1001374.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic and prognostic significance of CD47 expression and tumor-associated macrophages in endometrial carcinoma. Int J Gynecol Pathol. 2022;41:397-406.
- [CrossRef] [PubMed] [Google Scholar]
- The premetastatic lymph node niche in gynecologic cancer. Int J Mol Sci. 2023;24:4171.
- [CrossRef] [PubMed] [Google Scholar]
- The role of tumor-associated macrophages in the progression, prognosis and treatment of endometrial cancer. Front Oncol. 2023;13:1213347.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16:80.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of M1-and M2-polarized macrophages in glioma and as potential treatment targets. Brain Sci. 2023;13:1269.
- [CrossRef] [PubMed] [Google Scholar]
- The immunological landscape of M1 and M2 macrophages and their spatial distribution in patients with malignant pleural mesothelioma. Cancers (Basel). 2023;15:5116.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-associated macrophages promote epithelial-mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun Signal. 2022;20:92.
- [CrossRef] [PubMed] [Google Scholar]
- DLC1 is a prognosis-related biomarker correlated with tumor microenvironment remodeling in endometrial carcinoma. Front Oncol. 2022;12:823018.
- [CrossRef] [PubMed] [Google Scholar]
- An EMT-related genes signature as a prognostic biomarker for patients with endometrial cancer. BMC Cancer. 2023;23:879.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of E-cadherin and n-cadherin in the endocervix as a predictive factor in patients with endometrial cancer. Int J Mol Sci. 2024;25:3547.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic impact of EMT (epithelial-mesenchymal-transition)-related protein expression in endometrial cancer. Cancer Biol Ther. 2013;14:13-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immune modulatory properties of collagen in cancer. Front Immunol. 2021;12:791453.
- [CrossRef] [PubMed] [Google Scholar]
- A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2022;3:90-107.
- [CrossRef] [PubMed] [Google Scholar]
- Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat Commun. 2022;13:4587.
- [CrossRef] [PubMed] [Google Scholar]
- Collagen XII mediated cellular and extracellular mechanisms in development, regeneration, and disease. Front Cell Dev Biol. 2023;11:1129000.
- [CrossRef] [PubMed] [Google Scholar]
- METTL3 facilitates tumor progression by COL12A1/MAPK signaling pathway in esophageal squamous cell carcinoma. J Cancer. 2022;13:1972-84.
- [CrossRef] [PubMed] [Google Scholar]
- COL12A1, a novel potential prognostic factor and therapeutic target in gastric cancer. Mol Med Rep. 2019;20:3103-12.
- [CrossRef] [PubMed] [Google Scholar]
- Bioinformatics analysis identified MMP14 and COL12A1 as immune-related biomarkers associated with pancreatic adenocarcinoma prognosis. Math Biosci Eng. 2021;18:5921-42.
- [CrossRef] [PubMed] [Google Scholar]
- Bioinformatics analysis reveals most prominent gene candidates to distinguish colorectal adenoma from adenocarcinoma. Biomed Res Int. 2018;2018:9416515.
- [CrossRef] [PubMed] [Google Scholar]
- Palmatine protects against MSU-induced gouty arthritis via regulating the NF-κB/NLRP3 and Nrf2 pathways. Drug Des Devel Ther. 2022;16:2119-32.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun Signal. 2018;16:17.
- [CrossRef] [PubMed] [Google Scholar]
- Polarizing macrophages in vitro. Methods Mol Biol. 2018;1784:119-26.
- [CrossRef] [PubMed] [Google Scholar]
- Jian Yun Qing Hua Decoction inhibits malignant behaviors of gastric carcinoma cells via COL12A1 mediated ferroptosis signal pathway. Chin Med. 2023;18:118.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated bioinformatics analysis of expression and gene regulation network of COL12A1 in colorectal cancer. Cancer Med. 2020;9:4743-55.
- [CrossRef] [PubMed] [Google Scholar]
- PABPC1 promotes cell proliferation and metastasis in pancreatic adenocarcinoma by regulating COL12A1 expression. Immun Inflamm Dis. 2023;11:e919.
- [CrossRef] [PubMed] [Google Scholar]
- Features of the immunosuppressive tumor microenvironment in endometrial cancer based on molecular subtype. Front Oncol. 2023;13:1278863.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun (Lond). 2022;42:1112-40.
- [CrossRef] [PubMed] [Google Scholar]
- Perivascular tumor-associated macrophages and their role in cancer progression. Essays Biochem. 2023;67:919-28.
- [CrossRef] [PubMed] [Google Scholar]
- JMJD6 in tumor-associated macrophage regulates macrophage polarization and cancer progression via STAT3/IL-10 axis. Oncogene. 2023;42:2737-50.
- [CrossRef] [PubMed] [Google Scholar]
- d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma. Sci Adv. 2023;9:eadg2697.
- [CrossRef] [PubMed] [Google Scholar]
- The IL-4/13-induced production of M2 chemokines by human lung macrophages is enhanced by adenosine and PGE2. Int Immunopharmacol. 2024;128:111557.
- [CrossRef] [PubMed] [Google Scholar]
- Guiqi Baizhu prescription ameliorates cytarabine-induced intestinal mucositis by targeting JAK2 to inhibit M1 macrophage polarization. Biomed Pharmacother. 2023;164:114902.
- [CrossRef] [PubMed] [Google Scholar]
- Sitravatinib combined with PD-1 blockade enhances cytotoxic T-cell infiltration by M2 to M1 tumor macrophage repolarization in esophageal adenocarcinoma. Carcinogenesis. 2024;45:210-9.
- [CrossRef] [PubMed] [Google Scholar]
- TAM family kinases as therapeutic targets at the interface of cancer and immunity. Nat Rev Clin Oncol. 2023;20:755-79.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic targeting of the functionally elusive TAM receptor family. Nat Rev Drug Discov. 2024;23:201-17.
- [CrossRef] [PubMed] [Google Scholar]
- COL12A1 as a prognostic biomarker links immunotherapy response in breast cancer. Endocr Relat Cancer. 2023;30:e230012.
- [CrossRef] [PubMed] [Google Scholar]








