Translate this page into:
Is programmed death-ligand 1 positivity a predictor of poor survival in patients with different histological subtypes of pancreatic cancer?

*Corresponding author: Ceren Canbey, Department of Medicine Pathology, Bagcilar Research and Training Hospital, University of Health Sciences, Istanbul, Türkiye. drcerencanbey@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Canbey C, Şen S, Karabulut S, Özcan TB, Paşaoğlu E, Altun E, et al. Is programmed death-ligand 1 positivity a predictor of poor survival in patients with different histological subtypes of pancreatic cancer? CytoJournal. 2025;22:47. doi: 10.25259/Cytojournal_2_2025
Abstract
Objective
Pancreatic cancer is the cancer type with the highest mortality rate worldwide, and despite advances in treatment, molecular biomarkers are needed for both early diagnosis for developing targeted therapies and improving survival rates in this challenging malignancy. In our study, the contributions of programmed death-ligand 1 (PD-L1) expression to the determination of pancreatic cancer subtypes and patient prognosis and its impact on survival were investigated.
Material and Methods
Paraffin-embedded tissues from 92 patients diagnosed with pancreatic cancer were included in this study. Tumor-infiltrating lymphocytes (TILs) and lymphocytes in the stromal area within the tumor borders were scored as stromal TILs; lymphocytes in tumor islands were scored as intratumoral TILs. Staining in each area was scored as a percentage, and staining with a score of 5 or more in tumor and immune cells for PD-L1 was scored as positive.
Results
After staining, a score of 5 or more with tPD-L1 staining was used to identify one patient as having micropapillary adenocarcinoma, three patients as having ductal adenocarcinoma, and four patients as having signet ring cell carcinoma. When the clinical parameters and outcomes were compared, a statistically significant difference was found between the histopathologic type of signet ring cell carcinoma and poor differentiation and positivity of PD-L1 expression (P < 0.05). Survival was significantly influenced by tumor location, histopathological subtype, degree of differentiation, PD-L1 expression, and tumor size, with tumor size being the most critical factor (P < 0.05).
Conclusion
Our findings suggest that PD-L1 positivity is notably prevalent in signet ring cell carcinoma of the pancreas and is strongly associated with poor survival outcomes. Given these results, further studies with larger patient cohorts are warranted to validate these observations and explore potential therapeutic implications.
Keywords
Biomarker
Immunohistochemistry
Pancreatic cancer
Programmed cell death ligand 1
INTRODUCTION
According to GLOBOCAN 2020 data, the incidence of pancreatic cancer ranks 14th in the world and 7th in cancer-related deaths due to poor prognosis.[1] Pancreatic cancer, which is extremely fatal, has a 5-year survival rate of approximately 10% from the time of diagnosis. Approximately 80-85% of patients present to the hospital with inoperable metastatic disease.[2,3] In the small subgroup of patients diagnosed with a localized, resectable tumor, the survival rate 5 years after surgery is only approximately 20%. According to a study conducted in Türkiye in 2020, pancreatic cancer was one of the leading causes of cancer-related deaths.[4] Despite the progress made in recent years in diagnostic approaches, perioperative management, radiotherapy techniques, and systemic treatments for progressive disease, little progress has been made in terms of the progression and survival of patients with pancreatic cancer.[5-7] New strategies are needed to detect pancreatic tumors at early stages and to screen and treat high-risk patients.
The blockade of immune checkpoints has become one of the most promising approaches for activating therapeutic antitumor immunity. Programmed death-ligand 1 (PD-L1) is a transmembrane protein that, on binding to its receptor PD-1 on T cells, sends an inhibitory signal that reduces T-cell proliferation and cytokine production.[8] Many tumors upregulate PD-L1 to evade immune surveillance, making PD-L1 an important target for immune checkpoint inhibitors. To escape the immune system, tumor cells in the tumor microenvironment can upregulate PD-L1 expression and bind to PD-1 on the surface of T cells.[9] This allows them to inhibit the functions of T cells and cause T cells to lose their killing effect on tumor cells. Late diagnosis, rapid metastasis, and the limited effectiveness of conventional treatments require research into new treatment approaches. PD-L1, an immune checkpoint protein, plays a crucial role in tumor immune evasion by binding to the PD-1 receptor on T cells, thereby inhibiting their activity.[10] Pancreatic cancer is typically classified as a non-immunogenic tumor, which limits the effectiveness of PD-1/PD-L1 inhibitors, except in patients with microsatellite instability/deficient DNA mismatch repair (MSI/dMMR).[11] In a study examining the expression of the B7 family in pancreatic cancer, only PD-L1 was found to have prognostic significance, with higher PD-L1 expression associated with lower median survival.[12] Similarly, in a study involving 453 patients, PD-L1 positivity was linked to lymphocyte depletion, and these patients had shorter disease-free survival.[13] A meta-analysis revealed that high PD-L1 expression in pancreatic cancer was associated with advanced T stage, positive N stage, and poor differentiation but was not significantly correlated with M stage.[14] Conventionally, tumors have been classified solely on the basis of tumor histology, and classical chemotherapy protocols have been applied. At present, targeted therapies and immunotherapies are gradually being incorporated into treatment protocols, but due to the great diversity of carcinomas, further research is needed.[15] New studies should be conducted to predict which targeted or immunotherapeutic treatments will respond well.
These inhibitors have revolutionized the treatment landscape of many cancers, including melanoma and non-small cell lung cancer, but their role in pancreatic cancer is still under investigation. The PD-1/PD-L1 signaling pathway controls the induction and maintenance of immune tolerance in the tumor microenvironment. The activity of PD-1 and its ligands PD-L1 or PD-L2 is responsible for the activation, proliferation, and cytotoxic secretion of T cells against degenerative antitumor immune responses in cancer.[16] Therefore, examining the PD-L1 score through pathological examination is considered very important for determining the treatment method.
The aim of this study was to investigate the relationships between PD-L1 expression and various histopathological subtypes of pancreatic cancer, evaluating its associations with clinicopathological parameters such as tumor stage, lymph node involvement, differentiation status, and patient survival outcomes. In addition, this study sought to determine the prognostic significance of PD-L1 expression in a cohort of Turkish patients and assess its potential role in guiding immunotherapeutic treatment strategies.
MATERIAL AND METHODS
In this study, 92 patients with a clinical diagnosis of pancreatic cancer were included in the study. After all patients were informed about the aim of this study, they signed the voluntary informed consent form. Paraffin block samples were used from the volunteers who agreed to participate in this study. This study included patients aged 18 years and older who had a histopathologically confirmed diagnosis of pancreatic cancer and available formalin-fixed paraffin-embedded (FFPE) tissue samples suitable for PD-L1 immunohistochemical (IHC) analysis. Patients with complete clinical and pathological data, including tumor stage, histological subtype, and survival outcomes, were enrolled after providing written informed consent. Those with inadequate or poor-quality FFPE samples unsuitable for IHC evaluation were excluded from the study. In addition, patients with a history of other primary malignancies, except for nonmelanoma skin cancer or in situ carcinoma, as well as those who had received prior systemic immunotherapy before tissue collection, were not included in the study. Individuals with severe comorbidities that could affect the study assessments or those who declined to participate were also excluded from the study. PD-L1 expression was evaluated through immunohistochemistry by a team of expert pathologists, ensuring the reliability and accuracy of the results.
Preparation of samples and histomorphological evaluation
All specimens belonging to the selected cases were reevaluated through the tissue microarray method. The area that best represented the tumor and was free of necrosis and artifacts was determined and marked, and the regions corresponding to the tumor areas marked on the slides were separated from the paraffin block using a 6 mm diameter punch biopsy device (LOT No: A01020302, Kai Medical, Japan) used in dermatology clinics. The separated tissues were systematically embedded in new paraffin blocks, and new blocks were made to minimize costs. During the reblocking process, the biopsy protocol numbers were considered and are listed. A total of 92 new paraffin blocks were created. A control block was used for optimal IHC analysis.
FFPE samples were used to evaluate PD-L1 expression, with hematoxylin and eosin (H&E) (Cat #14-5983-82) (Thermo Fisher, USA) staining serving as the initial histopathological evaluation method. Tissue sections 4-5 µm thick were cut from FFPE blocks and mounted on glass slides. The slides were deparaffinized in xylene (LOT: BCCL9397) (Sigma Aldrich, USA) and rehydrated in a graded ethanol (LOT: 64-17-5) (Sigma Aldrich, USA) series. Hematoxylin staining was performed to highlight the nuclear morphology, followed by eosin staining to contrast the cytoplasmic and extracellular matrix components. After dehydration and mounting, the stained sections were examined under a light microscope to assess tissue integrity, morphological features, and the presence of immune and tumor cells. As a result of the examination, which was performed through a U-MDOB3 microscope (serial number: 8H16329) (Olympus, Japan), the H&E-stained samples were re-evaluated according to the World Health Organization 2020 histological classification criteria for pancreatic cancer.[17] H&E staining allowed the identification of tumor regions and immune cell infiltration, which were subsequently used for PD-L1 IHC (clone 22C3, LOT No: 11763243, pharmDx) analysis to allow a more accurate assessment of PD-L1 expression in tumor and immune cells. The staging was performed through the American Joint Committee on Cancer 2017 staging system.
Evaluation of PD-L1 expression through immunohistochemistry
The control blocks were used to ensure the accuracy of the IHC evaluations. One section for hematoxylin and eosin staining and seven sections for IHC analysis were prepared from paraffin blocks obtained through the tissue microarray method. The sections for IHC analysis were cut with a microtome to 2 µm thickness and placed on positively charged coverslips. The sections were deparaffinized in an oven at 60°C for one hour. PD-L1 was applied through a closed system for IHC staining. This was followed by a 30-min pretreatment at 95°C in Dako Pretreatment Solution (K800421-2 EnV FLEX TRS, high pH (50×) (Dako PT Link PT100 Slide Staine, USA). Counterstaining was performed with a Dako Autostainer. PD-L1 was used as the primary antibody. The incubation times of the antibodies were set to 25-30 min. Finally, all coverslips were treated with 96% ethyl alcohol for 3 × 1 min and then with xylene for 3 × 1 min, after which the coverslips were sealed. For PD-L1 evaluation, clone 22C3 was used, while the tonsil tissue served as control tissue. The IHC staining procedure was performed through a standardized protocol. The tissue sections were incubated at 97°C for 40 min under low pH conditions for antigen retrieval, followed by a washing step. Peroxidase blocking reagent and wash buffer were applied sequentially. Primary antibody incubation was carried out for 40 min, followed by washing. A mouse linker was applied for 10 min, followed by another washing step. The samples were then incubated with horseradish peroxidase (HRP) for 20 min, followed by washing. The substrate chromogen was applied for 5 min and then washed. Hematoxylin counterstaining was performed for 6 min, followed by a final wash to complete the procedure.
PD-L1 staining was evaluated separately for membrane and cytoplasmic localization in tumor cells (tPD-L1) and inhibitor PD-L1 (iPD-L1) in the tumor microenvironment. The results were categorized into four groups according to the staining rate. Due to the limited number of cases and the observed PD-L1 staining in the tumor in some cases, a threshold of score 5 was adopted. The percentage of staining in the tumor was classified as follows: ≤1% was regarded as negative, 1-5% as weakly positive, 5-10% as moderately positive, and 10-100% as strongly positive.[18] Accordingly, a score of <5 was considered negative, and a score of >5 was considered positive.
Statistical analysis
Chi-square tests (Pearson’s Chi-square test, Yates’ continuity correction, and Fisher’s exact test) and one-way analysis of variance (ANOVA) were performed through Statistical Package for the Social Sciences (SPSS) statistical software version 30.0 (SPSS Inc., USA) to compare clinical parameters and expression status. Categorical data are represented as n (%). When the expected frequency T ≥ 5 and N ≥ 40, the Pearson Chi-square test was used; when the expected frequency T ≥ 5 and N ≥ 40, the continuity-corrected Chi-square (Yates’ correction) test was applied; and when T < 1, N < 40, or zero samples were present, Fisher’s exact test was conducted. The Kolmogorov‒Smirnov test was applied to assess the normality of the age distribution. The effects of age and sex on expression levels were examined through linear regression analysis. Survival analysis was conducted through the Kaplan‒Meier method, and differences between survival curves were evaluated through the log-rank test. The relationships between survival times and independent variables were assessed through univariate and multivariate Cox proportional hazards regression analyses, with the results visualized through a forest plot. Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported for all Cox regression analyses. P < 0.05 was considered statistically significant.
RESULTS
The clinical parameters of the patients are shown in Table 1. The median age of patients diagnosed with pancreatic cancer was 56.5 years (minimum: 28-maximum: 88). A total of 55.4% of the patients were male (51/92), and 44.6% (41/92) were female. Histologic analysis revealed ductal adenocarcinoma in 52.2% (48/92), neuroendocrine adenocarcinoma in 15.2% (14/92), biliary adenocarcinoma in 14.1% (13/92), intestinal adenocarcinoma in 9.8% (9/92), micropapillary adenocarcinoma in 4.3% (4/92), and signet ring cell carcinoma in 4.3% (8/92) of the patients. Pancreatic tumors were observed in the pancreatic head in 43.5% (40/92), the ampulla in 22.8% (21/92), the pancreatic body in 19.6% (18/92), the pancreatic tail in 3.3% (3/92), the choledoch in 3.3% (3/92), and the pancreatic body in 19.6% (18/92) of the patients. The study revealed poor differentiation in 22.8% (21/92), moderate differentiation in 71.7% (66/92), and good differentiation in 5.4% (5/92) of the patients. Follow-up revealed that 81.5% (75/92) of patients died, and the median survival time was 16 months. In accordance with the 8th edition of the AJCC,[19] a general TNM classification was made on the basis of pathological stage. A total of 2.2% (2/92) of patients were in stage 1a, 8.7% (8/92) were in stage 1b, 29.3% (27/92) were in stage 2a, and 59.8% (55/92) were in stage 2b. A total of 60.9% (56/92) of the tumors were 4 cm or larger, 35.9% (33/92) were between 2 and 4 cm, and 3.3% (3/92) of the samples were smaller than 2 cm.
| Parameters | n | % |
|---|---|---|
| Age -median (min-max) | 56.5 (2-88) | |
| Gender | ||
| Male | 51 | 55.4 |
| Female | 41 | 44.6 |
| Anatomical involvement | ||
| Ampulla | 21 | 22.8 |
| Choledoch | 3 | 3.3 |
| Body | 18 | 19.6 |
| Head | 40 | 43.5 |
| Corpus | 7 | 7.6 |
| Tail | 3 | 3.3 |
| Histopathology | ||
| Ductal adenocarcinoma | 48 | 52.2 |
| Signet ring cell | 4 | 4.3 |
| Neuroendocrine | 14 | 15.2 |
| Intestinal type | 9 | 9.8 |
| Biliary type | 13 | 14.1 |
| Micropapillary adenocarcinoma | 4 | 4.3 |
| Tumor size | ||
| <2 cm | 3 | 3.3 |
| 2-4 cm | 33 | 35.9 |
| >4 cm | 56 | 60.9 |
| Degree of differentiation | ||
| Low | 21 | 22.8 |
| Medium | 66 | 71.7 |
| High | 5 | 5.4 |
| Overall stage | ||
| Stage Ia | 2 | 2.2 |
| Stage Ib | 8 | 8.7 |
| Stage IIa | 27 | 29.3 |
| Stage IIb | 55 | 59.8 |
| Survival status | ||
| Deceased | 75 | 81.5 |
| Alive | 17 | 18.5 |
Evaluation of PD-L1 expression by immunohistochemistry
In our study, PD-L1 staining was examined separately in tumor cells (tPD-L1) and immune cells in the immune microenvironment of the tumor (iPD-L1). A cutoff value of 5 was defined for the evaluation. According to this value, staining rates below score 5 were classified as negative, and rates above score 5 were classified as positive. Among the examined cases, tumor cell (tPD-L1) staining was detected in eight cases, and no staining was detected in 84 cases. One patient with tPD-L1 staining was identified as having micropapillary adenocarcinoma, three as having ductal adenocarcinoma, and four as having signet ring cell carcinoma. Some examples of positive PD-L1 staining are shown in Figure 1. Positive immunoreactivity with PD-L1 in lymphocytes in ductal adenocarcinoma is shown in Figure 1a; score 5 immunoreactivity with PD-L1 in tumor cells in mixed-type ductal adenocarcinoma and signet ring cells is shown in Figure 1b; score 5 immunoreactivity with PD-L1 in tumor cells in micropapillary adenocarcinoma is shown in Figure 1c; and score 10 immunoreactivity with PD-L1 only in tumor cells in ductal adenocarcinoma is shown in Figure 1d.
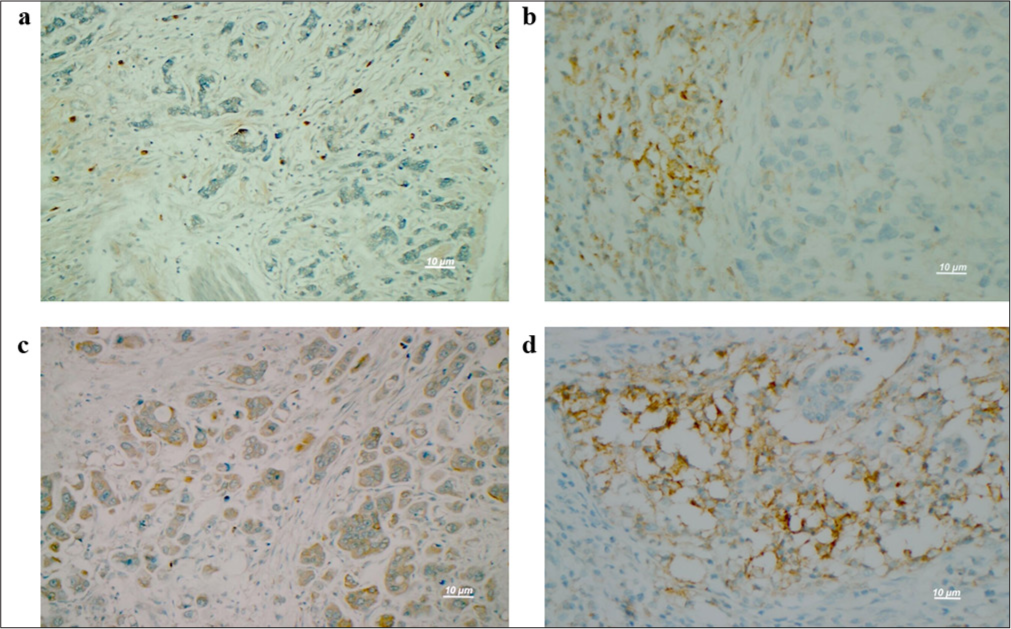
- Evaluation of immunoreactivity in pathological samples. (a) In ductal-type adenocarcinoma tumors, immunoreactivity with PD-L1 was detected only in lymphocytes (PD-L1 ×400). (b) In mixed-type adenocarcinoma with ductal and signet ring cells, immunoreactivity with a PD-L1 score of 5 was observed in tumor cells (PD-L1 ×400). (c) In micropapillary adenocarcinoma, tumor cells (PD-L1 ×400) with a score of 5 were immunoreactive for PD-L1. (d) In ductal-type adenocarcinoma, immunoreactivity to PD-L1 was indicated by a score of 10 or greater in tumor cells (PD-L1 ×400). Scale bar = 10 μm. PD-L1: Programmed death-ligand 1.
Hematoxylin and eosin staining at ×400 magnification revealed a mixed-type adenocarcinoma with ductal and signet ring cells, as shown in Figure 2a, and one carcinoma infiltration with local mucinous areas of ductal adenocarcinoma, as shown in Figure 2b. Figure 2c shows a carcinoma infiltration with local peritumoral lymphocytes of ductal adenocarcinoma, and Figure 2d shows a tumor with infiltration in the form of solid layers and nests of a neuroendocrine carcinoma.
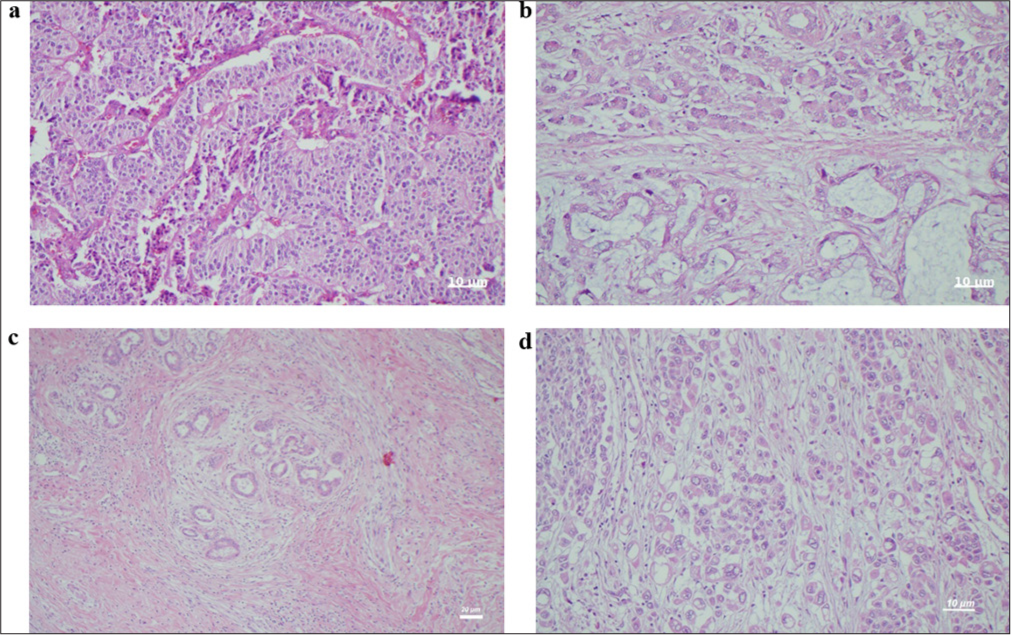
- Hematoxylin and eosin (H&E) staining of pathologic tissue samples at ×400. (a) Mixed-type adenocarcinoma with ductal and signet ring cells (H&E ×400). Scale bar = 10 μm. (b) Carcinoma infiltration with local mucinous areas of ductal adenocarcinoma (H&E ×400). Scale bar = 10 µm. (c) Carcinoma infiltration with local peritumoral lymphocytes of ductal adenocarcinoma (H&E ×100). Scale bar = 20 µm. (d) Neuroendocrine carcinoma tumor with infiltration in the form of solid layers and nests (H&E ×400). Scale bar = 10 µm.
Correlation of results with clinical parameters
Relationships between variables related to the presence or absence of PD-L1 expression were examined through the Chi-square test, and P-values and descriptive statistics obtained are presented in Table 2. The Chi-square test was used for >5 samples, and Fisher’s exact test was used for <5 samples. A statistically significant correlation was found between PD-L1 positivity and tumor location {(χ2 (1, N = 92) = 1.14, P < 0.001)}, histological subtype {(χ2 (5, n = 92) = 47.129, P < 0.001)}, and degree of differentiation {χ2(2, N = 92) = 7.950, P = 0.019)}. No statistically significant differences were found with respect to the other clinical parameters. According to the one-way ANOVA results, significant differences were found among the six histological subgroups in terms of the dependent variable {F(3.74, 3.56) = 18.07, P < 0.001, η2 = 0.51}. Post hoc analyses revealed a significant difference between the patient group with signet-ring cell carcinoma histology and the other five subtypes (P < 0.001). PD-L1 expression was observed in 30% of patients with poorly differentiated features. According to the ANOVA results, significant differences were found among the three differentiation degree groups in terms of the dependent variable {F(0.61-6.67)=4.21, P = 0.018, η2 =0.86}. Post hoc analyses revealed a statistically significant difference between the poorly differentiated group and the moderately differentiated group (P = 0.017).
| Parameters | n(%) | PD-L1 (−) | PD-L1 (+) | P-value |
|---|---|---|---|---|
| Age -median (min-max) | 56.5 (28-88) | |||
| Gender | ||||
| Male | 51 (55.4) | 48 | 3 | 0.285 |
| Female | 41 (44.6) | 36 | 5 | |
| Anatomical involvement | ||||
| Ampulla | 21 (22.8) | 20 | 1 | 0.466 |
| Choledoch | 3 (3.3) | 3 | 0 | |
| Body | 18 (19.6) | 17 | 1 | |
| Head | 40 (43.5) | 35 | 5 | |
| Corpus | 7 (7.6) | 7 | 0 | |
| Tail | 3 | 2 | 1 | |
| Histopathology | ||||
| Ductal adenocarcinoma | 48 (52.2) | 45 | 3 | <0.001✶ |
| Signet ring cell | 4 (4.3) | 0 | 4 | |
| Neuroendocrine | 14 (15.2) | 14 | 0 | |
| Intestinal type | 9 (9.8) | 9 | 0 | |
| Biliary type | 13 (14.1) | 13 | 0 | |
| Micropapillary adenocarcinoma | 4 (4.3) | 3 | 1 | |
| Tumor size | ||||
| <2 cm | 3 (3.3) | 3 | 0 | 0.624 |
| 2-4 cm | 33 (35.9) | 29 | 4 | |
| >4 cm | 56 (60.9) | 52 | 4 | |
| Degree of differentiation | ||||
| Low | 21 (22.8) | 16 | 5 | 0.019✶ |
| Medium | 66 (71.7) | 63 | 3 | |
| High | 5 (5.4) | 5 | 0 | |
| Overall stage | ||||
| Stage Ia | 2 (2.2) | 2 | 0 | 0.677 |
| Stage Ib | 8 (8.7) | 7 | 1 | |
| Stage IIa | 27 (29.3) | 26 | 1 | |
| Stage IIb | 55 (59.8) | 49 | 6 | |
| Survival | ||||
| Deceased | 75 (81.5) | 67 | 8 | 0.159 |
| Alive | 17 (18.5) | 17 | 0 | |
The Chi-square test was used for > 5 samples, and Fisher’s exact test was used for <5 samples.
The impact of the clinical characteristics of patients diagnosed with pancreatic cancer on overall survival was analyzed through the Kaplan‒Meier and Cox regression methods [Figure 3]. Survival analyses for all clinical parameters are shown in Table 3. According to the Kaplan‒Meier analysis, the median survival time for PD-L1-positive pancreatic cancer patients was 9 months (95% CI, 7.658-10.342), whereas for PD-L1-negative patients, it was 17 months (95% CI, 15.403-18.597). The difference between the two groups was statistically significant (log-rank test, P = 0.006). The median survival time for patients with signet-ring cell pancreatic cancer was 8 months (95% CI, 5.346-10.654), and the difference between this group and other histological subgroups was highly statistically significant (log-rank test, P < 0.001). When survival times were analyzed according to the anatomical location of tumor involvement, the median survival time for patients whose tumors were located in the pancreatic tail was 5.5 months (95% CI, 2.560-8.440). The difference in survival time compared with other anatomical regions was highly statistically significant (log-rank test, P < 0.001). The survival outcomes of pancreatic cancer patients were analyzed based on tumor differentiation grade. The median survival time for patients with well-differentiated tumors was 29 months (95% CI, 24.199-33.801), and this group was found to have significantly greater survival than the other groups (log-rank test, P = 0.005). The survival time was shorter for patients with larger tumors, but this difference was not statistically significant (P = 0.219). Although the risk ratio (HR = 15.574) was greater in patients with tumors larger than 4 cm than in the other groups, this result is not clear due to the wide confidence intervals. The survival rates decreased with stage 2b disease, but the difference between the groups was not statistically significant (P = 0.475). Although the risk ratio (HR = 15.868) was greater, the confidence intervals were wide.
| Parameters | n(%) | Estimated HR | Std. Error | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Anatomical involvement | ||||||
| Ampulla | 51 (55.4) | 15.714 | 1.652 | 12.476 | 18.953 | <0.001✶ |
| Choledoch | 41 (44.6) | 12.667 | 2.667 | 7.440 | 17.893 | |
| Body | 21 (22.8) | 15.722 | 1.873 | 12.051 | 19.393 | |
| Head | 3 (3.3) | 17.865 | 1.219 | 15.476 | 20.255 | |
| Corpus | 18 (19.6) | 19.571 | 2.768 | 14.147 | 24.996 | |
| Tail | 40 (43.5) | 5.500 | 1.500 | 2.560 | 8.440 | |
| Histopathology | ||||||
| Ductal adenocarcinoma | 7 (7.6) | 16.600 | 0.948 | 14.742 | 18.458 | <0.001✶ |
| Signet ring cell | 3 | 8.000 | 1.354 | 5.346 | 10.654 | |
| Neuroendocrine | 48 (52.2) | 13.000 | 3.733 | 5.684 | 20.316 | |
| Intestinal type | 4 (4.3) | 21.600 | 3.234 | 15.261 | 27.939 | |
| Biliary type | 14 (15.2) | 17.385 | 2.197 | 13.078 | 21.691 | |
| Micropapillary adenocarcinoma | 9 (9.8) | 21.750 | 1.493 | 18.824 | 24.676 | |
| Tumor size | ||||||
| <2 cm | 13 (14.1) | 12.000 | 0.000 | 12.000 | 12.000 | 0.219 |
| 2-4 cm | 4 (4.3) | 18.434 | 1.345 | 15.797 | 21.071 | |
| >4 cm | 3 (3.3) | 15.574 | 1.033 | 13.549 | 17.600 | |
| Degree of differentiation | ||||||
| Low | 33 (35.9) | 13.000 | 3.182 | 6.763 | 19.237 | 0.005✶ |
| Medium | 56 (60.9) | 16.000 | 1.122 | 13.800 | 18.200 | |
| High | 21 (22.8) | 29.000 | 2.449 | 24.199 | 33.801 | |
| Overall stage | ||||||
| Stage Ia | 66 (71.7) | - | - | - | - | 0.475 |
| Stage Ib | 5 (5.4) | 19.429 | 3.054 | 13.443 | 25.414 | |
| Stage IIa | 2 (2.2) | 17.217 | 1.540 | 14.199 | 20.236 | |
| Stage IIb | 8 (8.7) | 15.868 | 1.016 | 13.877 | 17.858 | |
| PD-L1 expression | ||||||
| No | 8 (8.7) | 17.000 | 0.815 | 15.403 | 18.597 | 0.006✶ |
| Yes | 84 (91.3) | 9.000 | 0.685 | 7.658 | 10.342 | |
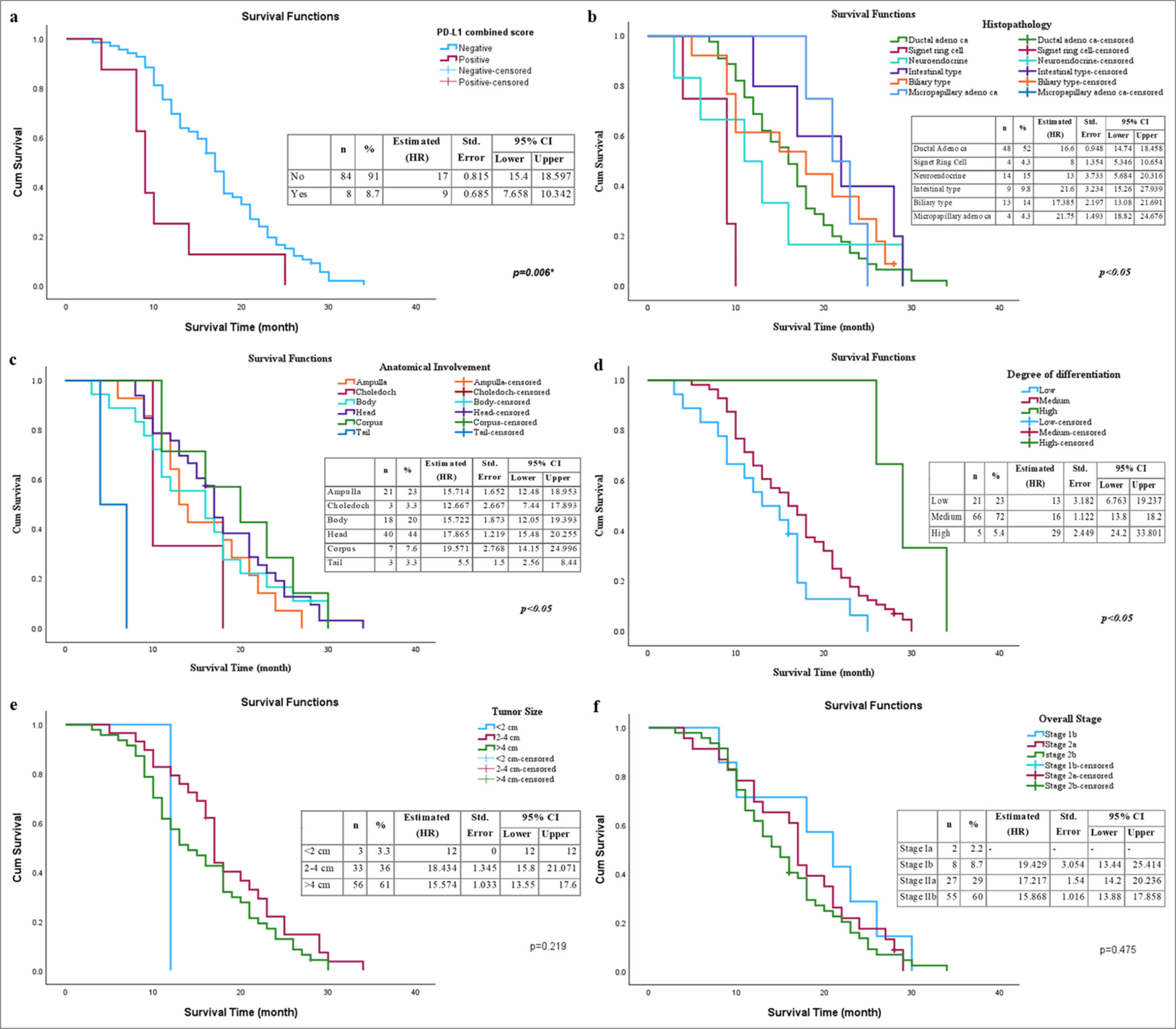
- Effects of clinical parameters and PD-L1 status on overall survival in patients with pancreatic cancer. (a) PD-L1 combined expression status; (b) anatomical involvement region; (c) histological subtype; (d) degree of differentiation; (e) tumor size; and (f) overall stage (✶P < 0.05 is statistically significant). PD-L1: Programmed death-ligand 1.
A forest plot was generated based on the univariate and multivariate analysis results of the survival status of pancreatic cancer patients [Figure 4]. In the univariate analysis [Figure 4a], tumor size (P = 0.010, HR = 3.801, CI: 1.372-10.530) and degree of differentiation (P = 0.050, HR = 0.579, 95% CI: 0.335-1.001) were significantly associated with survival. Other variables, including sex, location, histopathological type, PD-L1 score, and overall stage, were not statistically significant. In the multivariate analysis [Figure 4b], tumor location (P = 0.039, HR = 0.482, 95% CI: 0.241-0.963) and histopathological type (P = 0.016, HR = 0.636, 95% CI: 0.441-0.918) were independently and significantly associated with survival. However, the PD-L1 score, tumor size, overall stage, sex, and degree of differentiation were not significant in the multivariate analysis.
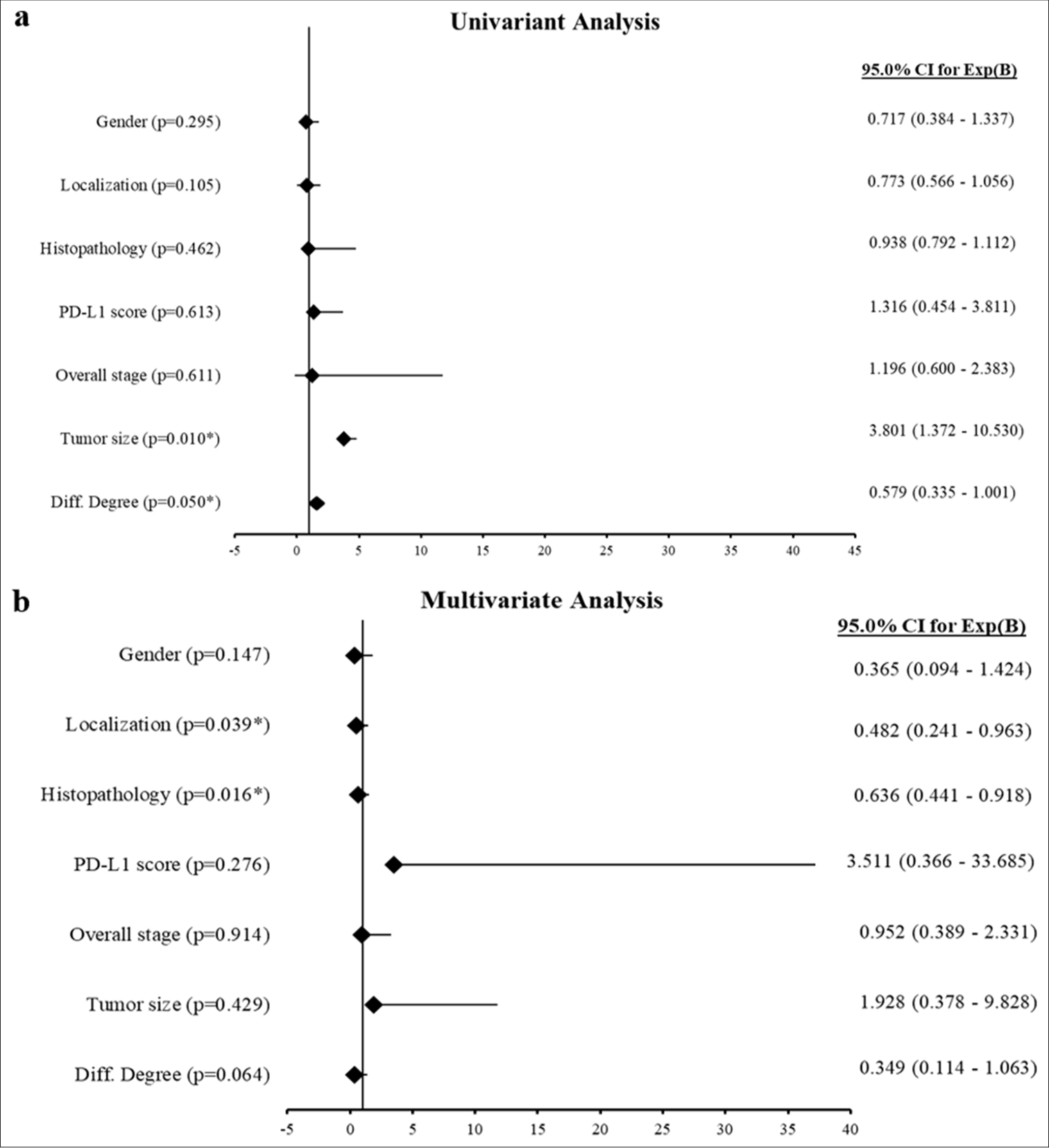
- Forest plots representing Cox regression analyses. (a) Univariate analysis: The forest plot displays the hazard ratios (HRs) with 95% confidence intervals (Cis) for the univariate Cox regression analysis of clinical variables. (b) Multivariate analysis: The forest plot illustrates the HRs with 95% CIs for the multivariate Cox regression analysis, evaluating the independent effects on survival (✶P < 0.05 is statistically significant). PD-L1: Programmed death-ligand 1.
DISCUSSION
Pancreatic carcinomas are heterogeneous cancers that comprise various subtypes and are often diagnosed in advanced stages. Despite the primary treatment methods of surgery and chemotherapy, the disease-free survival rate in these patients remains low. Local recurrences and metastases are frequently observed, and tumor cells often develop resistance to chemotherapy. New therapeutic strategies, such as immunotherapy, are therefore needed. The aim of this study was to investigate the relationships among PD-L1 expression, clinicopathologic parameters, and prognosis in patients with different histopathologic subtypes of pancreatic cancer. In addition, the potential use of immunotherapeutic agents currently used to treat carcinomas in other organs will be investigated in pancreatic carcinomas.
Several studies have investigated PD-L1 expression in pancreatic cancer through IHC techniques and have provided valuable information about its role in tumor biology and prognosis. Collectively, these studies support the importance of PD-L1 as a critical factor in pancreatic cancer pathology and its potential usefulness in guiding treatment strategies. Similar results have been obtained in the literature, indicating that PD-L1 expression in pancreatic cancer tissues is associated with poor prognosis. Nomi et al. investigated PD-L1 expression in 51 patients diagnosed with pancreatic cancer and reported that PD-L1-positive patients had a worse prognosis; therefore, the PD-L1/PD-1 pathway may be a critical regulator.[20] Similarly, in a study by Wang et al., PD-L1 was reported to inhibit the activation of CD4+ and CD8+ T cells in the tumor microenvironment and promote tumor growth, leading to a poor prognosis in PD-L1-positive patients.[21] Another study reported that PD-L1 positivity was predictive of poor prognosis in patients with pancreatic cancer and that its expression was significantly associated with tumor stage and the pre-operative serum CA19-9 level.[22] Geng et al. reported that PD-L1 overexpression in pancreatic carcinoma tissues may be associated with tumor progression and invasiveness and that this significantly correlated with poor overall survival.[23] Overall, PD-L1 expression is associated with poor clinical outcomes in patients with pancreatic cancer. Previous studies in the literature have shown that membranous PD-L1 expression is rare in malignant types of the pancreas.[21,24,25] The lack of PD-L1 expression is thought to be responsible for the ineffectiveness of anti-PD-L1/PD-L1 antibodies in treating pancreatic cancer. PD-L1 expression is activated by oncogenic signaling in tumor cells as a result of the adaptive immune response or by inflammatory cytokines, especially interferon-gamma.[26] Due to the cold tumor characteristic of pancreatic cancer, it has been reported in the literature that there is no inflammatory signaling required for effective T-cell infiltration, thus activating PD-L1 expression.[25,27,28]
Whether oncogenic signaling activates PD-L1 expression in pancreatic cancer and its subtypes has not yet been sufficiently investigated. Therefore, our study contributes to the literature on this subject. In a study by Yang et al., PDL1 expression levels were examined in pancreatic cancer tissues through IHC, and high PD-L1 expression levels were reported to be associated with poor prognosis. In this study, patients with high PD-L1 and B7-H4 expression constituted a new subgroup and exhibited an immune-cold phenotype that may not be suitable for immunotherapy.[29] In another study, PD-L1 and PD-1 statuses were examined through the IHC method in FFPE samples from 376 patients diagnosed with pancreatic cancer, and PD-L1 positivity was reported to be present in 3.2% of the patients, whereas PD-L1 positivity was observed in 7.5% of the patients.[30] In the same study, PD-L1 expression was associated with lymph node metastasis predominantly in membranous tumor cells, and as a result, PD-L1 positivity was proven to be an independent prognostic factor for poor prognosis.
The examination of PD-L1 expression in the tumor cells of pancreatic cancer patients provides critical insights into the molecular characteristics of different histological subtypes. Among the total cases evaluated, tPD-L1 staining was observed in only eight samples, with the majority exhibiting no staining. Notably, among the patients with positive tPD-L1 expression, one was classified as micropapillary adenocarcinoma, three as ductal adenocarcinoma, and four as signet-ring cell carcinoma. This distribution indicates that PD-L1 expression may be more prevalent in certain histological types, particularly in more aggressive variants, such as micropapillary and signet-ring cell carcinomas. The limited number of cases with positive staining suggests that PD-L1 may not be a common feature across all pancreatic cancer subtypes, raising questions about its role in tumor biology and potential implications for targeted therapies. Given that PD-L1 is often associated with immune evasion, understanding the context in which it is expressed could be crucial for developing effective immunotherapeutic strategies. Furthermore, these findings highlight the need for further research to explore the biological significance of tPD-L1 expression in pancreatic cancer and its potential as a biomarker for treatment response, particularly in patients with more aggressive tumor characteristics.
In this study, PD-L1 was positively detected in four signet ring cell tumors of the pancreas. According to the literature, pancreatic ring cell carcinoma is a rare histopathological variant of pancreatic cancer with an incidence of <1%. Pancreatic ring cell carcinoma is a rare and aggressive cancer with highly variable patient outcomes due to limited epidemiological data and a lack of standardized treatment strategies. Difficulties in the use of chemotherapy have also been reported in a case study.[31] Compared with pancreatic adenocarcinoma, signet ring cell carcinoma generally has a lower 5-year survival rate and is more likely to have distant metastases at the time of diagnosis.[32] PD-L1 positivity in pancreatic cancer has generally been associated with a poor prognosis.[33] The poor prognosis and insensitivity to immunotherapy in patients with signet ring cell carcinoma and PD-L1 positivity in the sample group in our study are consistent with the literature. No study showing PD-L1 positivity in signet ring cell carcinoma of the pancreas has been reported in the literature. Although the insufficient number of our signet ring cell carcinoma patients is an important factor, it is thought that studies conducted on a larger cohort will make a significant contribution.
In our study, the average survival time of the patients was approximately 13 months, and the poor progression of the patients was consistent with the literature.[34] The analysis of survival outcomes in pancreatic cancer patients revealed significant associations between clinical characteristics and overall survival, emphasizing the heterogeneity of this malignancy. The Kaplan‒Meier method demonstrated that PD-L1 expression is a critical prognostic factor, with PD-L1-positive patients exhibiting a notably shorter median survival time of 9 months than 17-month-old PD-L1-negative patients. When other studies in the literature were examined, PD-L1 expression was reported to be associated with low survival.[12-14] These findings suggest that PDL1 may play a role in tumor biology and the response to treatment, potentially guiding therapeutic strategies in this patient population. No study examining the differences in survival between signet ring cell pancreatic carcinoma and different anatomical regions of the pancreas has been reported in the literature. Our analysis also demonstrated that the histological subtype significantly influences survival. Among the different histological types, patients diagnosed with signet-ring cell carcinoma had the shortest median survival time (8 months, P < 0.001). This aggressive behavior of signet-ring cell carcinoma is consistent with previous reports, where this subtype has been associated with high metastatic potential and poor prognosis. In contrast, patients with micropapillary adenocarcinoma and intestinal-type adenocarcinoma had longer survival times, suggesting that histological classification could play a role in treatment stratification. Tumor localization is another crucial determinant of survival. Patients with tumors located in the pancreatic tail had a significantly shorter median survival time (5.5 months, P < 0.001) than those with other anatomical sites. The poor prognosis associated with pancreatic tail tumors might be due to their late-stage diagnosis, as tumors in this region often remain asymptomatic until they reach an advanced stage. This finding underscores the importance of early detection strategies, particularly for tumors arising in the pancreatic tail. When we examine patients with poor prognosis and low survival, it is noted that they not only are aggressive histological types of signet ring cell carcinoma but also poorly differentiated. Tumor differentiation has also emerged as a significant prognostic factor. Patients with well-differentiated tumors had the longest survival (29 months, P = 0.005), whereas poorly differentiated tumors were associated with worse outcomes. This finding is in accordance with prior studies, which have shown that tumor differentiation is directly correlated with biological aggressiveness and response to therapy. Interestingly, although tumor size was expected to influence survival, our findings did not reveal a statistically significant association (P = 0.219). Patients with tumors >4 cm had a higher HR = 15.574, but the wide confidence intervals indicate uncertainty in the risk estimation. These findings suggest that tumor size alone may not be a strong predictor of survival in patients with pancreatic cancer and should be considered alongside other factors, such as tumor differentiation and anatomical location. Similarly, the overall stage of the disease did not significantly impact survival (P = 0.475). Although stage 2b patients exhibited a lower survival rate, the wide confidence intervals for HR = 15.868 suggest variability in survival outcomes, which may be influenced by treatment modalities, molecular characteristics, or patient-specific factors. These insights underscore the need for a nuanced understanding of individual patient characteristics to optimize management strategies and improve survival outcomes in patients with pancreatic cancer. Finally, our study highlights the importance of PD-L1 expression, histological subtype, tumor differentiation, and tumor localization as key determinants of survival in pancreatic cancer patients. These findings provide a basis for risk stratification and targeted therapeutic approaches.
The presence of crossover points in survival curves has been widely discussed in the literature and is often an indication of non-proportional hazards. While the assumption of proportional hazards is commonly used in survival analysis, deviations from this assumption may require alternative modeling approaches, such as time-dependent covariates or landmark analyses. While PD-L1 was not identified as an independent prognostic factor in our multivariate analysis, its potential role in shaping immune interactions and therapy responses in pancreatic cancer warrants further investigations with larger cohorts and functional studies. Increasing the sample size and refining subgroup analyses could further clarify the impact of specific prognostic factors and reduce the influence of crossover points in survival curves. Future studies that incorporate time-varying covariates and alternative survival models could help improve the accuracy of survival predictions and gain deeper insights into the mechanisms of disease progression.
Our results revealed that only 8% of patients with pancreatic cancer are PD-L1 positive. However, pancreatic cancer is known for its poor prognosis and cold tumor status, making it poorly responsive to treatments, especially PD-L1-targeted therapies.[35] One limitation of our study is likely due to the use of the IHC method itself. Since there are no standardized guidelines for IHC staining, there are often conflicting results across institutions when different protocols and antibodies are used.[36] Problems with sensitivity and specificity can arise from variations in tissue fixation, slide thickness, and antigen retrieval, as well as inadequate quality control measures beyond antibodies.[37] To enable the systematic use of IHC-based biomarkers, detailed and standardized protocols are essential. Furthermore, a single biomarker is often not sufficient to reliably classify patients. The combination of IHC with genomic and transcriptomic methods can help to identify more accurate and predictive biomarkers. While IHC-based methods are powerful, their integration with other approaches or their further development into multiplex and quantitative methods could accelerate biomarker discovery. While our study provides valuable insights into the prognostic factors of pancreatic cancer, several limitations must be acknowledged. The retrospective nature of the study and the relatively small sample size, particularly for specific subgroups such as signet-ring cell carcinoma, may limit the generalizability of our findings. In addition, molecular and genetic factors were not extensively analyzed, which could further refine risk stratification. Future studies should focus on validating these findings in larger, multicenter cohorts and exploring the potential of PD-L1 inhibitors in the treatment of pancreatic cancer. Moreover, integrating molecular markers with clinical parameters may lead to more precise prognostic models and personalized treatment approaches.
SUMMARY
Our study emphasized the association between PD-L1 expression and poor clinical outcomes in patients with pancreatic cancer, particularly in aggressive subtypes such as signet ring cell carcinoma. The low prevalence of PD-L1 positivity underscores the challenges in treating this aggressive malignancy, which responds poorly to conventional therapies, including immunotherapy. Our results show that high PD-L1 expression is correlated with lower survival rates and poorer prognosis, especially for patients with tumors in the ampulla and tail regions. These findings emphasize the potential of PD-L1 as a biomarker for predicting treatment outcomes, particularly in high-risk patients. The incorporation of PD-L1 status into clinical decisions is critical for the development of targeted therapies to improve treatment efficacy and survival rates.
AVAILABILITY OF DATA AND MATERIALS
All data and materials used in this study are publicly available.
ABBREVIATIONS
AJCC: American Joint Committee on Cancer
ANOVA: One-way analysis of variance
Cis: Confidence intervals
dMMR: Deficient DNA mismatch repair
FFPE: Formalin-fixed paraffin-embedded
GLOBOCAN: Global Cancer Observatory
HRP: Horseradish peroxidase
HRs: Hazard ratios
IHC: Immunohistochemical
iPD-L1: Inhibitor PD-L1
MSI: Microsatellite instability
PD-L1: Programmed death-ligand 1
SPSS: Statistical Package for the Social Sciences
TILs: Tumor-infiltrating lymphocytes
TNM: Tumor, Node, Metastasis Staging System
tPD-L1: Tumor cell PD-L1
η2: Eta-squared
χ2: Chi-square value
AUTHORS’ CONTRIBUTIONS
CC: Conceptualization; CC: Methodology; CC and SŞ: Formal analysis and investigation; CC, SŞ, SK, TBÖ, EP, and EA: Writing – original draft preparation; and CC: Writing – review and editing. CC and TYK: Resources; and CC and FE: Supervision. All authors meet ICMJE authorship requirements.
ACKNOWLEDGMENT
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in its latest version) and was approved by the Clinical Research Ethics Committee of Kartal Kosuyolu Training and Research Hospital (Date: 14.12.2021/No: 2021/18/562). The authors certify that they have obtained all appropriate patient consent.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: Not applicable.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- The American cancer society's facts and figures: 2020 edition. J Adv Pract Oncol. 2020;11:135-6.
- [CrossRef] [PubMed] [Google Scholar]
- Has the cancer-related death trend been changing in Türkiye? An evaluation of the period between 2009 and 2019. Cancer Epidemiol. 2022;80:102228.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267:936-45.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cancer: A review of current treatment and novel therapies. J Invest Surg. 2023;36:2129884.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. Am Soc Clin Oncol. 2018;36:LBA4002.
- [CrossRef] [Google Scholar]
- Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer. 2024;23:108.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359-70.
- [CrossRef] [PubMed] [Google Scholar]
- A snapshot of the PD-1/PD-L1 pathway. J Cancer. 2021;12:2735-46.
- [CrossRef] [PubMed] [Google Scholar]
- PD-1/PD-L1 expression in pancreatic cancer and its implication in novel therapies. Med Pharm Rep. 2021;94:402-10.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98-109.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of PDL1 expression in pancreatic cancer. Oncotarget. 2016;7:71198-210.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of PD-L1 expression in patients with pancreatic cancer: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2019;98:e14006.
- [CrossRef] [PubMed] [Google Scholar]
- Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237-51.
- [CrossRef] [PubMed] [Google Scholar]
- The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182-188.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic and predictive significance of PD-L1 expression in non-small cell lung cancer patients: A single-center experience. Turk Patoloji Derg. 2021;37:239-48.
- [CrossRef] [PubMed] [Google Scholar]
- The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059-65.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of B7-H1 protein in human pancreatic carcinoma tissues and its clinical significance. Ai Zheng. 2009;28:1328-32.
- [CrossRef] [PubMed] [Google Scholar]
- B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134:1021-7.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616-31.
- [CrossRef] [PubMed] [Google Scholar]
- PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37.
- [CrossRef] [PubMed] [Google Scholar]
- CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518-27.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance and correlation of PD-L1, B7-H3, B7-H4, and TILs in pancreatic cancer. BMC Cancer. 2022;22:584.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 in pancreatic ductal adenocarcinoma: A retrospective analysis of 373 Chinese patients using an in vitro diagnostic assay. Diagn Pathol. 2018;13:5.
- [CrossRef] [PubMed] [Google Scholar]
- Primary signet ring cell carcinoma of the pancreatic head: A case report. Clin Case Rep. 2019;7:2235-8.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of epidemiological factors and treatment interventions on survival in patients with signet ring cell carcinoma of the pancreas. Am J Clin Oncol. 2018;41:1176-84.
- [CrossRef] [PubMed] [Google Scholar]
- Primary pancreatic signet ring cell carcinoma: A case report and review of the literature. J Pancreat Cancer. 2021;7:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis and survival analysis of patients with pancreatic cancer: Retrospective experience of a single institution. World J Surg Oncol. 2022;20:11.
- [CrossRef] [PubMed] [Google Scholar]
- Immunologic strategies in pancreatic cancer: Making cold tumors hot. J Clin Oncol. 2022;40:2789-805.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic and predictive immunohistochemistry-based biomarkers in cancer and immunotherapy. Hematol Oncol Clin North Am. 2019;33:291-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody identification for antigen detection in formalin-fixed paraffin-embedded tissue using phage display and naive libraries. Antibodies (Basel). 2021;10:4.
- [CrossRef] [PubMed] [Google Scholar]









