Translate this page into:
Liquid based material from fine needle aspirates from breast carcinomas offers the possibility of long-time storage without significant loss of immunoreactivity of estrogen and progesterone receptors
* Corresponding Author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Estrogen receptor (ER) status and progesterone receptor (PgR) status are strong prognostic and predictive markers in breast carcinomas. Steroid receptors are fragile and optimal handling of both cytological and histological material, including fixation, is crucial. Liquid based material offers the possibility to prepare a number of slides from one lesion and is increasingly being used for immunocytochemistry. It also offers the possibility to prepare several smears and to store these at different temperatures as well as storing residual material in the liquid.
Materials and Methods:
The samples consisted of fine needle aspirate material from 53 breast carcinomas. Direct smears and liquid based preparations were used in parallel for immunocytochemical detection of ER and PgR receptor status. Slides from liquid suspensions were stored at -20°C and -74°C for 3 and 6 months, respectively. Direct smears were fixed primarily in 4% formalin. Liquid based specimens were post-fixed in 4% formalin. All specimens were subjected to microwave-stimulated epitope retrieval. Antibody concentrations were ER 1:150 and PgR 1:200 for both preparation methods. The immunostaining program was identical for both the methods.
Results:
Liquid based specimens had a statistically non-significant higher percentage of positive cases compared to direct smears. Specimens prepared from liquid suspensions and stored at −20°C and −74°C for 3 and 6 months, respectively, showed a virtually unchanged ER and PgR reactivity (P = 0.002).
Conclusions:
Liquid suspensions and liquid based slide preparations seem to offer an optimal pre-fixation and preservation of ER/PgR in breast carcinoma cells. Post-fixation with 4% formalin followed by microwave-stimulated epitope retrieval before immunostaining is recommended. Long-time storage of liquid based specimens at -20°C or -74°C for at least 6 months without significant loss of immunoreactivity is feasible. They may be used as internal positive and negative controls.
Keywords
Breast carcinoma
estrogen receptor
fine needle aspiration cytology
liquid based preparation
long-time storage
INTRODUCTION
Estrogen receptor (ER) status and progesterone receptor (PgR) status are strong prognostic and predictive markers in breast carcinomas.[1]
Receptor status is most commonly assessed on histological material, but can be determined safely on fine needle aspiration cytology (FNAC) material, provided the institutions have expertise in doing this.[2]
Steroid receptors are fragile and optimal handling of both cytological and histological material, including fixation, is crucial. In histology, both are subject to rigorous quality control (QC) and standardizing. Some argue that immunocytochemistry (ICC) is more unreliable and unpredictable than immunhistochemistry (IHC), partly due to lack of QC and standardizing.
Reported studies show an overall good agreement between ER/PgR ICC and IHC,[3–7] with a concordance for ER of 91-99% and for PgR of 76-96%. In an earlier study, we demonstrated good agreement between our in-house method and biochemical determination of ER/PgR status.[8] We had noticed, however, that in a routine setting, there were occasional discrepancies with IHC, i.e., some ICC ER and/or PgR negative cases turned out positive when they were repeated on IHC.
Liquid based material offers the possibility to prepare a number of slides from one lesion and is increasingly being used for ICC. One aim of this study was to compare a method using liquid based preparations for ER/PgR determination with our in-house method using direct quick-frozen smears.
Liquid based preparations offer the ability to prepare several smears and to store these at different temperatures as well as storing residual material in the liquid. Our second aim was to investigate the effects of long-time storage of cytological material and eventual changes or reductions in the percentage of receptor positive tumor cell nuclei.
MATERIALS AND METHODS
The material consisted of FNAC material from 53 breast carcinomas. The aspirations had been done by a cytopathologist in all cases. Most of the tumors were from the symptomatic out-patient clinic; a few were screening detected lesions. Cases were collected consecutively during 4 months. Both palpable and non-palpable tumors were included in the material. Non-palpable lesions had been aspirated under ultrasound guidance. Only cases with a pure or dominant invasive growth pattern on histology were included in the series.
Air-dried Giemsa stained smears had been used for routine diagnostics. Whenever a rapid, preliminary stain or clinical and/or radiological findings indicated malignancy, additional smears were made for ER/PgR ICC determination. Direct smears were put in a box containing dry ice and then transferred to a freezer at -74°C until immunostaining.[8] Residual material in the syringe and/or needle was rinsed in 30 ml ThinPrep Cytolyt™ (Hologic, Crawley, West Sussex, UK). Usually, there were two needle passes per patient. In the laboratory, the material in Cytolyt solution was transferred to a vial containing ThinPrep Preservecyt (Hologic). Cell blocks were not used in this study.
Before ICC, the direct smears were fixed in 10% formalin for 10 minutes (pH 7.2−7.4) at room temperature (RT) and in MBO citrate buffer (pH 6). A jar with the buffer and the slides were kept in the microwave oven (at 750 W for 3.5 minutes, then at 160 W for 7 minutes). The jar was allowed to stand and cool for 10 minutes at RT and then in running water for 10 minutes. The slides were transferred to distilled water before they were placed in the Autostainer (DAKO, Glostrup, Denmark).
The suspensions were allowed to stand for at least 15 minutes before the smears were prepared on the Hologic ThinPrep 2000 (Hologic). Before ICC, the smears were immersed in 10% formalin (pH 7.2-7.4) for 10 minutes and in MBO TE (pH 9) (citrate buffer 3 minutes, TE 3.5 minutes). Microwave treatment was at 750 W for 3.5 min and then at 160 W for 7 minutes. The jar was allowed to stand and cool for 10 minutes at RT, followed by 10 minutes in running tap water. The slides were transferred to distilled water and then to TBS (tris buffered saline) for 5 minutes before they were put in the Autostainer (DAKO). An additional specimen was prepared and used as negative control.
Four smear preparations from the remnants in the PreserveCyt (Hologic) vial were stored at −20°C and −74°C for 3 and 6 months, respectively, or as many as there was sufficient material for (when less than 4).
Immunostaining was done on the DAKO Autostainer (DAKO) using antibodies (AB) from Novocastra (Newcastle, UK, clones ER: 6F11 NCL and PgR: 312 NCL). DAB+ was used for visualization and hematoxylin used as a counterstain. Antibody concentrations were ER 1:150 and PgR 1:200 for both the preparation methods. The staining program was identical for both methods and was as follows: blocking of endogenous peroxidase (15 minutes), incubation of AB 30 minutes, envision substrate for 30 minutes and DAB+ 2 × 5 minutes.
Routine immunostaining and ICC on the liquid based smears were done at different times and read separately. The routine ICC was reported as usual. Stored material from previous known positive cases was used as positive control. The evaluation of receptor status was done according to the recommendations of the Norwegian Breast Cancer Group (NBCG)(www.nbcg.no). It was identical irrespective of the preparation method and was done by a single cytopathologist. In brief, the tumor cells were evaluated as negative when less than 10% of the nuclei stained positive (this has recently been changed to less than 1%, but did not apply to this series). Correlation with tumor morphology was done to ascertain that only carcinoma cells were evaluated. Occasional benign epithelial ductal groups contained varying numbers of positively stained nuclei, whereas admixed apocrine cells, as a rule, were negative.
National recommendations in the Norwegian Breast Screening Program define inadequate material in the following way (www.kreftregisteret.no): “The material is scant, acellular or has preparation artifacts that prohibit morphological evaluation. The amount of cells necessary to make a diagnosis is subjective and will depend on the experience of the cytopathologist. Less than five groups of epithelial cells are sufficient to say that a specimen is inadequate”. Both cytological and histological specimens are accepted for evaluation of receptor status and a cytological result of more than 50% positivity does not warrant histological confirmation. If routine ER/PgR had inadequate cell material, is negative or less than 50% of the cells were positive, the procedure is repeated on the histological (surgical) specimen. In some cases, there was no residual carcinoma in the surgical specimen as a result of preoperative adjuvant chemotherapy and ER/PgR could not be repeated. The results of ER and PgR on the liquid based specimens were not reported to the clinicians.
Histological tumor subtype, ER and PgR as well as grading (a modified Bloom-Richardson) were retrieved from the pathology files. The stored specimens were immunostained after 3 and 6 months storage, respectively.
Statistical analyses were done using SPSS version 15.0. Pearson's Chi square was done to investigate an eventual significance of differences of the results between the two ICC methods and the stored specimens.
RESULTS
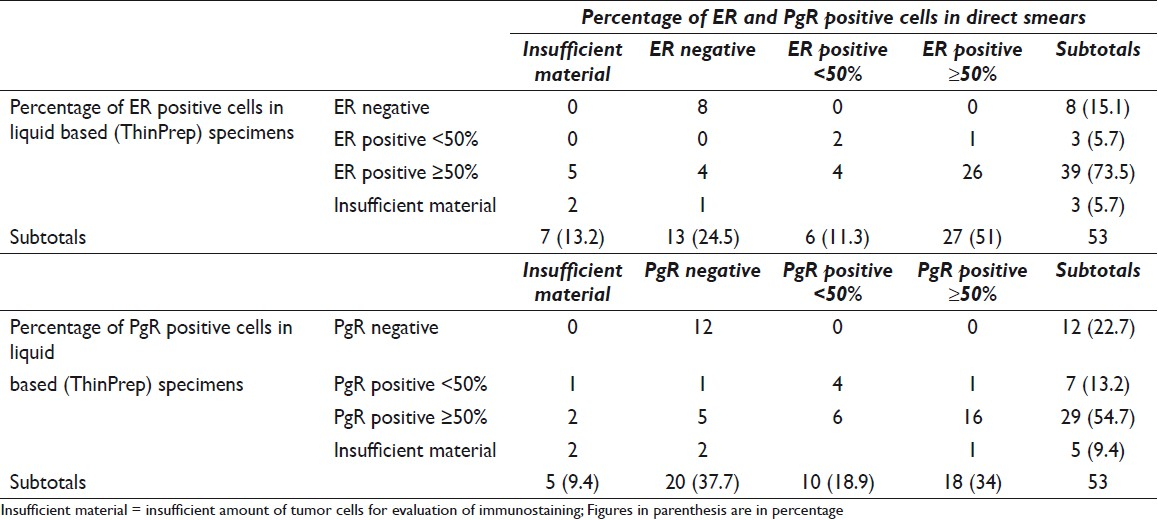
An overview of tumor subtypes and grading of the 53 cases is shown in Table 1. The comparison of ER and PgR evaluation on direct smears and liquid based preparations is shown in Table 2. The distinction ≥ or <50% of the cells reflects the recommendation flowchart of the NBCG (www.nbcg.no ) where women with tumors showing ER positivity in 50% of the tumor cell nuclei are eligible for anti-estrogen therapy. In four cases that were ER negative (4/13) on the direct smears, liquid based preparation revealed positivity in ≥50% of the tumor cell nuclei. Likewise, 5 (5/20) cases that were PgR negative on direct smears showed positivity in 50% of the tumor cell nuclei in the liquid bases preparations. Discrepancies in the percentage of positive tumor cell nuclei were found in 5 (ER) and 7 (PgR) cases, respectively, and 4 (ER) and 6 (PgR) of these were in favor of the liquid based specimens. The differences were not statistically significant (P = 0.320 for ER and P = 0.380 for PgR).
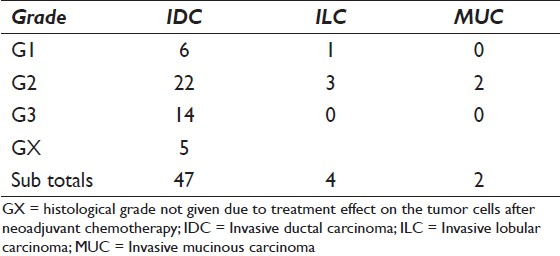
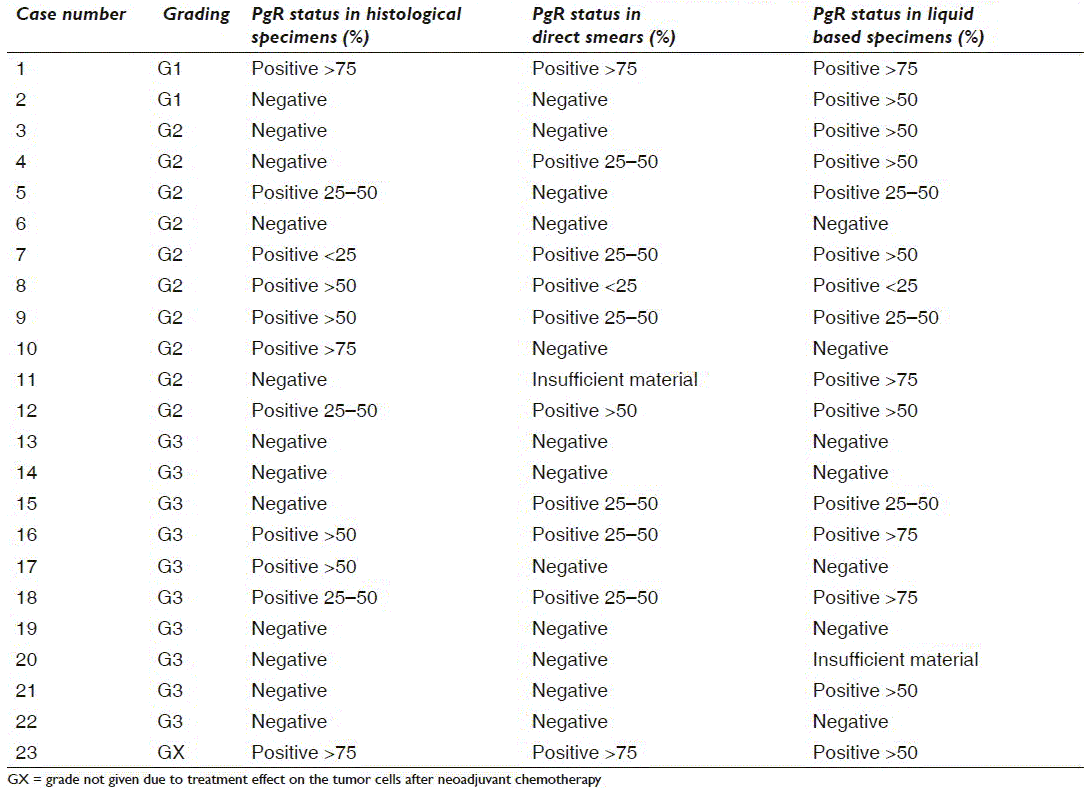
Tables 3a and 3b give a case-by-case overview of 23 cases with histological ER (n = 14) and/or PgR (n = 23) status, respectively, and direct comparison of the results in direct smears and liquid based specimens as well as grading. All were invasive ductal carcinomas (IDC). All lobular and mucinous carcinomas were ER/PgR positive in the liquid based preparations as well as all G1 and GX IDC. IDC G2 showed 1 ER−/PgR−, 4 ER+/PgR− and 20 ER+/PgR+, whereas 7 of the IDC G3 were ER+/PgR+ and 6 were ER−/PgR−
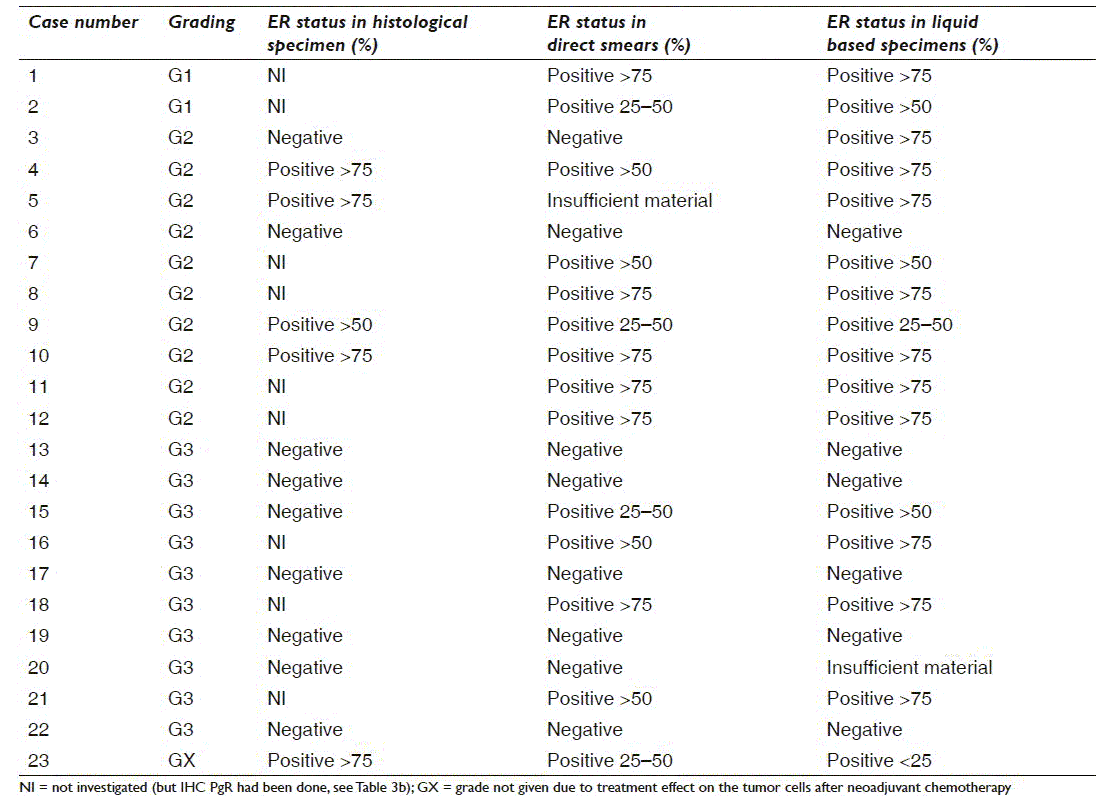
Discordance between in-house cytological ER and histological ER was found in three cases with representative cytological material for evaluation [Table 3a] (cases numbers 9, 15 and 23). Two were in favor of histology with cytological ER positive in <50% of the cells and ≥50% positive tumor cell nuclei in the histological specimen. None of the in-house cytological ER negative cases were positive on histology (7/13 cases) in this series. Likewise, four liquid based cytological ER cases [Table 3a] (cases numbers 3, 9, 15 and 23) with representative cytological material had discordant histological results. Two were in favor of the liquid based specimens (≥50% positivity vs. negative on histology). In these cases, histology might be regarded as false negative probably due to inadequate fixation of the tumor tissue.[9]
PgR had been repeated on the histological specimen in 23 cases [Table 3b], in one of these because the cytological material was insufficient. In-house cytological PgR analysis was discordant with histology in nine cases. Three were in favor of the cytological specimen (negative or less positive in histology) and six were in favor of the histological result (negative or less positive in the cytological specimens). Liquid based PgR was discordant with the histology in 13 cases; nine were in favor of the liquid based specimens (negative or less positive in histology) and 4 were in favor of histology (negative or less positive in the liquid based preparations).
Tables 4 and 5 show the results of ER and PgR, respectively, in liquid based preparations according to storing time and temperature. In 30 and 24 cases, respectively, there had been adjunct material for storing. All were from IDCs. The expression of ER and PgR was virtually unchanged in the stored specimens (P = 0.002) [Figure 1]. One case of ER and PgR staining turned negative, and one case each of ER and PgR ICC showed reduction of staining to <50% of the tumor cell nuclei. In addition, one case each of ER and PgR that were negative at time “zero” revealed a low percentage of positive tumor cell nuclei in the stored specimens, which probably represent a small tumor population that had not been sampled/included in all the preparations.
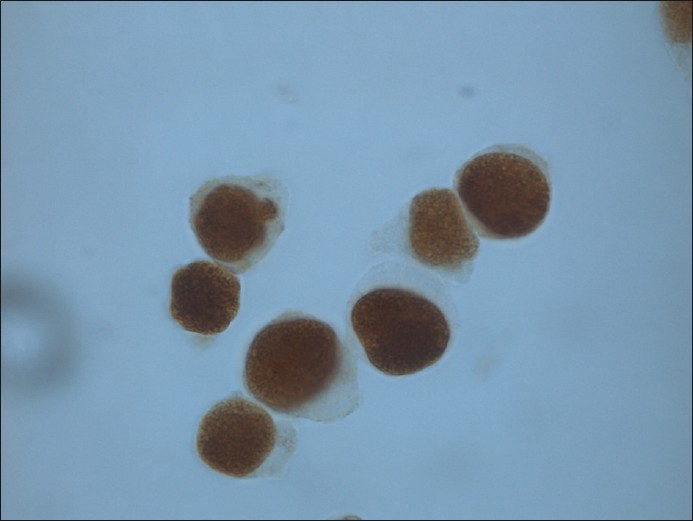
- Liquid based specimen stored at − 20°C for 6 months and then immunostained for ER (counterstain hematoxylin)
DISCUSSION
ER and PgR ICC can be determined preoperatively on FNAC from malignant breast lesions and in recurrences and metastases.[1] Several pre-procedural techniques and fixatives have been tested and advocated. Wet fixed or quick frozen direct smears, air-dried smears as well as cells suspended in a cell culture medium, physiological saline solution, a commercial transport fixative or a home-made solution have all been used for ER/PgR ICC.[810–17]
Most of the discordant cases of ER/PgR, both in our study and in the literature, are false negative ICC.[3–7] Several studies have found major loss of immunoreactivity in air-dried specimens.[14–17] Air-drying starts within a few seconds when making direct smears. Air-drying artifacts were often seen in the periphery of the smears using our old in-house method. Staining was often weaker or completely negative in the periphery of the smears. In contrast, drying out of the smears during the staining procedure led to a diffuse brown (false) staining of the cytoplasm. In a recent study, Gong et al.[12] states that air-drying is not recommended. Preparation methods where direct smears are used for ER/PgR ICC are all at risk for inadvertent air-drying. The risk can be avoided with liquid based preparations.
Cytopathology has a strong tradition for alcohol-based fixatives. Ethanol, methanol, sequential 10% formalin-methanol-acetone and Carnoy′s fixative have all been used in ER/PgR ICC.[78121318–20] In recen microwave-stimulated epitope retrieval is an excellent fixative for cytological specimens intended for ICC[12] or in situ hybridization (ISH). [21] The College of American Pathologists' (CAP) guidelines for ER/PgR FAQ [22] raises the following question: “Do the guidelines exclude testing of cytology specimens that have been fixed in 95% ethanol rather than formalin?” Their answer is no, other fixatives are not precluded, but labs performing testing on cytological specimens must document that they validated their methods.
Our laboratory has used 4% formalin as standard fixation for the last 7 years. Prior to this, sequential formalin-methanol-acetone fixation had been used. Internal validation was done comparing with the early biochemical method [8] and later with histology (unpublished).
When using liquid based suspensions, the primary fixation of the cells is whatever is used as preservative (if any) in the liquid (in ThinPrep it is methanol). In these cases, post-fixation in formalin and microwave-stimulated epitope retrieval should be done. We achieved an increase (statistically nonsignificant) in positivity using liquid based suspensions compared to our old in-house method. The major change was the liquid suspension instead of direct smears, whereas the differences in fixation were regarded as minor.
One of the major objections against using cytological examination of ER/PgR for treatment management is the lack of QC and standardization of fixatives and procedures as well as appropriate controls in cytology. Paraffin sections as well as residual smears and material from cell lines with known ER/PgR status have been used as positive and negative controls. Liquid based suspensions offer the possibility to store both liquid residual material as well as prepared specimens for QC purposes. Tabbara et al.[23] demonstrated good preservation of ER/PgR antigenicity in liquid suspensions and prepared specimens stored at RT and newly prepared specimens for up to 56 days. Our study demonstrated that prepared specimens stored at either −20°C or −74°C for 3 and 6 months, respectively, retained their immunoreactivity and the percentage of stained cells remained virtually unchanged [Tables 4 and 5]. The fixative(s) in Cytolyt and PreserveCyt thus seem to preserve the cells optimally, and the prepared specimens are suitable for long-time storing. This gives us the possibility to store residual material with known ER/PgR status for a longer period of time and use them as controls and for internal standardization and QC. Whether this also applies to storing the cells in the liquid has to be investigated.
Residual material from liquid based suspensions could potentially serve as basis for external QC schemes. Stored slides retain their immunoreactivity for at least 6 months. Whether these specimens can be sent by mail to QC participating laboratories and still retain their immunoreactivity remains to be investigated. The volume of such material will always be limited, though, and large-scale QC may not be possible. Carcinoma cells from cultured cell lines that have been immersed in liquid suspensions could be an option to test out.
In conclusion, liquid suspensions and liquid based slide preparations seem to offer an optimal pre-fixation and preservation of ER/PgR in breast carcinoma cells. Post-fixation with 4% formalin, followed by microwave-stimulated epitope retrieval before immunostaining is recommended. The slides can be stored in the freezer (−20°C or −74°C) for at least 6 months without significant loss of immunoreactivity. They can be used as internal positive and negative controls. Their potential use in external QC schemes remains to be investigated.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
No competing interest to declare by any of the authors.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version was read and approved. All authors qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) (or its equivalent) of all the institutions associated with this study. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model(authors are blinded for reviewers and reviewers are blinded for authors)through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2010/7/1/24/75665
REFERENCES
- American Society of Clinical Oncology 2007 Update of Recommendations for the Use of Tumor Markers In Breast Cancer. J Clin Oncol. 2007;25:5287-311.
- [Google Scholar]
- The role of breast FNAC in diagnosis and clinical management: a survey of current practice. Cytopathology. 2008;19:271-8.
- [Google Scholar]
- The role of ThinPrep cytology in the evaluation of estrogen and progesterone receptor content of breast tumors. Surg Oncol. 2006;15:257-66.
- [Google Scholar]
- Estrogen and progesterone receptor contents in ThinPrep-processed fine-needle aspirates of breast. Am J Clin Pathol. 1999;112:50-6.
- [Google Scholar]
- Hormone receptor status in breast cancer--a comparison between surgical specimens and fine needle aspiration biopsies. Cytopathology. 2003;14:136-42.
- [Google Scholar]
- Comparison of the results of immunocytochemical assays for biologic variables on preoperative fine-needle aspirates and on surgical specimens of primary breast carcinomas. Cancer Cytopathol. 2000;90:61-6.
- [Google Scholar]
- Correlation of immunocytochemical and immunohistochemical determination of estrogen and progesterone receptors in breast cancer. Acta Cytol. 2002;46:337-40.
- [Google Scholar]
- Assessing estrogen and progesterone receptor status in fine needle aspirates from breast carcinomas.results on six years of material and correlation with biochemical assay. Anal Quant Cytol Histol. 1998;20:122-6.
- [Google Scholar]
- Delay to formalin fixation effect on breast biomarkers. Mod Pathol. 2009;22:1457-67.
- [Google Scholar]
- Estimation of hormone receptor status in fine-needle aspirates and paraffin-embedded sections from breast cancer using the novel rabbit monoclonal antibodies SP1 and SP2. Diagn Cytopathol. 2003;29:207-11.
- [Google Scholar]
- Analysis of estrogen and progesterone receptors on preoperative fine-needle aspirates. Breast Cancer Res Treat. 1995;33:179-84.
- [Google Scholar]
- Optimal fixation conditions for immunocytochemical analysis of estrogen receptor in cytologic specimens of breast carcinoma. Cancer Cytopathol. 2003;102:34-40.
- [Google Scholar]
- Expression of estrogen and progesterone receptors in cytologic specimens using various fixatives. Diagn Cytopathol. 1996;15:78-83.
- [Google Scholar]
- Immunocytochemical detection of prognostic markers in breast cancer; technical considerations. Cytopathology. 1999;10:308-16.
- [Google Scholar]
- Presurgical detection of estrogen receptor status using immunocytochemically stained fine needle aspirate smears in patients with breast cancer. Cancer Res. 1987;47:6118-22.
- [Google Scholar]
- An evaluation of potentially suitable fixatives for immunoperixidase staining of estrogen receptors in imprints and frozen sections of breast carcinoma. Pathology. 1988;20:320-5.
- [Google Scholar]
- Estrogen and progesterone receptor status in breast carcinoma: comparison of immunocytochemistry and immunohistochemistry. Diagn Cytopathol. 2002;26:137-41.
- [Google Scholar]
- A reliable method of demonstrating HER-2/neu, estrogen receptors and progesterone receptors in routinely processed cytological material. Appl Immunohistochem Mol Morphol. 2001;9:352-7.
- [Google Scholar]
- Cytologic diagnosis of estrogen and progesterone receptors in breast omprints. Acta Cytol. 2002;46:1056-60.
- [Google Scholar]
- Immunostaining of estrogen receptor, progesterone receptor.MIB1 antigen and c-erbB-2 oncoprotein in cytologic specimens: a simplified method with formalin fixation. Diagn Cytopathol. 1997;17:127-33.
- [Google Scholar]
- Method development for DuoCISH HER-2 on FNAC material from breast carcinomas. Cytopathology. 2009;20:110.
- [Google Scholar]
- College of American Pathologists (CAP) Frequently Asked Questions About Breast Predictive Factors (HER2, ER, PgR). Reference Resources and Publications. College of American Pathologists. Available from: http://wwwcaporg/apps/capportal_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt%7BactionFormcontentReference%7D=laboratory_accreditation%2Fer_pgr_faqhtml&_state=maximized&_pageLabel=cntvwr
- [Google Scholar]
- The stability of estrogen and progesterone receptor expression on breast carcinoma cells stored as preservCyt suspensions and as ThinPrep slides. Cancer. 1998;84:355-60.
- [Google Scholar]








