Translate this page into:
Lysine acetyltransferase 5 contributes to diabetic retinopathy by modulating autophagy through epigenetically regulating autophagy-related gene 7

*Corresponding author: Shifang Zhuang, Department of Laboratory Clinical Laboratory, Ninth Hospital of Xi’an, Xi ’an, Shaanxi Province, China. zsfcmu@163.com
-
Received: ,
Accepted: ,
How to cite this article: Gao Q, Lai Y, He S, Wang Y, Zhang G, Zhu X, et al. Lysine acetyltransferase 5 contributes to diabetic retinopathy by modulating autophagy through epigenetically regulating autophagy-related gene 7. CytoJournal. 2025;22:22. doi: 10.25259/Cytojournal_187_2024
Abstract
Objective
Diabetic retinopathy (DR) is a prevalent and serious complication among individuals with diabetes, significantly compromising their visual acuity and overall quality of life. Lysine acetyltransferase 5 (KAT5), an essential catalytic subunit of the nucleosome acetyltransferase of the H4 complex, is implicated in the development of various diseases, including neurological disorders, breast cancer, and lung cancer. However, the function of KAT5 in DR remains poorly understood. This study aims to investigate the influence of KAT5 on autophagy (Atg) during DR.
Material and Methods
Experiments were conducted using streptozotocin (STZ)-treated rats to induce diabetes and observe changes in KAT5 expression and its effect on Atg. Retinal tissues and RF/6A cells were utilized to analyze the expression levels of various proteins and their involvement in Atg and apoptosis. KAT5 depletion and Atg7 knockdown were performed to further understand their roles in the process.
Results
The eyeballs of STZ-treated rats showed increased expression of KAT5. Depletion of KAT5 attenuated STZ-induced DR injury in rats. The retinal tissues of STZ-treated rats exhibited reduced expression of B-cell lymphoma-2 (Bcl-2) and increased levels of BCL-2-associated X protein and cleaved caspase 3, which could be reversed by KAT5 depletion. STZ treatment induced expression of Beclin-1 and microtubule-associated protein 1 light chain 3B in retinal tissues, and KAT5 knockdown blocked this effect. In monkey retinal choroidal endothelial ( RF/6A) cells, high glucose (HG) treatment decreased 5-ethynyl-2’-deoxyuridine-positivecells and increased terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive cells, which were reversed by KAT5 depletion. KAT5 depletion also attenuated HG-induced apoptosis and Atg in RF/6A cells. Mechanistically, KAT5 depletion reduced histone H3 lysine 27 acetylation and ribonucleic acid ( RNA) polymerase II enrichment on the Atg7 promoter, leading to a decrease in the messenger RNA ( mRNA) and protein expression of Atg7. Atg7 knockdown suppressed Atg in RF/6A cells under HG conditions and reversed the effect of KAT5 depletion on cell apoptosis and Atg.
Conclusion
The findings suggest that KAT5 contributes to DR by modulating Atg through epigenetic regulation of Atg7. KAT5 emerges as a valuable target for DR treatment, providing a fresh perspective on the disease’s pathogenesis and laying the foundation for the development of potential therapeutic strategies.
Keywords
Autophagy
Autophagy-related protein 7
Diabetic retinopathy
Lysine acetyltransferase 5
INTRODUCTION
Diabetic retinopathy (DR) is a prevalent ocular complication in diabetic patients, characterized by symptoms such as blurred vision, metamorphopsia, and ocular inflammation. In severe cases, it can progress to secondary glaucoma.[1,2] High glucose (HG) in diabetes causes the death of pericytes, endothelial cells, Müller cells, ganglion cells, and other retinal cells, leading to early lesions on the retina, including neuronal dysfunction, acellular capillaries, and glial activation.[3,4] At present, several death forms have been discovered in diabetic retinas, including apoptosis, necrosis, pyroptosis, and autophagic death.[5]
Autophagy (Atg) is a conserved intracellular catabolic pathway capable of degrading excess or damaged organelles and cell components for recycling, which maintains cellular homeostasis under certain damage and stress.[6] Atg is modulated by multiple signaling proteins such as the Atg family (Atg3, Atg5, and Atg7), Beclin-1, and microtubule-associated protein 1 light chain 3B (LC3B).[7] Studies have proven that autophagy dysfunction is closely associated with various diabetic complications, such as cardiomyopathy, retinopathy, and nephropathy.[8,9] Diabetes causes inflammation and oxidative stress in the retina and capillary cells, and autophagy activation is closely correlated with excessive reactive oxygen species.[10] The balance disruption between autophagy and oxidative stress could lead to retinal damage.
As the catalytic subunit of the nucleosome acetyltransferase of H4 complex,[11,12] lysine acetyltransferase 5 (KAT5) is capable of not only epigenetically regulating gene transcription but also directly targeting the non-histone proteins for acetylation. An aberrant level of KAT5 is found in various diseases, such as neurodegenerative diseases and diabetic embryopathy, and it is regarded as a promising therapeutic target.[13] Studies have reported the various and crucial functions of KAT5 in cell proliferation, apoptosis, metabolism, inflammatory responses, DNA damage, autophagy, and other cellular processes.[14-16] A notable detail is that KAT5 can affect the autophagy process by acetylating proteins such as serine/threonine-protein kinase ULK1, thereby regulating the progression of various diseases, including neurodegenerative diseases.[17]
This work emphasized the role of KAT5 during DR pathogenesis using in vivo and in vitro experiments, discovering KAT5 and Atg7 upregulation in diabetic retina and HG-stimulated retinal cells. According to the analysis of molecular mechanisms, KAT5 epigenetically upregulated the acetylation and expression of Atg7, promoted autophagy and retinal cell death, and caused retinopathy lesions. The findings demonstrated KAT5 as a promising target for treating DR.
MATERIAL AND METHODS
Cell culture and treatment
Rhesus endothelial cell line RF/6A (Delf-16736, Wanwu, Hefei, China) was purchased from Wanwu Biotechnology Co., Ltd. and cultured in Dulbecco’s Modified Eagle Medium (M4655, Merck, Darmstadt, Germany) added with 10% fetal bovine serum (A5256701, Gibco, New York, USA) under conditions of 37°C and 5% carbon dioxide. To verify the purity and authenticate the identity of the cell lines, mycoplasma detection and short tandem repeat profiling were performed. The cells underwent 48 h of treatment using HG concentration at 35 mM to mimic an in vitro diabetic model.
Cell transfection
The Atg7 overexpressing plasmid, the short hairpin RNA (shRNA) that targets Atg7 (AUAUUGAUCAAGAACUUUGGA), the shRNA that targets KAT5 (shKAT5: UAACUCUUGUUCUUACGUCCA), and the short hairpin negative controls (sh-NC: CGAGUAUCUUCAUCAUCGUUC) were all provided by GenePharma Co., Ltd (C01001, Shanghai, China). By following the instructions provided with the lipofectamine 2000 kit (11668500, Invitrogen, Carlsbad, USA), these vectors were transfected into the cells.
Diabetic rat model
180~200 g male Sprague–Dawley rats were provided by Beijing Huafukang Biotechnology Co., Ltd. (Beijing, China). These rats were housed in a 12-h light and 12-h dark alternating cycle environment. Streptozotocin (STZ, 18883-66-4, Sigma, St. Louis, USA) was intraperitoneally injected into rats (60 mg/kg body weight). The sham group was administered with citrate buffer injection. The blood glucose level was detected 72 h after STZ injection, and the value > 16.7 mmol/L was considered standard for diabetic rats. For treatment, the rats were anesthetized using a combination of ketamine (75 mg/kg, REF-K05004, Tonghui, Guangzhou, China) and xylazine (15 mg/kg, M8857, AbMole, Houston, USA). The adeno-associated virus shKAT5 was injected into the vitreous cavity. The treatment was performed 10 days after model establishment and repeated another two times at 10-day intervals. After anesthesia, the rats were humanely sacrificed by cervical dislocation, and the eyeballs were immediately collected for subsequent experiments. The animal experiments strictly adhered to relevant regulations and guiding principles. This animal experiment has obtained approval from the experimental animal ethics committee.
Histological analysis
The eyeballs were fixed with 4% paraformaldehyde (PFA, P1110, Solarbio, Beijing, China), dehydrated with sucrose solution, and embedded in optimal cutting temperature compound (O.C.T., 4583, SAKURA, Torrance, USA) to prepare 5 μm thick slices. The samples underwent one night of incubation using anti-KAT5 antibodies (DF13348, 1:1000, Affinity Biosciences, Changzhou, China) at 4°C and another 1 h of incubation using secondary antibodies (S0009, 1:5000, Affinity Biosciences, Changzhou, China) to determine the level of KAT5. Diaminobenzidine (DAB) staining was performed, followed by counterstaining with hematoxylin with the use of an immunohistochemistry kit (D41–18, Maxim, Fujian, China). Meanwhile, the embedded sections were stained according to the instructions of the hematoxylin and eosin staining kit (G1120, Solarbio, Beijing, China). Finally, all stained tissue sections were observed and analyzed using a microscope (CX33-LV2000, Olympus, Tokyo, Japan) and Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA).
Western blot
Radioimmunoprecipitation assay ( RIPA) lysis buffer (89901, Thermo Fisher Scientific, Rockford, USA) was used in obtaining total proteins from eyeballs and cells, and the bicinchoninic acid ( BCA) protein assay kit (P0011, Beyotime, Beijing, China) was used for quantification. Proteins (40 μg) divided by sodium dodecyl sulfatepolyacrylamide gel electrophoresis ( SDS-PAGE) were blotted to polyvinylidene fluoride ( PVDF) membranes (IPVH15150, Millipore, Billerica, USA). The blots received 2 h of blockage in 5% skim milk and another one night of incubation using primary antibodies against KAT5 (DF13348, 1:1000, Affinity Biosciences, Changzhou, China), LC3B (AF4650, 1:1000, Affinity Biosciences, Changzhou, China), Beclin-1 (AF5128, 1:1000, Affinity Biosciences, Changzhou, China), Atg7 (DF6130, 1:1000, Affinity Biosciences, Changzhou, China), B-cell lymphoma-2 (Bcl-2) (AF6139, 1:1000, Affinity Biosciences, Changzhou, China), BCL-2-associated X (Bax) (AF0120, 1:1000, Affinity Biosciences, Changzhou, China), cleaved caspase 3 (c-caspase-3) (AF6311, 1:1000, Affinity Biosciences, Changzhou, China), and β-actin (T0021, 1:3000, Affinity Biosciences, Changzhou, China) overnight at 4°C. Next, the blots were hatched with horseradish peroxidase ( HRP)-linked secondary antibodies (S0009, 1:5000, Affinity Biosciences, Changzhou, China). The enhanced chemiluminescence ECL reagent (1622301, Millipore, Billerica, USA) was used to visualize the target protein bands.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA extraction was performed using the TRIzol reagent (15596018CN, Invitrogen, Carlsbad, USA), followed by reverse transcription into complimentary DNA ( cDNA) utilizing the First Strand cDNA Synthesis Kit (K1642, Thermo Fisher Scientific, Rockford, USA). mRNA levels of Atg7 and KAT5 were then quantitatively assessed using SYBR Green Universal Master Mix (4344463, Thermo Fisher Scientific, Rockford, USA) with β-actin serving as the internal reference gene. The 2−ΔΔCt method was applied for RNA level calculation. The primer sequences for Atg7, KAT5 and β-actin are as follows: Atg7 5'-ACAGCCCTGCCATACTTCTT-3' (forward), 5'-AGGGTGCTGGGTTAGGTTAC-3' (reverse), KAT5 5'- GAGTGTATTGAGCTTGGCCG-3'(forward), 5'- CAGGTAGAGGACAGGTAGCG-3' (reverse), β-actin 5'- ACAGCCCTGCCATACTTCTT-3' (forward), 5'-AGGGTGCTGGGTTAGGTTAC-3' (reverse).
Cell proliferation and apoptosis
Cell proliferation was assessed through a 5-ethynyl-2’-deoxyuridine (EdU) assay (C10337, Invitrogen, Carlsbad, USA). In brief, cells underwent 2 h of incubation in EdU solution, 30 min of fixation in 4% PFA, and neutralization with glycine solution. 4’,6-diamidino-2-phenylindole ( DAPI) (62248, Thermo Scientific, Rockford, USA) was used to stain the nucleus. Positive staining was observed by fluorescence microscopy (Zeiss LSM 780, Carl Zeiss, Oberkochen, Germany). These cells were stained with Click-iT Plus Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (C10618, Invitrogen, Carlsbad, USA) according to the manufacturer’s guidelines.
The Annexin V/propidium iodide ( V/PI) apoptosis detection kit (V13242, Invitrogen, Carlsbad, USA) was used in detecting cell apoptosis in accordance with the producer’s protocol. In brief, the cell suspension was carefully mixed with Annexin V and PI labeled with fluorescent dyes, gently mixed, and incubated for 20 min at room temperature in the dark. After 400 μL of fluorescence-activated cell sorting buffer was added, the cell suspension was loaded into a flow cytometer (NovoCyte Quanteon, Agilent, Santa Clara, USA) for detection.
Chromatin immunoprecipitation (ChIP)
The protein–chromatin interaction was assessed through a ChIP assay using a ChIP chromatin immunoprecipitation kit (17–371, Millipore, Billerica, USA). The specific steps included: First, cross-linking chromatin in cells with 1% formaldehyde; then, fragmenting the chromatin to 200–500 bp by sonication. The magnetic beads were conjugated with antibodies against polymerase II, KAT5, and histone H3 acetylation on lysine 27 (H3K27ac) and incubated with the sonicated chromatin fragments at 4°C for 6 h. Next, the eluted samples underwent reverse crosslinking. The DNA levels were determined by qRT-PCR.
Statistics
Data presentation followed the mean ± standard deviation format, and data analysis was conducted through GraphPad Prism 7 (GraphPad, La Jolla, California, USA). Student’s t-test was used for two-group comparisons and one-way analysis of variance for multiple groups. P < 0.05 indicated statistical significance.
RESULTS
KAT5 is increased in DR rat model
A DR rat model was established to evaluate the association of KAT5 with DR by treating rats with STZ. The findings showed reduced body weight and enhanced blood glucose in rats [Figures 1a and b]. The expression of KAT5 in STZ-treated rats’ eyeballs significantly increased [Figure 1c-e].
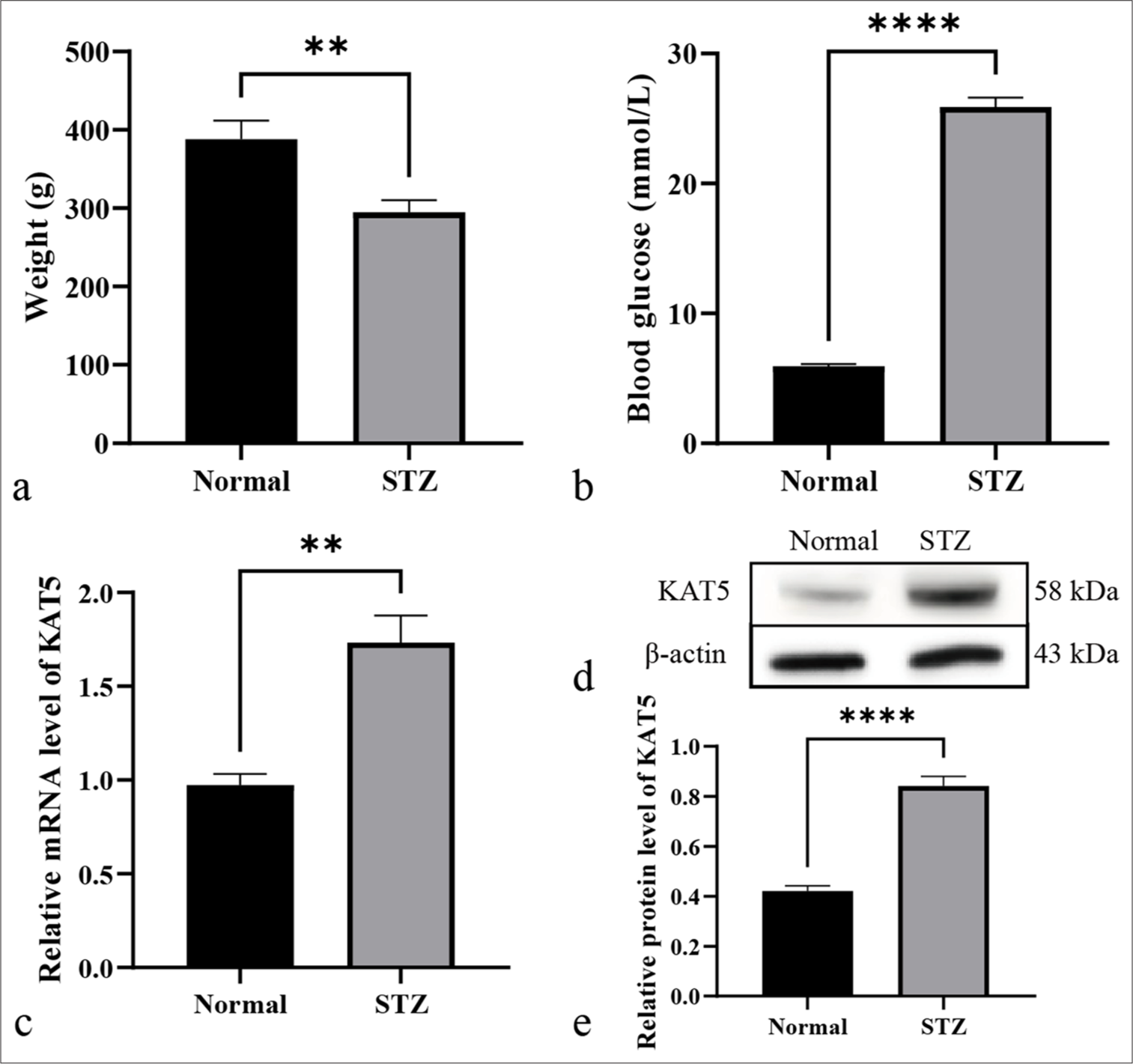
- KAT5 is increased in diabetic retinopathy rat model. (a-e) A diabetic retinopathy rat model was established by treating rats with STZ. (a) The weight of the rats was shown. (b) The blood glucose of the rats was analyzed. (c) The messenger RNA (mRNA) >expression of KAT5 was quantified using qRT-PCR in eyeballs. (d and e) The protein expression of KAT5 was quantified using Western blot analysis in eyeballs. ✶✶P < 0.01, ✶✶✶✶P < 0.0001. KAT5: Lysine acetyltransferase 5, STZ: Streptozotocin, qRT-PCR: Quantitative real-time polymerase chain reaction
Depletion of KAT5 attenuates autophagy in DR rat model
The modulating impact of KAT5 on DR was evaluated. Results demonstrated that KAT5 expression was upregulated in the retinal tissues of STZ-induced diabetic rats. In addition, KAT5 exhibited an inhibitory effect on DR-related autophagy and injury in these rats [Figure 2a and b]. STZ-treated rats showed reduced Bcl-2 expression along with elevated levels of Bax and cleaved caspase-3, and KAT5 depletion could reverse these phenotypes [Figure 2c and d]. Meanwhile, STZ treatment facilitated the protein level of Beclin-1 and LC3B, and this effect could be blocked by KAT5 knockdown [Figure 2c and d].
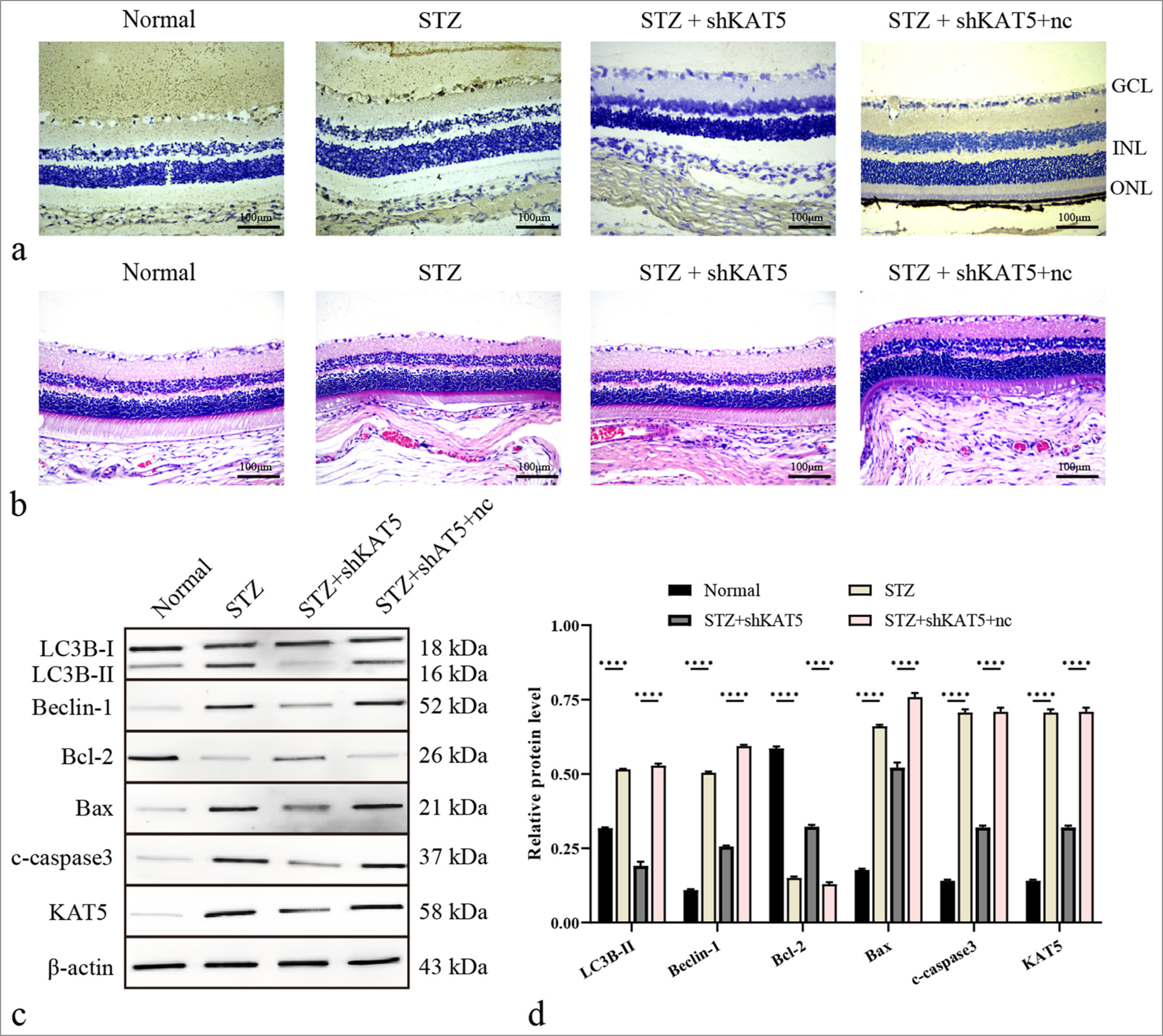
- Depletion of KAT5 attenuates autophagy in diabetic retinopathy rat model. (a) KAT5 expression levels were assessed using IHC in rat retinal tissues. Scale bar = 100 μm. (b) Diabetic retinopathy injury was assessed using H&E staining. Scale bar = 100 μm. (c and d) The expression levels of proteins including KAT5, Bcl-2, Bax, c-caspase-3, Beclin-1, and LC3B-II in rat retinal tissues were assessed through Western blot analysis. ✶✶✶✶P < 0.0001. GCL: Ganglion cell layer, INL: Inner nuclear layer, ONL: Outer nuclear layer, KAT5: Lysine acetyltransferase 5, STZ: Streptozotocin, Bcl-2: B-cell lymphoma-2, Bax: BCL-2-associated X protein, LC3B: Microtubule-associated protein 1 light chain 3B, H&E: Hematoxylin and eosin.
Knockdown of KAT5 represses autophagy in RF/6A cells under HG condition
To evaluate the impact of KAT5 on DR in vitro, rhesus endothelial RF/6A cells were subjected to HG treatment or co-treated with KAT5 shRNA and HG. The EdU-positive RF/6A cells were decreased [Figures 3a and b], and the TUNEL-positive RF/6A cells were increased by HG treatment, and the depletion of KAT5 reversed the numbers of the cells [Figures 3c and d]. Meanwhile, KAT5 depletion weakened RF/6A cell apoptosis due to HG treatment [Figure 3e and f]. Similarly, Bcl-2 was lowly expressed and Bax and c-caspase-3 were highly expressed due to HG treatment, and KAT5 depletion could reverse these phenotypes [Figures 3g and h]. HG treatment induced the expression of Beclin-1, and this effect could be blocked by KAT5 knockdown [Figures 3g and h].
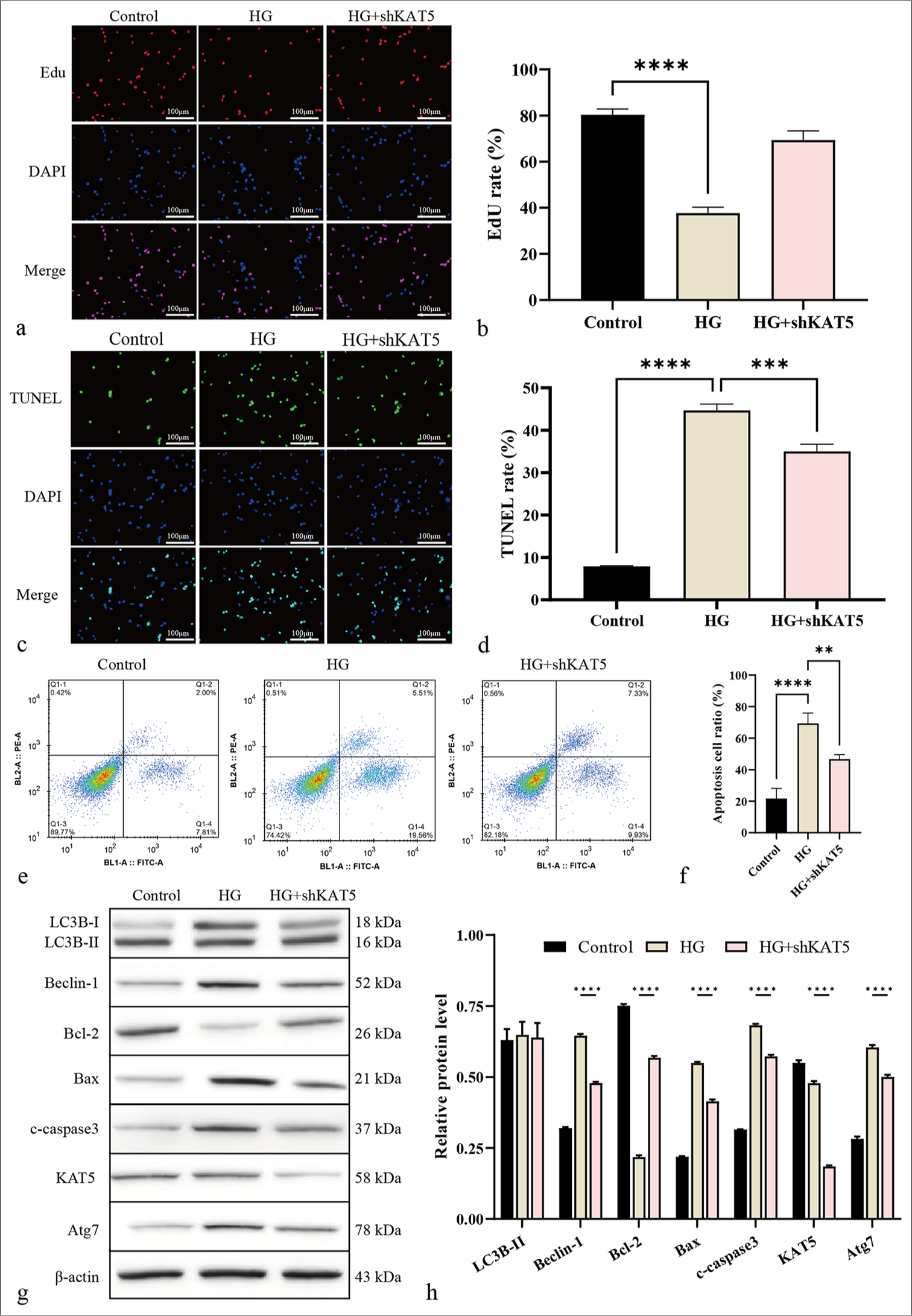
- Knockdown of KAT5 represses autophagy in RF/6A cells exposed to HG condition. (a and b) Cell proliferation was measured by EdU assay. Scale bar = 100 μm. (c and d) Cell apoptosis was analyzed by TUNEL. Scale bar = 100 μm. (e and f) Cell apoptosis was assessed using flow cytometry. (g and h) The expression levels of proteins of Atg7, KAT5, Bcl-2, Bax, c-caspase-3, Beclin-1, and LC3B-II were assessed through Western blot analysis. ✶✶P < 0.01, ✶✶✶P < 0.001, and ✶✶✶✶P < 0.0001. HG: High glucose, Atg7: Autophagy-related protein 7, KAT5: lysine acetyltransferase 5, Bcl-2: B-cell lymphoma-2, Bax: BCL-2-associated X protein, c-caspase-3: cleaved caspase-3, LC3B: Microtubule-associated protein 1 light chain 3, EdU: 5-ethynyl-2’-deoxyuridine, TUNEL: Terminal deoxynucleotidyl transferase dUTP nick-end labeling.
KAT5 epigenetically induces Atg7 expression in RF/6A cells
Next, the regulatory mechanism of KAT5 against autophagy in DR was explored. The depletion of KAT5 resulted in decreased enrichment of histone H3K27ac and RNA polymerase II less enriched at the Atg7 promoter [Figure 4a and b]. Meanwhile, the knockdown of KAT5 reduced mRNA and protein expression of Atg7 [Figure 4c-e].
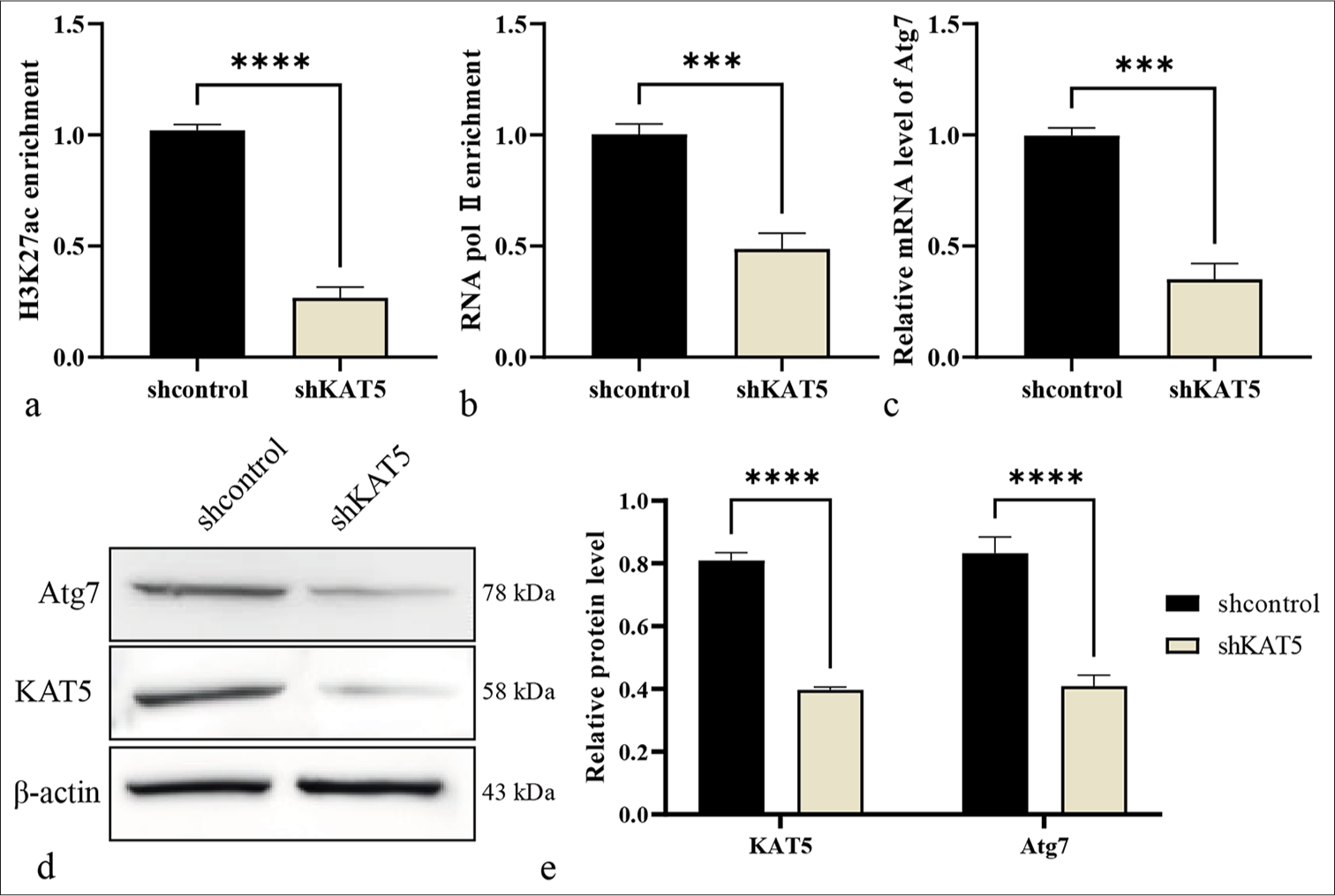
- KAT5 epigenetically induces Atg7 expression in RF/6A cells. (a and b) Enrichment of H3K27ac and ribonucleic acid (RNA) polymerase II at the Atg7 promoter was evaluated using a ChIP assay. (c) The mRNA expression of Atg7 was measured using qRT-PCR. (d and e) The protein levels of Atg7 and KAT5 were analyzed using Western blot analysis. ✶✶✶P < 0.001, ✶✶✶✶P < 0.0001. KAT5: Lysine acetyltransferase 5, Atg7: Autophagy-related protein 7, qRT-PCR: Quantitative real-time polymerase chain reaction, ChIP: Chromatin immunoprecipitation, H3K27ac: Histone H3 lysine 27 acetylation.
Knockdown of Atg7 suppresses autophagy in RF/6A cells under HG condition
Next, the function of Atg7 in the model was examined. The results showed that the EdU-positive RF/6A cells were reduced [Figure 5a and b] and the TUNEL-positive RF/6A cells were enhanced by HG treatment, and the depletion of Atg7 reversed the number of cells [Figure 5c and d]. Meanwhile, Atg7 depletion inhibited RF/6A cell apoptosis after HG treatment [Figure 5e and f]. Consistently, Bcl-2 was lowly expressed, and Bax and c-caspase-3 were highly expressed due to HG treatment, and these phenotypes were reversed by the depletion of Atg7 [Figure 5g and h]. In addition, HG treatment induced the expression levels of Beclin-1 and LC3B, and this effect could be blocked by the knockdown of Atg7 in the model [Figure 5g and h].
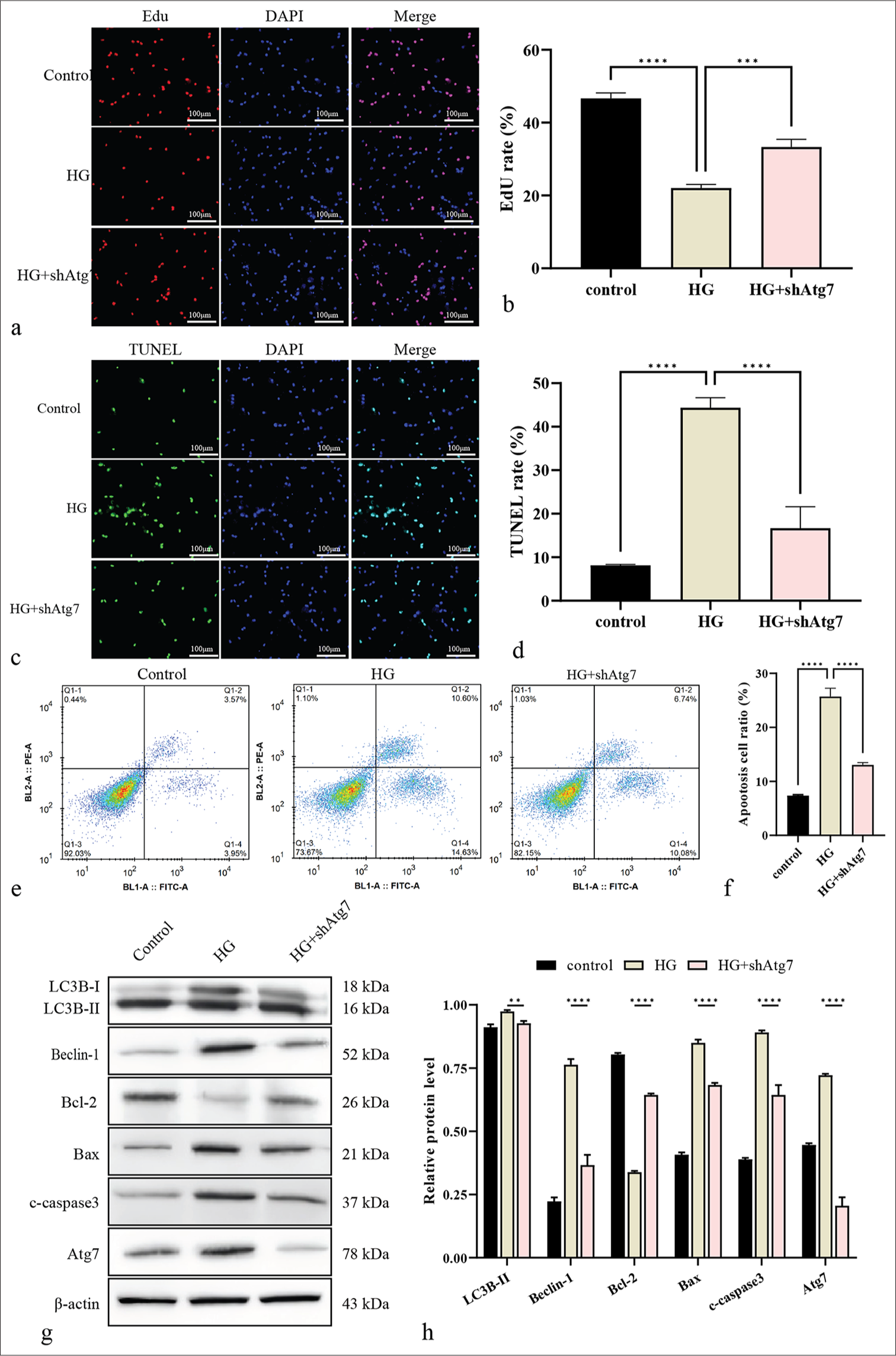
- Knockdown of Atg7 suppresses autophagy in RF/6A cells under HG. (a and b) Cell proliferation was measured by EdU. Scale bar = 100 μm. (c and d) Cell apoptosis was analyzed by TUNEL. Scale bar = 100 μm. (e and f) Cell apoptosis was quantified by flow cytometry. (g and h) The protein levels of Atg7, Bcl-2, Bax, c-caspase-3, Beclin-1, and LC3B were using Western blot. ✶✶P < 0.01, ✶✶✶P < 0.001, ✶✶✶✶P < 0.0001. HG: High glucose, KAT5: Lysine acetyltransferase 5, Atg7: Autophagy-related protein 7, Bcl-2: B-cell lymphoma-2, Bax: BCL-2-associated X protein, c-caspase-3: Cleaved caspase-3, LC3B: Microtubule-associated protein 1 light chain 3B, EdU: 5-ethynyl-2’-deoxyuridine, TUNEL: Terminal deoxynucleotidyl transferase dUTP nick-end labeling.
KAT5 contributes to autophagy in RF/6A cells under HG condition by targeting Atg7
The function of the KAT5/Atg7 axis in the model was analyzed. The results showed that the number of EdU-positive RF/6A cells were promoted [Figure 6a and b], whereas the TUNEL-positive RF/6A cells [Figure 6c and d] and apoptosis were inhibited by the depletion of KAT5 in HG-treated RF/6A cells. Such an effect could be reversed by the overexpression of Atg7 [Figure 6e and f]. Similarly, KAT5 knockdown in HG-treated rats led to upregulated Bcl-2 expression and downregulated levels of Bax and c-caspase-3, and the overexpression of Atg7 reversed the phenotypes in the cells [Figure 6g and h]. Besides, KAT5 knockdown in HG-treated rats led to reduced Beclin-1 expression, an effect that was reversed by Atg7 overexpression. [Figure 6g and h].
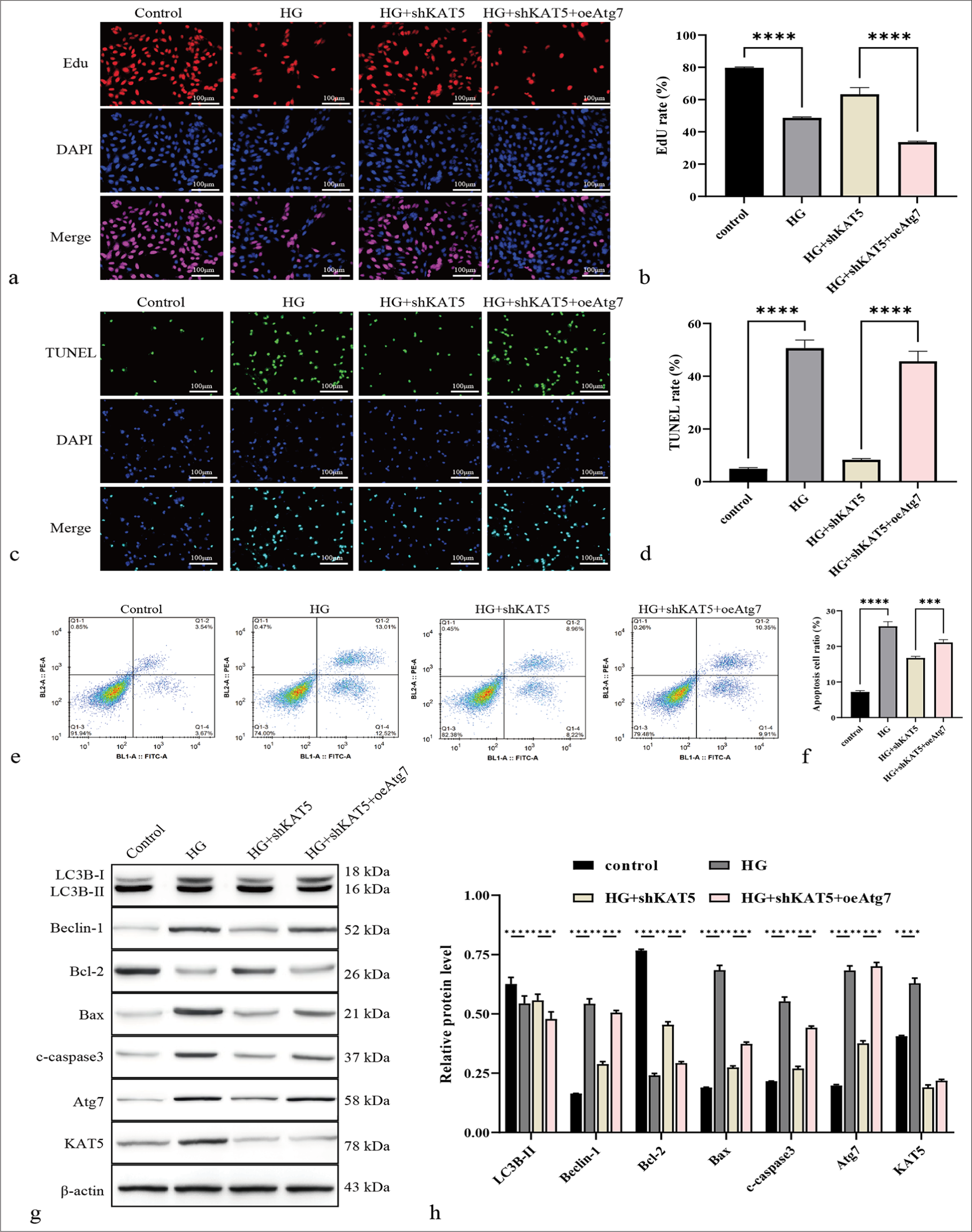
- KAT5 contributes to autophagy in monkey retinal choroidal endothelial cells (RF/6A) cells under HG condition by targeting Atg7. (a and b) Cell proliferation was measured by EdU. Scale bar = 100 μm. (c and d) Cell apoptosis was evaluated using by TUNEL. Scale bar = 100 μm. (e and f) Cell apoptosis was detected by flow cytometry. (g and h) The protein levels of Atg7, KAT5, Bcl-2, Bax, c-caspase-3, Beclin-1, and LC3B were assessed through Western blot. ✶✶✶P < 0.001, ✶✶✶✶P < 0.0001. KAT5: Lysine acetyltransferase 5, Atg7: Autophagy-related protein 7, Bcl-2: B-cell lymphoma-2, Bax: BCL-2-associated X protein, c-caspase-3: Cleaved caspase-3, LC3B: Microtubule-associated protein 1 light chain 3B, EdU: 5-ethynyl-2’-deoxyuridine, TUNEL: Terminal deoxynucleotidyl transferase dUTP nick-end labeling.
DISCUSSION
DR is a prevalent and serious complication among individuals with diabetes, significantly compromising their visual acuity and overall quality of life. KAT5 plays crucial roles in multiple diseases, but its functions have not been clearly explored. The new effect of KAT5 on autophagy during DR was determined in this study.
According to previous studies, KAT5 functions crucially in multiple diseases, causing cGMP-AMP synthase acetylation and enhancing innate immune response to DNA viruses.[18] KAT5 stabilizes Myc proto-oncogene in anaplastic thyroid cancer to benefit metastasis and invasion.[12] KAT5 depletion in kidney podocytes limits DNA repair and increases alterations in DNA methylation.[16] In this study, KAT5 expression was upregulated in the eyeballs of STZ-treated rats. The depletion of KAT5 attenuated DR injury in these rats. The retinal tissues of the STZ-treated rats exhibited reduced Bcl-2 levels along with elevated levels of Bax and c-caspase-3, and KAT5 depletion could reverse the phenotypes in the rats. Beclin-1 expression was induced by the treatment of STZ in the retinal tissues of the rats, whereas the knockdown of KAT5 blocked this effect in the model. Meanwhile, the EdU-positive RF/6A cells were decreased, and the TUNEL-positive RF/6A cells were increased by HG treatment, and the depletion of KAT5 reversed the number of the cells. Meanwhile, KAT5 depletion weakened the HG-induced RF/6A cell apoptosis and autophagy. Taken together, the results showed that KAT5 contributes to DR by regulating autophagy. Future studies are suggested to more deeply explore the relevant clinical significance.
Autophagy essentially impacts DR development, which can be modulated by histone HIST1H1C/H1.2, according to reports.[19] PG545, a heparan sulfate mimetic, attenuates DR by enhancing the autophagy of retinal Müller cells to repress the inflammatory response.[20] Notoginsenoside R1 alleviates DR by phosphatase and tensin homolog (PTEN)-induced putative kinase protein 1-related activation of autophagy.[21] In the current investigation, the depletion of KAT5 resulted in decreased enrichment of H3K27ac and RNA polymerase II at the Atg7 promoter. Meanwhile, KAT5 depletion reduced Atg7 expression at the mRNA and protein levels. Atg7 knockdown suppressed autophagy in RF/6A cells under HG concentration. The depletion of Atg7 reversed the effect of KAT5 knockdown on RF/6A cell apoptosis and autophagy. These findings assist in understanding the regulatory mechanism of KAT5 against autophagy in DR by targeting Atg7 from new perspectives. Future studies are suggested to pay attention to other potential downstream factors.
SUMMARY
KAT5 contributes to DR by modulating autophagy through epigenetically regulating Atg7, thereby serving as a valuable target for DR treatment.
AVAILABILITY OF DATA AND MATERIALS
The datasets used in this study are available from the corresponding author on reasonable request.
ABBREVIATIONS
ATC: Anaplastic thyroid cancer
Atg7: Autophagy-related protein 7
Bax: BCL-2-associated X protein
BCA: Bicinchoninic Acid
Bcl-2: B cell lymphoma-2
c-caspase-3: Cleaved caspase3
cDNA: Complementary DNA
cGAS: cGMP-AMP synthase
C-MYC: Myc proto-oncogene
DAB: Diaminobenzidine
DAPI: 4’,6-diamidino-2-phenylindole
DR: Diabetic retinopathy
EDU: 5-ethynyl-2’-deoxyuridine
ECL: Enhanced Chemiluminescence Reagent
HRP: Horseradish peroxidase
H3K27ac: histone H3 acetylation on lysine 27
KAT5: Lysine acetyltransferase 5
LC3B: Microtubule-associated proteins 1 light chain 3B
mRNA: Messenger RNA
PVDF: Polyvinylidene Fluoride
PI: Propidium Iodide
PTEN: Phosphatase and tensin homolog
PINK1: PTEN-induced putative kinase protein 1
RNA: Ribonucleic Acid
RIPA: Radio Immunoprecipitation Assay
SDS-PAGE: Sodium Dodecyl Sulfate-Polyacrylamide Gel
Electrophoresis
shRNA: Short Hairpin RNA
STZ: Streptozotocin
TUNEL: Terminal deoxynucleotidyl transferase dUTP Nick-
End Labeling
ULK1: Serine/threonine-protein kinase ULK1
AUTHOR CONTRIBUTIONS
SFZ and QG: Concepted and designed the research; QG, YJL, and SH: Acquired the data; YHW, GCZ, and XYZ: Analyzed and interpreted data; QG and YJL: Drafted the manuscript; SFZ: Revised manuscript for important intellectual content. All authors contributed to this present work. All authors read and approved the manuscript.
ACKNOWLEDGMENT
The authors thank all of our colleagues at Department of Laboratory Clinical Laboratory of Ninth Hospital of Xi’an for constructive discussions and technical help.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This animal experiment has obtained approval from the Biomedical Ethics Committee of Xi’an Ninth Hospital:No:202169). Patient consent not required as there are not patients in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: This project was supported by the Natural Science Basic Research Program of Shaanxi Province, General Project (Youth) (2020JQ-959).
References
- The pathology associated with diabetic retinopathy. Vision Res. 2017;139:7-14.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62:211-7.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8:337-47.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic retinopathy: Pathophysiology and treatments. Int J Mol Sci. 2018;19:1816.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers in diabetic retinopathy. Rev Diabet Stud. 2015;12:159-95.
- [CrossRef] [PubMed] [Google Scholar]
- Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16:345-57.
- [CrossRef] [PubMed] [Google Scholar]
- Autophagy and obesity and diabetes. Adv Exp Med Biol. 2020;1207:445-61.
- [CrossRef] [PubMed] [Google Scholar]
- Autophagy and mitochondria in obesity and type 2 diabetes. Curr Diabetes Rev. 2017;13:352-69.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting AMPK in diabetes and diabetic complications: Energy homeostasis, autophagy and mitochondrial health. Curr Med Chem. 2019;26:5207-29.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of autophagy in oxidative stress. Int J Mol Sci. 2020;21:3289.
- [CrossRef] [PubMed] [Google Scholar]
- KAT5 Negatively regulates the proliferation of prostate cancer LNCaP cells via the caspase 3-dependent apoptosis pathway. Anim Cells Syst (Seoul). 2019;23:253-9.
- [CrossRef] [PubMed] [Google Scholar]
- KAT5 promotes invasion and metastasis through C-MYC stabilization in ATC. Endocr Relat Cancer. 2019;26:141-51.
- [CrossRef] [PubMed] [Google Scholar]
- TIP60/KAT5 is required for neuronal viability in hippocampal CA1. Sci Rep. 2019;9:16173.
- [CrossRef] [PubMed] [Google Scholar]
- The KAT5-Acetyl-Histone4-Brd4 axis silences HIV-1 transcription and promotes viral latency. PLoS Pathog. 2018;14:e1007012.
- [CrossRef] [PubMed] [Google Scholar]
- DNA repair factor KAT5 prevents ischemic acute kidney injury through glomerular filtration regulation. iScience. 2021;24:103436.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased KAT5 expression impairs DNA repair and induces altered DNA methylation in kidney podocytes. Cell Rep. 2019;26:1318-32.e4.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of ER stress-induced autophagy by GSK3β-TIP60-ULK1 pathway. Cell Death Dis. 2016;7:e2563.
- [CrossRef] [PubMed] [Google Scholar]
- KAT5 acetylates cGAS to promote innate immune response to DNA virus. Proc Natl Acad Sci U S A. 2020;117:21568-75.
- [CrossRef] [PubMed] [Google Scholar]
- Histone HIST1H1C/H1.2 regulates autophagy in the development of diabetic retinopathy. Autophagy. 2017;13:941-54.
- [CrossRef] [PubMed] [Google Scholar]
- PG545 alleviates diabetic retinopathy by promoting retinal Müller cell autophagy to inhibit the inflammatory response. Biochem Biophys Res Commun. 2020;531:452-8.
- [CrossRef] [PubMed] [Google Scholar]
- Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells. 2019;8:213.
- [CrossRef] [PubMed] [Google Scholar]








