Translate this page into:
Metastatic Carcinoma in Effusions

*Corresponding author: Vinod B. Shidham, MD, FIAC, FRCPath, Department of Pathology, Wayne State University School of Medicine, Karmanos Cancer Center and Detroit Medical Center, Detroit, Michigan, United States. vshidham@med.wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Shidham VB. Metastatic carcinoma in effusions. CytoJournal 2022;19:4.
Abstract
Serous cavity may be involved by any neoplasm, including very rare examples of involvement by central nervous system tumors leading to a malignant effusion. The serous cavity lining is rich in lymphatics with lymphatic lacunae opening directly through narrow gaps (stoma) in the lining. Carcinomas mainly metastasize to serosa via the lymphatic vessels, which may be blocked leading to effusion. Primary carcinomas of organs such as lung, intestines, liver, ovary, etc., lined by serosal membranes may spread by direct extension, resulting in malignant effusions. As standard of practice, unless specified, cytopathologic examination of serous effusions implies detection of malignant cells.
As compared to a surgical biopsy from a small focal area of an extensive serosal surface, effusion fluid from respective cavity exfoliates the cells from the entire serosal surface with minimal chance of sampling artifact. Because of this, effusion fluid cytology generally provides a higher diagnostic yield as compared to biopsy of the serous lining, as demonstrated by some studies. However, various challenges related to effusion fluid cytology makes the interpretation of effusion fluid cytology a field with potential misinterpretations, especially for those without proper experience or training.
Developing and following a methodical approach is important for appropriate cytologic examination of effusion fluids. Proper approach may achieve definitive interpretation even without ancillary tests. However, lack of appropriate approach and processing may introduce a significant variation in interpretation due to combination of well-recognized diagnostic pitfalls, which may lead to lower reproducibility and even serious misinterpretations.
Current review discusses in brief appropriate approach to processing and evaluating effusion fluid cytology for metastatic carcinoma. At general level, this is comparable to that of other specimens; however, it is critical to modify with reference to the limitations associated with effusion cytology.
Keywords
Carcinoma
Metastasis
Effusion
Serous cavity
Fluid
Cytopathology

GENERAL FEATURES
• Any neoplasm, including rare examples of central nervous system tumors,[1] may involve a serous cavity and manifest as a malignant effusion. Amongst these, metastatic adenocarcinoma is, by far, the most common cause of malignant effusions [Figure 1].[2] The serous cavity lining is rich in lymphatic channels. The lymphatic lacunae open through narrow gaps (stoma) in the lining with virtual extension of the lymphatic system into the serous cavities.[3] Most of the carcinomas from various sites metastasize to serosa via the lymphatic vessels. Blockage of lymphatic channels by carcinoma cells also contribute to effusion. Primary carcinomas of organs covered with serosal membranes such as lung, intestines, liver, ovary, etc., can spread to serous cavities by direct extension, resulting in malignant effusions.
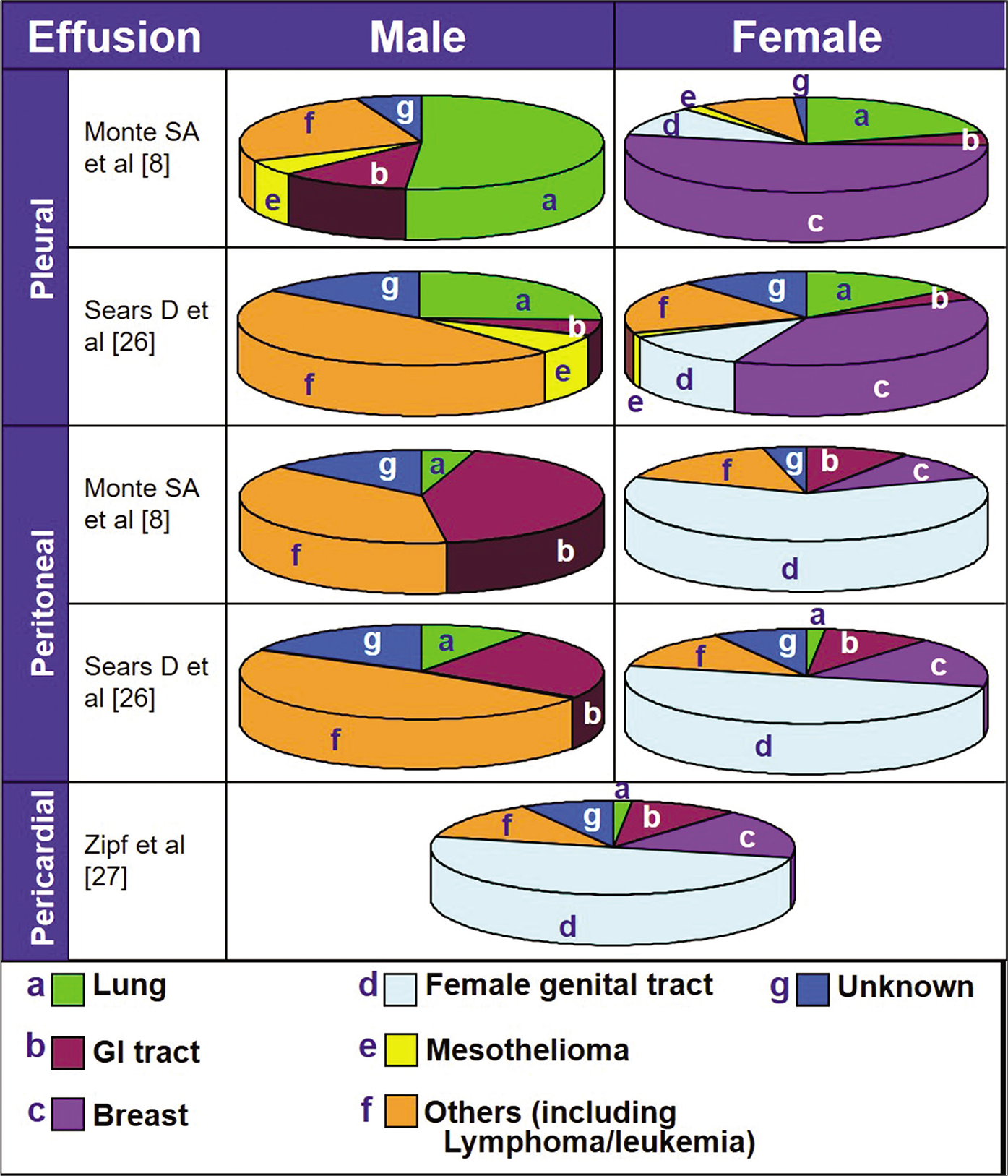
- Common sources of primary neoplasms causing malignant effusions (compiled from refs 8, 26 and 27).
Cytopathologic evaluation of effusions from serous cavities is usually focused on the detection of malignant cells.[2]
• The sampling benefit of effusion cytology generally provides a higher yield of diagnostic material than biopsy of the serous lining. In contrast to a focal biopsy from a small area of an extensive serosal surface, an effusion represents cells exfoliated from the entire serosal surface. In a series of 414 cases of malignant effusion, needle biopsy of the pleura was non-diagnostic in 13%; however, cytologic analysis of the effusions demonstrated higher diagnostic sensitivity.[4]
With the proper approach, cytologic examination of effusion fluids, with or without ancillary tests, is a highly valuable method for diagnosing cancer. However, there may be a significant variation in interpretation of effusion cytology. This, in combination with well-recognized diagnostic pitfalls in this field, may lead to a lack of reproducibility and even serious misinterpretation.
The approach to processing and evaluating effusion cytology for interpretation of metastatic carcinoma is comparable to that of other specimens. However, it is crucial not to ignore the need for incidental modifications with reference to the limitations associated with effusion cytology. A brief discussion about this, with reference to metastatic carcinoma, follows.
CLINICAL HISTORY
Clinical details may be important for cytologic interpretation of effusions. However, depending on the clinical scenario and cytologic findings, the clinical history may be misleading, especially for the beginners. In addition, some effusions may develop without any history of cancer and may present a diagnostic challenge.[5–7] In a series of 248 patients with malignant effusions, 10% of cases (25 effusion fluids: 18 pleural and 7 peritoneal) presented with effusion as the initial manifestation of cancer.[8]
• The clinical history plays a critical role in ensuring proper triaging and processing of effusion specimens; therefore, to facilitate appropriate processing of effusions, the clinical history should be provided in all the requisitions. This could avoid suboptimal cytopathologic interpretations. Depending on individual laboratory preferences, the cell-blocks should be prepared from all effusion specimens, especially from the cases with a history of malignancy and those cases with suspected malignant effusions. If the history is not available, the decision to make a cell-block may have to be based on cytologic findings. If indicated, the cell-block are prepared from the remaining effusion fluid stored in the refrigerator. However, this approach will increase the turnaround time and may compromise specimen quality, especially related to the integrity of immunoreactivity.
CYTOPATHOLOGY
Reactive mesothelial cells may have significant morphologic overlap with cancer cells. Such reactive mesothelial cells may be a major or a minor component of the malignant effusion. Some of these factors are significant diagnostic pitfalls, which may lead to false-positive interpretations. This is particularly applicable to cases with a previous history of carcinoma. Although a false-positive diagnosis may be difficult to disprove, it may subject the patient to improper management decisions and emotional distress.
On the other hand, adenocarcinoma cells may resemble reactive mesothelial cells, leading to false-negative interpretations. Some well-differentiated and low-grade adenocarcinomas show features such as intercellular spaces resembling ‘mesothelial windows’ [see Figures 2c,3],[9] hyperchromasia, high nucleocytoplasmic ratios, anisokaryosis, etc., overlapping with reactive mesothelial cells.
![Metastatic poorly differentiated adenocarcinoma of lung, pleural fluid. The DQ-stained preparation (a) demonstrates reactive mesothelial cells (arrowhead 1 in a) mixed with a ‘second population’ of cohesive groups of cells (arrow 2 in a) with eccentric nuclei that touch the periphery of the carcinoma cells (arrowheads in b,c,f). Some cells are less cohesive seen as scattered small groups or solitary carcinoma cells (arrows in d–f). Occasional intercellular spaces, resembling mesothelial windows may be present (arrowhead w in c). The patient had poorly differentiated adenocarcinoma of the lung. [a–c, DQ-stained Cytospin smear; d–f, PAP-stained SurePath smear (a, 10X; b,c, 100X; d, 10X; e,f, 100X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g003.png)
-
Metastatic poorly differentiated adenocarcinoma of lung, pleural fluid. The DQ-stained preparation (a) demonstrates reactive mesothelial cells (arrowhead 1 in a) mixed with a ‘second population’ of cohesive groups of cells (arrow 2 in a) with eccentric nuclei that touch the periphery of the carcinoma cells (arrowheads in b,c,f). Some cells are less cohesive seen as scattered small groups or solitary carcinoma cells (arrows in d–f). Occasional intercellular spaces, resembling mesothelial windows may be present (arrowhead w in c). The patient had poorly differentiated adenocarcinoma of the lung. [a–c, DQ-stained Cytospin smear; d–f, PAP-stained SurePath smear (a, 10X; b,c, 100X; d, 10X; e,f, 100X).]
![Metastatic ovarian adenocarcinoma, peritoneal fluid. The neoplastic cells show an Indian-file pattern. This arrangement is not specific for small cell carcinoma of lung [Figure 6] and may be seen in other non-small-cell carcinomas including metastatic ovarian and mammary adenocarcinoma. [a, PAP-stained SurePath smear; b, DQ-stained Cytospin smear (a,b, 100X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g004.png)
-
Metastatic ovarian adenocarcinoma, peritoneal fluid. The neoplastic cells show an Indian-file pattern. This arrangement is not specific for small cell carcinoma of lung [Figure 6] and may be seen in other non-small-cell carcinomas including metastatic ovarian and mammary adenocarcinoma. [a, PAP-stained SurePath smear; b, DQ-stained Cytospin smear (a,b, 100X).]
Changes secondary to irradiation usually show bizarre cells in effusions. The reported findings include:
cytomegaly with normal nucleocytoplasmic ratio
degenerative hyperchromasia with smudgy chromatin
cytoplasmic vacuoles deforming the nucleus
degenerative cytoplasmic changes with two-tone staining [shades of cyanophilia (blue-green) and eosinophilia (pink)].
mitotic figures may be present.[10]
These features may be present collectively in association with irradiation, but they are not specific. There are no consistent cytologic changes in effusions that can confirm the irradiation therapy in effusion cytology. The important concern is that these changes should not be misinterpreted as neoplastic, representing recurrence of initial disease for which the radiation therapy was administered.
Scanty, degenerated, poorly preserved cells and improperly processed specimens by inadequate protocols hinder proper interpretation. If the initial findings are equivocal for cancer cells, it is prudent to be extra cautious by recommending repeat cytologic evaluation on a new properly collected and processed specimen in appropriate quantity. If the initial effusion is caused by cancer, it usually reaccumulates rapidly and may contain unequivocal cancer cells with improved morphology.
The cytologic examination of effusions not only allows a definite diagnosis of metastatic carcinoma but may also help for identifying the primary site [Table 1]. Once malignant cells are confirmed in effusion fluid, the clinical history and radiologic findings may also assist in identifying the site of origin of the carcinoma. Depending on the clinical scenario, the exercise of attempting to identify the primary site may be just an exercise of intellectual curiosity, especially if it does not change the clinical management.
| Cytomorphological patterns | Possible primary |
|---|---|
| 1. Three dimensional round cell groups- proliferation spheres or ‘cannonballs’ | Breast adenocarcinoma Ovarian adenocarcinoma Mesothelioma of epithelioid type |
| 2. Acini / glands | Adenocarcinomas of breast, lung, colorectum, stomach, ovary, endometrium, etc. Mesothelioma of epithelioid type |
| 3. Predominantly scattered isolated malignant cells | Gastric adenocarcinoma Non-cohesive variant of lung adenocarcinoma Breast lobular carcinoma Adrenocortical carcinoma (Also Lymphoma, Melanoma, & Sarcoma) |
| 4. Carcinoma cells in chains and rows (‘Indian file’ pattern) | Breast- Lobular and ductal carcinoma Poorly differentiated small cell carcinoma Gastric adenocarcinoma Ovarian adenocarcinoma |
| 5. Extensive cytoplasmic vacuolization | Renal cell adenocarcinoma (glycogen,fat) Adrenocortical carcinoma (fat) Pancreatic adenocarcinoma (mucin) Ovarian adenocarcinoma (mucin) Lung adenocarcinoma Clear cell carcinoma endometrium |
| 6. Signet-ring cells | Gastric adenocarcinoma Colorectal adenocarcinoma |
| 7. Giant tumor cells | Lung large cell carcinoma-giant cell type Pancreatic adenocarcinoma Thyroid anaplastic carcinoma Squamous cell carcinoma (also melanoma and pleomorphic sarcoma) |
| 8. Targetoid intracytoplasmic vacuole containing secretion | Breast adenocarcinoma (especially lobular) Thyroid carcinoma (colloid) Ovarian carcinoma Pancreatic carcinoma |
| 9. Three-dimensional groups in papillary configurations | Bronchioloalveolar carcinoma Colonic adenocarcinoma Endometrial adenocarcinoma Mammary adenocarcinoma |
| 10. Three-dimensional papillary groups containing psammoma bodies | Ovarian carcinoma-serous papillary Thyroid papillary carcinoma Pancreatic papillary carcinoma |
| 11. Cell groups of tall columnar cells with a picket fence pattern | Colonic adenocarcinoma Pancreato-biliary carcinoma |
| 12. Cellular pleomorphism | Poorly differentiated carcinomas of lung, pancreas, ovary, thyroid, urothelium |
| 13. Large polyhedral cells | Hepatocellular carcinoma Transitional cell carcinoma Large-cell-type squamous cell carcinoma |
| 14. Sharp angulated cell borders with keratinization | Keratinizing squamous cell carcinoma |
| 15. Cytoplasmic pigment | Hepatocellular carcinoma: bile (melanoma: melanin) |
| 16. Prominent nucleoli | Hepatocellular carcinoma Renal cell carcinoma Prostatic adenocarcinoma (melanoma) |
(Compiled from references 2,11-14).
This chapter describes cytomorphologic and salient clinical features of different types of metastatic carcinoma in serous effusions. Various features are summarized in Table 1 and discussed in brief below. Although many cytomorphologic features are characteristic,[11,12] there is a significant overlap of some features amongst different neoplasms, which may lead to repetition of some features in the description. Features such as intercellular windows, papillary configurations, Indian file pattern, etc., are generally identified with specific primary sites; however, they may also be observed unexpectedly with other metastatic carcinomas [Figures 2-4].[13,14]
![Metastatic pancreatic adenocarcinoma, peritoneal fluid. The neoplastic cells (NC) are seen as loosely cohesive groups (a) or as solitary cells (b,e) with eccentric nuclei. PAP-stained preparations facilitate evaluation of cellular details in cohesive groups (c,d). A few cell groups show gland-like structures (arrow in d). ‘A second population’ (arrows in f–h) of neoplastic cells is highlighted distinctly in immunostained cell-block sections f–h (the neoplastic cells are immunoreactive for BerEP4 in f, non-immunoreactive for vimentin in g, and calretinin in h). As inbuilt corresponding positive controls, inflammatory and reactive mesothelial cells (arrowhead in g) are immunoreactive for vimentin and reactive mesothelial cells (arrowhead RM in h) are immunoreactive for calretinin. The patient had pancreatic adenocarcinoma. NC, neoplastic cell; RM, reactive mesothelial cell. [a,b, DQ-stained Cytospin smear; c–e, PAP-stained SurePath smear; f–h, immunostained cell-block sections (a–e, 100X; f–h, 40X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g005.png)
-
Metastatic pancreatic adenocarcinoma, peritoneal fluid. The neoplastic cells (NC) are seen as loosely cohesive groups (a) or as solitary cells (b,e) with eccentric nuclei. PAP-stained preparations facilitate evaluation of cellular details in cohesive groups (c,d). A few cell groups show gland-like structures (arrow in d). ‘A second population’ (arrows in f–h) of neoplastic cells is highlighted distinctly in immunostained cell-block sections f–h (the neoplastic cells are immunoreactive for BerEP4 in f, non-immunoreactive for vimentin in g, and calretinin in h). As inbuilt corresponding positive controls, inflammatory and reactive mesothelial cells (arrowhead in g) are immunoreactive for vimentin and reactive mesothelial cells (arrowhead RM in h) are immunoreactive for calretinin. The patient had pancreatic adenocarcinoma. NC, neoplastic cell; RM, reactive mesothelial cell. [a,b, DQ-stained Cytospin smear; c–e, PAP-stained SurePath smear; f–h, immunostained cell-block sections (a–e, 100X; f–h, 40X).]
Although, this review focuses on cytomorphological features of various metastases to serous cavities, some cases may require ancillary testing. The most frequently applied ancillary testing is immunohistochemistry with SCIP approach discussed in detail in other reviews in this review series.
CHARACTERISTIC FEATURES OF SOME METASTATIC CARCINOMAS FROM SPECIFIC PRIMARY SITES [TABLE 1]
LUNG CARCINOMAS[15–25]
Lung cancer is the most common cause of malignant pleural effusions in men and the second most common cause of malignant pleural effusions in women [Table 2, Figure 1]. About 50% of patients with disseminated lung cancer develop pleural effusions, which may be due to neoplastic obstruction of hilar lymph nodes, blockage of the pleural lymphatics by carcinoma cells, direct extension of neoplasm to the pleural surfaces, or a combination of these.
| Order of frequency | Pleural effusion | Peritoneal effusion | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 1 | Lung | Breast | GI | Ovary |
| 2 | GI | Lung | Pancreas | GI |
| 3 | Pancreas | Ovary | Lung | Pancreas |
GI, gastrointestinal tract.
Adenocarcinoma is the most common primary lung cancer leading to pleural effusion, followed by undifferentiated small cell carcinoma. This appears to be due to frequent peripheral location of pulmonary adenocarcinomas, which facilitates their early spread to the pleura.
Other subtypes of lung cancer encountered in pleural effusions include lepidic adenocarcinoma (bronchioloalveolar cell carcinoma) and large cell carcinoma. In contrast, although squamous cell carcinoma is a common subtype, it is found with far less frequency in serous effusions, especially the keratinizing subtype. Although giant cell carcinoma of the lung is rare, it is a fast-growing neoplasm with a rapid course, commonly involving the pleura, with pleural effusion at the time of initial presentation. The diagnostic cytologic features of these subtypes are described below.
ADENOCARCINOMA [see Figure 2,5]
![Metastatic adenocarcinoma—NOS, peritoneal fluid. Cohesive groups of cells in papillary-like configurations show eccentrically placed nuclei touching the periphery of cells (arrows in b,c,f). The cells in such groups are difficult to study at lower magnification (a). However, the cell morphology in such groups can be observed at the periphery, especially under higher magnification (arrow in b and c). Some groups show gland-like spaces (arrowheads in d,e,f). [a–c, PAP-stained SurePath smear; d–f, DQ-stained Cytospin smear (a, 10X; b, 40X; c, 100X zoomed; d, 10X; e, 40X; f, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g006.png)
-
Metastatic adenocarcinoma—NOS, peritoneal fluid. Cohesive groups of cells in papillary-like configurations show eccentrically placed nuclei touching the periphery of cells (arrows in b,c,f). The cells in such groups are difficult to study at lower magnification (a). However, the cell morphology in such groups can be observed at the periphery, especially under higher magnification (arrow in b and c). Some groups show gland-like spaces (arrowheads in d,e,f). [a–c, PAP-stained SurePath smear; d–f, DQ-stained Cytospin smear (a, 10X; b, 40X; c, 100X zoomed; d, 10X; e, 40X; f, 100X zoomed).]
Well-differentiated variant
The cells in smears of effusions show a second population of loosely cohesive clusters of medium to large-sized cells, which usually have round to oval hyperchromatic eccentric nuclei with fine to coarsely granular chromatin and variably conspicuous nucleoli [Figure 5]. They have a moderate amount of cytoplasm, which is usually vacuolated [see Figure 2]. Even when accompanied by a well-differentiated component, some of the neoplastic cells are easy to interpret unequivocally as malignant in PAP stained preparation. As an adjunct, mucicarmine stain or Periodic Acid -Schiff (PAS) stain after diastase digestion may demonstrate mucin in the cytoplasm of the neoplastic cells.
Non-cohesive variant
This variant predominantly shows poorly cohesive, isolated, medium to large cells with eccentrically located, round to oval, hyperchromatic nuclei with fine to coarsely granular chromatin, variably conspicuous nucleoli, and a variable amount of cytoplasm which may be vacuolated. This variant may be difficult to distinguish from the epithelioid type of mesothelioma and high-grade, poorly differentiated large cell lymphoma in effusions. The PAS stain (with diastase) and mucicarmine stain may help to confirm the presence of intracytoplasmic mucin in some of the cells. Immunophenotyping with immunocytochemistry may be extremely valuable in arriving at a correct diagnosis.
Poorly differentiated variant
The cytomorphology of this variant is easy to interpret as malignant. The smears show poorly cohesive groups of large carcinoma cells with variable amounts of cytoplasm which may show vacuoles and pleomorphic, round to oval to irregularly shaped, hyperchromatic nuclei with coarsely granular chromatin. The nucleoli are usually prominent in most of the cells.
POORLY DIFFERENTIATED SMALL CELL CARCINOMA [Figure 6]
![Metastatic small cell carcinoma, pleural fluid. Cancer cells are present as solitary cells (c,d), small groups (g–i), and large groups (j). The cells are small with high nucleocytoplasmic ratios (c,d,g–i). The nuclei are hyperchromatic with salt and pepper chromatin (c,d,i). Solitary cancer cells (NC in c,d) resemble lymphocytes (blue arrow Ly in f) and may be misinterpreted as lymphoma, especially in PAP-stained preparations. However, the presence of cohesive groups (g–j) with various patterns, including Indian-file pattern (g), favor carcinoma. The nuclear molding (arrows nm in g,i) distinguishes them from other poorly differentiated carcinomas. Mitotic figures (arrowhead in e) and apoptotic cancer cells (arrowheads in d,f,h) are also present. Rare reactive mesothelial cells (blue arrows RM in a,b,e) are present with a few chronic inflammatory cells (blue arrow Ly in f) in the background. Immunostained cell-block sections showed immunoreactivity for neuroendocrine immunomarkers (chromogranin, synaptophysin, and CD56). The patient had poorly differentiated small cell carcinoma of lung. NC, neoplastic cell; RM, reactive mesothelial cell, nm, nuclear molding, Ly, lymphocyte.[a–j, PAP-stained SurePath smear (a,b, 100X; c–j, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g007.png)
-
Metastatic small cell carcinoma, pleural fluid. Cancer cells are present as solitary cells (c,d), small groups (g–i), and large groups (j). The cells are small with high nucleocytoplasmic ratios (c,d,g–i). The nuclei are hyperchromatic with salt and pepper chromatin (c,d,i). Solitary cancer cells (NC in c,d) resemble lymphocytes (blue arrow Ly in f) and may be misinterpreted as lymphoma, especially in PAP-stained preparations. However, the presence of cohesive groups (g–j) with various patterns, including Indian-file pattern (g), favor carcinoma. The nuclear molding (arrows nm in g,i) distinguishes them from other poorly differentiated carcinomas. Mitotic figures (arrowhead in e) and apoptotic cancer cells (arrowheads in d,f,h) are also present. Rare reactive mesothelial cells (blue arrows RM in a,b,e) are present with a few chronic inflammatory cells (blue arrow Ly in f) in the background. Immunostained cell-block sections showed immunoreactivity for neuroendocrine immunomarkers (chromogranin, synaptophysin, and CD56). The patient had poorly differentiated small cell carcinoma of lung. NC, neoplastic cell; RM, reactive mesothelial cell, nm, nuclear molding, Ly, lymphocyte.[a–j, PAP-stained SurePath smear (a,b, 100X; c–j, 100X zoomed).]
In effusions, the neoplastic cells of this variant are usually present as loose groups or as isolated cells. These fragile cells are usually small with high nucleocytoplasmic ratios. • A most significant and reliable diagnostic feature of small cell carcinoma is insignificant nucleoli, which, in smears, are usually unrecognizable. However, as compared to cytology of small cell carcinomas in other specimens, effusion cytology may exhibit small, insignificant nucleoli in some nuclei. The chromatin is of ‘salt and pepper’ type with a mixture of fine and coarse chromatin dots in hyperchromatic nuclei [Figure 6]. Most cells may appear as stripped nuclei without visible cytoplasm or with a scant amount of poorly discernible cytoplasm in Papanicolaou (PAP)-stained smears. However, this scant rim of cytoplasm is highlighted better in Diff-Quik (DQ)-stained smears. Because the nuclei are delicate and cytoplasm is scanty, the nuclei often mold with each other. This nuclear molding feature may help in distinguishing them from other poorly differentiated carcinomas, but not from high-grade lymphoma cells which may also show nuclear molding. The cell groups have a tendency to undergo focal single cell necrosis with scattered apoptotic bodies, concurrently admixed with some mitotic figures. Long-standing and recurrent effusions may contain small proliferation spheres of carcinoma cells which appear to wrap around one another to impart an onion-skin appearance.
POORLY DIFFERENTIATED LARGE CELL CARCINOMA
The smears show a population of isolated carcinoma cells and loosely cohesive groups of cells with variable amounts of non-vacuolated cytoplasm. The cells have high nucleocytoplasmic ratios with large, ovoid to irregularly shaped hyperchromatic nuclei, with irregularly distributed, coarsely granular chromatin. Multinucleation is frequent, and most of the cells have prominent nucleoli. The cytomorphologic features of this neoplasm overlap with those of poorly differentiated adenocarcinoma; however, focal glandular differentiation and the presence of mucicarmine-positive cytoplasmic vacuoles are absent in poorly differentiated large cell carcinoma.
Giant cell variant of large cell carcinoma
This rapidly growing, rare variant of poorly differentiated large cell carcinoma is highly malignant and is often associated with pleural effusion. Isolated, giant cancer cells may be multinucleated or mononucleated with huge hyperchromatic nuclei and variable amounts of well-defined cytoplasm. The nuclei have coarsely granular, irregularly distributed chromatin with significant parachromatin clearing and prominent nucleoli.
LEPIDIC ADENOCARCINOMA (BRONCHIOLOALVEOLAR CELL CARCINOMA
Non-secretory variant [Figure 7]
![Metastatic lepidic adenocarcinoma (bronchioloalveolar cell carcinoma) of lung, pleural fluid. Cellular specimen (a) shows three-dimensional groups of carcinoma cells (arrowheads in a–f) mixed with reactive mesothelial cells (blue arrow RM in f,g). The two populations are demonstrated more distinctly with the DQ stain (f,g) than with the PAP stain (a–e). However, the morphologic details of individual neoplastic cells are more distinct in the PAP stain (a–e), especially under higher magnification (c–e). The individual cells show features of well-differentiated adenocarcinoma (red arrows NC in d,e,g). Although usually not conspicuous, some of the carcinoma cells have prominent nucleoli (d,e). The neoplastic cells demonstrate nuclear immunoreactivity for TTF-1 (arrowheads in h) consistent with a lung primary. The patient had lepidic adenocarcinoma (bronchioloalveolar cell carcinoma) of lung. NC, neoplastic cell; RM, reactive mesothelial cell; TTF-1, thyroid transcription factor-1. [a–e, PAP-stained SurePath smear; f–g, DQ-stained Cytospin smear; h, immunostained cell-block section (a, 10X; b, 40X; c, 100X; d–e, 100X zoomed; f, 40X; g, 100X; h, 40X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g008.png)
-
Metastatic lepidic adenocarcinoma (bronchioloalveolar cell carcinoma) of lung, pleural fluid. Cellular specimen (a) shows three-dimensional groups of carcinoma cells (arrowheads in a–f) mixed with reactive mesothelial cells (blue arrow RM in f,g). The two populations are demonstrated more distinctly with the DQ stain (f,g) than with the PAP stain (a–e). However, the morphologic details of individual neoplastic cells are more distinct in the PAP stain (a–e), especially under higher magnification (c–e). The individual cells show features of well-differentiated adenocarcinoma (red arrows NC in d,e,g). Although usually not conspicuous, some of the carcinoma cells have prominent nucleoli (d,e). The neoplastic cells demonstrate nuclear immunoreactivity for TTF-1 (arrowheads in h) consistent with a lung primary. The patient had lepidic adenocarcinoma (bronchioloalveolar cell carcinoma) of lung. NC, neoplastic cell; RM, reactive mesothelial cell; TTF-1, thyroid transcription factor-1. [a–e, PAP-stained SurePath smear; f–g, DQ-stained Cytospin smear; h, immunostained cell-block section (a, 10X; b, 40X; c, 100X; d–e, 100X zoomed; f, 40X; g, 100X; h, 40X).]
This variant usually shows monolayered sheets and three-dimensional groups of carcinoma cells. The individual cells have features of well-differentiated adenocarcinoma [Figure 7]. The carcinoma cells may also form proliferation spheres. Medium to large cells have a small to moderate amount of cytoplasm and relatively uniform, round to oval nuclei with fine chromatin. Although usually not conspicuous, some of the carcinoma cells may have prominent nucleoli, and some nuclei may have intranuclear pseudoinclusions. Psammoma bodies are occasionally seen in the clusters of carcinoma cells.
Secretory variant
The cytomorphologic features of this variant overlap those of the non-secretory variant, except that the cells have large amounts of clear to vacuolated cytoplasm.
The appearance of cytoplasm in all lepidic adenocarcinoma cells forming a particular group is identical. In contrast, other adenocarcinomas typically show variation in cytoplasmic appearances from one cell to another in the same group.
SQUAMOUS CELL CARCINOMA
Keratinizing type
Although frequently observed in sputum, the cells of keratinizing squamous cell carcinoma are uncommon in serous effusions. If detected in effusions, the cells of keratinizing squamous cell carcinoma are seen as rare solitary cells or in small loose groups. Keratinizing carcinoma cells with pyknotic nuclei are not proliferating; therefore, they do not form proliferation spheres. These carcinoma cells show moderate amounts of relatively dense, orangeophilic cytoplasm with medium to large-sized nuclei without recognizable nucleoli.
Non-keratinizing type
The cytomorphology of non-keratinizing squamous cell carcinoma in smears of effusions may be difficult to distinguish from those of poorly differentiated non-mucinous adenocarcinoma and invasive urothelial carcinoma. Medium to large carcinoma cells may be observed as loosely cohesive, rather flat groups or even as three-dimensional cell groups. They have vaguely round to oval but usually irregularly shaped hyperchromatic nuclei, with fine to coarsely granular, irregularly clumped chromatin with parachromatin clearing. In contrast to the prominent nucleoli often seen in smoothly oval to round nuclei of adenocarcinoma cells, the nucleoli in non-keratinizing squamous cell carcinoma, although present, are not conspicuous. The dense cytoplasm is variable in amount and is without keratinization, yet cytoplasm of PAP stained cells may occasionally exhibit fine concentric lines around the nucleus, which is evidence of squamous differentiation. As compared to the curvilinear cell borders of adenocarcinoma cells, the cell borders of squamous cell carcinoma cells are usually straight and sharp with angulated cell borders.
BREAST CARCINOMA[28–35]
Carcinoma of the breast is the most common cause of malignant pleural effusions in women [see Table 2, Figure 1]. Up to one-half of patients with breast carcinoma present with a pleural effusion during the course of their disease. Most of the pleural effusions (50–80%) are on the same side as the primary cancer, but about 10% are bilateral.
DUCTAL CARCINOMA
Non-cohesive cell pattern [Figure 8]
![Metastatic mammary carcinoma, pleural fluid. a–g, Proliferation spheres (red arrows NC) with mostly reactive mesothelial cells (blue arrows RM) and inflammatory cells in the background, clearly separated out in the immunostained cell-block sections (a,e). The reactive mesothelial cells (blue arrows RM) stand out distinctly from the neoplastic cells (red arrow NC) in the DQ stain (b,d). However, the reactive mesothelial cells in the PAP stain (blue arrows in g) are difficult to distinguish from neoplastic cells (compare with case in h–j). h–j, A different patient with metastatic mammary carcinoma. The effusion predominantly contains solitary adenocarcinoma cells, as highlighted by the BerEP4 immunostained section (red arrows NC in h). The carcinoma cells (red arrows NC in j) can be distinguished easily from reactive mesothelial cells (blue arrow RM in j) in the DQ stain (j), but not in the PAP stain (i). A mitotic figure is present (blue arrow MF in i). Note the resemblance of reactive mesothelial cells in f,g to neoplastic cells in i. Without the help of DQ stain and immunocytochemistry, such fluids, with predominantly one type of cell population, may easily be misinterpreted. MF, mitotic figure; NC, neoplastic cell; RM, reactive mesothelial cell. [a,e,h, immunostained cell-block sections; f,g,I, PAP-stained SurePath smear; b,c,d,j, DQ-stained Cytospin smear (a, 40X; b, 100X; c,d, 100X zoomed; e, 40X; f, 100X; g, 100X zoomed; h, 40X zoomed; i, 100X; j, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g009.png)
-
Metastatic mammary carcinoma, pleural fluid. a–g, Proliferation spheres (red arrows NC) with mostly reactive mesothelial cells (blue arrows RM) and inflammatory cells in the background, clearly separated out in the immunostained cell-block sections (a,e). The reactive mesothelial cells (blue arrows RM) stand out distinctly from the neoplastic cells (red arrow NC) in the DQ stain (b,d). However, the reactive mesothelial cells in the PAP stain (blue arrows in g) are difficult to distinguish from neoplastic cells (compare with case in h–j). h–j, A different patient with metastatic mammary carcinoma. The effusion predominantly contains solitary adenocarcinoma cells, as highlighted by the BerEP4 immunostained section (red arrows NC in h). The carcinoma cells (red arrows NC in j) can be distinguished easily from reactive mesothelial cells (blue arrow RM in j) in the DQ stain (j), but not in the PAP stain (i). A mitotic figure is present (blue arrow MF in i). Note the resemblance of reactive mesothelial cells in f,g to neoplastic cells in i. Without the help of DQ stain and immunocytochemistry, such fluids, with predominantly one type of cell population, may easily be misinterpreted. MF, mitotic figure; NC, neoplastic cell; RM, reactive mesothelial cell. [a,e,h, immunostained cell-block sections; f,g,I, PAP-stained SurePath smear; b,c,d,j, DQ-stained Cytospin smear (a, 40X; b, 100X; c,d, 100X zoomed; e, 40X; f, 100X; g, 100X zoomed; h, 40X zoomed; i, 100X; j, 100X zoomed).]
Newly developed effusions in older patients with ductal carcinoma usually show the appearance of isolated carcinoma cells [Figure 8h–j]. The effusions contain isolated or small loose clusters of small to medium-sized carcinoma cells having scant cytoplasm with a large, single, cytoplasmic vacuole in some of the cells. Their hyperchromatic round to oval nuclei have finely granular chromatin and small inconspicuous nucleoli. A ‘cell-within-a-cell’ arrangement is common. Some of the neoplastic cells wrap around another carcinoma cell and impart a false appearance of small epithelial pearls. In long-standing or recurrent effusions with continued proliferation of cancer cells in the effusion, the isolated cell pattern may change into a cohesive cell pattern with proliferation spheres [Figures 8a, 9].
![Metastatic mammary adenocarcinoma, pleural fluid. The specimen predominantly shows proliferation spheres (red solid arrows in a and d). Rare reactive mesothelial cells (blue arrows RM) in DQ-stained preparations (e) are easily distinguished from carcinoma cells (red arrows). The nuclei of cancer cells (red arrows in b,e) are mostly eccentric and touch the cell membrane. Mitotic figures (yellow arrow MF in b,e) are present along with apoptotic cells (yellow arrow AP in b,e). Details of proliferation spheres are better seen at the periphery under high magnification (c,f). The patient had mammary carcinoma. AP, apoptotic cancer cell; MF, mitotic figure; RM, reactive mesothelial cell. [a–c, PAP-stained SurePath smear; d–f, DQ-stained Cytospin smear (a, 10X; b, 100X; c, 100X zoomed; d, 10X; e, 100X; f, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g010.png)
-
Metastatic mammary adenocarcinoma, pleural fluid. The specimen predominantly shows proliferation spheres (red solid arrows in a and d). Rare reactive mesothelial cells (blue arrows RM) in DQ-stained preparations (e) are easily distinguished from carcinoma cells (red arrows). The nuclei of cancer cells (red arrows in b,e) are mostly eccentric and touch the cell membrane. Mitotic figures (yellow arrow MF in b,e) are present along with apoptotic cells (yellow arrow AP in b,e). Details of proliferation spheres are better seen at the periphery under high magnification (c,f). The patient had mammary carcinoma. AP, apoptotic cancer cell; MF, mitotic figure; RM, reactive mesothelial cell. [a–c, PAP-stained SurePath smear; d–f, DQ-stained Cytospin smear (a, 10X; b, 100X; c, 100X zoomed; d, 10X; e, 100X; f, 100X zoomed).]
Cohesive cell pattern [Figure 9]
As mentioned above, a cohesive cytologic pattern is usually seen in association with long-standing or recurrent malignant effusions secondary to ductal carcinoma of the breast [see Figure 8a–g]. Characteristically, as a result of proliferation of carcinoma cells in nutrient-rich effusion fluids, the cytologic picture is that with numerous proliferation spheres of various sizes. These three-dimensional proliferation spheres are composed of cells with scant, ill-defined, non-vacuolated cytoplasm [Figure 9]. Their hyperchromatic, round to oval nuclei have finely granular chromatin and relatively inconspicuous nucleoli, with a lengthwise arrangement of nuclei along the periphery of the proliferation spheres [see Figures 9c,f]. Occasionally, conglomerations of several proliferation spheres may lead to papillary-like configurations.
Large cell pattern
This pattern is usually seen in association with effusions caused by poorly differentiated ductal carcinomas. These cells have a tendency to be scattered singly or distributed as loosely cohesive groups. Although proliferation spheres may rarely be seen, papillary configurations are absent. The large carcinoma cells with high nucleocytoplasmic ratio have a small to moderate amount of non-vacuolated cytoplasm. The nuclei are round to oval and hyperchromatic, with fine to coarsely granular chromatin, and frequently show prominent nucleoli.
LOBULAR CARCINOMA
Isolated scattered carcinoma cells with scant to moderate amount of cytoplasm have subtly hyperchromatic to normochromatic, oval to irregular, relatively low-grade, eccentrically placed nuclei with fine to coarsely granular chromatin and variable but usually inconspicuous nucleoli. Small cell groups with an ‘Indian file’ pattern may be seen. This pattern in effusion cytology is not specific for any particular primary neoplasm and is observed with other metastatic carcinomas14 [see Table 1]. A few cells have large cytoplasmic targetoid vacuoles with secretions. Although binucleated carcinoma cells are not uncommon, more than two nuclei are infrequent.
These tumor cells have significant cytomorphological overlap with floridly reactive mesothelial cells. Because of this some cases, especially those predominantly with tumor cells without detectable second foreign population may be misinterpreted falsely as negative. Application of ancillary studies including immunohistochemistry usually allows objective confirmation of metastatic disease.
MEDULLARY CARCINOMA
The cells of this type of breast carcinoma overlap with poorly differentiated carcinomas in general, with scattered isolated cells. The effusions exhibit a second population consisting of single or loosely cohesive groups of carcinoma cells in a background of lymphocytes. Medium-sized to large carcinoma cells with a high nucleocytoplasmic ratio have a moderate amount of cytoplasm and round to oval, eccentrically placed hyperchromatic nuclei with coarsely granular chromatin. The nuclei of some cancer cells have prominent nucleoli. In general, binucleation of neoplastic cells may be observed, but more than two nuclei are infrequent.
These tumor cells have significant cytomorphological overlap with floridly reactive mesothelial cells. Because of this some cases, especially those predominantly with tumor cells without detectable second foreign population may be misinterpreted falsely as negative, comparable to that with metastatic lobular carcinoma. Application of ancillary studies including immunohistochemistry usually allows objective confirmation of metastatic disease.
CARCINOMAS OF THE GASTROINTESTINAL TRACT[36–45]
Gastrointestinal carcinomas develop malignant effusions in 20–30% of cases. In men they are the most common cause and in women the second most common cause of malignant peritoneal effusions. After lung cancer, gastrointestinal carcinomas are the second most common cause of malignant pleural effusions in men [see Table 2, Figure 1]. They rarely present as pericardial effusions except in esophageal carcinoma, usually secondary to esophagopericardial fistula.[44,45] Various cytomorphologic features useful for interpreting different types of gastrointestinal carcinomas in effusions are described below.
ADENOCARCINOMA OF COLON [see Figure 10]
Non-secretory variant
In effusions, this carcinoma shows significant morphologic overlap with other adenocarcinomas. The smears show closely packed cohesive groups or papillary-like structures of medium-sized carcinoma cells with scant cytoplasm. Usually small, ovoid or fusiform hyperchromatic nuclei are with finely granular chromatin and generally small unrecognizable nucleoli. Rarely, the nuclei may be of medium to large size. A characteristic cytologic feature of the non-secretory variant of adenocarcinoma of the colon is elongated, palisading nuclei with parallel arrangements along the periphery of cell groups [Figure 10d]. Apoptois, responsible for ‘dirty necrosis’, is usually present.
![Metastatic adenocarcinoma of colon, peritoneal fluid. Cohesive groups of cells (a) show high nucleocytoplasmic ratios and eccentric nuclei touching the periphery of the cell (arrow in b). Some adenocarcinoma cells show cytoplasmic vacuoles containing mucin (arrowhead in the inset of b). The cell groups in papillary configurations are difficult to study at lower magnification (c). Peripheral palisading is better observed under higher magnification (d). The patient had colonic adenocarcinoma. [a,b, DQ-stained Cytospin smear; c,d, PAP-stained SurePath smear (a, 40X; b, 100X; c, 40X; d, 100X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g011.png)
-
Metastatic adenocarcinoma of colon, peritoneal fluid. Cohesive groups of cells (a) show high nucleocytoplasmic ratios and eccentric nuclei touching the periphery of the cell (arrow in b). Some adenocarcinoma cells show cytoplasmic vacuoles containing mucin (arrowhead in the inset of b). The cell groups in papillary configurations are difficult to study at lower magnification (c). Peripheral palisading is better observed under higher magnification (d). The patient had colonic adenocarcinoma. [a,b, DQ-stained Cytospin smear; c,d, PAP-stained SurePath smear (a, 40X; b, 100X; c, 40X; d, 100X).]
Secretory variant
Cohesive groups of carcinoma cells with a small to moderate amount of clear to vacuolated cytoplasm are frequently seen in a papillary configuration. Similar to the non-secretory variant, although these carcinomas are not papillary adenocarcinomas, they often demonstrate papillary-like aggregates in effusions [Figure 10d], which may be mistaken for papillary carcinomas [see Table 1]. The carcinoma cells have medium-sized, round, ovoid, elongated, or fusiform nuclei with finely granular chromatin and generally small or unrecognizable nucleoli. The PAS (after diastase) stain or mucicarmine stain may help to highlight the mucin in some cells.
Mucinous variant
In this variant, the cohesive groups of carcinoma cells demonstrate large nuclei and a lot of clear to vacuolated cytoplasm; however, they do not show a papillary configuration. Intracytoplasmic mucin vacuoles usually distort the hyperchromatic nuclei. The nuclei in some cells have prominent nucleoli. Foamy macrophages with cytoplasmic mucin are frequently seen in effusions in association with this variant. Most of the carcinoma cells in effusions secondary to mucinous adenocarcinomas have a large amount of cytoplasmic mucin. Although a mucinous background is usually seen in fine-needle aspirates of this carcinoma, it is seldom a significant feature in effusion preparations.
Signet-ring variant
The cytomorphology of this tumor may overlap with signet ring variants of other adenocarcinomas, including gastric adenocarcinoma. The smears show single or loosely cohesive groups of carcinoma cells with a large amount of vacuolated cytoplasm. Some of the cells have large, round to oval, hyperchromatic nuclei with fine to coarsely granular chromatin and prominent nucleoli. Other cells have peripheral, crescent-shaped nuclei displaced by a single, large, mucin-containing cytoplasmic vacuole. These two types of adenocarcinoma cells are usually mixed with each other.
ADENOCARCINOMA OF THE STOMACH
Well-differentiated variant
The cytomorphologic features of this variant in effusions overlap with well-differentiated adenocarcinomas of the small intestine, colon, rectum, pancreas, and lung. Loosely cohesive groups of medium to large carcinoma cells with a moderate amounts of vacuolated cytoplasm have round to oval hyperchromatic nuclei with fine to coarsely granular chromatin. The nucleoli are usually small but may be conspicuous in some cells. The PAS (after diastase) stain or mucicarmine stains may demonstrate cytoplasmic mucin.
Poorly differentiated variant
Effusions associated with this variant have cytomorphological features that overlap with other poorly differentiated adenocarcinomas [Figure 5]. Cells with variable amount of cytoplasm may be seen as isolated cells, in loose groups or, rarely, in cohesive clusters. Their hyperchromatic nuclei are large, round to oval, or irregularly shaped with variably clumped, coarsely granular chromatin. The nucleoli are usually prominent, and multinucleation is frequent. The PAS (after diastase) stain or mucicarmine stain may show cytoplasmic vacuoles with mucin in some of the carcinoma cells.
Signet-ring variant
This variant diffusely infiltrates the gastric wall with extensive desmoplasia (linitis plastica). Neoplastic cells are usually seen as isolated cells; however, occasionally loosely cohesive groups may be present. The crescent-shaped nucleus shows finely granular chromatin. A large amount of vacuolated cytoplasm displaces the nuclei peripherally. The cytoplasmic vacuoles are usually positive for mucin with the PAS (after diastase) stain or mucicarmine stain. The nucleoli are usually not conspicuous. The cytomorphology of these carcinoma cells may overlap those of signet-ring-like reactive mesothelial cells with degenerative intracytoplasmic vacuoles. They can be distinguished from mesothelial cells, since they do not fall in the morphologic spectrum of reactive mesothelial cells with or without vacuolation; instead they stand out as a second population. In difficult cases, immunocytochemical evaluation could be performed on cell-block sections to verify their nonmesothelial and non-histiocytic nature with the SCIP approach and demonstrating immunoreactivity for BerEP4, claudin-4, mCEA, and/or B72.3, consistent with adenocarcinoma.
Anaplastic variant [Figure 11]
This variant also infiltrates the gastric wall diffusely with extensive desmoplasia (linitis plastica) and overlaps cytomorphologically with other anaplastic tumors. The smears contain a second population of numerous, medium-sized, singly scattered, pleomorphic carcinoma cells having scant cytoplasm and high nucleocytoplasmic ratios [Figure 11]. Hyperchromatic, irregularly shaped, eccentric nuclei have coarsely granular chromatin with prominent nucleoli. Multinucleation of tumor cells may be present. Some of these features may overlap with other types of neoplasms such as large cell lymphomas, germ cell tumors, and melanoma, which also have the propensity to be seen as isolated cells [Figure 11a,c].
![Metastatic gastric adenocarcinoma, peritoneal fluid. The specimen contained a predominance of solitary neoplastic cells (red arrows in a,c) with rare reactive mesothelial cells (blue arrow RM in a). Most of the cancer cells have eccentric nuclei touching the periphery of the cells (b,d). The reactive mesothelial cells (blue arrow RM) are identifiable more easily in the DQ-stained (a) than in the PAP-stained (c) preparation. The solitary carcinoma cells may be misinterpreted as high-grade lymphoma cells, especially in PAP-stained preparations (c,d). Apoptotic cancer cells (yellow arrow ‘e’ in c) and mitotic figures (yellow arrow ‘f ’ in c) are also present. The predominance of solitary neoplastic cells (a–j) is confirmed in the HEstained (g,h) and BerEP4 (i) immunostained cell-block sections. A few CK 7 immunoreactive mesothelial cells, as intrinsic positive control, are present amongst many neoplastic cells (j). Although the adenocarcinoma cells in this case were non-immunoreactive for CK 7 (j), a significant proportion of gastric adenocarcinomas are immunoreactive for CK 7. The patient had linitis-plastica-type diffuse anaplastic gastric adenocarcinoma. AP, apoptotic cancer cell; CK 7, cytokeratin 7; MF, mitotic figure; NC, neoplastic cell; RM, reactive mesothelial cell. [a–b, DQ-stained Cytospin smear; c–f, PAPstained SurePath smear; g,h, HE-stained cell-block section; i,j, immunostained cell-block sections (a, 100X; b, 100X zoomed; c, 100X; d–f, 100X zoomed; g, 40X; h–j, 100X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g012.png)
-
Metastatic gastric adenocarcinoma, peritoneal fluid. The specimen contained a predominance of solitary neoplastic cells (red arrows in a,c) with rare reactive mesothelial cells (blue arrow RM in a). Most of the cancer cells have eccentric nuclei touching the periphery of the cells (b,d). The reactive mesothelial cells (blue arrow RM) are identifiable more easily in the DQ-stained (a) than in the PAP-stained (c) preparation. The solitary carcinoma cells may be misinterpreted as high-grade lymphoma cells, especially in PAP-stained preparations (c,d). Apoptotic cancer cells (yellow arrow ‘e’ in c) and mitotic figures (yellow arrow ‘f ’ in c) are also present. The predominance of solitary neoplastic cells (a–j) is confirmed in the HEstained (g,h) and BerEP4 (i) immunostained cell-block sections. A few CK 7 immunoreactive mesothelial cells, as intrinsic positive control, are present amongst many neoplastic cells (j). Although the adenocarcinoma cells in this case were non-immunoreactive for CK 7 (j), a significant proportion of gastric adenocarcinomas are immunoreactive for CK 7. The patient had linitis-plastica-type diffuse anaplastic gastric adenocarcinoma. AP, apoptotic cancer cell; CK 7, cytokeratin 7; MF, mitotic figure; NC, neoplastic cell; RM, reactive mesothelial cell. [a–b, DQ-stained Cytospin smear; c–f, PAPstained SurePath smear; g,h, HE-stained cell-block section; i,j, immunostained cell-block sections (a, 100X; b, 100X zoomed; c, 100X; d–f, 100X zoomed; g, 40X; h–j, 100X).]
ADENOCARCINOMA OF SMALL INTESTINE
Since this carcinoma is about 50 times less common than adenocarcinoma of the large intestine, effusion secondary to adenocarcinoma of the small intestine is rarely encountered in practice. The cytomorphology of this carcinoma in effusions overlaps with other well-differentiated adenocarcinomas. The loosely cohesive groups of medium to large cells have round to oval hyperchromatic nuclei with fine to coarsely granular chromatin and variably conspicuous nucleoli. The moderate amounts of vacuolated cytoplasm may show mucicarmine or PAS (after diastase) positivity.
CARCINOMAS OF THE PANCREAS, LIVER, BILE DUCT, AND GALLBLADDER[43,46-54]
After carcinoma of the gastrointestinal tract, carcinoma of pancreas is one of the most common causes of malignant peritoneal effusions in men. In women, it is the third most common cause of malignant peritoneal effusions [see Table 2, Figure 1].
Although obstruction of the portal vein by invading tumor may lead to peritoneal effusion without malignant cells, hepatocellular carcinoma and cholangiocarcinoma may occasionally cause malignant peritoneal effusions. Rarely, adenocarcinoma of the gallbladder may cause malignant peritoneal effusions, especially in patients over 70 years of age.
CARCINOMAS OF THE PANCREAS
Adenocarcinoma [Figure 4]
Well-differentiated adenocarcinoma
The cytomorphology of this carcinoma overlaps that of other well-differentiated adenocarcinomas.
Ductal carcinoma
The smears may show closely packed cohesive clusters of medium-sized adenocarcinoma cells with scant cytoplasm, and without mucin. Their ovoid, hyperchromatic nuclei have finely granular chromatin, and nucleoli are mostly inconspicuous [see Figure 4]. Occasional groups with papillary configuration in effusion preparations may be misinterpreted as true papillary carcinoma of the pancreas [Figure 4d]. This neoplasm is usually indistinguishable from cholangiocarcinoma.
Papillary carcinoma
The smears show three-dimensional cohesive clusters in papillary configurations comprising of medium-sized neoplastic cells with high nucleocytoplasmic ratios, scant cytoplasm, and ovoid hyperchromatic nuclei with fine to coarsely granular chromatin. The nucleoli are usually less conspicuous. Superficially, the morphologic features in the effusions may resemble the ductal variant of pancreatic adenocarcinoma; however, the three-dimensional papillary formations associated with papillary carcinoma are numerous. In contrast, the papillary formations associated with the ductal variant of pancreatic adenocarcinoma are relatively flat and are less frequently seen in effusions.
Poorly differentiated small cell carcinoma
There is significant overlap in the morphologic features of poorly differentiated small cell carcinoma of the lung and the pancreas. The smears show isolated small cells or loose groups of small cells with high nucleocytoplasmic ratios, scant cytoplasm, hyperchromatic nuclei with ‘salt and pepper’ chromatin, and unrecognizable or inconspicuous nucleoli. In contrast to poorly differentiated small cell carcinoma of the lung, the nuclei in these carcinoma cells are relatively regular in shape, without significant nuclear molding, and proliferation spheres are uncommon.
Pleomorphic giant cell carcinoma
This is a highly aggressive but rare variant which metastasizes early, and is commonly associated with malignant effusions. The smears show numerous, large, isolated, mononucleated or multinucleated neoplastic giant cells with a moderate to large amount of well-defined cytoplasm without mucin. Their large, pleomorphic, hyperchromatic nuclei with irregularly distributed coarsely granular chromatin have prominent nucleoli. Effusions do not demonstrate the osteoclast-like giant cells seen in histologic sections of some of these carcinomas. The cytomorphology overlaps with other pleomorphic carcinomas and sarcomas.
HEPATOCELLULAR CARCINOMA
Hepatocellular carcinomas of any type is a rare cause of malignant effusion. Hepatocellular carcinomas have higher tendancy to be associated with floridly reactive mesothelial cells which may lead to atypical interpretations with higher frequency.[53] The smears contain loosely cohesive groups of medium-sized carcinoma cells with moderate amounts of granular cytoplasm and large, round to oval, hyperchromatic nuclei with fine to coarsely granular chromatin and variably prominent nucleoli.[54] Some cancer cells in groups may contain inspissated bile (which appear yellow yellow even in DiffQuik stained preparations). The poorly differentiated lesions show many poorly cohesive cells with prominent nucleoli and frequent multinucleation.
ADENOCARCINOMA OF THE BILIARY TRACT (CHOLANGIOCARCINOMA) [Figure 12]
![Metastatic cholangiocarcinoma, peritoneal fluid. Cancer cells (red arrows in a,h) are present mostly as solitary cells with eccentric nuclei touching the periphery of the cells (b,d,i,k,l) with occasional loosely cohesive groups of cancer cells (a,f) and reactive mesothelial cells (c,e,j and arrowheads in a,h). A few cells show cytoplasmic vacuoles (i) with secretion (yellow arrow in l), which is positive for mucicarmine in cell-block sections (n). Some apoptotic cancer cells (yellow arrow AP) are also present (g,m). The morphologic features overlap those of other mucinous adenocarcinomas. The patient had cholangiocarcinoma with a mucinous pattern. AP, apoptotic cancer cell; NC, neoplastic cell; RM, reactive mesothelial cell. [a–g: DQ-stained Cytospin smear; h–m, PAP-stained SurePath preparation; n, mucicarmine-stained cell-block section. (a, 100X; b–g, 100X zoomed; h, 100X; i–m, 100X zoomed; n, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g013.png)
-
Metastatic cholangiocarcinoma, peritoneal fluid. Cancer cells (red arrows in a,h) are present mostly as solitary cells with eccentric nuclei touching the periphery of the cells (b,d,i,k,l) with occasional loosely cohesive groups of cancer cells (a,f) and reactive mesothelial cells (c,e,j and arrowheads in a,h). A few cells show cytoplasmic vacuoles (i) with secretion (yellow arrow in l), which is positive for mucicarmine in cell-block sections (n). Some apoptotic cancer cells (yellow arrow AP) are also present (g,m). The morphologic features overlap those of other mucinous adenocarcinomas. The patient had cholangiocarcinoma with a mucinous pattern. AP, apoptotic cancer cell; NC, neoplastic cell; RM, reactive mesothelial cell. [a–g: DQ-stained Cytospin smear; h–m, PAP-stained SurePath preparation; n, mucicarmine-stained cell-block section. (a, 100X; b–g, 100X zoomed; h, 100X; i–m, 100X zoomed; n, 100X zoomed).]
Intrahepatic and extrahepatic cholangiocarcinomas have similar cytomorphologic features, and they overlap significantly with the ductal variant of pancreatic adenocarcinoma [see Figure 4]. The cohesive groups or papillary-like formations of medium-sized cells show scant cytoplasm and ovoid, hyperchromatic nuclei with finely granular chromatin. The nucleoli are usually not prominent. Only proper clinical correlation and imaging techniques can demonstrate the actual origin of these neoplasms. The non-secretory variant of cholangiocarcinoma is more frequently recognized in effusion cytology. However, mucin-producing [see Figure 12] and papillary cholangiocarcinomas may also be manifested as metastatic adenocarcinoma in malignant effusions.
ADENOCARCINOMA OF THE GALLBLADDER
Adenocarcinoma of the gallbladder is uncommon before 70 years of age. Spread to the peritoneal cavity is rapid in poorly differentiated neoplasms, resulting in peritoneal effusion. Because of its low prevalence, metastatic adenocarcinoma of the gallbladder is rarely encountered in effusion cytology. The adenocarcinoma cells are indistinguishable from other pancreatobiliary adenocarcinomas [see Figure 4].
Effusions contain loose groups or isolated large carcinoma cells with a small amount of vacuolated cytoplasm. Pleomorphic, hyperchromatic nuclei with finely granular chromatin have frequently prominent nucleoli. Multinucleation of these cells is common. Occasional cells may contain cytoplasmic mucin, which can be demonstrated by PAS (after diastase digestion) or mucicarmine staining.
CARCINOMAS OF OVARY, PERITONEUM, ENDOMETRIUM, AND UTERINE CERVIX[55–77]
In women, ovarian cancer is the most common cause of malignant peritoneal effusions [see Table 2, Figure 1]. The commonly encountered neoplasms responsible for these effusions are papillary serous adenocarcinoma, papillary mucinous adenocarcinoma, and granulosa cell tumor. However, other types of ovarian tumors may also cause malignant peritoneal effusions. Ovarian Sertoli–Leydig cell germ cell tumors, such as endodermal sinus tumor and dysgerminoma, may cause effusions. A high incidence of positivity has been reported in up to 60% of patients with dysgerminoma in peritoneal fluid.[74] A study evaluating cytomorphologic features of these neoplasms in ascitic fluid recommends careful correlation of cytomorphology with histomorphology in cell-block sections and immunocytochemistry for proper interpretation.[75] Poorly cohesive isolated neoplastic cells of dysgerminoma should be distinguished from cells of large cell lymphomas and non-cohesive variants of poorly differentiated carcinomas.
Endometrial carcinoma may spread by direct invasion into the peritoneal cavity. Although relatively uncommon, other malignant tumors such as mixed Müllerian tumor, clear cell carcinoma, and high-grade stromal sarcoma may also present with malignant effusion. Cervical cancer may metastasize to the retroperitoneal space and spread to the serosal surfaces, leading to ascites.
In effusion cytology, the morphology of well-differentiated variants of ovarian epithelial neoplasms overlaps with the wide spectrum of reactive mesothelial cells. This may render the interpretation challenging because of difficulty in identifying the neoplastic cells as a second population. This difficulty may be experienced even with ancillary tests, especially if the specimen contains relatively few neoplastic cells.
OVARIAN CARCINOMA AND PRIMARY PERITONEAL CARCINOMA
Papillary serous adenocarcinoma [Figure 13]
![Metastatic ovarian serous papillary cystadenocarcinoma, peritoneal fluid. Psammoma bodies (red arrows PSM in a,b,c,e,f,i) are present, isolated, and in association with papillary clusters (d,f) of adenocarcinoma cells without stromal cores (g,h). Some carcinoma cells show degenerative vacuoles (blue arrows VAC in h), which should not be misinterpreted as mucinous. Some apoptotic neoplastic cells (blue arrow AP in i) are present. AP, apoptotic cancer cell; NC, neoplastic cell; PSM, psammoma body; VAC, vacuole. [a–I, PAP-stained SurePath smear (a, 20X; b, 40X; c–e, 100X, f–i, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g014.png)
-
Metastatic ovarian serous papillary cystadenocarcinoma, peritoneal fluid. Psammoma bodies (red arrows PSM in a,b,c,e,f,i) are present, isolated, and in association with papillary clusters (d,f) of adenocarcinoma cells without stromal cores (g,h). Some carcinoma cells show degenerative vacuoles (blue arrows VAC in h), which should not be misinterpreted as mucinous. Some apoptotic neoplastic cells (blue arrow AP in i) are present. AP, apoptotic cancer cell; NC, neoplastic cell; PSM, psammoma body; VAC, vacuole. [a–I, PAP-stained SurePath smear (a, 20X; b, 40X; c–e, 100X, f–i, 100X zoomed).]
Effusions contain cohesive clusters or papillations composed of medium to large cells with relatively scant, non-vacuolated cytoplasm and round to oval hyperchromatic nuclei with fine to coarsely granular chromatin [see Figure 13]. Their small nucleoli are usually inconspicuous. These cells may be difficult to distinguish from groups of reactive mesothelial cells. The papillary structures may be complex with many branches. Other groups appear flat and monolayered, but demonstrate frequent palisading along the periphery. Morphologically similar peritoneal neoplasms, such as primary peritoneal carcinoma [Figure 14] and papillary epithelioid mesothelioma, have been reported in the absence of any detectable primary ovarian neoplasm.[76,77]
![Primary peritoneal carcinoma, peritoneal fluid. The specimen shows a predominance of adenocarcinoma cells in papillary configurations (a,d) without stromal cores (g). Solitary neoplastic cells (red arrows NC in a,b,d,e) are easily distinguished from the rare reactive mesothelial cells (blue arrowheads RM in c,d,e). However, in PAP-stained preparation (d), reactive mesothelial cells (arrowhead RM in e) have significant morphologic overlap with neoplastic cells (red arrows NC in d,e). Mitotic figures (yellow arrow MF in a) and apoptotic cells (yellow arrows AP in d,f) are present concurrently. Some cells show degenerative vacuolation (a,b). These vacuoles may resemble secretory vacuoles and lead to misinterpretation as mucinous adenocarcinoma. The cancer cells do not show nuclear immunoreactivity for calretinin (h), but they are immunoreactive for BerEP4 (i). The neoplastic cells show nuclear (and cytoplasmic) immunoreactivity for WT-1(j). The patient had ascites with diffuse peritoneal involvement with omental caking. The ovaries were not enlarged. AP, apoptotic cancer cell; MF, mitotic figure; NC, neoplastic cell; RM, reactive mesothelial cell. [a–c, DQ-stained Cytospin smear; d–f, PAP-stained SurePath smear; g, HE-stained cell-block section; h–j, immunostained cell-block sections (a, 100X; b,c, 100X zoomed; d, 100X; e,f, 100X zoomed; h–j, 100X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g015.png)
-
Primary peritoneal carcinoma, peritoneal fluid. The specimen shows a predominance of adenocarcinoma cells in papillary configurations (a,d) without stromal cores (g). Solitary neoplastic cells (red arrows NC in a,b,d,e) are easily distinguished from the rare reactive mesothelial cells (blue arrowheads RM in c,d,e). However, in PAP-stained preparation (d), reactive mesothelial cells (arrowhead RM in e) have significant morphologic overlap with neoplastic cells (red arrows NC in d,e). Mitotic figures (yellow arrow MF in a) and apoptotic cells (yellow arrows AP in d,f) are present concurrently. Some cells show degenerative vacuolation (a,b). These vacuoles may resemble secretory vacuoles and lead to misinterpretation as mucinous adenocarcinoma. The cancer cells do not show nuclear immunoreactivity for calretinin (h), but they are immunoreactive for BerEP4 (i). The neoplastic cells show nuclear (and cytoplasmic) immunoreactivity for WT-1(j). The patient had ascites with diffuse peritoneal involvement with omental caking. The ovaries were not enlarged. AP, apoptotic cancer cell; MF, mitotic figure; NC, neoplastic cell; RM, reactive mesothelial cell. [a–c, DQ-stained Cytospin smear; d–f, PAP-stained SurePath smear; g, HE-stained cell-block section; h–j, immunostained cell-block sections (a, 100X; b,c, 100X zoomed; d, 100X; e,f, 100X zoomed; h–j, 100X).]
Psammoma bodies may be observed, with varying frequency, from scant to numerous, in about 40% of the effusions [see Figure 13]. • Although usually associated with papillary serous adenocarcinomas of the ovary, psammoma bodies may be observed even in reactive processes in the pelvic cavity (more frequent in association with pelvic/peritoneal washings due to Mullerian rests) and should not be equated with malignancy. It is not uncommon to observe rare vacuolated carcinoma cells containing mucin in malignant effusions due to metastatic ovarian serous papillary adenocarcinoma [Figure 13h]. This finding should not be misinterpreted as mucinous cystadenocarcinoma.
Instillation of chemotherapeutic agents for treating such malignant effusions leads to nuclear pyknosis of neoplastic cells in papillations with distortion. These changes are consistent with a chemotherapy response.
As the carcinoma becomes poorly differentiated, the cells are present as solitary cells or as loosely cohesive groups with rare papillary configurations, which, if present, are simple and poorly formed without any peripheral palisading. These are relatively easy to identify as a second population, different from reactive mesothelial cells. The carcinoma cells have high nucleocytoplasmic ratios, scant cytoplasm, and large, round to oval, hyperchromatic nuclei with coarsely granular chromatin. Nucleoli are frequent and prominent. Psammoma bodies are rare in effusions due to poorly differentiated ovarian papillary serous adenocarcinoma.
Papillary serous borderline tumor
Up to 15% of ovarian serous neoplasms in young women have features of papillary serous adenocarcinoma without definite stromal invasion. Borderline papillary serous tumors are often associated with peritoneal effusions in 30–50% of women with extraovarian peritoneal implants. However, the prognosis of patients with this neoplasm is good, and survival rate is up to 95%.
Effusions contain medium to large neoplastic cells with a small to moderate amount of cytoplasm. They are usually present in papillary configurations or as cohesive clusters. The uniformly regular, oval, hyperchromatic nuclei with fine chromatin have small or inconspicuous nucleoli. Very large papillary structures and flat monolayered cohesive groups usually show peripheral palisading, generally with numerous psammoma bodies. There is morphologic overlap with reactive mesothelial cells, and these specimens, especially washings, may be difficult to interpret. This overlap may also be noted at the level of immunoprofile, and the interpretation challenge may persist even after ancillary immunocytochemical evaluation. • In this situation, morphological comparison of these cells in effusion fluid with those in the hematoxylin and eosin (HE)-stained cell-block sections with that of histomorphology of the primary neoplasm is a simple, yet highly effective approach.
Papillary mucinous adenocarcinoma
This carcinoma has a greater tendency to implant on the peritoneum and in neighboring tissues. In some cases, the abdominal cavity is studded with gelatinous nodules associated with mucoid peritoneal effusion (pseudomyxoma peritonei), which may result in intestinal obstruction. Mucinous adenocarcinomas of the appendix have been demonstrated to be the predominant cause of pseudomyxoma peritonei as compared to ovarian mucinous adenocarcinoma. Presence of lubricant material in most washing specimens should not be misinterpreted as mucin.
Cohesive clusters of cells with large amounts of vacuolated cytoplasm may show poorly formed papillary structures without peripheral palisading [Figure 15]. Slightly hyperchromatic neoplastic nuclei are large, round to oval, with fine to coarsely granular chromatin. Nucleoli may be inconspicuous to slightly prominent [see Figure 3] Psammoma bodies are absent.
![Metastatic ovarian mucinous cystadenocarcinoma, peritoneal fluid. Cohesive groups of neoplastic cells with high nucleocytoplasmic ratios and eccentric nuclei touching the periphery of the cells (arrow in b) are present. Some carcinoma cells have cytoplasmic vacuoles (a,b). The cell groups in papillary-like configurations are difficult to study at lower magnification (c). However, the cell morphology in such groups can be observed at the periphery of the groups, especially under higher magnification (arrow in d). The patient had ovarian mucinous cystadenocarcinoma. [a,b, DQ-stained Cytospin smear; c,d, PAP-stained SurePath smear (a, 40X; b, 100X; c, 40X; d, 100X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g016.png)
-
Metastatic ovarian mucinous cystadenocarcinoma, peritoneal fluid. Cohesive groups of neoplastic cells with high nucleocytoplasmic ratios and eccentric nuclei touching the periphery of the cells (arrow in b) are present. Some carcinoma cells have cytoplasmic vacuoles (a,b). The cell groups in papillary-like configurations are difficult to study at lower magnification (c). However, the cell morphology in such groups can be observed at the periphery of the groups, especially under higher magnification (arrow in d). The patient had ovarian mucinous cystadenocarcinoma. [a,b, DQ-stained Cytospin smear; c,d, PAP-stained SurePath smear (a, 40X; b, 100X; c, 40X; d, 100X).]
Granulosa cell tumor
This neoplasm, composed of closely packed cohesive clusters of medium-sized neoplastic cells with scant cytoplasm, may exfoliate its cells into the peritoneum with an effusion. Apart from solitary cells, the neoplasm may be represented by proliferation spheres and sometimes their papillary-like combinations. The hyperchromatic ovoid nuclei have fine to coarsely granular chromatin with nuclear grooves and usually small, inconspicuous nucleoli. Rosette-like cell arrangements, forming the so-called Call–Exner bodies, may be seen within the cohesive clusters.
ENDOMETRIAL CARCINOMA
Adenocarcinoma [Figure 16]
![Metastatic carcinoma of endometrium, pleural fluid. Papillary-like cohesive groups (blue arrow in a) of columnar cells (red arrow in d). The patient had endometrial carcinoma of endometrioid type. [a–d, PAP-stained SurePath smear (a, 10X; b, 40X; c,d, 100X).]](/content/105/2022/19/1/img/Cytojournal-19-4-g017.png)
-
Metastatic carcinoma of endometrium, pleural fluid. Papillary-like cohesive groups (blue arrow in a) of columnar cells (red arrow in d). The patient had endometrial carcinoma of endometrioid type. [a–d, PAP-stained SurePath smear (a, 10X; b, 40X; c,d, 100X).]
The loosely cohesive to closely packed groups of mediumsized carcinoma cells with relatively scant cytoplasm in papillary-like configurations may show some morphologic overlap with reactive mesothelial cells on the one hand, and well-differentiated adenocarcinoma on the other [see Figure 16]. The secretory variant may have vacuolated cytoplasm. Their hyperchromatic, oval nuclei show fine to coarsely granular chromatin, generally with inconspicuous nucleoli.
Clear cell carcinoma
Cohesive clusters of large carcinoma cells with well-defined cell borders imparting a honeycomb appearance are present. Three-dimensional proliferation spheres may also be present. The cells have a large amount of clear cytoplasm with round to oval hyperchromatic nuclei, coarsely granular chromatin, and frequently prominent nucleoli. The abundant, glycogen-rich, clear cytoplasm of these vacuolated carcinoma cells is better highlighted in DQ and other Romanowsky-stained smears.
Mixed Müllerian tumor
This endometrial neoplasm may demonstrate a mixture of both carcinomatous and sarcomatous components in various proportions. Because of the relative inability of well-differentiated sarcomas to exfoliate into effusions, a sarcomatous component is usually scant or absent in such effusions. Poorly differentiated sarcoma cells may be present in the effusion fluids. However, the surface tension related phenomenon modifies the morphology of most of the sarcoma cells in the effusion fluids into polyhedral shapes. Due to this, it may not be possible to interpret them morphologically as sarcoma cells.
Generally, the predominant component in effusions is the carcinomatous, which may be present either as loosely cohesive groups or as individually scattered large cells. These cells have scant, ill-defined cytoplasm and large, pleomorphic, hyperchromatic nuclei with coarsely granular chromatin. The nucleoli are frequently prominent.
The poorly differentiated sarcoma cells have ill-defined cytoplasm and oval, fusiform, or irregularly shaped hyperchromatic nuclei, with fine to coarsely granular chromatin without recognizable nucleoli. These features overlap with the cytomorphologic features of poorly differentiated carcinoma in general, limiting the role of cytology for final specific interpretation of this neoplasm in effusions without clinical details.
CARCINOMAS OF THE UTERINE CERVIX
Squamous cell carcinoma
Non-keratinizing, poorly differentiated squamous cell carcinomas of the uterine cervix may be associated with a peritoneal effusion. The smears show a second population of loosely cohesive groups of medium to large carcinoma cells (sometimes as proliferation spheres), with moderate to large amounts of relatively dense cytoplasm. Their oval to irregularly shaped hyperchromatic nuclei show irregularly distributed, coarsely granular chromatin, with variable prominence of nucleoli.
Adenocarcinoma
The smears show loosely cohesive groups of carcinoma cells with vacuolated cytoplasm and large, round to oval, usually eccentrically placed hyperchromatic nuclei with coarsely granular chromatin. Nucleoli, in at least some of the cells, are prominent. The cytomorphology overlaps that of other adenocarcinomas [see Figure 5].
CARCINOMAS OF THE KIDNEY, URINARY TRACT, AND PROSTATE[41,43,50,78-82]
Malignant effusions caused by urogenital carcinomas (including renal cell carcinoma, urothelial carcinoma, and prostatic adenocarcinoma) are infrequent. Renal cell carcinoma associated with malignant effusions, usually peritoneal, is reported in only 2% of cases. As these carcinomas often metastasize to the lung and mediastinum, they may present with malignant pleural effusions.
RENAL CELL CARCINOMA
The effusion preparation shows a second population of loosely cohesive clusters of moderate to large neoplastic cells with abundant clear to foamy vacuolated cytoplasm, and round to oval hyperchromatic nuclei with finely granular chromatin. Cells of clear cell and papillary subtypes cannot be distinguished in effusions.[79] Air-dried smears stained with DQ are valuable in assessing the cytoplasmic details, in the form of multiple, small, cytoplasmic, punched-out lipid or glycogen vacuoles. Cytoplasmic lipid vacuoles may also be demonstrated in air-dried smears stained with oil red O as red cytoplasmic globules. Vacuolation of cytoplasm may vary from patient to patient. Nucleolar prominence, although variable, is usually present. Three-dimensional proliferation spheres are common. The cytomorphology overlaps with clear cells of adrenocortical carcinoma.
Pleomorphic cell type
Higher-grade carcinoma shows isolated cells or loosely cohesive groups of medium to large cells with variably foamy, well-defined cytoplasm, and large, bizarre, eccentrically placed hyperchromatic nuclei with irregularly distributed, coarsely granular chromatin. Nucleoli are prominent and some cells show multinucleation.
UROTHELIAL CARCINOMA (TRANSITIONAL CELL CARCINOMA) OF THE URINARY TRACT [Figure 17]
![Metastatic urothelial carcinoma of bladder, pleural fluid. The specimen has a predominance of solitary neoplastic cells (red arrows in a,b) and loosely cohesive groups (brown arrow in c) of cancer cells. The cells in loosely cohesive groups show angulated outlines with straight borders (c) and may resemble poorly differentiated squamous cell carcinoma. Solitary neoplastic cells (red arrows in a,b) are easily distinguished from rare reactive mesothelial cells in the DQ-stained preparation (blue arrowheads in a) as compared to the PAP-stained preparation (blue arrowheads in b,c). The nuclei of cancer cells are hyperchromatic compared to the pale nuclei of reactive mesothelial cells in the PAP-stained preparations (b–d). Rare cells show degenerative vacuolation (blue arrow ‘vac’ in a,b), which should not be confused with secretory vacuoles of mucinous adenocarcinoma. Although urothelial carcinoma cells have a tendency to round up in cytology preparations, some urothelial carcinoma cells (yellow arrows in b, including arrow ‘d’) have their nucleus at one end of the cell and a non-tapering cytoplasmic tail at the other (d). The cells are known as cercariform cells (d) and are termed after their perceived resemblance to cercarial larvae of trematodes such as Schistosoma. They are best recognized in PAP-stained preparations (yellow arrows in b) and are consistent with the urothelial nature of primary cancer. The patient had high-grade invasive urothelial carcinoma of the bladder with metastasis to bone (e). [a, DQ-stained Cytospin smear; b–d, PAP-stained SurePath smear; e, HE-stained cell-block section (a–c, 100X; d, 100X zoomed; e, 100X)].](/content/105/2022/19/1/img/Cytojournal-19-4-g018.png)
-
Metastatic urothelial carcinoma of bladder, pleural fluid. The specimen has a predominance of solitary neoplastic cells (red arrows in a,b) and loosely cohesive groups (brown arrow in c) of cancer cells. The cells in loosely cohesive groups show angulated outlines with straight borders (c) and may resemble poorly differentiated squamous cell carcinoma. Solitary neoplastic cells (red arrows in a,b) are easily distinguished from rare reactive mesothelial cells in the DQ-stained preparation (blue arrowheads in a) as compared to the PAP-stained preparation (blue arrowheads in b,c). The nuclei of cancer cells are hyperchromatic compared to the pale nuclei of reactive mesothelial cells in the PAP-stained preparations (b–d). Rare cells show degenerative vacuolation (blue arrow ‘vac’ in a,b), which should not be confused with secretory vacuoles of mucinous adenocarcinoma. Although urothelial carcinoma cells have a tendency to round up in cytology preparations, some urothelial carcinoma cells (yellow arrows in b, including arrow ‘d’) have their nucleus at one end of the cell and a non-tapering cytoplasmic tail at the other (d). The cells are known as cercariform cells (d) and are termed after their perceived resemblance to cercarial larvae of trematodes such as Schistosoma. They are best recognized in PAP-stained preparations (yellow arrows in b) and are consistent with the urothelial nature of primary cancer. The patient had high-grade invasive urothelial carcinoma of the bladder with metastasis to bone (e). [a, DQ-stained Cytospin smear; b–d, PAP-stained SurePath smear; e, HE-stained cell-block section (a–c, 100X; d, 100X zoomed; e, 100X)].
Malignant effusions secondary to low-grade urothelial papillary carcinoma are rare. However, high-grade nonpapillary urothelial carcinoma may be associated with malignant effusions. In effusions caused by non-papillary urothelial carcinoma, the smears show a second population of cohesive clusters of medium to large cells with high nucleocytoplasmic ratios, a moderate amount of cytoplasm, and oval to irregularly shaped hyperchromatic nuclei with coarsely granular irregularly distributed chromatin. The nucleoli are usually not prominent. Proliferation spheres are usually absent.
The morphologic features of urothelial carcinoma cells overlap with other poorly differentiated carcinomas, including poorly differentiated, non-keratinizing squamous cell carcinoma [see Figures 17c]. The ‘cercariform’ cell is a urothelial carcinoma cell with nucleated globular body having a fishtail-like, non-tapering cytoplasmic process with a flat or bulbous end[80] [Figure 17b-d]. This terminology originates from their perceived resemblance to cercarial larvae of trematodes such as Schistosoma. These cells have been reported to distinguish urothelial cell carcinoma cells from neoplastic squamous cells as well as spindle cells of mesenchymal origin in fine-needle aspiration cytology.[81] A reported observation suggests that the morphology of these cells is not a result of smearing and processing artifact.[82] They may also be observed in effusions secondary to metastatic urothelial carcinoma (personal experience) [Figure 17b,d].
ADENOCARCINOMA OF THE PROSTATE [Figure 18]
![Metastatic prostatic adenocarcinoma, peritoneal fluid. Cohesive small groups of cancer cells (red arrows in a,e,f,i,j) mostly with eccentric nuclei (with prominent nucleoli i and j) touching the periphery of the cells, with some solitary cancer cells (red arrows NC in a,c,d,f,g,h). Reactive mesothelial cells (blue arrows RM in a,b) are more easily and distinctly identifiable in DQ-stained (a) than in PAP-stained (f) smears. Most cancer cells were poorly cohesive, manifested as the presence of small groups (e,i,j) or solitary cells (c,d,g,h). Some cells show vacuolation (f–j) which may be in-vitro degenerative or functional with secretion. The patient had prostatic adenocarcinoma with colonic metastasis. The primary tumor also showed a few vacuolated cancer cells in tissue sections. NC, neoplastic cell; RM, reactive mesothelial cell. [a–e, DQ-stained Cytospin smear; f–j, PAP-stained SurePath smear (a, 100X; b–e, 100X zoomed; f, 100X; g–j, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g019.png)
-
Metastatic prostatic adenocarcinoma, peritoneal fluid. Cohesive small groups of cancer cells (red arrows in a,e,f,i,j) mostly with eccentric nuclei (with prominent nucleoli i and j) touching the periphery of the cells, with some solitary cancer cells (red arrows NC in a,c,d,f,g,h). Reactive mesothelial cells (blue arrows RM in a,b) are more easily and distinctly identifiable in DQ-stained (a) than in PAP-stained (f) smears. Most cancer cells were poorly cohesive, manifested as the presence of small groups (e,i,j) or solitary cells (c,d,g,h). Some cells show vacuolation (f–j) which may be in-vitro degenerative or functional with secretion. The patient had prostatic adenocarcinoma with colonic metastasis. The primary tumor also showed a few vacuolated cancer cells in tissue sections. NC, neoplastic cell; RM, reactive mesothelial cell. [a–e, DQ-stained Cytospin smear; f–j, PAP-stained SurePath smear (a, 100X; b–e, 100X zoomed; f, 100X; g–j, 100X zoomed).]
The smears show loosely cohesive groups of medium to large neoplastic cells with ill-defined, scant cytoplasm and round, sometimes oval, hyperchromatic, usually eccentric nuclei with finely granular chromatin [see Figure 18]. Proliferation spheres may be present. Higher-grade carcinomas have prominent nucleoli, but they are variable in well-differentiated carcinomas. Some cells show cytoplasmic vacuoles, which may be degenerative or real secretory vacuoles [Figure 18g–j].
MISCELLANEOUS CARCINOMAS: THYROID, SALIVARY, AND ADRENAL GLANDS[83-91]
Carcinomas of the thyroid, salivary gland, and adrenal glands are rarely associated with malignant effusions, which are generally observed late in the disease, when pulmonary metastatic deposits have developed.
CARCINOMAS OF THYROID GLAND
Follicular carcinoma
Cohesive clusters of medium to large carcinoma cells have small to moderate amounts of cytoplasm, and round to oval, hyperchromatic nuclei with coarsely granular chromatin. Some neoplastic cells may have prominent nucleoli, and others may exhibit Hürthle cell change with abundant granular cytoplasm.
Papillary carcinoma [Figure 19]
![Metastatic papillary carcinoma of thyroid, pleural fluid. ‘Second population’ of cohesive papillary groups (red arrows NC in b,e,f) of cells associated with psammoma bodies with concentric lamination (red arrow PSM in g2,h2) are seen amongst a few reactive mesothelial cells (blue arrow RM in b,i). d1, single tumor cells with eccentric nuclei touching the periphery (red arrow) in DQ-stained preparation. d2, single cells may have cytoplasmic vacuoles with colloid (arrowhead) in DQ-stained preparation. j1, single tumor cells with eccentric nuclei in PAP-stained preparation. j2, compare with reactive mesothelial cell (binucleate) with central nuclei in PAP-stained preparation. The patient had papillary carcinoma of thyroid. NC, neoplastic cell(s); PSM, psammoma body; RM, reactive mesothelial cell(s). [a–d, DQ-stained Cytospin smear; e–j, PAP-stained SurePath smear. (a, 40X; b,c, 100X; d1,d2, 100X zoomed; e, 10X; f, 40X; g1,g2,h1,h2,i, 100X; j1,j2, 100X zoomed).]](/content/105/2022/19/1/img/Cytojournal-19-4-g020.png)
-
Metastatic papillary carcinoma of thyroid, pleural fluid. ‘Second population’ of cohesive papillary groups (red arrows NC in b,e,f) of cells associated with psammoma bodies with concentric lamination (red arrow PSM in g2,h2) are seen amongst a few reactive mesothelial cells (blue arrow RM in b,i). d1, single tumor cells with eccentric nuclei touching the periphery (red arrow) in DQ-stained preparation. d2, single cells may have cytoplasmic vacuoles with colloid (arrowhead) in DQ-stained preparation. j1, single tumor cells with eccentric nuclei in PAP-stained preparation. j2, compare with reactive mesothelial cell (binucleate) with central nuclei in PAP-stained preparation. The patient had papillary carcinoma of thyroid. NC, neoplastic cell(s); PSM, psammoma body; RM, reactive mesothelial cell(s). [a–d, DQ-stained Cytospin smear; e–j, PAP-stained SurePath smear. (a, 40X; b,c, 100X; d1,d2, 100X zoomed; e, 10X; f, 40X; g1,g2,h1,h2,i, 100X; j1,j2, 100X zoomed).]
Although papillary carcinoma of the thyroid is common, metastasis to the lung with pleural effusion is rare. Cohesive clusters or papillary-like structures composed of small to mediumsized carcinoma cells have high nucleocytoplasmic ratios with a scant to moderate amount of cytoplasm and round to oval nuclei with fine chromatin. Nucleoli are inconspicuous. Nuclear grooves and intranuclear pseudoinclusions may be present. Psammoma bodies are usually frequent [see Figure 19]. Some cells may show cytoplasmic targetoid vacuoles with colloid [Figure 19d2].
Anaplastic carcinoma
Although anaplastic carcinomas of the thyroid are rare, they are aggressive and may metastasize to lungs and lead to pleural effusion.[85] Effusion fluid cytology is similar to that of other metastatic, poorly differentiated carcinomas.
CARCINOMA OF THE SALIVARY GLAND
Malignant effusions caused by carcinomas of a salivary gland are rare and, if present, are observed in patients with metastasis to lungs.[86–88] Depending on the grade of the carcinoma, the cytomorphology overlaps with other adenocarcinomas [see Figure 5]. As an example, malignant effusion due to metastatic acinic cell carcinoma contains isolated scattered carcinoma cells to loosely cohesive groups. Medium to large carcinoma cells have abundant, finely granular to foamy cytoplasm. Hyperchromatic, eccentrically placed nuclei are round to oval, with finely granular chromatin and variably conspicuous nucleoli.
ADRENOCORTICAL CARCINOMA
Although adrenocortical carcinomas are rare, they are aggressive and often metastasize widely with associated malignant effusions.[89–91] These cells overlap morphologically with other carcinomas, especially renal cell carcinoma of clear cell type, and show scattered isolated cells or loosely cohesive small groups of large cells with high nucleocytoplasmic ratios. Their moderate amounts of foamy to vacuolated cytoplasm resemble that of renal cell carcinoma cells and are highlighted better in DQ-stained air-dried smears. A lipid stain shows small cytoplasmic lipid vacuoles. Their variably sized, round to oval, hyperchromatic nuclei are usually eccentrically placed and often have prominent nucleoli with irregularly distributed, fine to coarsely granular chromatin. Some cases may demonstrate multinucleated carcinoma cells.
ABBREVIATIONS IN ALPHABETIC ORDER
CK – Cytokeratin
DQ – Diff-Quik
MF – Mitotic figure
NC – Neoplastic cells
NOS – Not otherwise specified
PAS – Periodic-acid Schiff
PAP – Papanicolaou
PSM – Psammoma body
RM – Reactive mesothelial cells
VAC – Vacuole.
References
- Disseminated primary diffuse leptomeningeal gliomatosis: a case report with liquid based and conventional smear cytology. Cytojournal. 2005;2:16. Free full text available at: http://www.cytojournal.com/content/2/1/16
- [CrossRef] [PubMed] [Google Scholar]
- Effusion cytopathology In: Atkinson BF, Silverman JF, eds. Atlas of Difficult Diagnoses in Cytopathology (1st edn). Philadelphia: WB Saunders; 1998. p. :165-199.
- [Google Scholar]
- Serous membranes In: Sternberg SS, ed. Histology for Pathologists (2nd edn). Philadelphia: Lippincott-Raven; 1997. p. :223-239.
- [Google Scholar]
- Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc. 1985;60:158-164.
- [CrossRef] [Google Scholar]
- Cytomorphologic features of immature ovarian teratoma in peritoneal effusion: a case report. Diagn Cytopathol. 2005;33:39-42.
- [CrossRef] [PubMed] [Google Scholar]
- Primary ovarian angiosarcoma presenting as malignant cells in ascites: case report and review of the literature. Diagn Cytopathol. 2005;32:307-309.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant pleural effusion in a patient with multiple myeloma. Diagn Cytopathol. 2005;32:171-172.
- [CrossRef] [PubMed] [Google Scholar]
- Positive effusion cytology as the initial presentation of malignancy. Acta Cytol. 1987;31:448-452.
- [Google Scholar]
- Occurrence of intercellular spaces (windows) in metastatic adenocarcinoma in serous fluids: a cytomorphologic, histochemical, and ultrastructural study. Diagn Cytopathol. 1999;20:115-119.
- [CrossRef] [Google Scholar]
- Determination of primary site by examination of cancer cells in body fluids. Am J Clin Pathol. 1972;58:479-488.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of types and primary sites of malignant tumors by examination of exfoliated tumor cells in serous fluids. Acta Cytol. 1985;29:753-774.
- [Google Scholar]
- Cytodiagnosis of malignant effusions and determination of primary site. J Cytol. 2005;22:107-110.
- [Google Scholar]
- Indian file pattern of adenocarcinoma cells in effusions. Acta Cytol. 2004;48:385-386.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant pleural effusions due to small cell carcinoma of the lung: an immunocytochemical cell-surface analysis of lymphocytes and tumor cells. Acta Cytol. 1990;34:497-501.
- [Google Scholar]
- The unique cytologic picture of oat cell carcinoma in effusions. Acta Cytol. 1976;20:298-302.
- [Google Scholar]
- Cells of squamous cell carcinoma in pleural, peritoneal and pericardial fluids: origin and morphology. Acta Cytol. 1989;33:245-253.
- [Google Scholar]
- Malignant cells in serous effusions complicating bronchial carcinoma. Thorax. 1954;9:26-34.
- [CrossRef] [PubMed] [Google Scholar]
- Oat cell bronchial carcinoma: identification of cells in pleural fluid. Acta Cytol. 1976;20:525-529.
- [Google Scholar]
- Light and electron microscopic analysis of intranuclear inclusions in papillary adenocarcinoma of the lung. Acta Cytol. 1981;25:523-532.
- [Google Scholar]
- Alveolar cell carcinoma of the lung: an ultrastructural study of the cancer cells detected in the pleural fluid. Acta Cytol. 1972;16:63-69.
- [Google Scholar]
- Immunocytochemical diagnosis of oat cell carcinoma in pleural effusion. Acta Cytol. 1984;28:425-430.
- [Google Scholar]
- Relation between cell composition of pleural effusions in patients with pulmonary carcinomas and their clinical courses. Acta Cytol. 1976;20:537-541.
- [Google Scholar]
- The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol. 1987;31:85-97.
- [Google Scholar]
- The role of cytology in the evaluation of pericardial effusions. Chest. 1972;62:593-596.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of tumor cells from metastatic carcinoma of the breast in effusions. Acta Cytol. 1975;19:509-518.
- [Google Scholar]
- Pleural effusion in breast cancer: a review of 105 cases. Cancer. 1981;47:2087-2092.
- [CrossRef] [Google Scholar]
- The value of immunoperoxidase slide assay in the diagnosis of malignant pleural effusions in breast cancer. Acta Cytol. 1988;32:188-192.
- [CrossRef] [Google Scholar]
- A morphologic analysis of the cells of ductal carcinoma of the breast and of adenocarcinoma of the ovary in pleural and abdominal effusions. Acta Cytol. 1987;31:441-447.
- [Google Scholar]
- Malignant effusions in recurrent breast cancer. Cancer. 1980;45:1609-1614.
- [CrossRef] [Google Scholar]
- Pleural effusion in breast carcinoma: analysis of 122 cases. Cancer. 1981;48:2524-2527.
- [CrossRef] [Google Scholar]
- Intracellular mucous inclusions: a feature of malignant cells in effusions in the serous cavities, particularly due to carcinoma of the breast. J Clin Pathol. 1975;28:929-936.
- [CrossRef] [PubMed] [Google Scholar]
- Light and electron microscopic characteristics of signet-ring adenocarcinoma cells in serous effusions and their distinction from mesothelial cells. Acta Cytol. 1985;29:211-218.
- [Google Scholar]
- Immunocytochemical presentation of alpha-fetoprotein-producing gastric cancer in ascitic fluid. Diagn Cytopathol. 1988;4:116-120.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic Cytology and Its Histopathologic Bases (4th edn). Philadelphia: JB Lippincott; 1992.
- [Google Scholar]
- Electron microscopy in the cytological examination of metastatic pleural effusions. Thorax. 1976;31:443-449.
- [CrossRef] [PubMed] [Google Scholar]
- Electron microscopic identification of the colorectal origins of tumor cells in pleural fluid. Acta Cytol. 1983;27:45-48.
- [Google Scholar]
- The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol. 1987;31:85-97.
- [Google Scholar]
- Atlas of Serous Fluid Cytopathology: A Guide to the Cells of Pleural, Pericardial, Peritoneal and Hydrocele Fluids. London: Kluwer Academic Publishers; 1989
- [CrossRef] [Google Scholar]
- [Purulent pericarditis as an initial manifestation of esophageal carcinoma] Dtsch Med Wochenschr. 1999;124:381-385. [in German]
- [CrossRef] [PubMed] [Google Scholar]
- [Cardiac tamponade as the first manifestation of carcinoma of the esophagus] Med Clin (Barc). 1992;98:661-662. [in Spanish]
- [Google Scholar]
- Malignant Effusions: A Multimodal Approach to Cytologic Diagnosis. New York: Igaku-Shoin; 1994.
- [CrossRef] [Google Scholar]
- Cytologic studies for the diagnosis of pancreatic cancer. Cancer. 1981;47:1652-1655.
- [CrossRef] [Google Scholar]
- Pleomorphic carcinoma of the pancreas: a rare case report of combined histologic features of pleomorphic adenocarcinoma and giant cell tumor of the pancreas. Diagn Cytopathol. 1988;4:316-322.
- [CrossRef] [PubMed] [Google Scholar]
- The Cytology of Effusions: Pleural, Pericardial and Peritoneal, and of Cerebrospinal Fluid, 2nd edn. London: William Heinemann; 1968
- [CrossRef] [Google Scholar]
- Ultrastructure of hepatoma cells detected in peritoneal fluid. Acta Cytol. 1974;18:130-136.
- [Google Scholar]
- Cytopathology of clear cell hepatocellular carcinoma in ascitic fluid. Acta Cytol. 1985;29:911-913.
- [Google Scholar]
- Routine review of ascites fluid from patients with cirrhosis or hepatocellular carcinoma is a low-yield procedure: An observational study. Cytojournal. 2009;6:16.
- [CrossRef] [PubMed] [Google Scholar]
- Serous borderline tumors of the peritoneum. Am J Surg Pathol. 1990;14:230-239.
- [CrossRef] [PubMed] [Google Scholar]
- Benign and borderline serous lesions of the peritoneum in women. Pathol Annu. 1989;24:1-21.
- [Google Scholar]
- Ultrastructural studies of cells in body cavity effusions. Acta Cytol. 1985;29:226-238.
- [Google Scholar]
- Cytology of granulosa cell tumor of the ovary. Am J Clin Pathol. 1986;85:402-406.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of pleural effusion in ovarian cancer. Am J Obstet Gynecol. 1970;106:312-313.
- [CrossRef] [Google Scholar]
- Occurrence of cilia in exfoliated ovarian adenocarcinoma cells. Diagn Cytopathol. 1985;1:228-231.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphocyte subpopulations in malignant ascites of serous papillary ovarian adenocarcinoma. An immunocytochemical study. Acta Cytol. 1988;32:811-815.
- [Google Scholar]
- Clinical and cytologic aspects of primary fallopian tube carcinoma: a report of ten cases. Acta Cytol. 1987;31:834-840.
- [Google Scholar]
- The exfoliative cytology of endometrial stromal sarcoma in peritoneal fluid. Acta Cytol. 1981;25:277-281.
- [Google Scholar]
- Cytologic features of ovarian tumors of low malignant potential in peritoneal fluids. Acta Cytol. 1986;32:513-518.
- [Google Scholar]
- Papillary tumors of the peritoneum in women: mesothelioma or papillary carcinoma. Am J Obstet Gynecol. 1977;127:306-313.
- [CrossRef] [Google Scholar]
- Cytologic findings in peritoneal fluids from patients with ovarian serous adenocarcinoma. Acta Cytol. 1986;2:13-16.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic findings of ascites from patients with ovarian dysgerminoma. Acta Cytol. 1983;27:59-62.
- [Google Scholar]
- Prevalence of primary papillary-peritoneal neoplasms in patients with ovarian carcinomas. S Afr Med J. 1985;67:1005-1007.
- [Google Scholar]
- Ascitic fluid cytologic features of a malignant mixed mesodermal tumor of the ovary. Acta Cytol. 1987;31:63-67.
- [Google Scholar]
- Ascitic fluid cytology in a case of metastatic malignant mixed mesodermal tumor of the ovary. Acta Cytol. 1986;30:173-176.
- [Google Scholar]
- Ascitic positive cytology and intraperitoneal metastasis in ovarian dysgerminoma. J Obstet Gynaecol Res. 1996;22:89-94.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal cytology of uncommon ovarian tumors. Diagn Cytopathol. 1992;8:98-106.
- [CrossRef] [PubMed] [Google Scholar]
- The differential diagnosis of primary peritoneal papillary tumors. Arch Gynecol Obstet. 1992;251:139-144.
- [CrossRef] [PubMed] [Google Scholar]
- Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005;47:231-247.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate and phosphatase immunoperoxidase staining of cytologically positive effusions associated with adenocarcinomas of the prostate and neoplasms of undetermined origin. Acta Cytol. 1985;29:274-278.
- [Google Scholar]
- 'Cercariform' cells: a clue to the cytodiagnosis of transitional cell origin of metastatic neoplasms? Diagn Cytopathol. 1995;13:15-21.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of metastatic transitional cell carcinoma. Diagn Cytopathol. 2005;32:226-228.
- [CrossRef] [PubMed] [Google Scholar]
- Cercariform cells: are they specific for transitional cell carcinoma? Cancer. 1999;87:69-74.
- [CrossRef] [Google Scholar]
- Specific tumor markers in diagnostic cytology: immunoperoxidase studies of carcinoembryonic antigen, lysozyme and other tissue antigens in effusions, washes and aspirates. Acta Cytol. 1983;27:408-413.
- [Google Scholar]
- [Extensive pulmonary tumor embolisms of anaplastic thyroid gland carcinoma as a rare cause of hemorrhagic pleural effusion in an 88-year-old patient] Pneumologie. 1993;47:593-596. [in German]
- [Google Scholar]
- Cytomorphology of adenocarcinoma of lung presenting as submandibular salivary gland mass: report of a case diagnosed by fine-needle aspiration biopsy. Diagn Cytopathol. 1997;17:374-378.
- [CrossRef] [Google Scholar]
- Massive pleural effusion as isolated manifestation of metastatic spread of salivary adenoid cystic carcinoma. Respir Med. 1997;91:169-170.
- [CrossRef] [Google Scholar]
- Mucoepidermoid carcinoma of the salivary gland in pleural fluid. A case report. Acta Cytol. 1983;27:525-528.
- [Google Scholar]
- Adrenocortical carcinoma in two female children. Pediatr Surg Int. 2001;17:71-74.
- [CrossRef] [PubMed] [Google Scholar]
- Adrenocortical carcinoma with a giant pericardial mass. Intern Med. 1993;32:438-440.
- [CrossRef] [PubMed] [Google Scholar]
- Echocardiography in patients with malignant metastatic neoplasms of the heart and great vessels. J Cardiol. 1990;20:377-384.
- [Google Scholar]








