Translate this page into:
Noninvasive carcinoma ex pleomorphic adenoma of the parotid gland: A difficult diagnosis on fine needle aspiration
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Carcinoma ex pleomorphic adenoma (CXPA) is a rare epithelial malignancy that arises from a primary or recurrent pleomorphic adenoma (PA). It may be noninvasive (NI) or invasive. NI CXPA is extremely rare. Preoperative diagnosis on fine needle aspiration (FNA) of CXPA may be difficult and poses a diagnostic challenge to clinicians and pathologists. Herein, we describe the FNA findings of a case of NI-CXPA. A 69-year-old woman presented with rapid enlargement of a stable parotid mass of 25 years. Cytologically, malignant cells were focally associated with metachromatic fibromyxoid matrix that was homogeneous and dense with a vague fibrillary quality. There were cell groups, papillary-like clusters and single malignant cells. The nuclei were pleomorphic with irregularly dispersed chromatin, and the cytoplasm was ill-defined and granular. Nucleoli were small to inconspicuous. Mitoses and necrosis were not seen. Cytological features were not specific for any type of salivary gland carcinoma. The FNA diagnosis was primary high-grade adenocarcinoma of the parotid gland, not otherwise specified. Facial nerve-sparing total parotidectomy was performed, which histologically showed PA interspersed with ducts and nests composed of pleomorphic atypical nuclei surrounded by extensive hyalinization. Single cells were also noted. No capsular infiltration was seen in the entirely sampled tumor. Immunohistochemistry for Ki-67 showed a higher proliferation rate in the malignant ducts and p63 positive cells focally surrounded some of the malignant ducts. Histological diagnosis was NI-CXPA. Accurate diagnosis is important for proper surgical management; however, the preoperative diagnosis of NI-CXPA is difficult to make on FNA.
Keywords
Fine needle aspiration
noninvasive carcinoma ex pleomorphic adenoma
parotid gland
INTRODUCTION
Pleomorphic adenoma (PA) is the most common benign parotid gland neoplasm comprising 50% of all parotid tumors. Primary parotid carcinomas, on the other hand, are rare accounting for 1–3% of all head and neck malignancies. Carcinoma expleomorphic adenoma (CXPA) is a rare epithelial malignancy that arises from a primary or recurrent PA. Depending on the extent of invasion of the carcinomatous component, CXPA can be divided into noninvasive (NI) or invasive.[1234]
Most often, the carcinomatous component of CXPA is adenocarcinoma not otherwise specified (NOS). But any other histological sub-types of carcinomas may be seen with similar findings on fine needle aspiration (FNA).[125]
The specificity and diagnostic accuracy of salivary gland FNA are high (86–98%) and the sensitivity varies in the range from 64% to 94%.[678] However, the sensitivity in detecting CXPA on FNA is lower and has been reported to be as low as 29%.[9] The low sensitivity is mostly attributed to sampling error and may subsequently prevent the appropriate surgical planning.
Literature on FNA findings of CXPA including NI-CXPA of the parotid gland is scarce. Herein, we present a case of NI-CXPA, which was interpreted as high-grade adenocarcinoma, NOS on FNA.
CASE REPORT
Clinical presentation
A 69-year-old woman presented to the Head and Neck Clinic with slight odynophagia associated with rapid growth of a right parotid mass that had persisted for 25 years and recently doubled in size to 2.5 cm. Her past medical history was significant for Sjogren's disease and other autoimmune conditions. She also reported treatment of her tonsils with radiation as a child.
Magnetic resonance imaging findings
A magnetic resonance imaging of the head and neck showed a heterogeneously enhancing hypointense mass, centered at the superficial right parotid gland with overall dimensions of 1.8 cm. The medial aspect of the mass was hazy and irregular, and abutted the expected plane of the facial nerve suggesting a malignancy. There was no cervical lymphadenopathy.
Cytological findings
The FNA was performed using a 25-gauge needle. Four air-dried Diff-Quik direct smears were examined. The cellular specimen showed a high-grade tumor arranged in cohesive and loosely-cohesive clusters, papillary-like groups and as single cells. Small fragments of fibromyxoid matrix, some of which were closely associated with tumor cells, were present in the background. The matrix was densely homogeneous with focal vague fibrillary quality. The tumor cells were pleomorphic, round, polygonal to oval and displayed a high nuclear-to-cytoplasmic ratio. The nuclei were large, pleomorphic, with relatively smooth membranes, irregularly-clumped chromatin and small to inconspicuous nucleoli. The cytoplasm was scant-moderate, ill-defined and had a finely granular texture. Mitoses or necrosis were not identified. The cytological findings were not characteristic of any specific parotid gland malignancy, and a diagnosis of primary high-grade adenocarcinoma was rendered [Figure 1a–d].
![(a) Group of cohesive and pleomorphic tumor cells associated with a small amount of metachromatic fibromyxoid stroma with a vague fibrillary quality. This was a focal finding (Diff-Quik [DQ] stain, ×100). (b) A cluster of cohesive and three-dimensional pleomorphic tumor cells showing small fragments of homogeneous metachromatic fibromyxoid stroma in the background. The nuclei are enlarged, hyperchromatic and angulated. Nucleoli are not evident (DQ stain, ×100). (c) Loosely-cohesive cluster of tumor cells with large, somewhat irregular nuclei with occasional nucleoli (top of the group). Cytoplasm is scant-moderate and ill-defined with a finely granular texture (DQ stain, ×100). (d) Adenocarcinoma cells on a ThinPrep (TP) display coarse irregularly clumped chromatin, occasional nucleoli and dense to granular cytoplasm (TP, Pap stain, ×100)](/content/105/2015/12/1/img/CJ-12-7-g001.png)
- (a) Group of cohesive and pleomorphic tumor cells associated with a small amount of metachromatic fibromyxoid stroma with a vague fibrillary quality. This was a focal finding (Diff-Quik [DQ] stain, ×100). (b) A cluster of cohesive and three-dimensional pleomorphic tumor cells showing small fragments of homogeneous metachromatic fibromyxoid stroma in the background. The nuclei are enlarged, hyperchromatic and angulated. Nucleoli are not evident (DQ stain, ×100). (c) Loosely-cohesive cluster of tumor cells with large, somewhat irregular nuclei with occasional nucleoli (top of the group). Cytoplasm is scant-moderate and ill-defined with a finely granular texture (DQ stain, ×100). (d) Adenocarcinoma cells on a ThinPrep (TP) display coarse irregularly clumped chromatin, occasional nucleoli and dense to granular cytoplasm (TP, Pap stain, ×100)
The patient underwent a facial nerve-sparing right total parotidectomy and a right selective neck dissection.
Gross and histological findings
Grossly, a 2.2 cm well-demarcated tumor was seen. The cut-surface was firm, yellow-white, with minute areas of hemorrhage [Figure 2a]. Initial histologic evaluation, at low magnification, demonstrated a PA with extensive hyalinization and an intact fibrous capsule [Figure 2b]. Careful evaluation showed that the PA was interspersed with ductal structures and single cells with cellular anaplasia, exhibiting pleomorphic nuclei and eosinophilic cytoplasm. Mitotic figures were identified, and apoptic cells were present [Figure 2c]. No extracapsular invasion was present. Immunohistochemical stain for Ki-67 showed ~ 10% proliferation rate in the malignant ducts. The benign PA ducts were negative for Ki-67. A p63 stain showed focal staining in some of the ductal structures indicating an intact myoepithelial layer and the presence of an in-situ component. The histologic diagnosis was NI-CXPA with the carcinomatous component a high-grade adenocarcinoma, NOS. Thirteen level II lymph nodes and one external jugular lymph node were negative for carcinoma.
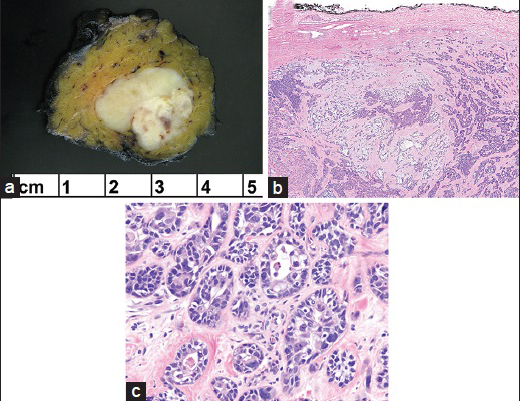
- (a) The resection specimen showed a well-demarcated tumor with a yellow-white cut-surface and minute areas of hemorrhage. (b) Low magnification of carcinoma ex pleomorphic adenoma (PA) admixed with PA. Note the uninvolved intact capsule of PA (H and E, ×4). (c) High magnification reveals highly atypical ductal structures and single cells with cellular anaplasia, exhibiting pleomorphic nuclei and eosinophilic cytoplasm (H and E, ×40)
DISCUSSION
Malignancies in a PA are uncommon. CXPA is a rare epithelial malignancy that arises from a primary or recurrent PA. Overall, CXPA comprises 3.6% of all salivary gland neoplasms and approximately 12% of all salivary gland malignancies. It usually occurs in the sixth decade, is slightly more common in women and is mostly asymptomatic. The classification and histological details of all types of CXPA have been previously published.[125]
Briefly, there are three main categories of CXPA based on the degree of invasion of the tumor: (1) NI-CXPA in which the carcinoma is confined within the PA either as an intraductal/in-situ component (surrounded by an intact layer of myoepithelial cells) or intracapsular malignant cells extending beyond the ducts but still confined within the PA (2) Minimally invasive CXPA (<1.5 mm invasion beyond the capsule) (3) Invasive CXPA (>1.5 mm invasion beyond the capsule). Prognosis of NI-CXPA is good and similar to that of PA, while invasive CXPA is usually a high-grade carcinoma with a poor prognosis.[25]
Molecular studies have shown that malignant transformation of PA follows a multi-step model of carcinogenesis and specific genes including mutation or loss of p53, deregulation of p16 and overexpression of PLAG1 and Her2 are associated with the progression of CXPA.[10] The role of radiation in the pathogenesis of CXPA, as in our patient, remains speculative.
Preoperative diagnosis of a parotid gland neoplasm is based on history, clinical findings, imaging and FNA findings. With the high specificity and diagnostic accuracy of salivary gland FNA (86–98%) and a sensitivity ranging from 64% to 94%, a FNA diagnosis of PA is reliable and has a high positive predictive value.[678] However, CXPA can be mistaken for a PA or other benign/malignant salivary gland tumors. To avoid diagnostic pitfalls, all three elements of PA that is three-dimensional cohesive clusters of ductal cells, background of myoepithelial cells and dense fibrillary metachromatic matrix with partially attenuated entrapped myoepithelial cells should be noted.[11] Although occasional cytological atypia may be encountered in some PA, the presence of numerous atypical cells, an abnormal chromatin pattern, and necrosis are features important in distinguishing PA from malignant tumors including CXPA.[3712] Utilizing a combination of clinical and radiologic features can help in making a preoperative diagnosis. A long standing mass with a recent growth spurt as seen in our patient should raise the suspicion for CXPA. In addition to being mistaken for a benign neoplasm, CXPA may be misdiagnosed as other malignancies including salivary duct carcinoma and metastasis. Metastasis to the parotid gland can be distinguished from CXPA by review of the primary tumor and use of an appropriate immunohistochemical panel.
The preoperative diagnosis of CXPA by FNA is difficult with low sensitivity and accuracy. A few studies have examined the cytologic features of CXPA. In the series reported by Zbären et al.,[4] FNA was performed on 16 CXPA and compared to FNA in 147 PA. For CXPA cases, the FNA diagnosis was a true-positive in seven (44%) and false-negative in nine cases (PA in seven, benign salivary gland tissue in one, nondiagnostic in one case). The sensitivity of FNA for diagnosing CXPA was only 47%, much lower than the 91% sensitivity for PA. Nouraei et al.[9] identified malignancy in 4 of 14 (29%) cases, and Klijanienko et al.[3] in 13 of 26 (50%) cases of CXPA on FNA. The authors in these studies contribute the false-negative diagnoses most likely to sampling errors as extent of the carcinomatous component in CXPA can vary considerably. Zbaren reported only 8 CXPAs with a greater than 66% carcinomatous component. Klijanienko noted that the diagnosis of malignancy was more likely to be rendered if the carcinomatous component was high grade and composed a significant proportion of the PA. In our case, the carcinomatous component made up a significant proportion of the tumor (>50%) and a diagnosis of high-grade adenocarcinoma NOS was made. However, the stromal component of the underlying PA on FNA was scant and not recognized as PA.
The low sensitivity of FNA may hamper appropriate planning of the extent of surgery. Although, the diagnosis of a NI-CXPA would require less aggressive surgery, cytologically, it is difficult to almost impossible to distinguish between NI-CXPA and invasive CXPA. As this case demonstrates, while the diagnosis of malignancy can be made, the infiltrative nature of the malignancy may not be possible to discern.
CONCLUSION/SUMMARY
Preoperative diagnosis of NI-CXPA on FNA is difficult and poses a diagnostic challenge to clinicians and pathologists. High-grade salivary adenocarcinoma, NOS should raise the possibility of CXPA especially in the clinical context of recent growth in a long standing mass. Accurate diagnosis is important for proper surgical management.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author.
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
TS and RH helped to draft the manuscript and provided the images. WK, RJ, ST, and MR helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent).
LIST OF ABBREVIATIONS
CXPA = Carcinoma Expleomorphic Adenoma
DQ = Diff-Quik
FNA = Fine Needle Aspiration
NI = Noninvasive
NOS = Not Otherwise Specified
PA = Pleomorphic Adenoma
TP = ThinPrep
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Carcinoma ex pleomorphic adenoma: A comprehensive review of clinical, pathological and molecular data. Head Neck Pathol. 2012;6:1-9.
- [Google Scholar]
- Carcinoma ex pleomorphic adenoma: Pathologic analysis of 73 cases. Hum Pathol. 2001;32:596-604.
- [Google Scholar]
- Fine-needle sampling findings in 26 carcinoma ex pleomorphic adenomas: Diagnostic pitfalls and clinical considerations. Diagn Cytopathol. 1999;21:163-6.
- [Google Scholar]
- Carcinoma ex pleomorphic adenoma: Diagnostic difficulty and outcome. Otolaryngol Head Neck Surg. 2008;138:601-5.
- [Google Scholar]
- Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: A clinicopathologic study of 43 cases. Hum Pathol. 2010;41:927-34.
- [Google Scholar]
- Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope. 2001;111:1989-92.
- [Google Scholar]
- Fine needle aspiration of parotid tumors: Diagnostic utility from a clinical perspective. J Oral Maxillofac Surg. 2013;71:1278-82.
- [Google Scholar]
- Fine-needle aspiration cytology of salivary gland: A review of 341 cases. Diagn Cytopathol. 2000;22:139-46.
- [Google Scholar]
- Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg. 2005;116:1206-13.
- [Google Scholar]
- Establishment and characterization of pleomorphic adenoma cell systems: An in-vitro demonstration of carcinomas arising secondarily from adenomas in the salivary gland. BMC Cancer. 2009;9:247.
- [Google Scholar]
- Pleomorphic adenoma: A diagnostic pitfall in the diagnosis of salivary gland lesions on FNAC: Case reports with review of the literature. Cytojournal. 2010;7:17.
- [Google Scholar]
- Cytologic diagnostic accuracy in pleomorphic adenoma of the salivary glands during 2 periods. A comparative analysis. Acta Cytol. 2007;51:16-20.
- [Google Scholar]








