Translate this page into:
NPC intracellular cholesterol transporter 2 regulates the anti-apoptotic protein baculoviral inhibitor of apoptosis repeat containing 3 and affects drug resistance in gastric cancer

*Corresponding author: Fengli Liu, Department of Gastroenterology, Shandong Provincial Third Hospital, Shandong University, Jinan, China. lflisme@126.com
-
Received: ,
Accepted: ,
How to cite this article: Xu Y, Wang Y, Zhao W, Liu F. NPC intracellular cholesterol transporter 2 regulates the anti-apoptotic protein baculoviral inhibitor of apoptosis repeat containing 3 and affects drug resistance in gastric cancer. CytoJournal. 2025;22:7. doi: 10.25259/Cytojournal_116_2024
Abstract
Objective:
Cisplatin (DDP)-based chemotherapy medications are frequently used as the initial line of treatment for cancer patients, including those with stomach cancer. At present, DDP resistance is a frequent problem in chemotherapy for advanced gastric cancer (GC). This study aimed to investigate the function of NPC intracellular cholesterol transporter 2 (NPC2) in GC cells.
Material and Methods:
The expression of NPC2 and baculoviral inhibitor of apoptosis repeat containing 3 (BIRC3) in gastric epithelial cells-1, BGC823, and BGC823/DDP cells was determined by Western blotting and quantitative real-time polymerase chain reaction, respectively. Subsequently, the proliferative capacity and viability of BGC823 cells were assessed by 3-(4,5-dimethylthiazol2-yl)-2.5-diphenyl-2-tetrazolium bromide, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling assay, and colony-formation assay. Finally, the association of NPC2 and BIRC3 with the nuclear factor kappa-B (NF-κB) pathway was determined by Western Blot.
Results:
In GC cells, NPC2 transcription increased, and DDP-resistant cells showed higher NPC2 expression levels than their parental cells (P < 0.001). In terms of mechanism, compared with parental cells, overexpressing NPC2, DDP-resistant cells showed resistance to DDP. Knocking down NPC2 increased the apoptotic response of DDP-resistant cells to DDP and blocked the cancer cells resistant to DDP exhibiting BIRC3, thereby promoting GC cell apoptosis (P < 0.001). Importantly, involving NF-κB signaling overturned the NPC2-mediated DDP resistance.
Conclusion:
NPC2 regulated BIRC3 and affected drug resistance in GC. Therefore, NPC2 and BIRC3 may be new targets for cancer patient treatment following DDP therapy and act as roadblocks to overcome chemotherapy resistance in GC.
Keywords
Baculoviral inhibitor of apoptosis repeat containing 3
Gastric cancer
Nuclear transcription factor-κB
INTRODUCTION
Globally, the overall survival (OS) rates for people with gastric cancer (GC) are low. GC is a highly invasive tumor that is the fifth leading cause of cancer-related fatalities despite significant progress in early diagnosis.[1] Cisplatin (DDP), whether used alone or in combination with other chemotherapy drugs, is the preferred first-line drug for advanced GC.[2] The recurrence of the patient’s tumor is due to drug resistance.[3] Therefore, exploring new strategies to reverse drug resistance and further investigating the mechanism of chemotherapy resistance in GC is necessary.
NPC intracellular cholesterol transporter 2 (NPC2) is a lipid-recognition structure containing protein-coding genes and binds to and transports cholesterol between NPC1 and the lysosome. NPC2 has been proven to be related to lipoprotein metabolism and innate immune pathways. The high protein expression of NPC2 has been detected in a variety of breast cancers, thyroid, and lung.[4] For instance, NPC2 expression in the thyroid has been described, especially with high levels in papillary thyroid carcinoma.[5] However, no detailed information regarding drug resistance of NPC2 in GC lesions is currently available.
Baculoviral inhibitor of apoptosis (IAP) repeat (BIR) domains, which are significantly upregulated in glioblastoma multiforme, are what define IAPs. IAPs are frequently regarded as pro-oncogenic proteins linked to the advancement of the cell cycle, proliferation, and cancer-cell evasion from death processes.[6] Therefore, IAP has become a hot topic in improving drug-resistance targets in cancer treatment. The IAP family comprises eight structural components.[7] The BIR-containing (BIRC)2 and BIRC3 proteins play indirect roles in tumor necrosis factor-α signaling and nuclear factor kappa-B (NF-κB) signaling.[8] As a major regulator of apoptosis, BIRC3 has recently been found to regulate the NF-κB signaling pathway and participate in the occurrence of oral squamous cell carcinoma, glioblastoma, pancreatic cancer, and other cancers.[9,10] Studies have shown that NF-κB regulates intracellular cholesterol transport by controlling NPC2 expression, thereby confirming a certain correlation between NF-κB and NPC2.[11] In the study of DDP resistance in nasopharyngeal carcinoma, BIRC3 was found to appear in DEG analysis results, but it was less correlated with platinum-resistance pathways. This result also suggests that BIRC3 may play a key role in DDP resistance in other cancers.[12] Several clinical studies published in recent years have shown the adverse effects of BIRC3 gene silencing or downregulation in cancer patients. This evidence indicates that in complex situations of the body, the expression of these genes is regulated, so careful consideration is needed when designing treatment plans.
In the present study, the relationship between NPC2 and BIRC3 and their effects on GC cells and DDP-resistant GC cells was revealed by silencing or overexpressing them. In addition, the key proteins of the NF-κB signaling pathway were determined. The relationship of NPC2 and BICR3 with these key proteins was revealed, as well as the possible mechanism of action of both in GC cells.
MATERIAL AND METHODS
Cell lines and culture
Human gastric epithelial cells (GES-1) (iCell-h062) were purchased from Shanghai Cellverse Biotechnology Co., Ltd. (Shanghai, China). The American Type Culture Collection (Rockville, MD, USA) provided the GC cell lines (BGC823). All cells were grown in complete culture media Roswell Park Memorial Institute 1640 (11875093, Gibco, Grand Island, NY, USA), which also contained 1% penicillin and streptomycin (C0222, Beyotime, Shanghai, China) and 10% fetal bovine serum (C0251, Beyotime, Shanghai, China). According to previous reports, BGC823 cells were treated with 5 μg/mL DDP to establish BGC823/DDP cells.[13] Incubation conditions for all cells were 5% carbon dioxide (CO2) at 37°C. The cell lines used were subjected to mycoplasma detection, and the results indicated the absence of mycoplasma contamination. All cell lines were authenticated through short tandem repeat analysis, confirming their identity.
Cell viability evaluation
Cell viability was evaluated using 3-(4,5-dimethylthiazol2-yl)-2.5-diphenyl-2-tetrazolium bromide (MTT) assays (G020-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). GC cells were seeded with 4000 cells/well at the beginning onto a 96-well plate and grown for 24 h at 37°C and 5% CO2. After adding 10 μL of MTT solution to each well, the cells were incubated for another 4 h. Following the removal of the medium, 100 μL/well of dimethyl sulfoxide was used to dissolve the formazan crystals. After shaking at room temperature for 30 min, the absorbance value at 570 nm was measured with a microplate reader (Synergy H1/HX, BioTek Instruments, Winooski, VT, USA). Cell viability was calculated as the ratio of the experimental group’s OD value to the blank group’s OD value multiplied by 100%.
Colony-formation assay
BGC823 and BGC823/DDP cells were seeded onto six-well plates at a density of 1000 cells/well, ensuring even distribution. The plates were incubated in a 37°C and 5% CO2 environment for 1 week, with regular medium changes. After the incubation period, we removed the medium from the wells, gently washed the cells with phosphate buffer saline (PBS), and fixed them with 4% paraformaldehyde (P1110, Solarbio, Beijing, China). The cells were stained with crystal violet (C0121, Beyotime, Shanghai, China) for 15 min and washed with water to remove excess dye. The stained cell colonies were observed and photographed using a microscope (CX53, Olympus, Tokyo, Japan) and counted with Image J software (v1.34, National Institutes of Health, Bethesda, MD, USA).
Cell transfection and plasmid construction
The prepared complementary DNA (cDNA) was digested with restriction enzymes and cloned into pCMV plasmids to obtain the recombinant pCMV-BIRC3. The plasmids used were pCMV (as control vectors), pCMV-FLAG-BIRC3, and pCMV-FLAG-NPC2. They were supplied by Invitrogen (Stealth RNAi). GC cells with a logarithmic growth phase were used for transfection experiments.
Cells were divided into five groups as follows: Cells treated with shRNA (negative control [NC]; 5' -CAAGTACTACGACGACGAGAA-3'), shRNA-NPC2 (5'-GATCCGTGGTATCCAGTGATTCGTTCTCAAG AGAAACGAATCACTGGATACCATTTTTGGAAA-3' and 5'-AGCTTTTCCAAAATGGTATCCAGTGATTC GTTTCTCTTGAGAACGAATCACTGGATACCACG-3'), NPC2 overexpression vector (the sequence is shown in Supplementary 1), BIRC3 overexpression vector (ov-BIRC3), forward primer: 3'- CACCGTATTTCAGTTCAAACGTGT-5', reverse primer: 3'-AAACACACGTTTGAACTGAAATAC-5'), or an empty vector (vector) group. We placed cells on a six-well plate at a density of 1 × 106 cells/well. When the cell density reached 70%, the cells were transfected according to the instructions of the transfection kit manufacturer, respectively. After 12 h, the original medium was replaced with a fresh one, 12.5 μM DDP was added, and the medium was allowed to stand for 24 h.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Utilizing TRIzol (15596018CN, Life Technologies, Carlsbad, CA, USA), total RNA was extracted. Thermo Scientific’s Nanodrop 2000 was used to measure the amount of ribonucleic acid (RNA). An iScript cDNA synthesis kit (1708891, Bio-Rad, Hercules, CA, USA) and 1 μg of total RNA were used to create cDNA. The polymerase chain reaction program was as follows: 95°C for 10 min, 1 cycle; 95°C for 15 s, 64°C for 30 s, 72°C for 30 s 30 cycles; 72°C for 10 min, 1 cycle. qRT-PCR analysis was conducted using Bio-Rad CFX96 Touch (Hercules, CA, USA). The changes in ploidy at the gene level were measured by the 2−ΔΔCT method, with actin as the normalization gene. The BIRC3 forward primer (F) was AGGTGTTGGGAATCTGGAGAT, and the reverse primer (R) was GCAGCATTAATCACAGGAGTA. The NPC2 forward primer (F) was TCCTGGCAGCTAC ATTCCTG, and the reverse primer (R) was ACAGAACC GCAGTCCTTGAAC. β-Actin served as an internal control (β-Actin F: ACCCCAAAGCCAACAGA, R: CCAGAGTCCATCACAATACC).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
Cells were fixed with 4% paraformaldehyde (P1110, Solarbio, Beijing, China) for 10 min, and excess fixative was removed with PBS. After permeabilizing the cells using 0.1% Triton X-100 (T8200, Solarbio, Beijing, China) in 0.1% citrate solution for 5 min, a TUNEL staining kit (A598363, ALADDIN, Shanghai, China) was used according to the manufacturer’s instructions. Cell nuclei were treated with 4',6-diamidino-2-phenylindole (C1005, Beyotime Biotechnology, Shanghai, China) at room temperature for 10 min. Finally, the staining results were observed using a fluorescence microscope (Zeiss LSM 880, Carl Zeiss AG, Oberkochen, Germany).
Western blotting
Cells with phenylmethanesulfonyl fluoride (1 mM) were lysed with radioimmunoprecipitation assay (RIPA) for 30 min and centrifuged at 4°C for 10 min. The supernatant was collected, and the protein concentrations were determined with a bicinchoninic acid assay kit (BL521A, Biosharp Life Science, Hefei, Anhui, China). The soluble protein was added to sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) loading buffer at a ratio of 1:10 and heated at 100°C for 5 min to denature the protein. The denatured proteins were separated by 15% SDS-PAGE electrophoresis and transferred onto a PVDF membrane (YA1700, Solarbio, Beijing, China). The membranes were blocked with 5% bovine serum albumin for 1 h, and the primary antibody was incubated overnight at 4°C. The primer antibody included the following: Glyceraldehyde-3-phosphate dehydrogenase (1:2000, 10494-1-AP), BIRC3 (1:2000, 24304-1-AP), NPC2 (1:1000, 19888-1-AP), phosphoprotein 65 (p65) (1:1000, 10745-1-AP), phosphorylated p65 (1:1000, 39675), inhibitor of nuclear factor kappa B alpha (IκB) (1:1000, 10268-1-AP), and phosphorylated-IκB (1:1000,82349-1-RR). All primary antibodies were purchased from Proteintech (Wuhan, Hubei, China). Then, the membrane was washed three times with tris buffered saline with tween-20, and the secondary antibody (1:1000, SA00001-2, Proteintech, Wuhan, Hubei, China) was incubated for 1 h at room temperature. The protein bands were then displayed with enhanced chemiluminescence kits (BL520b, Biosharp Life Science, Hefei, Anhui, China) and a chemiluminescence apparatus (Image Quant LAS4000, GE Healthcare, Chicago, IL, USA) and finally analyzed using ImageJ software (v1.54, National Institutes of Health, Bethesda, MD, USA).
Statistics
All experiments were performed in triplicate using GraphPad Prism software (version 9.0, GraphPad Software, San Diego, CA, USA). Data analysis involved two-way analysis of variance (ANOVA) with subsequent Bonferroni correction, and one-way ANOVA followed by Tukey’s test for additional multiple comparisons. OS was assessed using the Kaplan–Meier approach. A P < 0.05 was deemed statistically significant.
RESULTS
NPC2 upregulation in DDP-resistant GC cells
We initially assessed NPC2 expression in GC cells. The results in Figure 1a-c revealed a notable increase in NPC2 mRNA and protein levels in BGC823 cells compared with GES-1 cells (P < 0.001). Furthermore, NPC2 mRNA and protein levels in BGC823/DDP cells were significantly higher than those in BGC823 cells (P < 0.001) [Figure 1a-c].
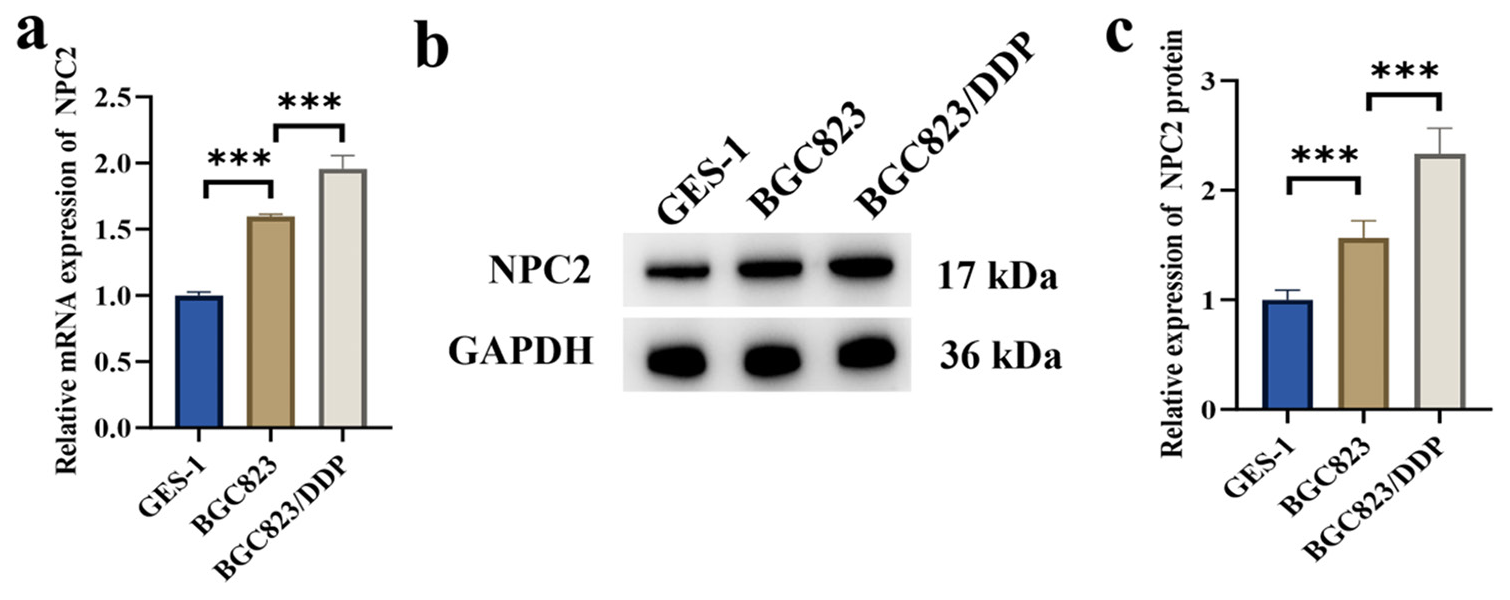
- Expression of NPC2 in GC cells. (a) mRNA levels of NPC2 in GES-1 and GC cells. (b and c) Protein levels of NPC2 in GES-1 and GC cells. (n=3). (✶✶✶P < 0.001). NPC2: NPC intracellular cholesterol transporter 2, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, GES-1: Gastric epithelial cells, GC: Gastric cancer; mRNA, messenger Ribonucleic Acid.
NPC2 overexpression promoted the DDP resistance of GC cell proliferation
As shown in Figure 2a, compared with the corresponding parental cells (BGC823 cell), DDP-resistant cells (BGC823/DDP) showed considerable resistance to DDP. Subsequently, NPC2 was overexpressed in BGC823/DDP cells. Figures 2b and c show that NPC2 overexpression was effective. To further investigate the potential role of NPC2 in DDP-resistant GC cells, we transfected the recombinant NPC2 overexpression plasmid into DDP-resistant cells. Results showed that NPC2 was successfully overexpressed in DDP-resistant cells (P < 0.001) [Figure 2d]. We examined as well the effect of NPC2 overexpression on the viability and colony-formation ability of DDP-resistant cells. NPC2 overexpression was found to significantly enhance the viability and colony-formation ability of BGC823/DDP cells (P < 0.001) [Figure 2e-g]. In addition, we assessed the impact of NPC2 overexpression on the apoptosis of BGC823/DDP cells. As shown in Figures 2h and i, NPC2 overexpression significantly inhibited apoptosis in BGC823/DDP cells compared with the overexpression NC group (P < 0.01).
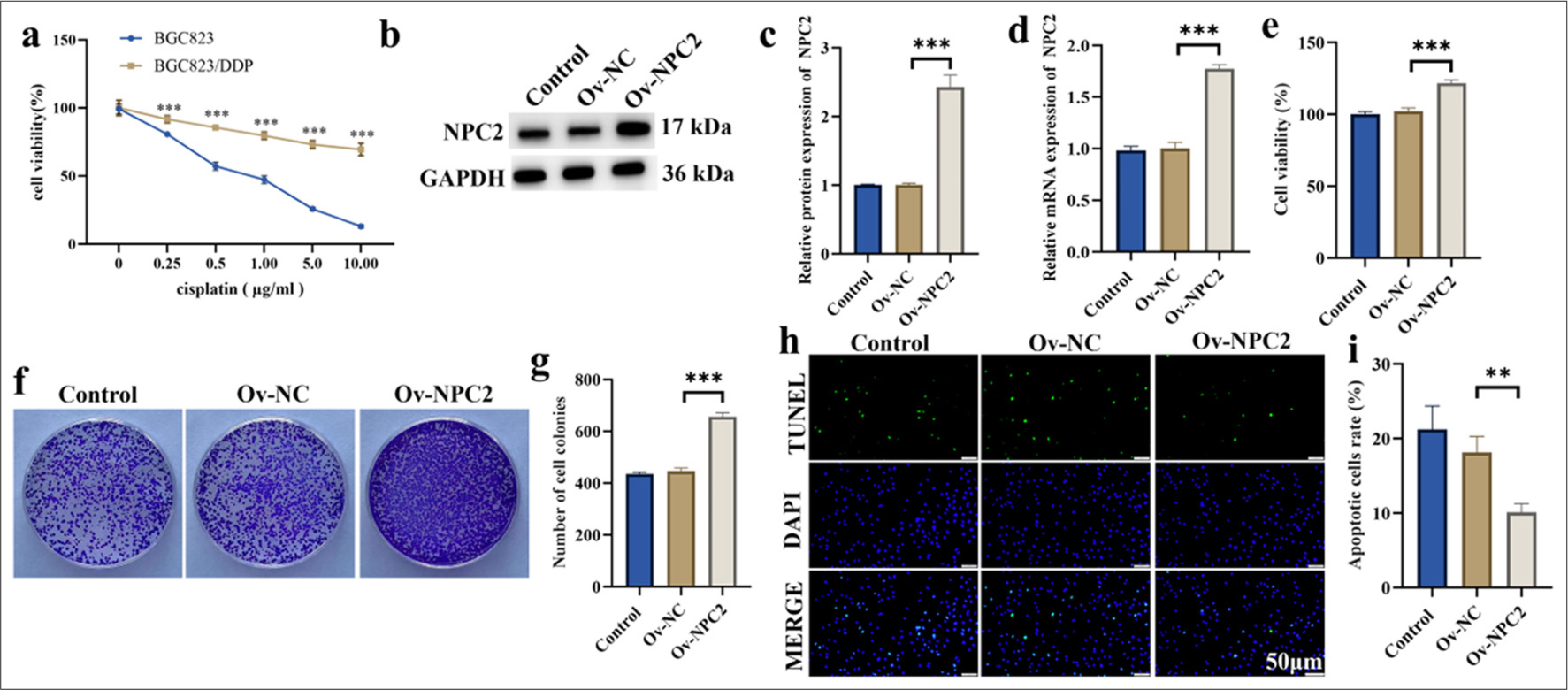
- NPC2 overexpression promotes the growth of DDP-resistant cells. (a) Cell viability was assessed using the MTT assay. (b and c) The efficiency of NPC2 overexpression was validated by Western blotting. (d) The efficiency of NPC2 overexpression was validated by qRT-PCR. (e) Cell viability was assessed. (f and g). Cell colony formation. (h and i) Cell apoptosis was measured by TUNEL assay. (n = 3). (✶✶P < 0.01, ✶✶✶P < 0.001). NPC2: NPC intracellular cholesterol transporter 2, Ov-NC: Overexpression negative control, Ov-NPC2: Overexpression NPC intracellular cholesterol transporter 2, TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling, DAPI: 4',6-diamidino-2-phenylindole, MTT: 3-(4,5-dimethylthiazol2-yl)-2.5-diphenyl-2-tetrazolium bromide, qRT-PCR: Quantitative real-time polymerase chain reaction, DDP: Cisplatin.
Knocking down NPC2-inhibited BIRC3 expression in DDP-resistant cells
To delve deeper into the expression pattern of BIRC3 in BGC823/DDP cells, we utilized qRT-PCR and Western blot analyses to assess mRNA and protein levels. Figures 3a-c demonstrate that BIRC3 expression in BGC823/DDP cells markedly exceeded that of the control and BGC823 groups. Next, we proceeded to knock down NPC2 expression in BGC823/DDP cells using ShRNA. The results in Figure 3d confirmed that the mRNA levels of NPC2 were significantly reduced in the ShNPC2 group (P < 0.001). The results in Figures 3e and f showed that silencing NPC2 was successful. The results in Figure 3g-i indicated that NPC2 knockdown significantly inhibited the mRNA and protein expression of BIRC3 in BGC823/DDP cells (P < 0.001).
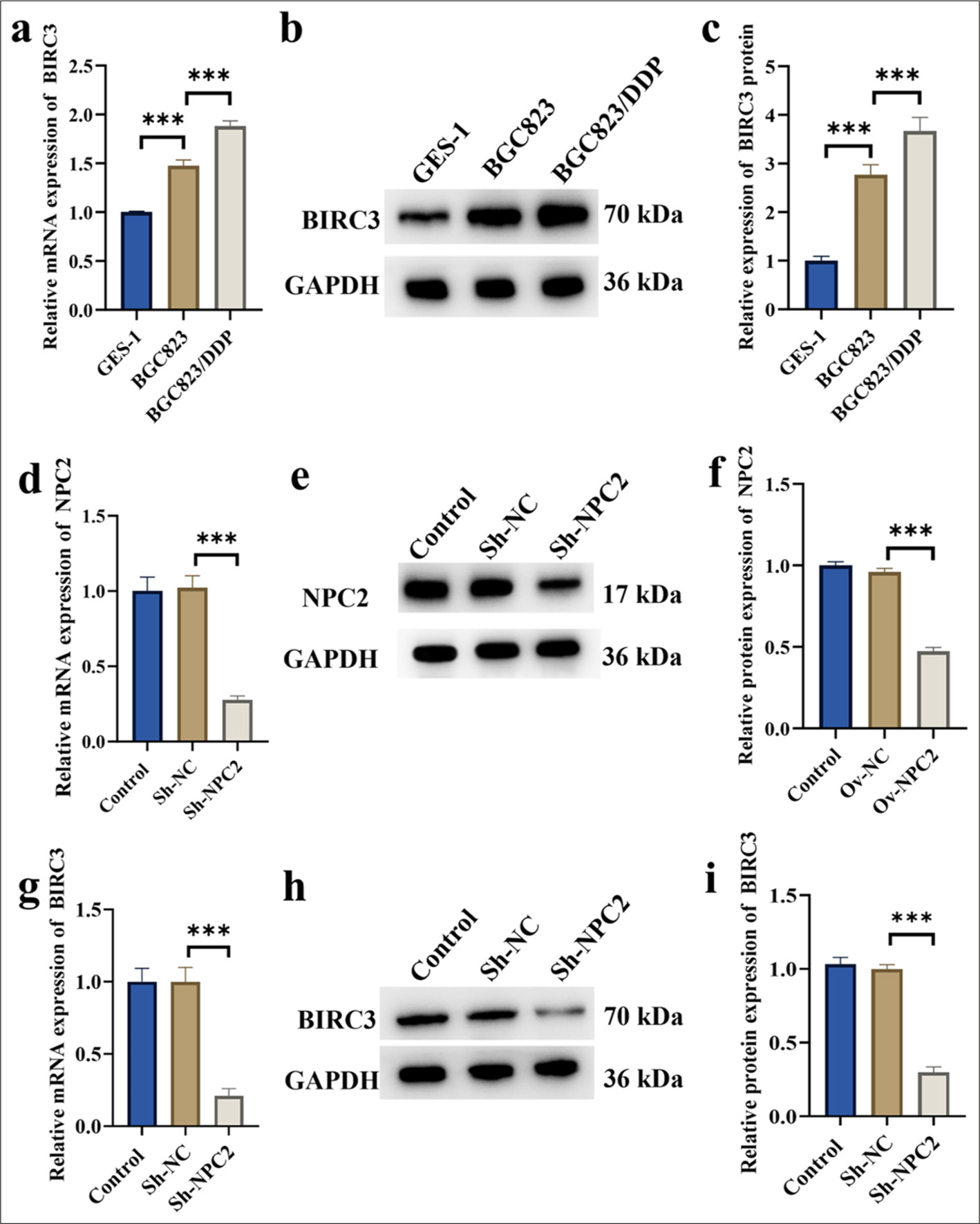
- NPC2-regulated BIRC3 genes in DDP-resistant cells. (a) BIRC3 mRNA expression in DDP-resistant cells. (b-c) BIRC3 protein expression in DDP-resistant cells. (d) The efficiency of NPC2 knockdown was verified by qRT-PCR. (e-f) The efficiency of NPC2 knockdown was verified by Western blotting. (g) Impact of NPC2 knockdown on BIRC3 mRNA expression in BGC823/DDP cells. (h-i) Impact of NPC2 knockdown on BIRC3 protein expression in BGC823/DDP cells. (n = 3). (✶✶✶P < 0.001). NPC2: NPC intracellular cholesterol transporter 2, BIRC3: Baculoviral inhibitor of apoptosis repeat containing 3, Sh-NC: ShRNA negative control, Sh-NPC2: ShRNA NPC intracellular cholesterol transporter 2, qRT-PCR: Quantitative real-time polymerase chain reaction, DDP: Cisplatin.
ov-BIRC3 reversed the decreased cell viability caused by NPC2 knockdown in BGC823/DDP cells
To further validate whether BIRC3 was involved in regulating the biological role of NPC2 in BGC823/DDP cells, we co-transfected ShNPC2 and ov-BIRC3 plasmids into BGC823/DDP cells. We verified the success of silencing NPC2 and overexpressing BIRC3 by Western blotting [Figure 4a and b]. The results in Figure 4c-g showed that NPC2 knockdown significantly decreased the activity and colony formation of BGC823/DDP cells. Conversely, ov-BIRC3 significantly rescued the reduced cell activity and colony formation caused by NPC2 knockdown (P < 0.001). NPC2 knockdown markedly increased apoptosis in BGC823/DDP cells. Conversely, ov-BIRC3 significantly attenuated the increased apoptosis levels caused by NPC2 knockdown (P < 0.001).
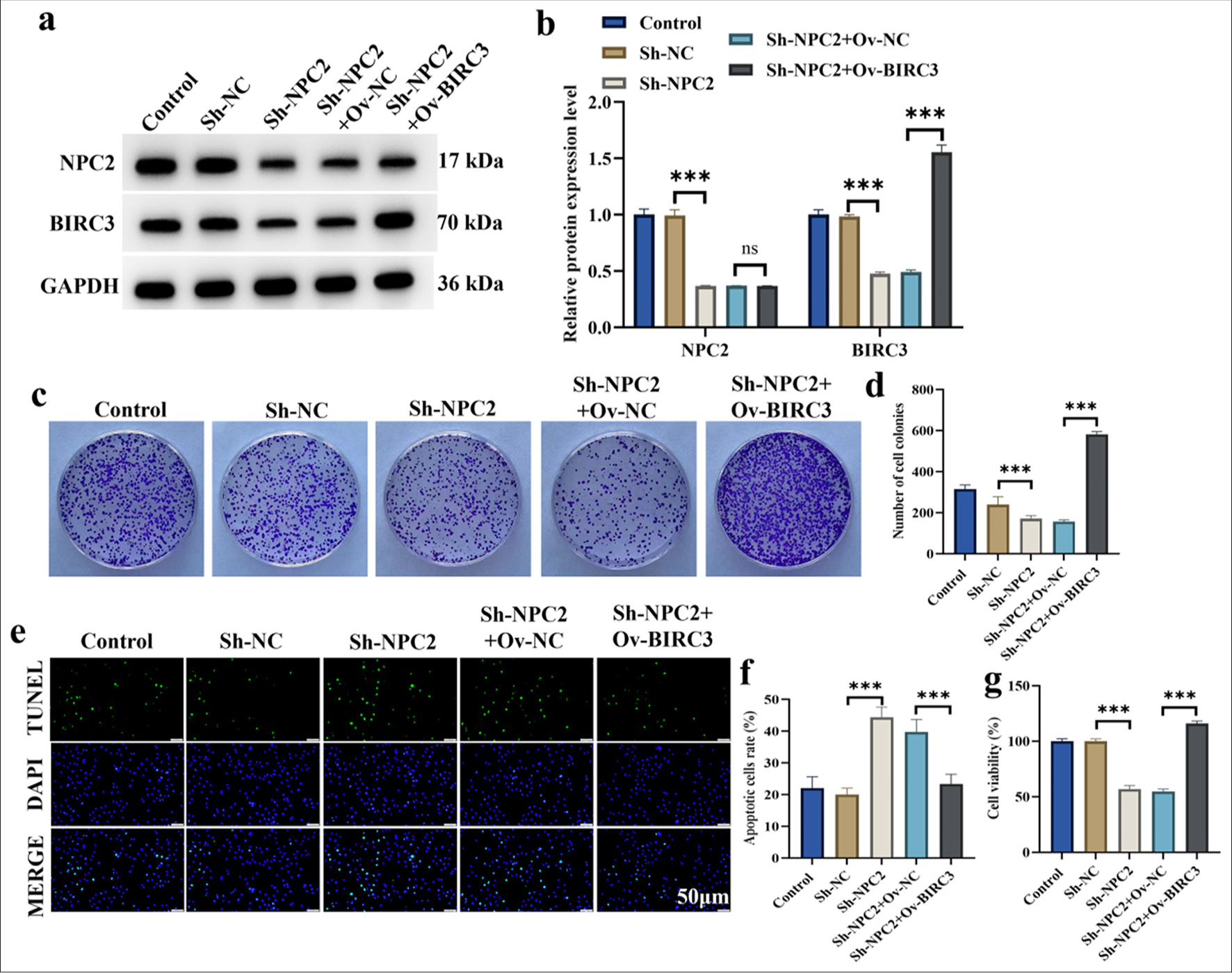
- BIRC3 overexpression restored the cell viability reduced by NPC2 knockdown in BGC823/DDP cells. (a and b) The efficiency of NPC2 knockdown and BIRC3 overexpression was verified by Western blotting. (c) Pictures of cell colonies. (d) Statistical analysis of cell-colony formation assay. (e) Pictures of TUNEL assay. (f) Statistical analysis of TUNEL assay. (g) Cell viability. (n = 3). (ns: No significant difference; ✶✶✶P < 0.001). NPC2: NPC intracellular cholesterol transporter 2, BIRC3: Baculoviral inhibitor of apoptosis repeat containing 3, DDP: Cisplatin, TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
NPC2/BIRC3 participated in activating the NF-κB signaling pathway in BGC823/DDP cells
As shown in Figure 5a-c, NPC2 knockdown notably suppressed the phosphorylation levels of p65 and IκBα. Conversely, ov-BIRC3 significantly enhanced the reduced phosphorylation levels of p65 and IκBα resulting from NPC2 knockdown (P < 0.001).
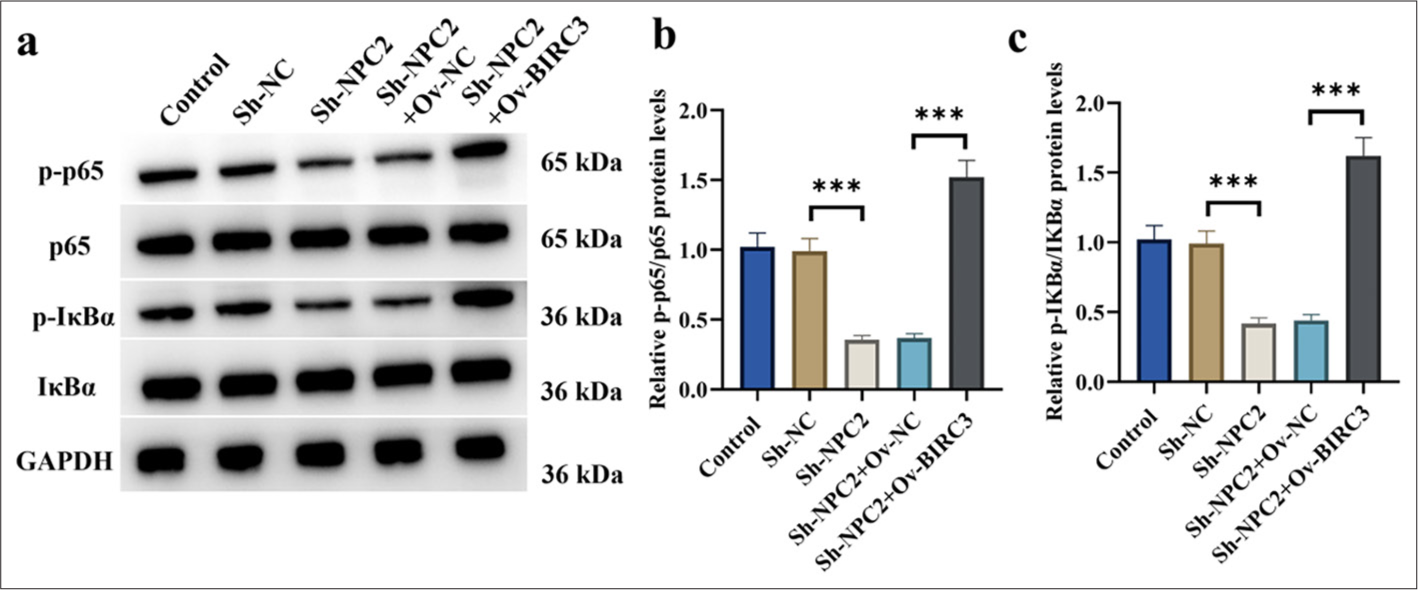
- NPC2 and BIRC3 were involved in activating the NF-κB signaling pathway in BGC823/DDP cells. (a-c) The protein levels of p65, p-p65, IκBα, and p-IκBα in BGC823/DDP cells were determined by Western blotting. (n = 3) (✶✶✶P < 0.001). p65: Phosphoprotein 65, p-p65: Phosphorylated p65, IκBα: Inhibitor of nuclear factor kappa B alpha, p-IκBα: Phosphorylated IκBα, NF-κB: Nuclear factor kappa-B, NPC2: NPC intracellular cholesterol transporter 2, BIRC3: Baculoviral inhibitor of apoptosis repeat containing 3, DDP: Cisplatin.
DISCUSSION
Each year, a significant number of people are affected by GC, a heterogeneous disease with unsolved clinical issues.
To our knowledge, NPC2 plays a key role in chemotherapy resistance in ovarian cancer. NPC2 is a protein-coding gene that serves as a new immune-related target, and its response to the prognosis and treatment of glioblastoma has been validated.[14] In this study, our results confirmed an increase in NPC2 in GC cells compared with GES-1 cells, suggesting that NPC2 may have a potential GC-promoting function.
To verify the drug-resistance treatment response of NPC2 to GC cells, the majority of GC patients were treated primarily with palliative chemotherapy. However, GC patients have been noted to develop drug resistance, which frequently leads to a low 5-year survival rate, and poor or even no response to chemotherapy. The potential of NPC2 for DDP resistance was demonstrated in this work by the construction of a cell model of DDP resistance and confirmation that the rise of NPC2 in DDP-resistant cells was related to its parental cells. Evidence suggests that DDP induces apoptosis in lung cancer cells,[15] and our findings also proved this. We also found that NPC2 overexpression promoted the apoptosis of DDP-resistant GC cells through the NF-κB pathway. Therefore, NPC2 downregulation was a new mechanism that made GC cells sensitive to DDP therapy.
BIRC3 is a member of the IAP family. It is typically upregulated in various cancers and inhibits apoptotic cell death by interfering with caspase activation.[6] Our most recent findings on how NPC2 controlled BIRC3 expression in GC confirmed that unusual NPC2 expression in GC cells primarily contributed to chemotherapy resistance. By preventing cell apoptosis, BIRC3 increased the resilience of cancer cells to DDP.
However, IAPs also affect a wide range of other physiological functions. For instance, NF-κB activation is controlled by ubiquitin-dependent signaling, which affects the expression of the genes necessary for inflammatory response and living cells.[16] Research shows that NF-κB inhibition enhances the sensitivity of GC chemotherapy. NF-κB was located upstream of BIRC3, so apoptosis escape signals from this pathway were likely to converge on BIRC3. NPC2 knockdown prevented NF-κB signaling activation as expected and decreased BIRC3 expression in gastric cells. This finding suggested that ovBIRC3 by NPC2 in GC was also mediated by the NF-κB signaling pathway. Our experimental results further revealed that in GC cells, knocking down NPC2 prevented NF-κB signal activation and reduced BIRC3 expression, indicating that NPC2 promoted BIRC3 elevation, presumably through the NF-κB signaling pathway.
SUMMARY
NPC2 was more highly expressed in GC cells. Recent studies have focused on a novel finding that increased NPC2 inhibits NF-κB signal transduction, induces the production of BIRC3, and renders cells resistant to the chemotherapy drug DDP previously susceptible to it. Therefore, NPC2 may be a promising novel therapeutic target to overcome treatment resistance in GC.
ACKNOWLEDGMENT
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
BCA: Bicinchoninic acid assay
BIR: Baculoviral IAP repeat
BIRC3: Baculoviral IAP repeat containing 3
DDP: Cisplatin
ECL: Enhanced chemiluminescence
FBS: Fetal bovine serum
GC: Gastric cancer
MTT: 3-(4,5-dimethylthiazol2-yl)-2.5-diphenyl-2-tetrazolium bromide
NF-κB: Nuclear transcription factor-κB
NPC2: NPC intracellular cholesterol transporter 2
qRT-PCR: Quantitative real-time polymerase chain reaction
AUTHOR CONTRIBUTIONS
YNX and YBW: Designed the research study; FLL: Performed the research; WYZ: Collected and analyzed the data. All authors have been involved in drafting the manuscript and all authors have been involved in revising it critically for important intellectual content. All authors give final approval of the version to be published. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval and consent to participate is not required as this study does not involve animal or human experiments.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin as first-line therapy in patients with advanced gastric cancer (SOLAR): A randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1045-56.
- [CrossRef] [PubMed] [Google Scholar]
- Medical management of gastric cancer: A 2017 update. Cancer Med. 2018;7:123-33.
- [CrossRef] [PubMed] [Google Scholar]
- The role of NPC2 gene in glioma was investigated based on bioinformatics analysis. Sci Rep. 2024;14:19134.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated analysis of fine-needle-aspiration cystic fluid proteome, cancer cell secretome, and public transcriptome datasets for papillary thyroid cancer biomarker discovery. Oncotarget. 2018;9:12079-100.
- [CrossRef] [PubMed] [Google Scholar]
- BIRC3 and BIRC5: Multi-faceted inhibitors in cancer. Cell Biosci. 2021;11:8.
- [CrossRef] [PubMed] [Google Scholar]
- IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239-52.
- [CrossRef] [PubMed] [Google Scholar]
- Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439-52.
- [CrossRef] [PubMed] [Google Scholar]
- BIRC3 alterations in chronic and B-cell acute lymphocytic leukemia patients. Oncol Lett. 2016;11:3240-6.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of microRNA-124 in regulation of Hepatocellular carcinoma through BIRC3 and the NF-κB pathway. J Cancer. 2018;9:3006-15.
- [CrossRef] [PubMed] [Google Scholar]
- The non-canonical NF-κB pathway promotes NPC2 expression and regulates intracellular cholesterol trafficking. Sci China Life Sci. 2018;61:1222-32.
- [CrossRef] [PubMed] [Google Scholar]
- IAP-1 promoted cisplatin resistance in nasopharyngeal carcinoma via inhibition of caspase-3-mediated apoptosis. Am J Cancer Res. 2021;11:640-67.
- [Google Scholar]
- Epigallocatechin gallate enhances inhibition effect of DDP on the proliferation of gastric cancer BGC-823 cells by regulating p19Arfp53-p21Cip1 signaling pathway. Asian Pac J Cancer Prev. 2021;22:1263-70.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of histologically classified oligodendrogliomas reveals characteristic molecular differences between subgroups. BMC Cancer. 2018;18:399.
- [CrossRef] [PubMed] [Google Scholar]
- Cordycepin reverses cisplatin resistance in non-small cell lung cancer by activating AMPK and inhibiting AKT signaling pathway. Front Cell Dev Biol. 2021;8:609285.
- [CrossRef] [PubMed] [Google Scholar]
- The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021;28:423-6.
- [CrossRef] [PubMed] [Google Scholar]








