Translate this page into:
Pap Smear Collection and Preparation: Key Points

*Corresponding author: Meherbano Kamal, Department of Pathology, Government Medical College, Nagpur, Maharashtra, India. dr.mmkamal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kamal M. Pap Smear Collection and Preparation: Key Points. CytoJournal 2022;19:24.
Abstract
Cytology is the science of study of cells. It is derived from the Greek word “cytos” which means cells. The cells of the cervicovaginal epithelium are continuously evolving. The mature cells reach the surface and are then exfoliated. Initially, these exfoliated cells were collected from the posterior fornix, which showed cells from endocervix, ectocervix, and the vaginal epithelium. Hence, it was known as the exfoliative vaginal cytology. But now, the cells are taken directly by scraping the ecto and the endocervix. A variety of sampling devices are available in the market. The basic aim is to augment sampling of the complete transformation zone (TZ) as well as the squamocolumnar junction (SCJ) and cause least possible trauma to the cervical and endocervical epithelium during its use. The SCJ is of crucial significance for cervical cancer pathogenesis. Most of the precancerous changes take place within the TZ and at the SCJ. Hence, the collection of cells from this area is of utmost importance. The reliability of cervical cytology for the detection of precancerous lesions also strongly depends on immediate wet fixation of the smear. Therefore, the gynecologists or the paramedics who perform the conventional Pap smears must not only be trained in the art of cell collection and smearing of the material onto the glass slides but also learn immediate wet fixation of the cervical cells. Liquid-based preparations have made all these steps relatively easy for them as the design of the Cervex brush is such that it mostly ensures the sampling of the complete TZ and the SCJ. Pre-fixation of cells occurs in the vial containing a weak fixative and the transfer of cells onto the glass slide is standardized by the automated stations designed for this purpose. This chapter gives an in depth description of the prerequisites and precautions while collecting and preparing a Pap smear with different devices, especially for settings where conventional smears are still the norm. Instructions for women undergoing Pap smear and the medical personnel who conduct this test are also highlighted.
Keywords
Conventional Pap smear -collection and preparation
Do’s and don’ts for women undergoing Pap smear
Tips of Pap smear collection for medical personnel
Limitations of Ayer’s spatula

This Chapter Includes:
Brief anatomy of the female genital tract
Pathophysiology of the transformation zone (TZ) and the squamocolumnar junction (SCJ)
Types of cervicovaginal smears
Collection devices
-
Prerequisites and precautions while collecting a Pap smear
Basic equipment required for Pap smear collection
Do’s and do not for women undergoing Pap smear
Do’s and do not of Pap smear collection for the medical personnel
Technique of collecting a Pap smear
Tips for gynecologists.
INTRODUCTION
The “Pap test saves lives.” In 1940’s, Dr. G. N. Papanicolaou first developed the technique of collecting, fixation, and staining of cervical cells (Pap smear). Today, it has become a revolutionary technique and a method of choice for the diagnosis of precancerous and cancerous changes in the uterine cervix. No other test has been as successful in stamping out cervical cancer, through the detection of treatable precursor lesions, provided screening is done regularly. A long window of opportunity exists during which regular screening, detection, diagnosis, and management of precursor lesions leads to prevention of invasive cancers and related deaths.
The Pap smear can be used as a screening test for:
Population screening by organizing camps in urban and rural areas.
Opportunistic screening method – meaning that all eligible women visiting any hospital or any gynecology outpatient department for any other reason undergoes a Pap smear.
Pap test is included in regular corporate/non-corporate health checkups.
The first two are more commonly adopted in developing countries where organized screening programs do not exist.
Screening using a Pap test – either conventional or liquid-based cytology (LBC) has stood the test of time. A systematically collected smear from the SCJ, well fixed, and well stained is still the best method to detect abnormalities in the cervical epithelium preceding invasive cancer. These abnormalities can be then confirmed through a directed biopsy, collected during colposcopy, and later ablated either by cryotherapy or loop electrosurgical excision procedure/large loop excision of the TZ.
BRIEF ANATOMY OF THE FEMALE GENITAL TRACT
The female genital tract is composed of the vulva, the vagina, the uterus, the fallopian tubes, and the ovaries [Figure 1]. The fallopian tubes, the uterus, and the upper two-third of the vagina develop from two embryonal structures, the Mullerian (paramesonephric) ducts. The caudal portion of the two Mullerian ducts fuses to form the uterus and proximal vagina. The upper part however remains separated to form the fallopian tubes. Imperfect fusion of the ducts results in formation of vagina septus and uterus septus. The fused ducts contact the urogenital sinus forming the lower vagina and vestibule.
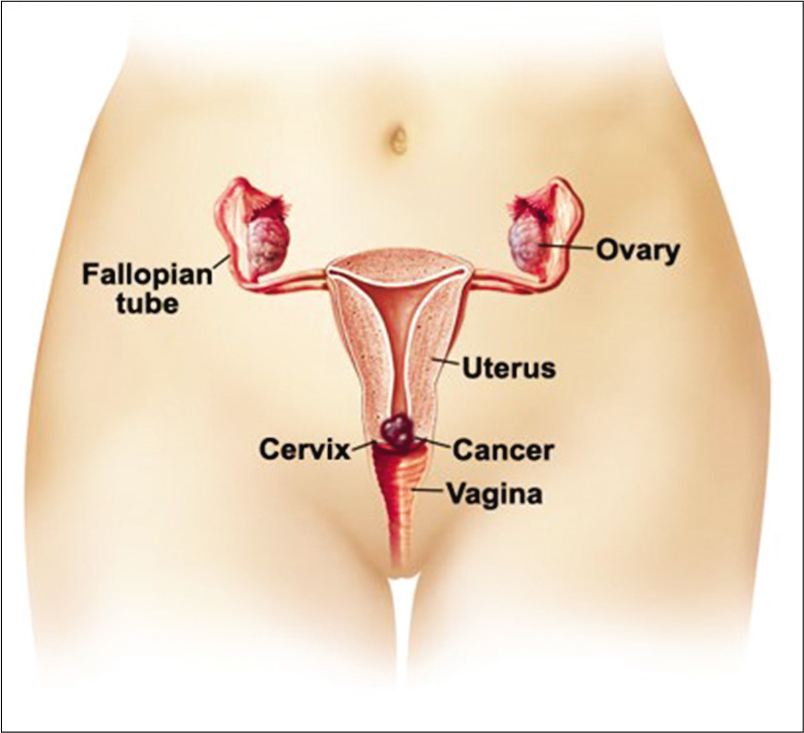
- Anatomy of female genital tract and the location of cervical cancer.
The vulva
The vulva is the external portal of entry to the female genital tract. It comprises labia majora and minora, both covered with keratinizing squamous epithelium. The outer surface of labia majora is hair bearing and inner surface contains numerous sebaceous and apocrine glands. The outer surfaces of the labia minora have sebaceous glands, whereas the inner surfaces blend with the vagina. The labia show pigmentation from the age of puberty, diminishing on the inner aspect of the labia minora where only a thin layer of keratin is present. This epithelium extends to cover the vestibule as far as the hymen. Located anteriorly between the labia minora is the female counterpart of the penis, the clitoris, provided with a retractile, prepuce-like structure. Located about 1 cm behind the clitoris is the opening of the urethra, the terminal portion of the urinary tract. The lymphatic drainage of the vulva is to the inguinal lymph nodes, which are the primary site of metastases in malignant tumors of the vulva.
The vagina
The vagina opens into the vestibule of the vulva. Mucin secreting glands are present on either side of the vaginal introitus, including Bartholin’s glands, which are situated in the lower vaginal wall, providing protection and lubrication. The vagina is lined by non-keratinized squamous epithelium. It is hormonally sensitive undergoing cyclic changes in reproductive age group and atrophies with menopause.
The cervix
The cervix is a cylindrical structure of approximately 4 cm in length and 3 cm in diameter. It has outer surface embedded within the vagina called as ectocervix or portio vaginalis visible during vaginal examination. It is covered by stratified squamous epithelium. The rest of it is continuous with the body of uterus. The parts of cervix include ectocervix, endocervix (the canal inside the cervix), external os (opening of endocervix in the vagina), and the internal os (opening of endocervix within the uterus). The endocervix is lined by simple columnar glandular epithelium. The protrusion of the cervix into the vagina creates folds of tissues known as fornices with one anterior, two lateral, and one posterior fornices. Exfoliated cells tend to pool in the secretions collected in these fornices. Inside the endocervix deep infoldings of the mucosa called as plicae palmatae travel up to the stroma forming crypts that look like branching glands. The canal is narrow measuring only 2–3 mm and often filled with mucus plug the physical properties of which changes with the ovulation timing.
PATHOPHYSIOLOGY OF THE TZ AND THE SCJ– [Figure 2]
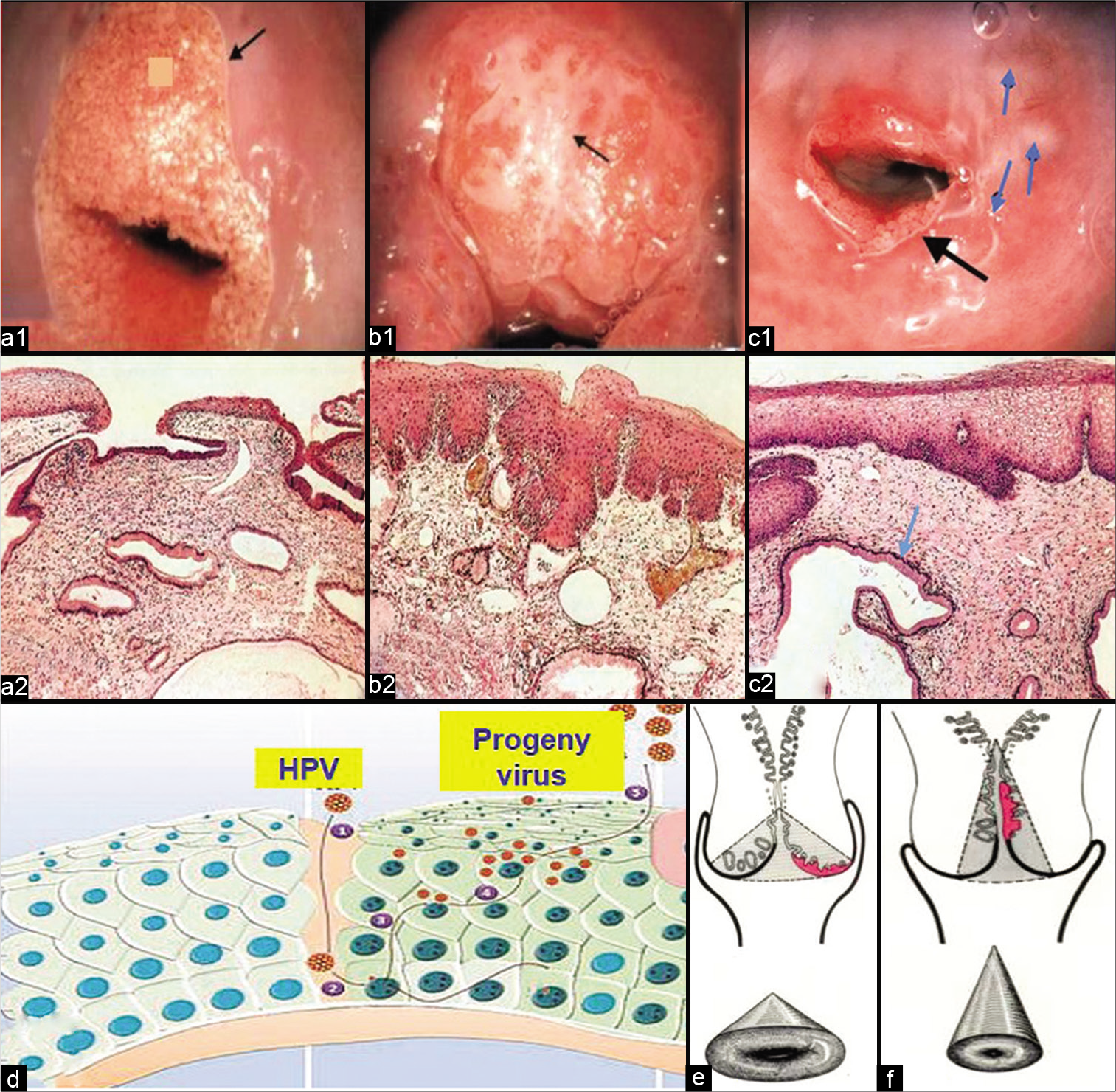
- (a) Cervical changes after puberty (ectropion) (a1) and histopathology of normal squamocolumnar junction (SCJ) (H and E) (a2). (b) Islands of metaplastic changes within the ectropion in cervix leads to formation of new SCJ (b1). Histopathology showing metaplastic squamous epithelium overlying the glands (H and E) (b2). (c) Macroscopically rounded nodules on ectocervix (blue arrows), which are distended mucous glands (nabothian follicles). Black arrow is showing the new SCJ after the healing of ectopy (c1). Histopathology showing the distended mucous glands (blue arrow) (H and E) (c2). (d) Entry of human papillomavirus through breach in the squamous epithelium. (e and f) Position of SCJ and transformation zone before and after menopause, respectively.
The SCJ is the point at which the cervical squamous and glandular epithelium meet. The location of the junction is more dependent on the age of the patient. Before puberty, the junction is normally at the external os. After puberty, the cervix changes its shape and the endocervical mucosa is exposed on the portio vaginalis, where it is visible as a red zone known as ectropion or eversion. It is sometimes mistakenly called an erosion, although no actual ulceration is present and the condition is essentially physiological [Figure 2a1 and a2, b1 and b2, c1 and c2].
Healing of the exposed endocervical epithelium (ectopy) occurs under the influence of acidic pH of the vagina. The glandular epithelium undergoes metaplastic change to squamous epithelium by differentiation of the reserve cells to a more sturdy squamous epithelium leading to the formation of a new SCJ [Figure 2b1 and b2]. This can also be due to recurring infections and repeated trauma of parturition.
The SCJ is of crucial significance for cervical cancer pathogenesis. The area between the native SCJ/or previous SCJ and the newly formed SCJ is known as the TZ. The reparative/regenerative changes that take place within the TZ and especially at the newly formed SCJ make this region prone to not only infections but also to the entry for the human papillomavirus [Figure 2c1 and c2]. Most of the precancerous and cancerous changes take place within the TZ and at the SCJ. Hence, the collection of cells from this area is of crucial importance. Glands with orifices obstructed by the metaplastic process are prone to distend with mucus, even becoming visible macroscopically as rounded nodules on the ectocervix, known as nabothian follicles [Figure 2d].
After the menopause, the cervix shrinks and the SCJ recedes higher up into the endocervical canal [Figure 2e and f]. The reliability of cervical cytology for the detection of precancerous lesions strongly depends on the proper sampling of the TZ and SCJ, immediate wet fixation, and staining of the smear.
TYPES OF CERVICOVAGINAL SMEARS
-
Pancervical smear: This is the most common and widely accepted method of collection. In this, cells are collected from the complete SCJ and the adjacent TZ. However, fallacies remain in the adequate sampling of cervices of:
Postmenopausal women (in whom the SCJ recedes higher up in the canal) [Figure 2f]
Nulliparous women with pinpoint external os
Patients who have undergone lower segment cesarean section (LSCS) and thereby have a pinpoint external os
And when endocervical brush alone is used as a sampler. In the latter case, the brush may not collect cells from the SCJ and TZ
Posterior vaginal pool smear: This helps in studying cells rolling down the entire female genital tract including cells from ovarian tumors
Lateral vaginal wall smear: It is collected from the junction of upper one-third and lower two-thirds of vagina. It is recommended for hormonal assessment
Endocervical smear: It is collected from the endocervical canal only
Vaginal vault smear: It is collected from patients who have undergone hysterectomy
Blind vaginal smear: It is collected from nulliparous, virgins, or patients having stenosed vagina
Endometrial aspiration smear: It is collected from patients complaining of postmenopausal bleeding or dysfunctional uterine bleeding (DUB) or suspected of having endometrial carcinoma.
COLLECTION DEVICES [Figure 3][1]
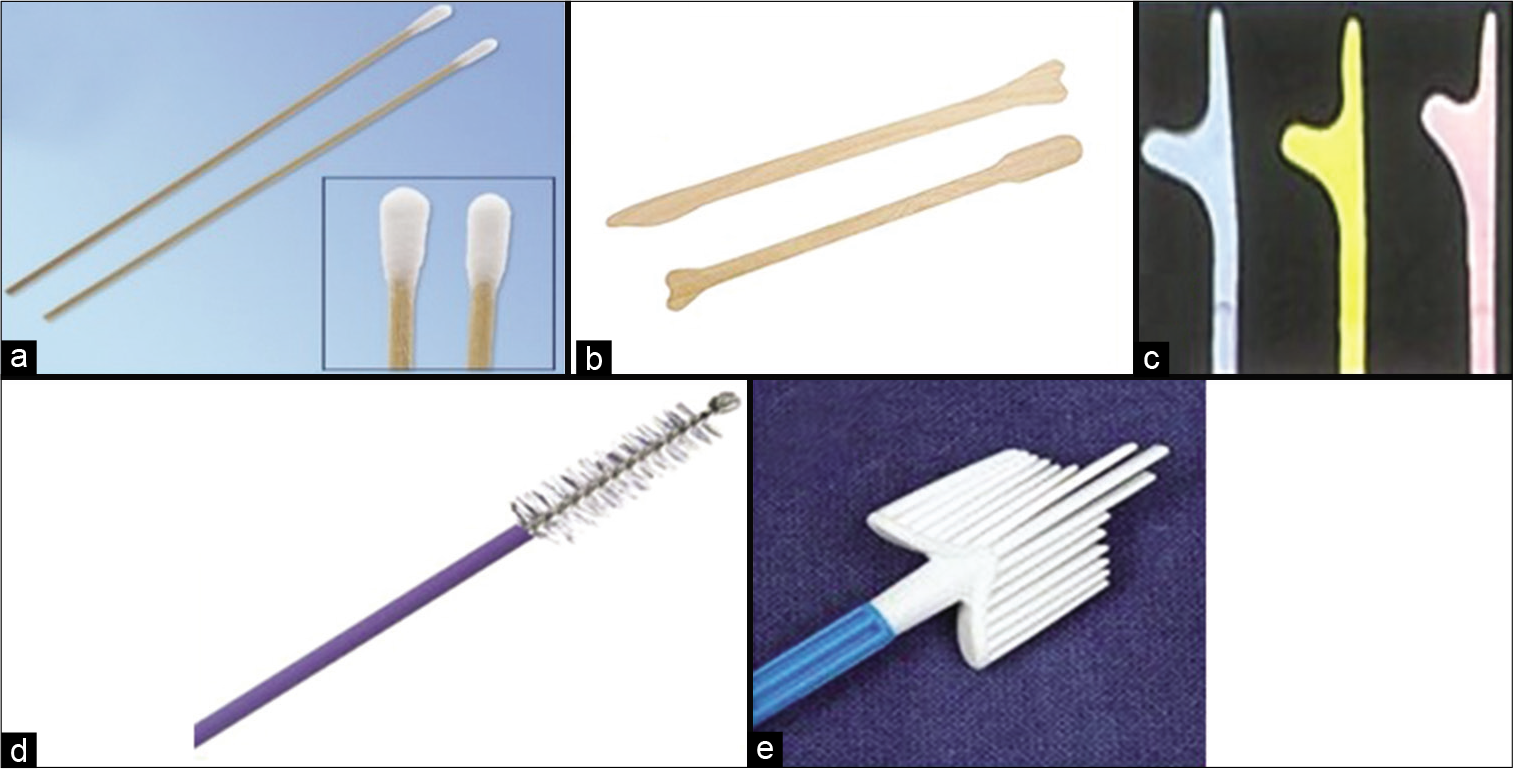
- (a) Cotton swabs for collection of smear from the transformation zone, (b) Ayer’s spatula, (c) Szalay’s spatula of different sizes. (d) Cytobrush, (e) Cervex brush.
A host of collection devices is available for collection of cells from the cervix, for example, the Ayer’s spatula, the Szalay plastic spatulas of different sizes, cotton swabs are also used at some centers and cytobrushes and brooms of different varieties and sizes are available in the market. Any one of these may be used as per the personal choice of the gynecologists, requirement of that particular cervical lesion, and also the cost and availability of the device. The basic aim is to augment sampling of the complete TZ as well as the SCJ and cause least possible trauma to the cervical and endocervical epithelium during its use. However, each of these devices has its own advantage and disadvantage.
-
Cotton swab [Figure 3a]: The applicator sticks are thin bamboo sticks, approximately 12 inches long
A moist cotton swab (preferably non-absorbable cotton) should be used
If cotton swab is too wet, the cells in smear acquire an exploded appearance and
when too dry, the cells are mechanically damaged by the friction between dry cotton swab and glass slide during smear preparation.
Ayer’s spatula [Figure 3b]: It is a bifid wooden or plastic spatula with one end longer than the other. It is the most commonly used device. Similar such device is Szalay’s spatula [Figure 3c].
Disadvantage: Occasionally traumatic to the cervical/ endocervical epithelium and also if the tip is not fit properly into the external os of the cervix, it may fail to scrape cells from TZ. Causes bleeding when cervix is inflamed.
-
Endocervical brushes: It includes cytobrush and Cervex brush.
Cytobrush [Figure 3d]: It is used for sampling of cells from the endocervical canal. It has a narrow tip covered with delicate bristles and is easy to introduce in the endocervical canal
Cervex brush [Figure 3e]: It is a broom-like device composed of soft plastic filaments. The central bristles of the broom are inserted into endocervical canal whereas lateral bristles bend against the ectocervix.
PREREQUISITES AND PRECAUTIONS WHILE COLLECTING A PAP SMEAR
Basic equipment required for Pap smear collection [Figure 4]
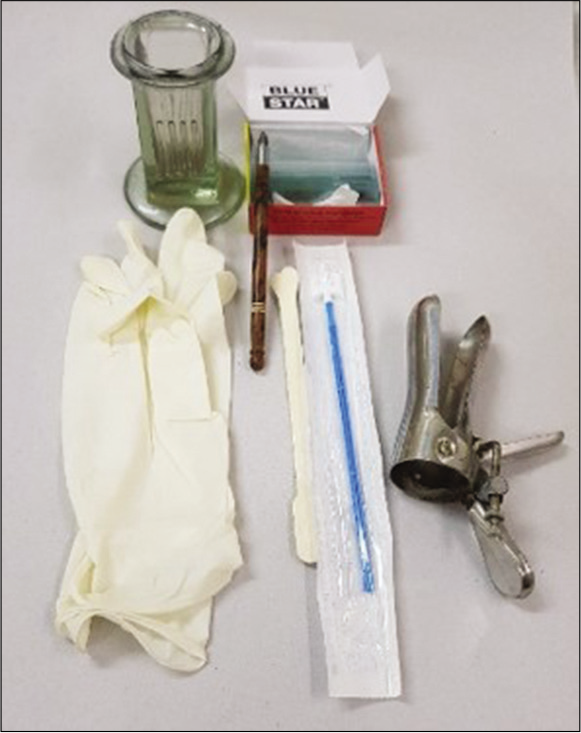
- Equipment required for Pap smear collection.
Sterilized dry bivalve Cusco speculum
Clean glass slides labeled with a diamond pencil
Sterilized cotton tipped applicator/sticks/Ayres spatula or a Cervex brush
A wide mouth specimen bottle with fixative (absolute alcohol) or commercial instant spray fixative
Normal saline
Glass marking diamond pencil
Hanging drop slide and 22 × 22 mm glass coverslips to check for Trichomonas vaginalis
Curved metal cannula or a disposable plastic endometrial aspirator (in case of endometrial cytology).
Do’s and do not for women undergoing Pap smear: Instruct the women
To come during the postmenstrual period or best between the 14th and 28th day of cycle (premenstrual period)
Not to come when there is active bleeding. First 3–4 days of menstruation are best avoided although not a contraindication
To avoid intercourse on the previous day as the contamination with semen material like sperms [Figure 5] can obscure squamous cell morphology and large seminal vesicle cells can mimic atypical squamous cells, as these cells are large and bizarre looking with large polypoid smudged nuclei and almost black chromatin. The clue to identify these cells is presence of cytoplasmic golden brown lipochrome pigment and accompanying sperms
To avoid douching with any antiseptic solution
To avoid use of pessary
Not to insert any vaginal tablets as a part of ongoing treatment (need to be withheld or smear collection is done after completion of treatment)
Not to come if a per vaginal examination is done in the past 24 h
Or a cervical biopsy or cryotherapy or any other procedure has been done in the past 6 weeks. Regeneration atypia in squamous cells can be a cause of overinterpretation of epithelial abnormalities.
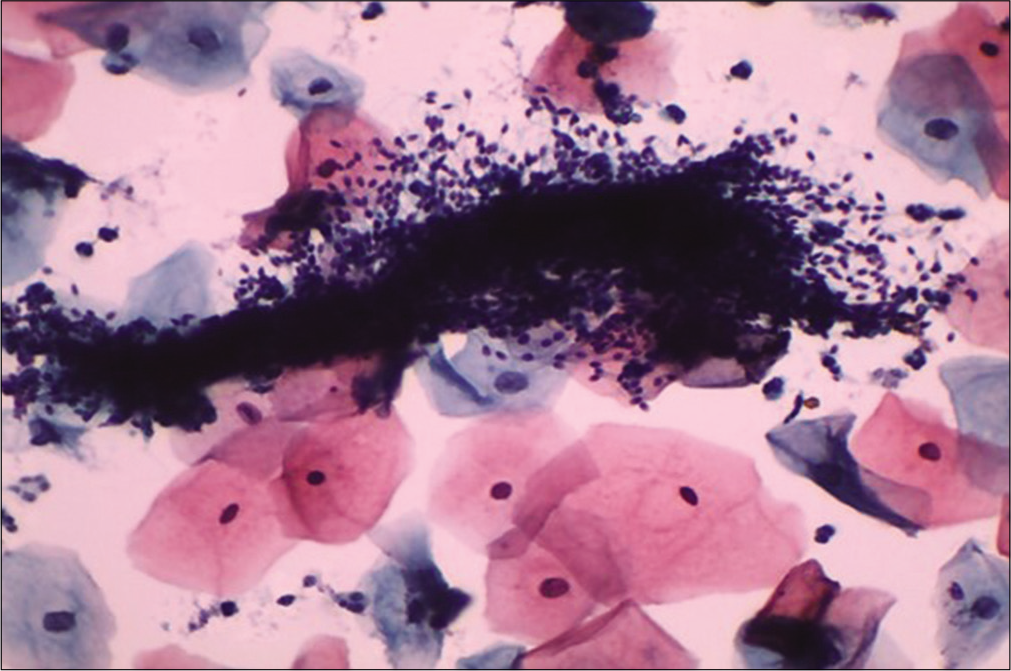
- Liquid-based cytology preparation: Smear showing clumps of sperms obscuring the squamous cell morphology (×40).
Do’s and do not for the medical personnel engaged in Pap smear collection
-
In addition to sociodemographic details, the cytology requisition form must include
Contact details such as mobile number and address
Age of the patient
Clinical history in short like parity, last child birth, biopsy, previous cytology or histopathology reports, and colposcopy findings (if any)
Date of last menstrual period and menstrual history
History of contraception such as use of intrauterine device, oral pills, and barrier contraceptives
Per speculum and per vaginal findings
Exact anatomical site of smear and type of device used
Do not use lubricated Speculum. The lubricant jelly can cause artifacts in the smear
Smear is collected as the first step after inserting and positioning of the speculum and before any vaginal examination is done. The glove powder can come as contaminant in the smears and may mimic abnormal cells like koilocytes [Figure 6]
Use spatula of suitable size and shape as per the size and shape of the cervix.
Smear should not be collected immediately after curettage or biopsy
The glass slide, speculum, and spatula should be absolutely dry as the presence of water or moisture on these destroys the cells and hampers cellular details
Immediate wet fixation is mandatory [Figure 7]
Slides must be fixed, that is, dropped immediately in the bottle containing the fixative to avoid air drying artifacts in the smear. Partial or complete air drying of cells because of delay in fixation leads to poor staining of nucleus and the cytoplasm. A bottle of absolute alcohol needs to be kept ready or LBC vial has to be ready at hand so that there is no delay in immersing the slide in alcohol or brush in the vial respectively
If for any reason there is delay in fixation of the smear, do not put it in alcohol. Instead it is safe to allow the Slide to DRY COMPLETELY and sent to the laboratory (wrapped in a paper). This also avoids the hurry and worry of immediate wet fixation that sometimes leads to the spillage of alcohol from jars.[2]
Smears should be uniformly thin and the material collected should be evenly distributed.
Avoid smearing purulent material alone on the slide [Figure 8a and b]. Too many inflammatory cells obscure morphologic details of epithelial cells and also result in altered staining of the squamous cells [Figure 9a]
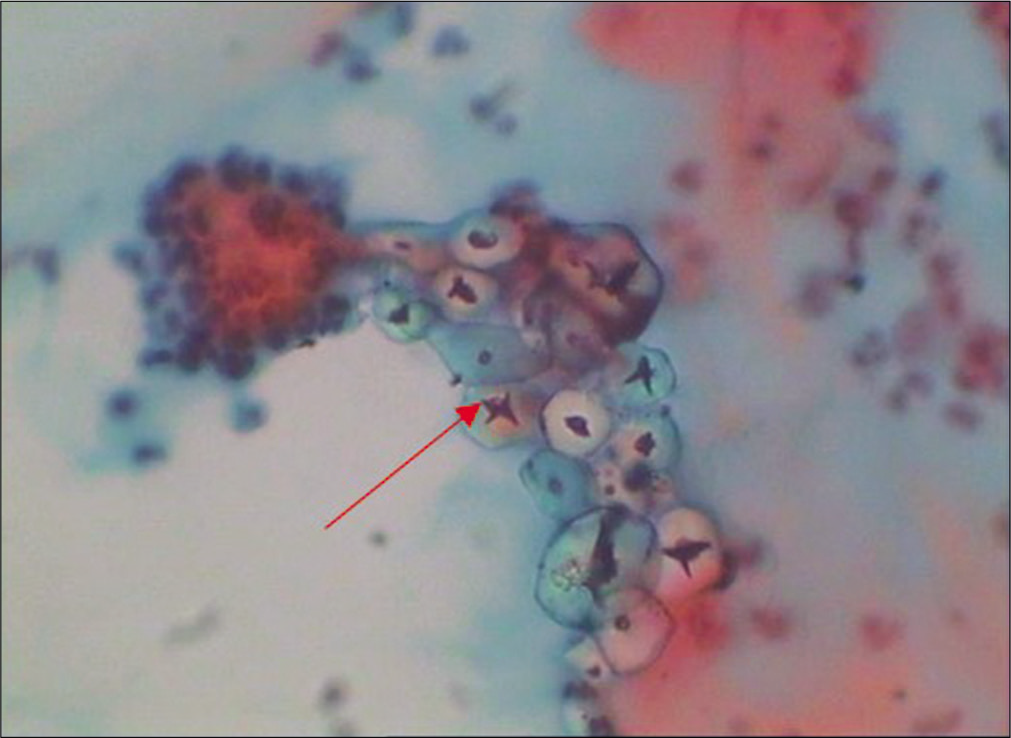
- Conventional Pap smear – Glove powder may resemble koilocytes (red arrow) (×40).

- Immediate wet fixation of slide in a Coplin jar containing 95% absolute alcohol is the most crucial step.
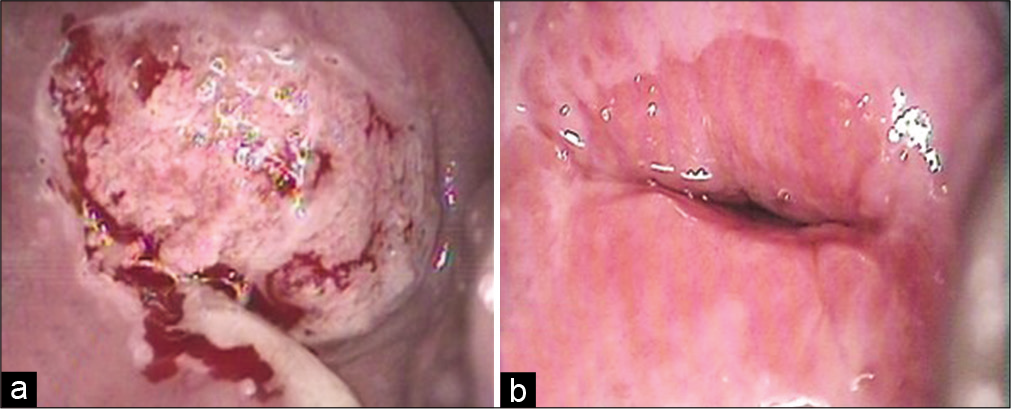
- (a) Inflamed cervix with purulent discharge on the surface, (b) same cervix after treatment of infection showing a well-demarcated squamocolumnar junction.
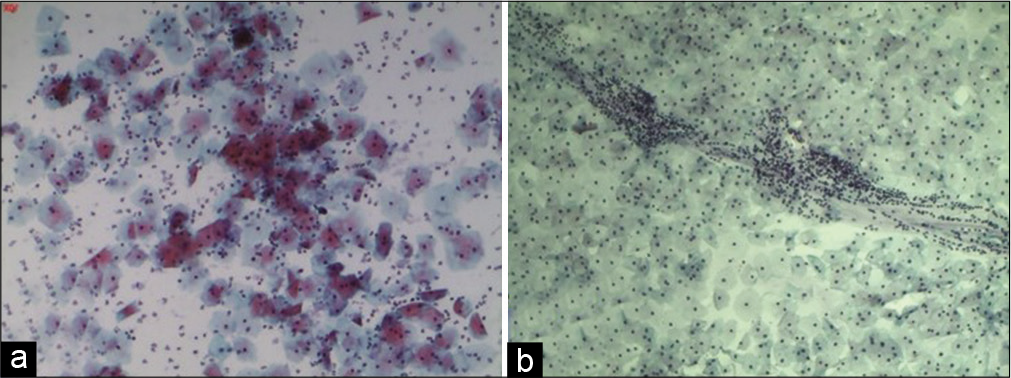
- (a) Conventional Pap smear showing plenty polymorphonuclear leukocytes and altered staining in the form of polychromasia of squamous cells when the purulent material is spread on the slide. (b) Normal Pap smear showing leukocytes in a streaming pattern with normal squamous cells and a leukocyte free background.
Note: Leukocytes are however present even in normal Pap smears [Figure 9b]. The healthy white discharge comprises of leukocytes and is seen in the conventional Pap smear in a linear or circular fashion (used to prepare the smear). The background shows squamous cells free of polymorphs. The polymorphs are healthy and do not show degenerative changes like cytoplasmic vacuolation that is seen in an inflammatory smear.
TECHNIQUE OF COLLECTING A PAP SMEAR [Figure 10][3]
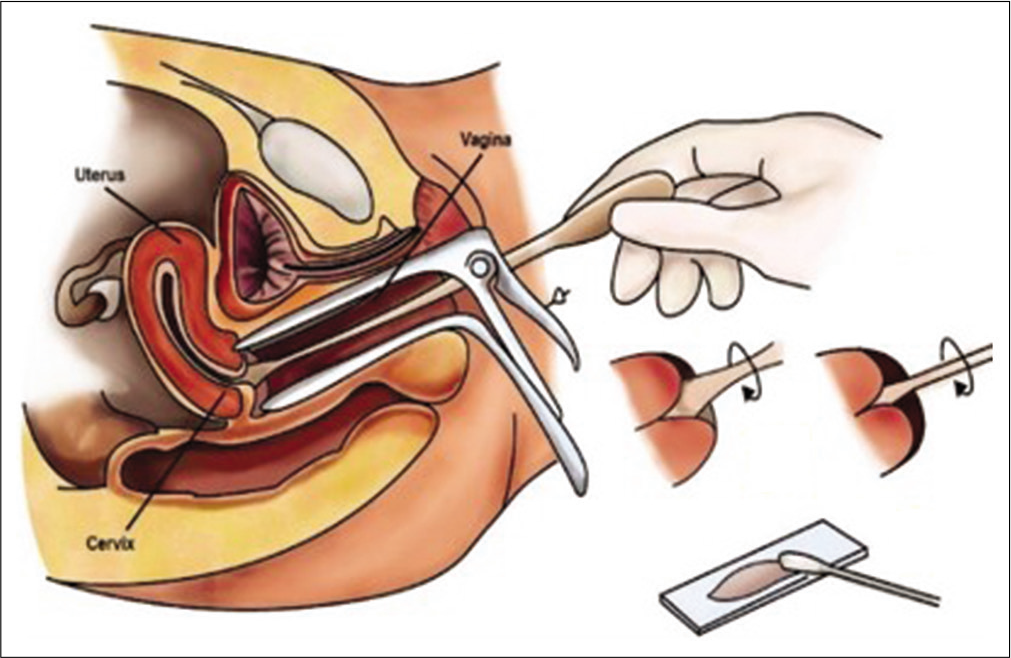
- Diagrammatic representation of the method of Pap smear collection.
Explain the procedure to the patient and take a verbal or informed consent
The patient is instructed to sleep in a lithotomy position – ideally on a PV table
A Sim’s speculum or a bivalve Cusco’s speculum is inserted in the vagina and the cervix is exposed with gentle maneuvering of the speculum. Use of Cusco speculum makes one “hands free.”
The long end of the tip of any of the spatulas mentioned above or the tip of the brush is put in the endocervical canal and fixed gently but firmly and then rotated clockwise through 360° 2–3 times [Figure 11a and b] and 5 times if the Cervex brush of Surepath [Figure 11c] is used. With the cytobrush and Cervex brush –reverse rotation during the procedure is strictly prohibited as it may leave behind those cells that are collected in the previous rotations
The material is spread in a circular or linear fashion in the central 2/3rd area of the glass slide leaving the edges of the glass slide free of material [Figure 12a and b].
For LBC preparation, the Cervex brush is broken away or detached from the stick and tip of the Cervex brush is dropped in the LBC vial containing fixative [Figure 13a and b]
The conventional smear is immediately fixed in absolute alcohol. Delay in fixation causes air drying artifacts and there is clouding of morphological details
However, if there is shortage of alcohol or constant failure to provide “good quality wet fixed smears,” then the smear may be totally air dried. The air dried smears are rehydrated in the laboratory
For spray fixing of the slide, spray should be kept at an angle of 45° and at a distance of 6 inches from the slide [Figure 14]
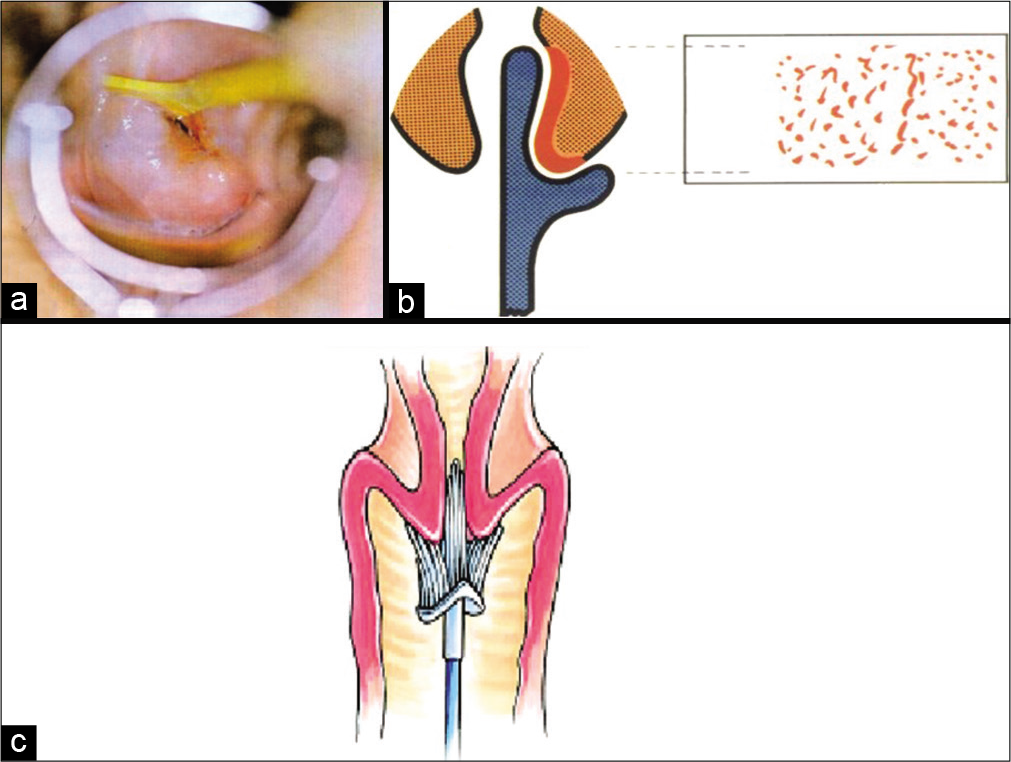
- The method of Pap smear collection. (a) Long end of the Szalay’s plastic spatula is seen fixed firmly in the endocervical canal and rotated clockwise through 360°, (b) The long end collects cells from the squamocolumnar junction even if it is higher up in the canal. (c) Soft bristles of Cervex brush of SurePath fit snugly on the contours of the cervix and when it is rotated 5 times through 360°, it collects cells from the complete transformation zone and also from the endocervical mucosa lining the canal.
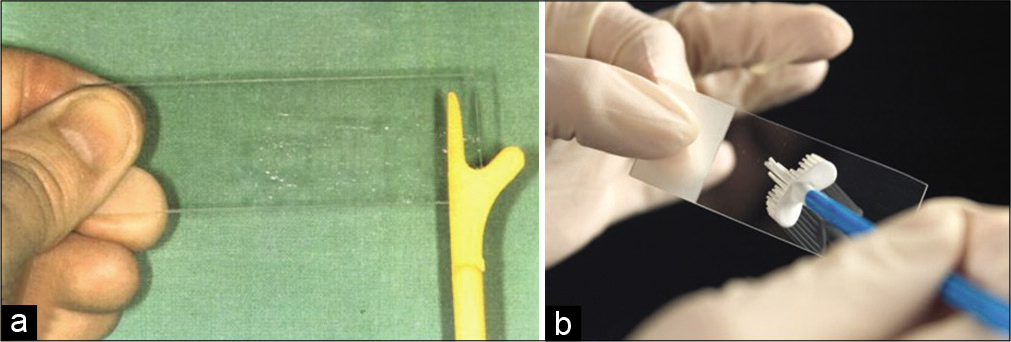
- (a and b) Material is spread in a circular or linear fashion on a glass slide for preparing conventional smears.

- a. SurePath TM ( superscript TM)LBC vial. b.Head of Cervex brush being detached. c. LBC vial with brush dropped into the fixative.
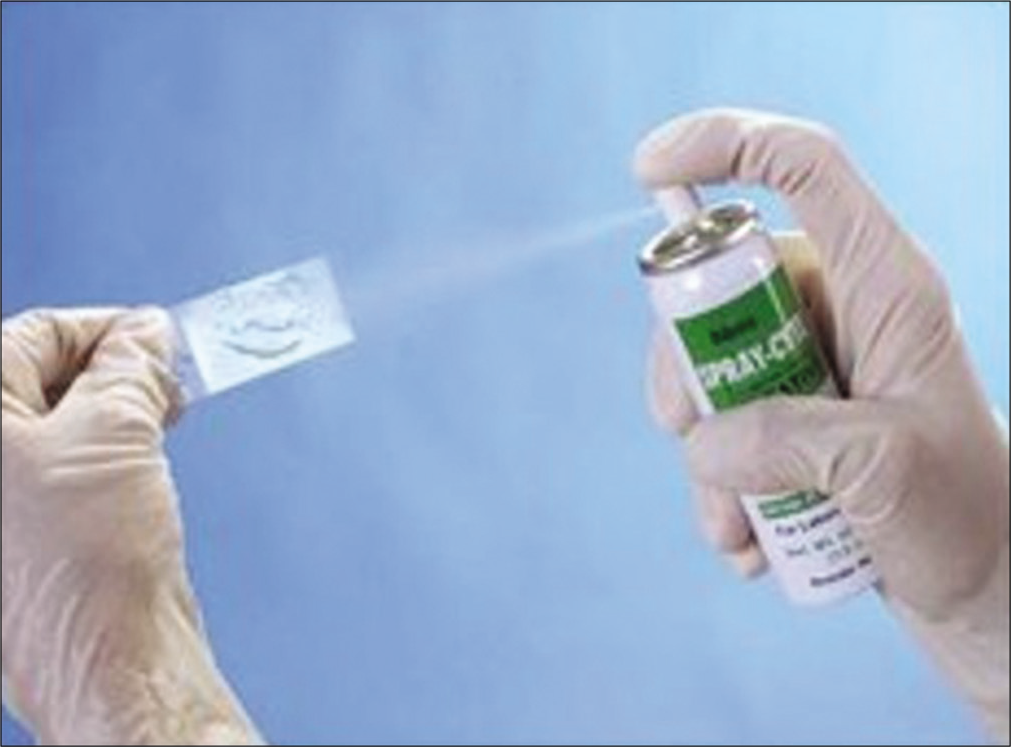
- Spray fixation. Spray the slide before it gets dry. Note the angle and the distance of the spray.
The requisition form must mention the mode of preservation of the slide as the steps in the processing of the smear in the laboratory are different depending on the mode of fixation of the smear.
Tips for the gynecologists
The clinician or the paramedical personnel are the best judge as to whether or not the cervix can be scraped, for example, when it is highly inflamed and angry looking or covered with copious purulent discharge as seen in Figure 8, it is better to call the patient 6 weeks after treatment with antibiotics. Repair of the epithelium leads to clear visualization of the SCJ and TZ and there are less chances that the smear will be unsatisfactory for evaluation due to obscuring inflammation or blood and less number of squamous cells. However, a fair judgment needs to be taken that the patient will return after a course of antibiotics, lest we may lose the chance of screening a high-risk patient.
Similarly when attempting to collect smear from a post-menopausal woman [Figure 16], or from a patient of prolapse uterus [Figure 17], if the cervix is dry and the epithelium is thin and parchment like [Figure 18], scanty material is scraped and transferred on the glass slide. In such a situation, local application of estrogen cream is advised for at least 3 weeks before taking a repeat smear. This enhances both – the maturation of the epithelium and exfoliation of cells.[4]
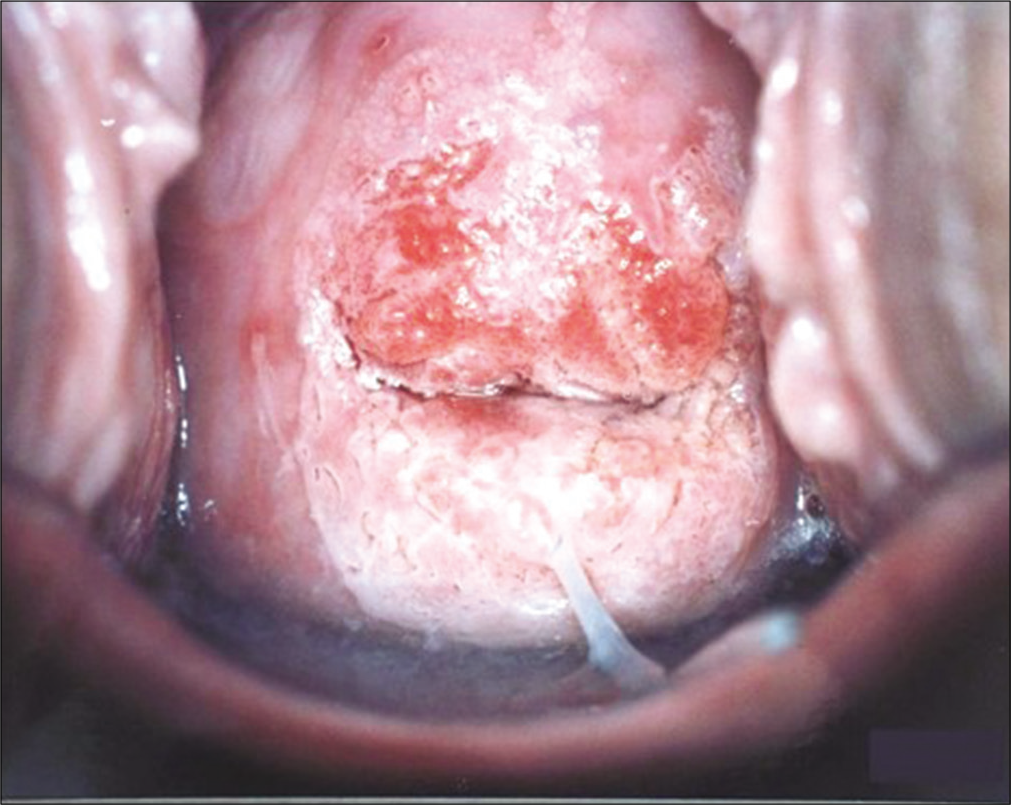
- Colposcopic appearance of Inflamed and angry looking cervix after application of 5% acetic acid. The smears from such a cervix show partial or totally obscuring blood and inflammatory cells.
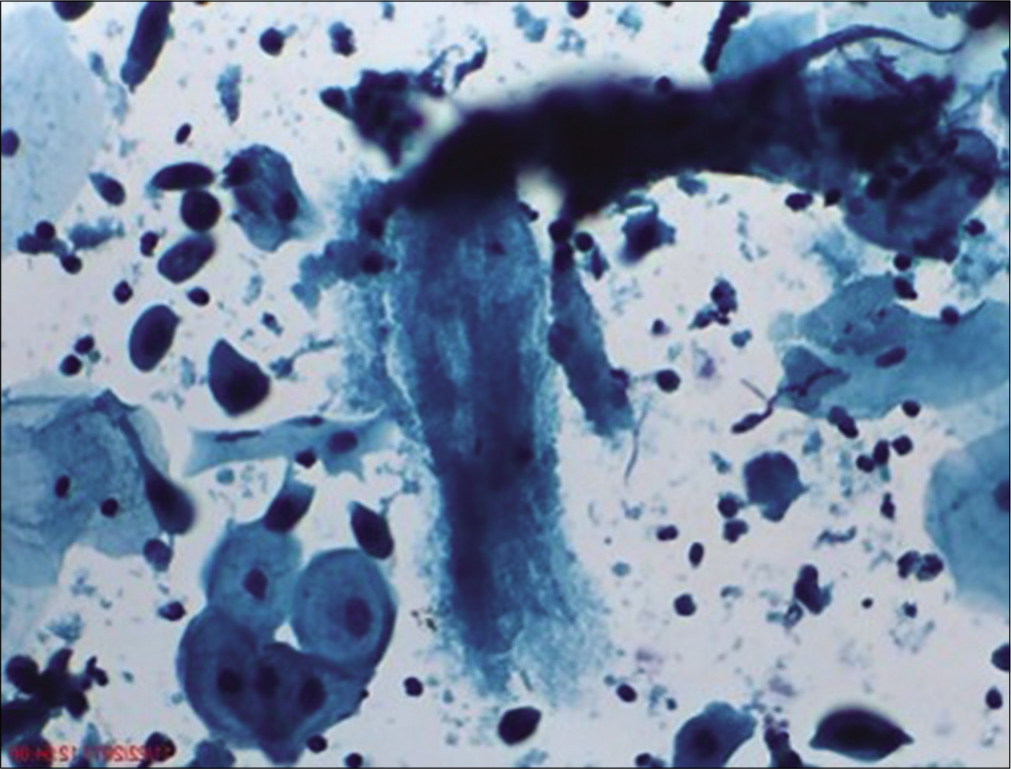
- Liquid-based cytology preparation: The atrophic smear at times shows less number of squamous cells and significant amount of granular cytolytic material in the background. This is because of dryness of the squamous epithelium.
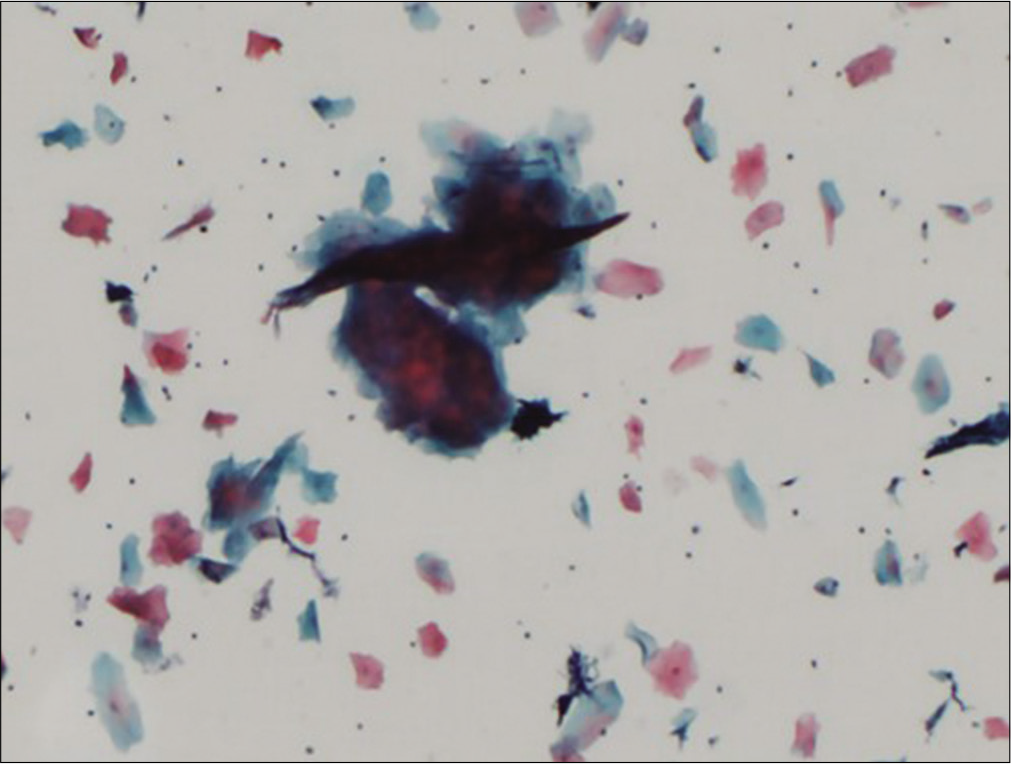
- Liquid-based cytology preparation: Smear from prolapsed cervix, showing scanty cellularity and anucleate squames mostly singly and in compact clusters.
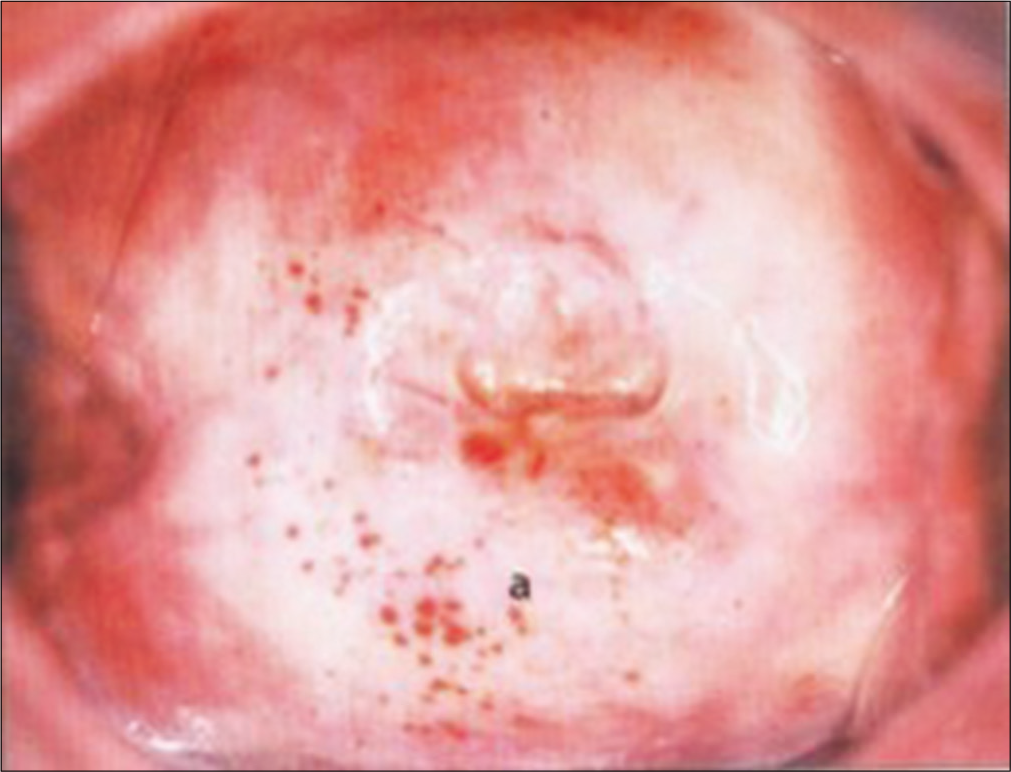
- Hemorrhagic spots beneath the dry and thin epithelium of an atrophic cervix.
Acknowledgments
I would like to acknowledge Dr. Prajakta Sathawne for her valuable help in the completion of this chapter.
ABBREVIATIONS (In alphabetic order)
DUB -Dysfunctional Uterine bleeding
LBC - Liquid based Cytology
LSCS - Lower segment cesarean section
PV - Per vaginum
SCJ - Squamocolumnar junction
TZ - Transformation zone
References
- The normal female genital tract In: Koss L, Melamed M, eds. Koss’ Diagnostic Cytology and its Histopathologic Bases (5th ed). Philadelphia, PA: Lippincott Williams and Wilkins; 2006. p. :183-225.
- [Google Scholar]
- Laboratory techniques In: Koss L, Melamed M, eds. Koss’ Diagnostic Cytology and its Histopathologic Bases (5th ed). Philadelphia, PA: Lippincott Williams and Wilkins; 2006. p. :1569-634.
- [Google Scholar]
- Pap test In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations (3rd ed). Boston: Butterworths; 1990. Ch. 178
- [Google Scholar]
- Available from: http://www.bccancer.bc.ca/screening/Documents/CCSP_GuidelinesManual_PapSampling_Technique.pdf [Last accessed on 2021 Sep 12]








