Translate this page into:
Prominin 2 knockdown inhibits the growth, migration, and invasion of non-small cell lung cancer cells by repressing phosphatidylinositol 3 kinase/protein kinase B pathway

*Corresponding author: Xiaoyong Pang, Department of Oncology, Guangdong Gaozhou People’s Hospital, Maoming, Guangdong Province, China. 13828608773@163.com
-
Received: ,
Accepted: ,
How to cite this article: He B, Chen Z, Zhong L, Pang X. Prominin 2 knockdown inhibits the growth, migration, and invasion of non-small cell lung cancer cells by repressing phosphatidylinositol 3 kinase/protein kinase B pathway. CytoJournal. 2025;22:21. doi: 10.25259/Cytojournal_118_2024
Abstract
Objective
The prognosis of patients with non-small cell lung cancer (NSCLC) is poor, and this malignancy represents a grievous danger to human health due to its high rates of recurrence and metastasis. Previous studies have linked prominin 2 (PROM2) to certain cancers. However, the impact of PROM2 on the biological behavior of NSCLC cells and regulatory pathways has rarely been explored. Therefore, the study aims to elucidate the roles and regulatory mechanisms of PROM2 in the cell function of NSCLC by interfering with PROM2.
Material and Methods
PROM2 messenger ribonucleic acid (mRNA) and protein expression levels in NSCLC cells were analyzed by applying quantitative real-time polymerase chain reaction and Western blot analysis. Phosphatidylinositol 3 kinase (PI3K), protein kinase B (AKT), and phosphorylated-AKT (p-AKT) protein levels were evaluated through Western blot analysis. Cell counting kit-8 and Transwell assays were used to evaluate NSCLC cell proliferation, migration, and invasion.
Results
PROM2 mRNA protein levels drastically increased in NSCLC tissues and cells. High PROM2 mRNA level was related to the poor prognosis of patients with NSCLC. PROM2 silencing remarkably repressed NCI-H520 and A549 cell proliferation, migration, and invasion. Furthermore, PI3K and p-AKT protein levels clearly decreased after PROM2 silencing. Importantly, rescue experiments elucidated that PI3K/AKT pathway activation could reverse the inhibitory effect of PROM2 silencing on the proliferation, migration, and invasion of NCI-H520 and A549 cells.
Conclusion
This study verified that PROM2 knockdown inhibits the growth, migration, and invasion of NSCLC by repressing the PI3K/AKT pathway.
Keywords
Carcinoma
Cell proliferation
Metastasis
Non-small cell lung
Prominin 2
INTRODUCTION
Lung cancer (LC) is a malignancy with high incidence and lethality.[1,2] Non-small cell LC (NSCLC) was the predominant LC type, accounting for approximately 80%. Given the failure of prophase indications, metastatic NSCLC is found in the vast majority of patients at the first diagnosis and thus results in poor prognosis and grievously threatens human health.[3,4] Despite improvements in immunotherapy and targeted treatments of NSCLC, patients’ prognosis is still unsatisfactory.[5] Considering the complex mechanisms involved in NSCLC tumorigenesis and progression,[6] finding new targeted therapies, as well as expounding the potential mode of action regarding the tumorigenesis of NSCLC, is essential to improve the clinical outcomes of patients with NSCLC.
Prominin 2 (PROM2) is abundant in plasma membrane protrusions.[7] It is restricted to epithelial cells and might be associated with an arrangement of plasma membrane microregions. Several studies have revealed that PROM2 is elevated in LC with metastatic melanoma.[8,9] PROM2 overexpression may serve as the novel biomarker in invasive breast cancer, lung adenocarcinoma, and endometrial carcinoma for diagnosis and prognostic prediction.[10] In addition, PROM2 is linked to drug resistance in pancreatic cancer.[11] In NSCLC, high PROM2 expression acts as a potential prognostic biomarker and enhances resistance to cisplatin.[12,13] However, the function and related action of PROM2 in NSCLC are not fully understood. The abnormal phosphatidylinositol 3 kinase/protein kinase B pathway (PA-P), which was the representative survival mode, is generally associated with NSCLC carcinogenesis.[14] Nonetheless, whether PROM2 is involved in the regulation of PA-P in NSCLC is unclear.
Herein, we first estimated PROM2 expression in NSCLC. Next, we assessed the action of PROM2 in NSCLC cells. Finally, we verified whether PROM2 regulates NSCLC cell behavior through PA-P.
MATERIAL AND METHODS
Cell culture and transfection
Five NSCLC cell lines, namely NCI-H520 (CL-0402, Procell, Wuhan, China), H69AR (YS08924B, YaJi cells, Shanghai, China), PC14/b (Bio-131650, Biowe, Beijing, China), A549 (SCSP-503, BioVector NTCC, Beijing, China), and HCC-44 (JY871, Shanghai Jinyuan Biotechnology Co., Ltd, Shanghai, China), and normal lung epithelial cells (BEAS-2B, CL0044, Fenghui, Changsha, China) were kept in Roswell park pemorial institute-1640 (SH30809.01B, Hyclone, South Logan, UT, the USA) supplemented with 10% fetal bovine serum (SH30087.01, Hyclone, South Logan, UT, USA). Cell lines were authenticated by the Genomics Unit at the center for integrated molecular analysis using short tandem repeat profiling (AmpFLSTR® ldentifler® Plus polymerase chain reaction (PCR) Amplification Kit, 4427368, Thermo Fisher Scientific, Waltham, MA, the USA). Mycoplasma in the cell was analyzed every 2 weeks using MycoAlert Mycoplasma Detection Kit (11296744001, Roche, Basel, Switzerland). The cell was kept at 37°C with 5% Carbon dioxide. Small interfering ribonucleic acid (siRNAs) against PROM2 (si-PROM2) and small interfering negative control (si-NC) (siB06525141922-1-5) were obtained from Guangzhou RiboBio (Guangzhou, China). The sequences of the siRNAs (5’–3’) are si-PROM2-1: CCACTGCCGGGCTTTGCTTC, si-PROM2-2: CTGAAGGGTTCCC GGGCTTG, si-PROM2-3: ATCCTGAGGAATGTGAGTGA, and si-NC: TTCTCCGAACGTGTCAC GTTT. Cell transfection was implemented with Lipofectamine 2000 Reagent (11668019, Invitrogen, Carlsbad, CA, the USA) in accordance with the method provided by the manufacturer.
Quantitative real-time PCR (qRT-PCR)
Total ribonucleic acid (RNA) was acquired with TRIzol Reagent (15596026CN, Invitrogen), then converted into complementary deoxyribonucleic acid (cDNA) using a reverse transcriptase reagent kit (RR037Q, TaKaRa, Dalian, China). Subsequently, the cDNA was executed using qRT-PCR on an Applied Biosystems Inc. 7500 PCR system (Applied Biosystems, Foster City, CA, the USA) using SYBR Green (RR820A, Takara). The following primers were used: PROM2 forward: 5’-TCAGGAGCTCCGTGTACCCC-3’, PROM2 reverse: 5’-AGCTCTACCACT GTAGCATT-3’, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward: 5’-GCTCATTTGCAGGGGGGAG-3’, and GAPDH reverse: 5’-GTTGGTGGTGCAGGAGGCA-3’. Gene expression was measured using the 2−ΔΔCT method. GAPDH serves as the internal control.
Western blot analysis
Total protein collected from cells was isolated using radioimmunoprecipitation assay lysis buffer (P0013B, Beyotime, Beijing, China). After sodium dodecyl sulfatepolyacrylamide gel electrophoresis, the proteins were electrotransferred onto polyvinylidene fluoride membranes (88585, Thermo Fisher Scientific, Waltham, MA, the USA). After stabilization, it was incubated in a solution containing the anti-PROM2 antibody (1:900, ab74997, Abcam, Cambridge, MA, the USA); anti-Phosphatidylinositol 3 kinase (PI3K) antibody (1:900, ab40776, Abcam), anti-phosphorylated-protein kinase B (p-AKT) antibody (1:900, 9271, Cell Signaling Technology, Danvers, MA, the USA), anti- protein kinase B (AKT) antibody (1:900, 9272, Cell Signaling Technology), or anti-GAPDH antibody (1:1,500, ab181602, Abcam) overnight at 4°C. Next, it was incubated with horseradish peroxidase-labeled antibody (1:1,600, ab205718, Abcam) at 25°C for 2 h. After tris-buffered saline tween washing, the proteins were quantified using enhanced chemiluminescence (36222ES60, Yeasen, Shanghai, China) with the ChemiDoc XRS system (Bio-Rad, Hercules, CA, the USA). Protein band intensity was quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Springs, MD, the USA). GAPDH is the internal control.
Cell counting kit-8 (CCK-8) assays
Cell proliferation assays were conducted by applying the CCK-8 method (C0037, Beyotime). Cells that transfected si-PROM2 were seeded into plates with three replicate wells in each group and then kept at 37°C for 0, 24, 48, and 72 h. Thereafter, 10 µL of CCK-8 solution was added to each well, and the cells were further incubated for 2 h at 37°C. The optical density at 450 nm was measured using a Multiscan MK3 microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).[15]
Transwell assays
Transwell chambers from Corning were utilized for assessing the migration and invasion capabilities of NCI-H520 and A549. Chambers (REF353097, BD, San Diego, CA, USA) were precoated with Becton, Dickinson and Company (BD) Matrigel (356234, BD) when it came to invasion or had no BD Matrigel when it was about migration. After transfection, cells were seeded into the top chamber, which either contained or did not contain Matrigel in a serum-free medium. The lower chamber was filled with medium containing 10% FBS. Following 48 h of incubation, NCI-H520 and A549 were fixed using 100% methanol. Thereafter, non-migrated/invaded cells in the top chamber were removed. Next, NCI-H520 and A549 on the surface of the bottom membrane were colored using crystal violet. Five random fields were selected for counting using a microscope (CKX41, OLYMPUS, Tokyo, Japan).
Statistical analysis
PROM2 messenger RNA (mRNA) and protein levels in tissues were analyzed using gene expression profiling interactive analysis (GEPIA, http://gepia2.cancer-pku.cn/#index) and the human protein atlas (HPA, https://www.proteinatlas.org/ ) website. The association between PROM2 mRNA and the overall survival in NSCLC patients was analyzed by employing the GEPIA website. In addition, data analysis was executed by applying the IBM Statistical Package for the Social Sciences 22.0 (Armonk, NY, USA). Data were shown as means ± standard deviation. To assess differences between more than two groups, one-way analysis of variance was applied, followed by post hoc analysis using Tukey’s multiple comparisons test. A P < 0.05 was considered statistically significant.
RESULTS
PROM2 expression was upregulated in NSCLC cells
To observe and explore PROM2’s function in NSCLC, we first analyzed the difference between PROM2 expression in normal and NSCLC lung tissues and cells. PROM2 mRNA expression in NSCLC tissues was analyzed using the GEPIA website, which showed that PROM2 mRNA levels in NSCLC lung tissue had dramatically increased compared with those in normal lung tissue [Figure 1a]. The HPA website showed that PROM2 protein was not detected in the normal control group but was detected in NSCLC tissues. This finding showed that PROM2 protein in NSCLC tissues had discernibly increased [Figure 1b]. High PROM2 mRNA expression was linked to the poor prognosis of NSCLC patients [Figure 1c]. PROM2 mRNA and protein levels in the five NSCLC cell lines, especially in NCI-H520 and A549, had dramatically increased compared with those in BEAS-2B cells [Figure 1d-f]. Therefore, the NCI-H520 and A549 cell lines were chosen for further study.
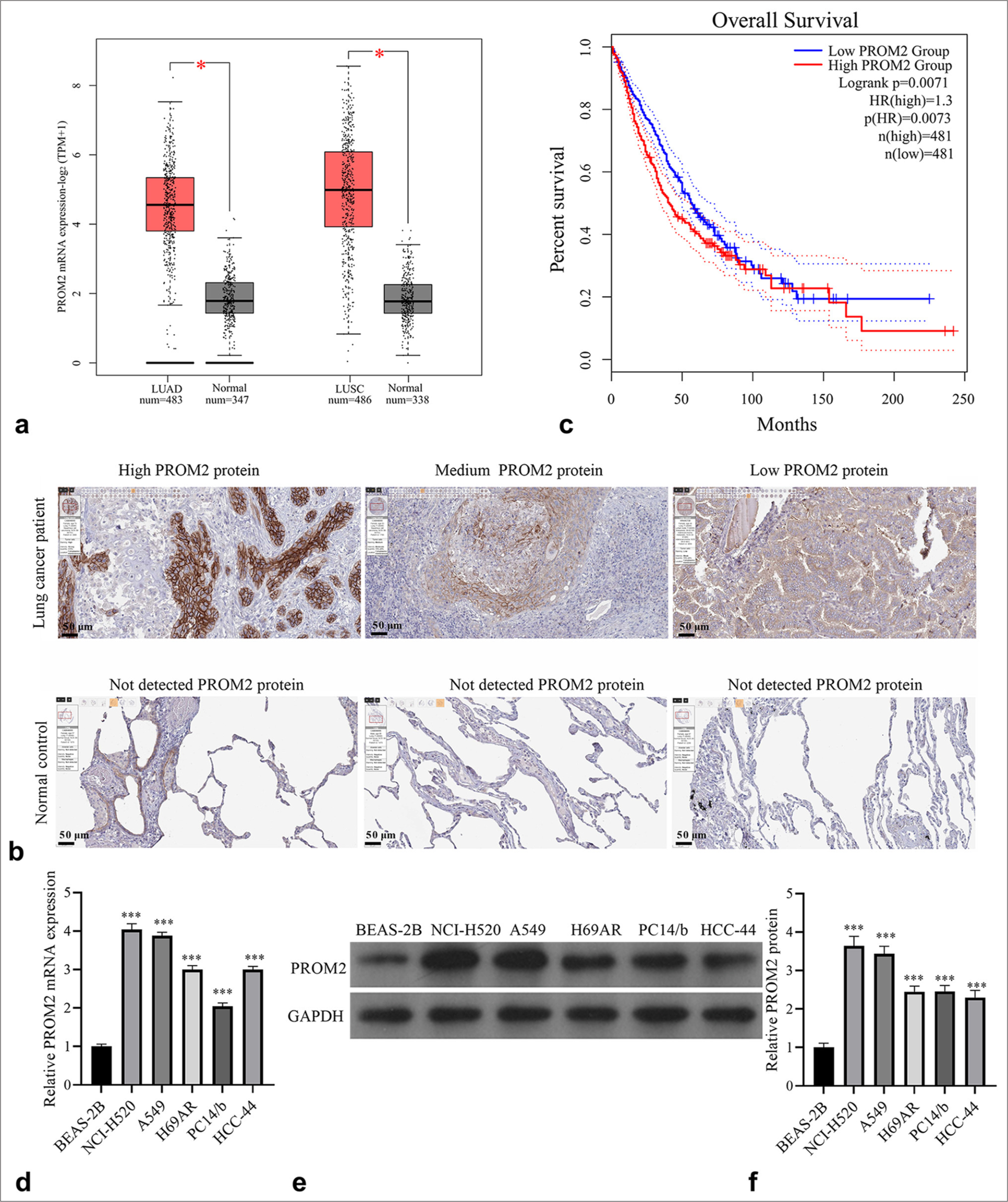
- PROM2 expression enhanced in NSCLC patients. (a) PROM2 mRNA expression enhanced in LUAD and LUSC (✶P<0.05). (b) The PROM2 protein in NSCLC and normal control tissues was analyzed using the HPA website (magnification, 100×). (c) The relationship between PROM2 mRNA expression and overall survival was analyzed using GEPIA. (d-f) qRT-PCR and Western blot analysis were applied to evaluate PROM2 mRNA (d) and protein (e and f) levels in normal (BEAS-2B) and NSCLC (NCI-H520, A549, H69AR, PC14/b, and HCC-44) cells. n = 3. Data are shown as means ± standard deviation. One-way ANOVA was utilized to analyze differences. (✶✶✶P < 0.001). LUAD: Lung adenocarcinoma, LUSC: Lung squamous cell carcinoma, PROM2: Prominin 2, HPA: Human protein atlas, GEPIA: Gene expression profiling interactive analysis, NSCLC: Non-small cell lung cancer, mRNA: Messenger ribonucleic acid, qRT-PCR: Quantitative real-time polymerase chain reaction, ANOVA: Analysis of variance.
Silencing of PROM2 expression in NCI-H520 and A549
To scout the functional effects of PROM2 on NSCLC cells, NCI-H520 and A549 were transforming infection, three siPROM2 sequences (si-PROM2-1, -2, and -3) or si-NC. The PROM2 mRNA and protein expression in the two kinds of NSCLC cells transfected with si-PROM2-1, -2, and -3, especially those in cells transfected with si-PROM2-1 and -3, were markedly inhibited relative to those in si-NC transfected cells [Figure 2a and 2b]. Therefore, si-PROM2-1 and -3 were chosen for further study.
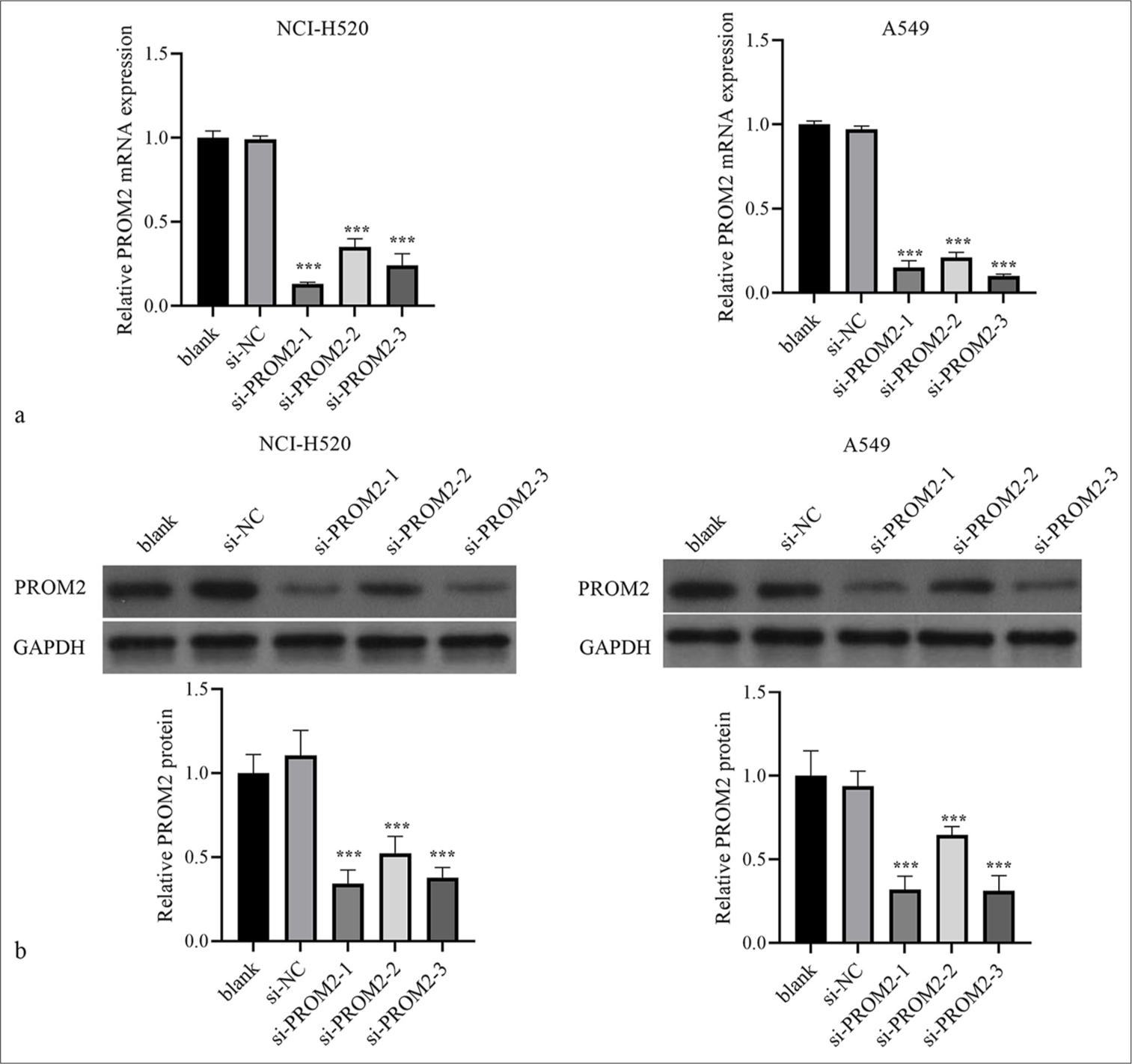
- Silencing of PROM2 expression in NCI-H520 and A549. (a and b) The PROM2 protein and mRNA levels in the two types of NSCLC cells transfected with si-PROM2 or si-NC were measured by applying Western blot analysis with qRT-PCR. n = 3. Data are shown as means ± standard deviation. One-way ANOVA was utilized to analyze differences (✶✶✶P < 0.001, vs. si-NC). PROM2: Prominin 2, mRNA: Messenger ribonucleic acid, NSCLC: Non-small cell lung cancer, qRT-PCR: Quantitative real-time polymerase chain reaction, ANOVA: Analysis of variance, si-PROM2: siRNAs against PROM2; si-NC: siRNAs against negative control.
PROM2 silencing suppressed cell function of NCI-H520 and A549
Functionally, PROM2 silencing blocked the proliferation of the two kinds of NSCLC cells at 48 and 72 h [Figure 3a]. Moreover, PROM2 silencing could depress the migration and invasion of the two types of NSCLC cells [Figure 3b and c].
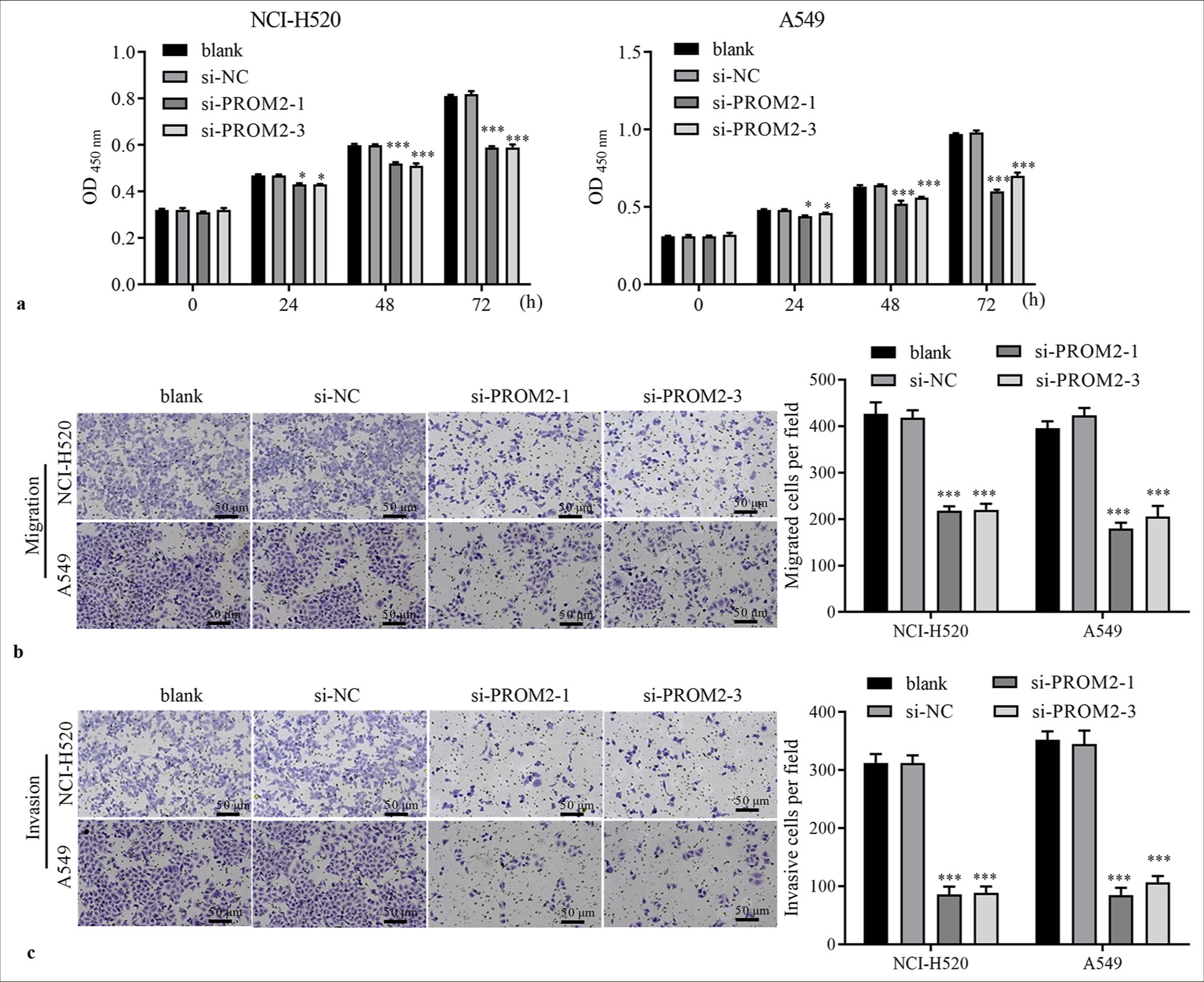
- Function of PROM2 silencing in NSCLC cells. (a) The proliferation of NCI-H520 and A549 transfected with si-PROM2 or si-NC was examined using the CCK-8 assay. (b and c) The migration and invasiveness of NSCLC cells transfected with si-PROM2 or si-NC were evaluated using Transwell assays (Crystal violet stain, ×100). n = 3. Data are shown as means ± standard deviation. One-way ANOVA was utilized to analyze differences. (✶P < 0.05 and ✶✶✶P < 0.001, vs. si-NC). PROM2: Prominin 2, NSCLC: Non-small cell lung cancer, CCK-8: Cell counting kit-8, ANOVA: Analysis of variance, si-PROM2: siRNAs against PROM2; si-NC: siRNAs against negative control.
PROM2 silencing impeded PA-P in NSCLC cells
PA-P has been proven to be a dynamic pathway of NSCLC carcinogenesis, regulating various key cell functions.[16] Hence, whether PROM2 regulated PA-P was examined. As expected, Western blot analysis revealed that PROM2 silencing remarkably reduced PI3K and p-AKT expression levels in the two kinds of NSCLC cells [Figure 4]. However, AKT expression did not remarkably change in si-PROM2-1/2- and si-NC-transfected cells [Figure 4].
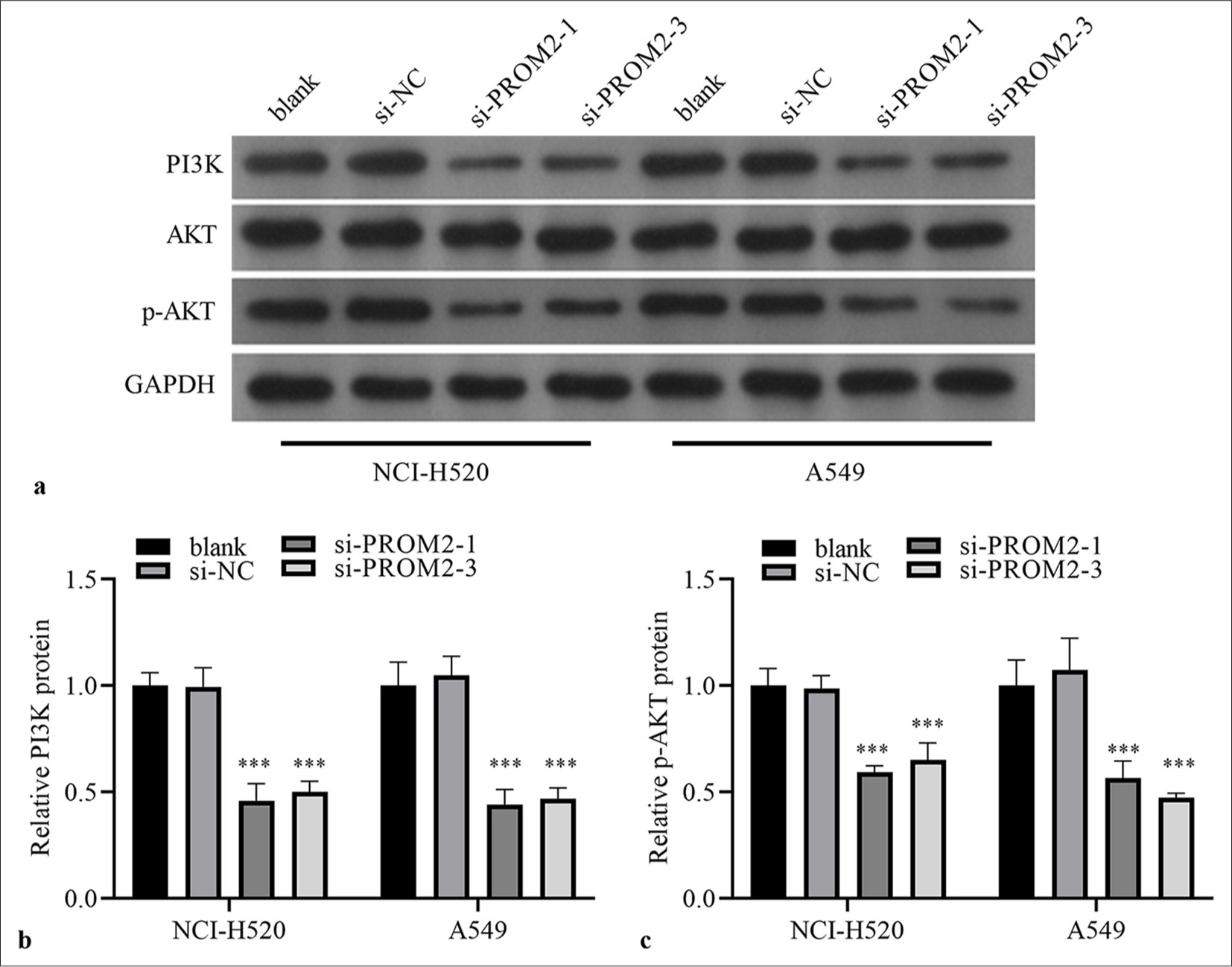
- PI3K and p-AKT/AKT protein in the two kinds of NSCLC cells after PROM2 silencing were evaluated. (a) PI3K protein and p-AKT/AKT were measured by employing Western blot analysis. (b–c) Histogram representing PI3K and p-AKT protein levels. n = 3. Data are shown as means ± standard deviation. One-way ANOVA was utilized to analyze differences. (✶✶✶P < 0.001, vs. si-NC). PROM2: Prominin 2, NSCLC: Non-small cell lung cancer, PI3K: Phosphatidylinositol 3 kinase, AKT: Protein kinase B, p-AKT: PhosphorylatedAKT protein, ANOVA: Analysis of variance.
PA-P activation could reverse the inhibitory effect of PROM2 silencing in NSCLC
Next, the role of PROM2 silencing through PA-P in NSCLC cells was verified. First, the two kinds of NSCLC cells with PROM2 silencing were treated with a PA-P activator (100 ng/ mL insulin-like growth factor-1). PI3K and p-AKT protein levels in the two kinds of NSCLC cells subjected to PROM2 silencing remarkably increased [Figure 5]. Subsequently, whether PA-P activation could reverse the effect of PROM2 silencing in the two kinds of NSCLC cells, was examined. PA-P activation could strikingly boost the proliferation, migration, and invasion of the two kinds of NSCLC cells with PROM2 silencing [Figure 6].
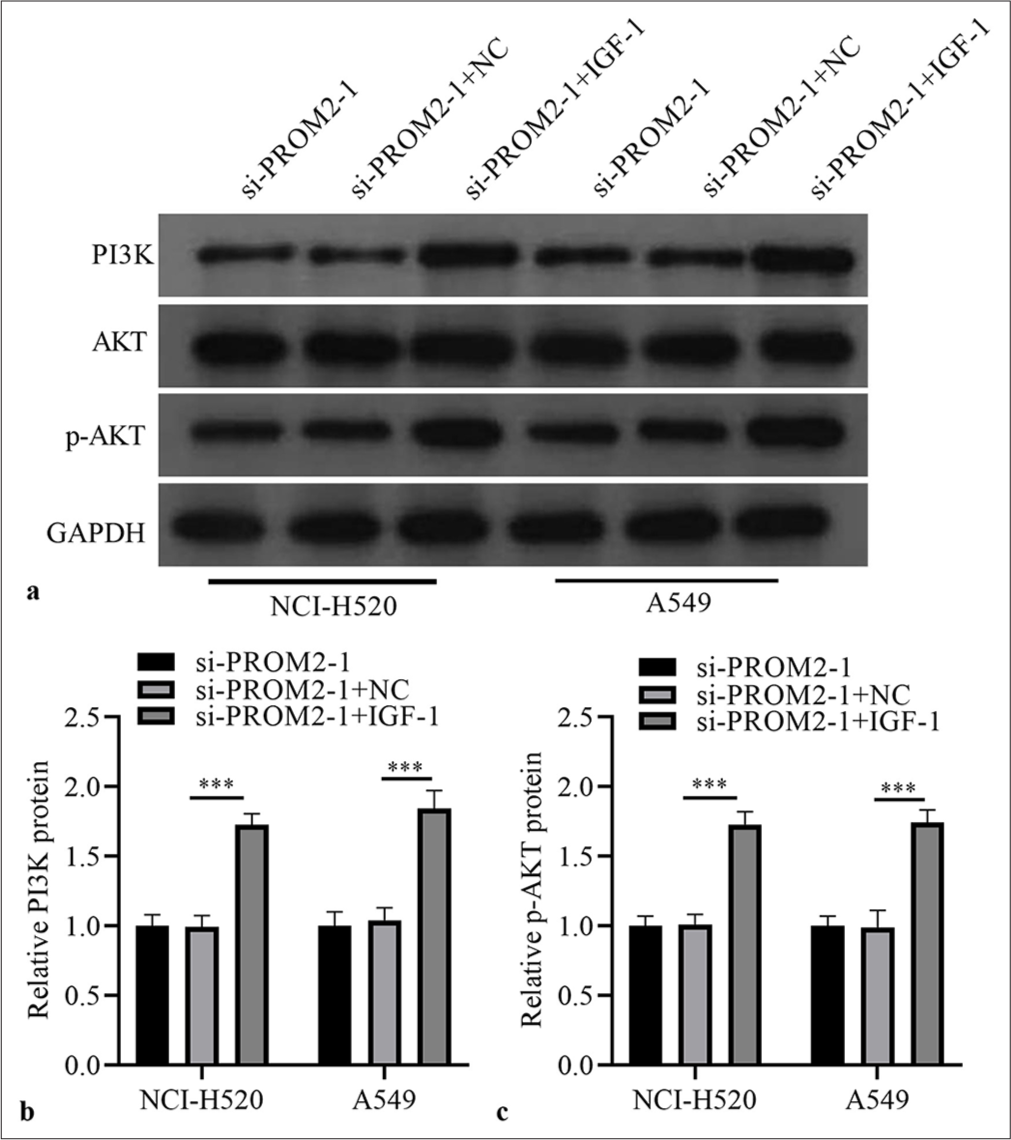
- IGF-1 activated PA-P in PROM2-silenced NSCLC cells. (a) Protein in the two kinds of NSCLC cells subjected to PROM2 silencing and treated with 100 ng/mL IGF-1 were evaluated through Western blot analysis. (b and c) Histogram representing PI3K and p-AKT protein levels. n = 3. Data are shown as means ± standard deviation. One-way ANOVA was utilized to analyze the differences. (✶✶✶P < 0.001). PROM2: Prominin 2, NSCLC: Non-small cell lung cancer, PI3K: Phosphatidylinositol 3 kinase, AKT: Protein kinase B, p-AKT: Phosphorylated-AKT protein, IGF-1: Insulin-like growth factor-1, ANOVA: Analysis of variance.
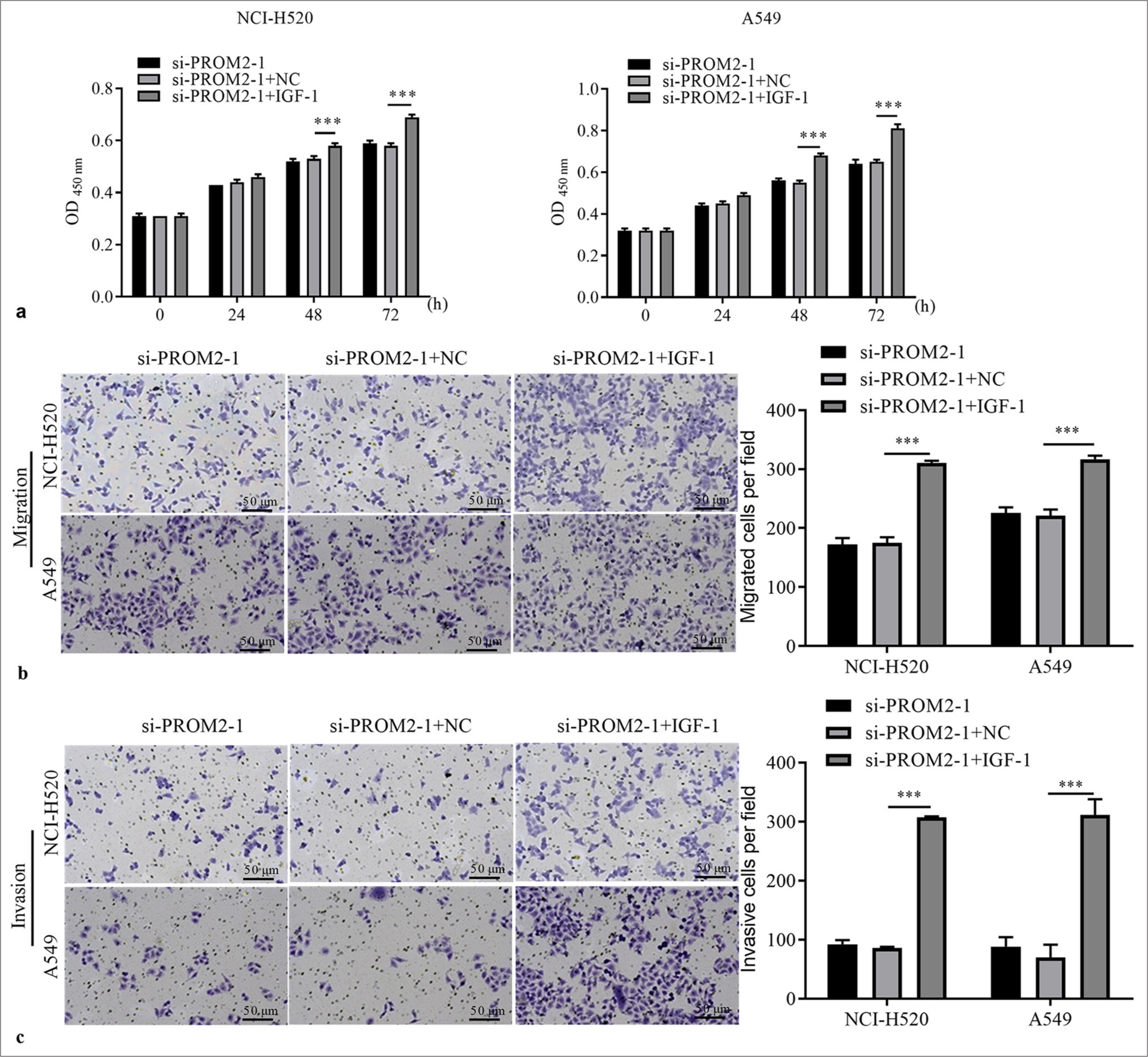
- PA-P activation boosted the function of two kinds of NSCLC cells with PROM2 silencing. (a) The proliferation of the two kinds of NSCLC cells with PROM2 silencing after IGF-1 treatment was evaluated through the CCK-8. (b and c) Migration and invasiveness in the two kinds of NSCLC cells with PROM2 silencing were evaluated through the Transwell assay (Crystal violet stain, ×100). n = 3. Data are shown as means ± standard deviation. One-way ANOVA was utilized to analyze the differences (✶✶✶P < 0.001). PROM2: Prominin 2, NSCLC: Non-small cell lung cancer, CCK-8: Cell counting kit-8, IGF-1: Insulin-like growth factor-1, ANOVA: Analysis of variance, PA-P: PI3K/AKT pathway.
DISCUSSION
Although numerous headways have been made in early diagnosis and minimally invasive biotargeted treatment approaches, the survival rate of patients with NSCLC is still dismal. Therefore, explaining the theory of NSCLC progression is important for developing targeted treatment strategies for this cancer. Herein, we revealed that PROM2 was abnormally upregulated in NSCLC cells; PROM2 acted as a tumor-promoting factor in NSCLC. PROM2 silencing remarkably repressed the proliferation, migration, and invasion of NSCLC cells and enhanced PA-P. In addition, PA-P activation could reverse the inhibitory effect of PROM2 silencing on the proliferation and metastasis in NSCLC.
The rapid development of molecular biology has expedited the investigations on tumor progression at the molecular level. Various studies have confirmed that tumor progression is usually accompanied by genetic mutations and aberrant protein expression.[17] Sun et al. reported that NCAPG protein was elevated in NSCLC; hence, NCAPG acts as a new oncogenic driver promoting the oncogenesis and progression of NSCLC.[18] Moreover, NCAPG expression was inversely correlated with NSCLC survival. Chen et al. indicated that overexpression of BZW2 protein was inversely linked to prognosis in NSCLC and that BZW2 could accelerate the development of NSCLC.[19] PROM2 was the key member of the pentaspan transmembrane family. However, studies on its expression in cancer and its associations are still limited. Previous studies have indicated that PROM2 is connected with overall survival in LC and ovarian cancers.[20] PROM2 is highly expressed in pancreatic cancer, and PROM2 overexpression promotes gemcitabine chemoresistance and results in a high recurrence rate in patients with pancreatic cancer.[11] In addition, PROM2 plays a vital role in altering the ferroptosis of cancer cells in breast cancer.[21] Luo et al.[22] reported that in bladder cancer, the increase in PROM2 cells is connected with reduced iron export and decreased ferroptosis due to abnormal mitochondrial reduction and involved in tumorigenesis and ferroptosis resistance. Herein, similar to its expression profile in LC,[23] PROM2 was highly expressed in NSCLC cells and PROM2 silencing could restrain the proliferation and metastasis of NSCLC cells.
PA-P has been found to perform essential functions in cell activities, such as growth, survival, and migration and was an extensively studied biological mechanism in NSCLC.[24] Recent studies have demonstrated that PA-P activation could modulate the stem cell function of NSCLC cells, such as proliferation and invasion. Zou et al.[25] discovered that PA-P could be activated by NMT1 to promote the metastasis and cisplatin resistance of NSCLC. Zhang et al. reported that PA-P could be regulated by KIFC3 to expedite the proliferation, migration, and invasion of NSCLC cells.[26] In this work, PROM2 silencing could block PI3K and p-AKT protein expression. Furthermore, our supplementation experiments indicated that PA-P activation could reverse the inhibitory effect of PROM2 silencing on the proliferation and metastasis of NSCLC cells, indicating that PROM2 expedited the function of NSCLC cells by activating PA-P.
Our study still has certain deficiencies. First, we did not test the function of PROM2 in the carcinogenesis of xenograft tumors. Second, we have not made further efforts to probe the upstream and downstream molecular mechanisms of PROM2 regulation.
SUMMARY
PROM2 knockdown blocks the proliferation and metastasis of NSCLC by repressing PA-P, which may have considerable value and therapeutic relevance for future NSCLC treatment.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
ABBREVIATIONS
AKT: Protein kinase B
CCK-8: Cell Counting Kit-8
GEPIA: Gene expression profiling interactive analysis
LC: Lung cancer
NSCLC: Non-small cell lung cancer
p-AKT: Phosphorylated-AKT
PA-P: PI3K/AKT pathway
PI3K: Phosphatidylinositol 3 kinase
PROM2: Prominin 2
ACKNOWLEDGMENT
Not applicable.
AUTHOR CONTRIBUTIONS
BH, ZC, LZ, XYP: Participated in the design of the study, all the experiments, statistical analysis; BH: Participated in writing draft manuscript; XYP: Participated in the revision of draft manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Patient data came from database, so ethics approval and consent to participate was not applicable to this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING Not applicable.
References
- Effect of microrna-143-3p-mediated CTNND1 on the biological function of lung cancer cells. Biocell. 2020;44:81-8.
- [CrossRef] [Google Scholar]
- Comprehensive metabolomic analysis of lung cancer patients treated with fu zheng fang. Curr Pharm Anal. 2022;18:881-91.
- [CrossRef] [Google Scholar]
- Molecular testing and personalized treatment of lung cancer. Curr Mol Pharmacol. 2014;7:22-32.
- [CrossRef] [PubMed] [Google Scholar]
- The mediating role of mir-451/etv4/mmp13 signaling axis on epithelialmesenchymal transition in promoting non-small cell lung cancer progression. Curr Mol Pharmacol. 2024;17:e210723218988.
- [CrossRef] [PubMed] [Google Scholar]
- FGD5 as a novel prognostic biomarker and its association with immune infiltrates in lung adenocarcinoma. Biocell. 2023;47:2503-16.
- [CrossRef] [Google Scholar]
- lncRNA SLC9A3-AS1 promotes oncogenesis of NSCLC via sponging microrna-760 and may serve as a prognosis predictor of NSCLC patients. Cancer Manag Res. 2022;14:1087-98.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem. 2003;278:8586-96.
- [CrossRef] [PubMed] [Google Scholar]
- ATF1 promotes ferroptosis resistance in lung cancer through enhancing mrna stability of prom2. Exp Cell Res. 2024;442:114190.
- [CrossRef] [PubMed] [Google Scholar]
- PROM2 overexpression induces metastatic potential through epithelial-to-mesenchymal transition and ferroptosis resistance in human cancers. Clin Transl Med. 2024;14:e1632.
- [CrossRef] [PubMed] [Google Scholar]
- Bioinformatics, rna sequencing, and targeted bisulfite sequencing analyses identify the role of PROM2 as a diagnostic and prognostic biomarker. Am J Transl Res. 2023;15:5389-407.
- [Google Scholar]
- PROM2 promotes gemcitabine chemoresistance via activating the akt signaling pathway in pancreatic cancer. Exp Mol Med. 2020;52:409-22.
- [CrossRef] [PubMed] [Google Scholar]
- Prominin 2 decreases cisplatin sensitivity in non-small cell lung cancer and is modulated by ctcc binding factor. Radiol Oncol. 2023;57:325-36.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of tumorigenesis and ferroptosis in non-small cell lung cancer by a novel BBOX1-AS1/miR-326/PROM2 axis. Mol Cell Biochem. 2024;479:2143-55.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC) Thorac Cancer. 2020;11:511-8.
- [CrossRef] [PubMed] [Google Scholar]
- An in vitro study to explore the role of prolylcarboxypeptidase in non-small cell lung cancer. Biocell. 2020;44:19-26.
- [CrossRef] [Google Scholar]
- LncRNA TBX5-AS1 regulates the tumor progression through the PI3K/AKT pathway in non-small cell lung cancer. Onco Targets Ther. 2020;13:7949-61.
- [CrossRef] [PubMed] [Google Scholar]
- LINC00184 plays an oncogenic role in non-small cell lung cancer via regulation of the miR-524-5p/HMGB2 axis. J Cell Mol Med. 2021;25:9927-38.
- [CrossRef] [PubMed] [Google Scholar]
- NCAPG promotes the oncogenesis and progression of non-small cell lung cancer cells through upregulating LGALS1 expression. Mol Cancer. 2022;21:55.
- [CrossRef] [Google Scholar]
- PRDX2 promotes the proliferation and metastasis of non-small cell lung cancer in vitro and in vivo. Biomed Res Int. 2020;2020:8359860.
- [CrossRef] [PubMed] [Google Scholar]
- PROM1 and PROM2 expression differentially modulates clinical prognosis of cancer: A multiomics analysis. Cancer Gene Ther. 2020;27:147-67.
- [CrossRef] [PubMed] [Google Scholar]
- Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. 2019;51:575-86.e4.
- [CrossRef] [PubMed] [Google Scholar]
- LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis. 2021;12:1043.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic roles of mRNA expression of peroxiredoxins in lung cancer. Onco Targets Ther. 2018;11:8381-8.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between the micrornas and pi3k/akt/mtor axis: Focus on non-small cell lung cancer. Pathol Res Pract. 2022;239:154093.
- [CrossRef] [PubMed] [Google Scholar]
- NMT1 enhances the stemness of NSCLC cells by activating the PI3K/Akt pathway. Pharmacology. 2022;107:486-94.
- [CrossRef] [PubMed] [Google Scholar]
- Kifc3 promotes the progression of non-small cell lung cancer cells through the pi3k/akt pathway. Thorac Cancer. 2024;15:2356-64.
- [CrossRef] [PubMed] [Google Scholar]








