Translate this page into:
Serous cavity metastasis: Evaluation of unknown primary

*Corresponding author: Liron Pantanowitz, MD, MHA Department of Pathology, University of Michigan, Ann Arbor, Michigan, United States. lironp@med.umich.edu
-
Received: ,
Accepted: ,
How to cite this article: Jhala N, Arriola A, Pantanowitz L. Serous cavity metastasis: Evaluation of unknown primary. CytoJournal 2022;19:16.
HTML of this article is available FREE at: https://dx.doi.org/10.25259/CMAS_02_11_2021
Abstract
Malignant effusions can occur in patients with neoplasia. Once a metastatic diagnosis is confirmed, the primary site of origin of malignancy needs to be ascertained. This task can be challenging without a prior history of malignancy. In some patients their effusion may be the initial presentation of an underlying malignancy. Metastases usually present with a dual population of mesothelial and malignant cells. Combining cytomorphologic examination with ancillary testing such as immunocytochemistry can help identify the origin of the foreign malignant cell population. Helpful architectural clues include a single cell pattern, solid cell ball pattern, single file arrangement, papillary formation, psammoma bodies and background mucin. Useful cellular features include the presence of signet ring cells, small cells, pleomorphic and multinucleated giant cells, squamous cells, spindle cells and pigmentation. Rarely, despite an extensive work-up the primary site of origin for a malignant effusion may remain unresolved. This review article will be incorporated finally as one of the chapters in CMAS (CytoJournal Monograph/Atlas Series) #2. It is modified slightly from the chapter by the initial authors in the first edition of Cytopathologic Diagnosis of Serous Fluids.
Keywords
Adenocarcinoma
cytology
effusion
immunohistochemistry
metastasis
tumor of unknown origin

INTRODUCTION
The accumulation of excess fluid (effusion) in any body cavity is always pathologic. About 15% of effusions are due to involvement by a malignancy,[1] indicating that malignant effusions are a likely occurrence in patients with neoplastic disease. Effusions can be caused by primary or secondary neoplastic disease. Examples of a primary neoplasm involving the body cavity are mesothelioma and primary effusion lymphoma (PEL). Secondary involvement, which is more common, can be due to direct extension of neoplasia involving the serosal lining or from distant metastasis. The development of a malignant effusion including ascites usually indicates a grave prognosis for patients with cancer; the mean survival time in afflicted patients is usually measured in weeks or months. Therefore, the primary indication for cytologic examination of exfoliated cells in body cavity fluids is to rule out malignancy.
Once a diagnosis of malignancy is established, the next step is to determine the primary tumor’s site of origin. Despite the grave prognosis, it is clinically important to identify the primary site of origin of the malignancy because this may dictate subsequent management. For example, malignant effusions caused by lymphoma, metastatic breast cancer, ovarian cancer, and small cell lung carcinoma may all respond to targeted systemic chemotherapy or hormonal therapy, whereas effusions secondary to more therapy-resistant cancers such as mesothelioma, non-small-cell lung carcinoma or certain sarcomas may instead be treated with palliative measures, such as pleurodesis or a pleuroperitoneal shunt.[2,3]
This chapter provides an approach to determine the primary site of origin of a malignant effusion, once a diagnosis of malignant mesothelioma is ruled out.
CLINICAL FINDINGS
In most instances, patients who are found to have malignant cells in their body cavity fluids have a known history of a malignant neoplasm. Since metastatic tumors originating from a recognized primary tumor often resemble each other, the answer to the origin of these malignant cells is usually straightforward by doing a comparative evaluation of their cytomorphology. The task for the cytologist is to accordingly ascertain that the cytologic morphology of the malignant cells in the current fluid specimen corresponds to that of the primary neoplasm. If available, review of any prior surgical and/or cytologic material would hence greatly aid this process. However, it is important to be aware that metastatic tumors may differ from primary tumors because (i) tumor cells, even those that were originally spindled, when present within body fluids have a tendency to round up and hence appear epithelioid, (ii) metastases may be more aggressive and thus could be less differentiated and/or vary in tumor grade, and (iii) their immunophenotype may be altered, typically due to loss of particular biomarker expression.
When a clinical history of a prior malignancy is not available, determining the origin of the primary neoplasm can be a challenge. Furthermore, in a small proportion of patients, a malignant effusion may be the initial presentation of an underlying malignant neoplasm.[4] In a series of 154 cases with malignant ascites and 174 malignant pleural effusions, researchers reported that ascites and pleural effusions were the presenting sign of cancer in 7% and 14% of patients, respectively.[5] The patients’ sex and age, as well as the location of the effusion (e.g. above/below the diaphragm), and pertinent laboratory test values (e.g. serum tumor markers) can sometimes help narrow the list of differential diagnoses. For example, papillary serous ovarian carcinoma should be at the top of the list of differential diagnoses in elderly female patients who present with malignant ascites as the first evidence of cancer,[6] especially if their serum CA-125 levels are elevated. When malignant ascites is the initial presentation of cancer in male adult patients, a tumor of gastrointestinal origin should be suspected.[7] For malignant pleural effusions, lung cancer is usually the most likely primary tumor to present as the first sign of cancer in male adult patients.[8-9] In female adult patients, breast cancer is the most common metastatic tumor encountered in pleural fluid. Of note, breast ductal carcinoma typically metastasizes to pleural effusions, whereas lobular breast carcinoma is seen more often in peritoneal fluid.
Malignant effusions are uncommon in children. In a published series of 103 pediatric specimens obtained from various body cavities, 14% were classified as malignant.[10] Most of these malignant effusions were caused by lymphoma and leukemia followed by the so-called small round blue cell tumors (SRBCTs) of childhood such as neuroblastoma, Wilms tumor, and rhabdomyosarcoma.[10-11] Germ cell tumors, metastatic osteosarcoma, and medulloblastoma may also cause malignant effusions in children and young adults.
CYTOLOGY
Metastatic malignancy usually presents with a dual population including reactive mesothelial cells and a foreign malignant population of atypical cells. A practical and logical approach to help figure out the possible tumor of origin for a malignant effusion is based on the cytologic cellular pattern and individual cell appearance of the tumor cells.[12] Table 1 summarizes the differential diagnosis of a malignant effusion based on such cytologic features.
| Cytologic features | Most likely primary tumor sites | Others possible tumor sites |
|---|---|---|
| Architecture | ||
| Single cell pattern | Lymphoma, leukemia, melanoma | Breast, lung, gastric, & renal carcinoma |
| Cannonball pattern | Breast (ductal) and ovarian carcinoma | Lung, stomach, & prostate carcinoma |
| Papillary formation and/or psammoma bodies | Ovarian carcinoma | Thyroid, lung, & kidney carcinoma, mesothelioma |
| Single file pattern | Breast (lobular) carcinoma | Breast (ductal) carcinoma, small cell carcinoma |
| Pseudomyxoma peritonei | Mucinous neoplasms of ovary & appendix | Pancreas, colon, endocervix, & breast carcinoma |
| Cellular features | ||
| Signet ring cells | Breast (lobular) & ovarian carcinoma | Gallbladder, colon, pancreas, & ovarian carcinoma |
| Pigmented cells | Melanoma | Hepatocellular carcinoma |
| Small cells | Small cell carcinoma (lung, Merkel), breast (lobular) carcinoma, non-Hodgkin lymphoma (low-grade) |
Undifferentiated and high-grade sarcomas (Ewing, Ewing-like, others), melanoma, Wilms tumor, neuroblastoma, medulloblastoma |
| Squamous cells | Squamous cell carcinoma | Urothelial & NUT carcinoma |
| Spindle cells | Sarcomas | Spindle cell carcinoma, melanoma, mesothelioma (sarcomatoid) |
CELL ARRANGEMENT
Single cell pattern
Malignant lymphomas, both Hodgkin and non-Hodgkin, as well as leukemia, typically present with a single cell (dyshesive) pattern without any cohesive groups of cells [Figure 1]. The presence of cohesive groups of cells usually excludes malignant lymphoma. Except for Hodgkin lymphoma, effusion specimens usually consist of a relatively monotonous population of lymphoid cells with or without reactive mesothelial cells. The diagnosis of Hodgkin lymphoma requires the identification of Reed–Sternberg cells and their variants against a background of mixed lymphocytes, eosinophils, and plasma cells. Patients with lymphoma, leukemia or myeloma seldom present with malignant effusions and ascites without a known history of such malignancy. Only rarely, are body cavities the initial site of presentation of systemic disease or the location of a primary malignancy (e.g. PEL). The latter is more common in patients who are immunosuppressed and seropositive for human immunodeficiency virus (HIV).[13-15] Marked chronic inflammation may be difficult to distinguish from malignant lymphoma. The presence of a mixed polymorphic lymphoid population favors a reactive process. Immunophenotyping is particularly helpful in differentiating a reactive lymphoid process from a neoplastic one. In addition, this also helps subclassify non-Hodgkin lymphomas and leukemias.
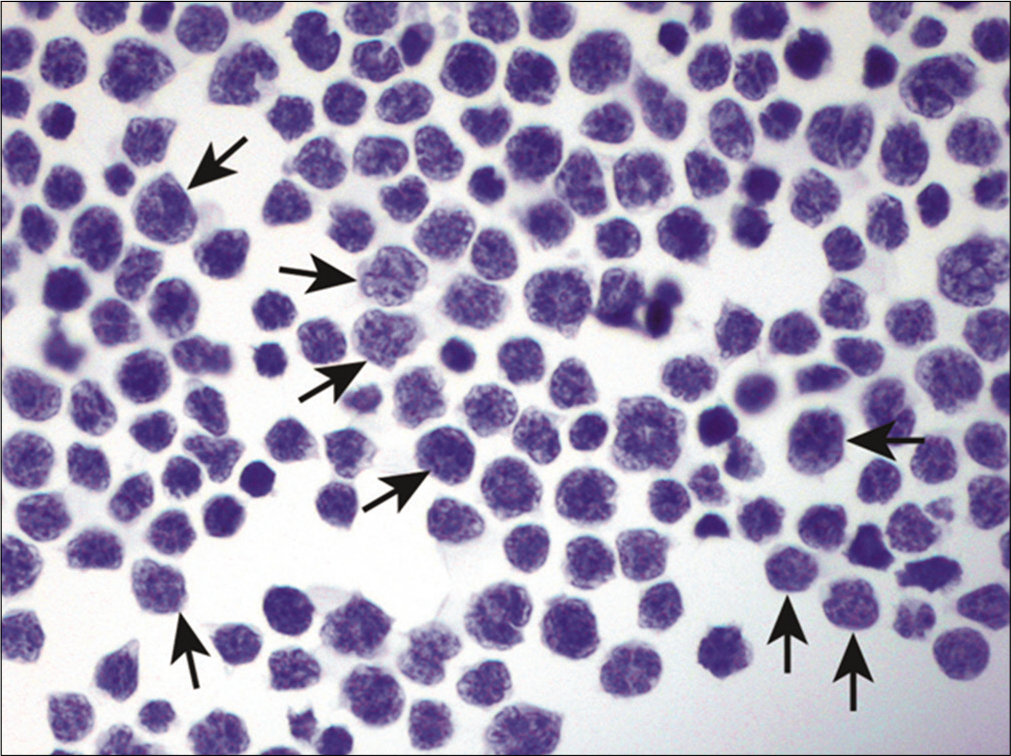
- Single-cell pattern in a malignant lymphoma. This specimen consists of a monotonous population of dyscohesive lymphocytes (arrows) with irregular nuclear contours and hyperchromasia. (Cytospin preparation, PAP stain, 400X.)
Epithelial malignancies can sometimes also present as singly scattered population. This so-called mesothelial-like malignant effusion pattern is more frequently encountered in carcinomas of the breast, stomach, kidney, and prostate. In these cases, there is a predominance of single round cells [Figure 2]. The tumor cells are often larger than mesothelial cells. Poorly differentiated, non-keratinizing squamous cell carcinomas can also shed single cells into body cavity fluids, that may resemble reactive mesothelial cells. With metastatic melanoma tumor cells in an effusion also tend be mostly single.[16-17] When cytoplasmic melanin pigment is present, the diagnosis is often straightforward. Small, round to oval, non-cohesive cells are also characteristic of SRBCTs, which includes Ewing sarcoma and neuroblastoma, among other such tumors.
- (a) Metastatic adenocarcinoma with a mesothelioma-like pattern characterized by many isolated tumor cells. (Cytospin preparation, DQ stain, 400X). (b) Metastatic adenocarcinoma with a mesothelioma-like pattern. (Cell-block preparation, HE stain, 400X). (c) Metastatic adenocarcinoma with a mesothelioma-like pattern showing strong BerEP4 immunoreactivity of almost all tumor cells. (Cell-block preparation, IHC stain, 200X). (d) Metastatic adenocarcinoma with a mesothelioma-like pattern showing only rare calretinin positive mesothelial cells present among the many negative tumor cells. (Cell-block preparation, IHC stain, 400X.)
Solid cell ball pattern
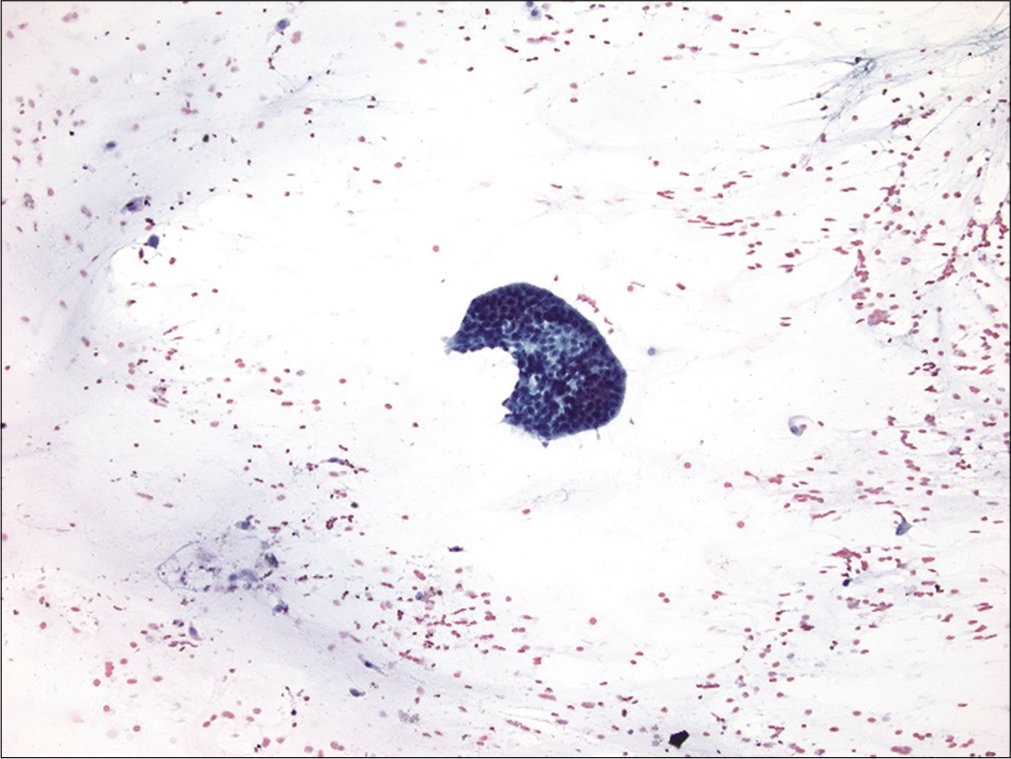
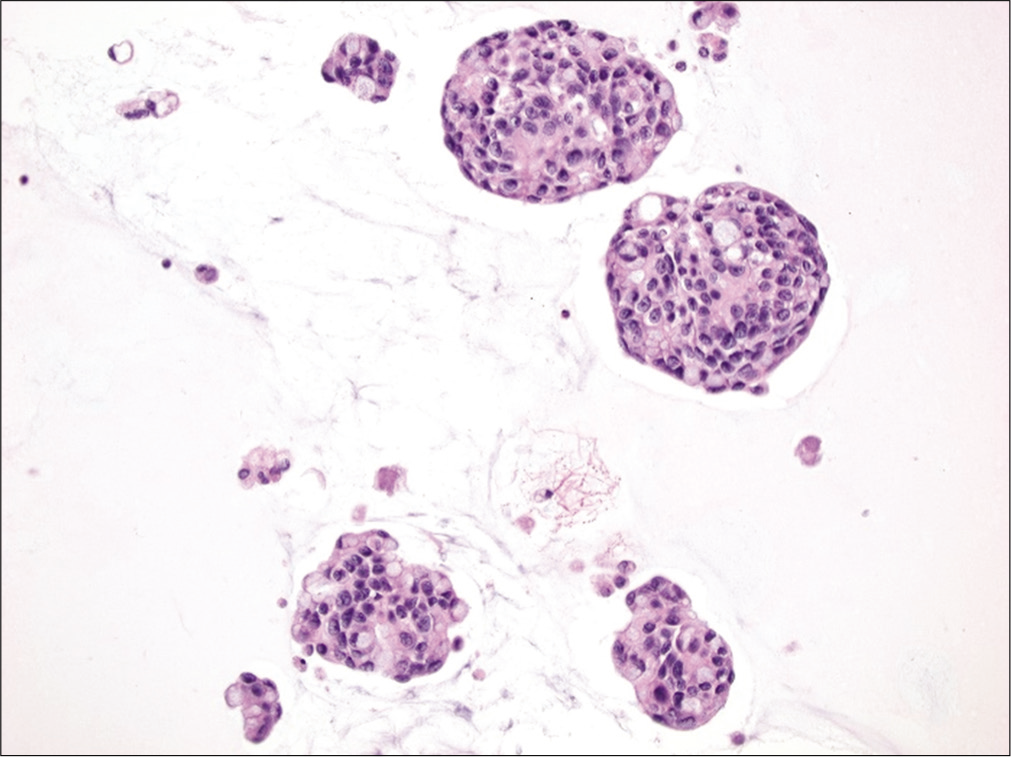
Also known as the cannonball pattern or malignant effusions with proliferation spheres. This pattern is characterized by the presence of three-dimensional, cohesive, tightly packed clusters of cells, with smooth community borders. These cellular globules are quite obvious at low-power examination [Figure 3]. The number of cells present in these proliferation spheres ranges from a few to several hundred. In cell-block sections the center of these cannonballs may contain a central lumen filled with necrotic debris but without stromal core [Figure 4]. This pattern is typically seen in carcinomas of the breast and ovary, and therefore is commonly encountered in elderly women. In fact, very large proliferation spheres are characteristic of the ductal variant of infiltrating mammary carcinoma in fluids.[18] Other malignancies that can present with a cannonball pattern include metastatic carcinoma of the lung, stomach, and prostate. Malignant mesothelioma, and rarely mesothelial hyperplasia, can also demonstrate a cannonball pattern in body cavity fluids. However, the cell globules of mesothelial origin typically demonstrate a scalloped or knobby border and may show stromal core.[19]
- Cannonball pattern in metastatic breast carcinoma. This effusion specimen consists of numerous three-dimensional tightly cohesive cell clusters (arrows). (Cytospin preparation, PAP stain, 100X.)
- Cannonball pattern in metastatic breast carcinoma. The cell-block section illustrates tumor balls with a central lumen filled with necrosis. (Cell-block preparation, HE stain, 100X.)
Papillary formation and psammoma bodies
Papillae are three-dimensional, cohesive structures that consist of a fibrovascular core or have one axis longer than the others [Figures 5 and 6]. Individual cells are usually polarized; i.e. their nuclei are arranged perpendicular to the long axis or the fibrovascular core. Papillae are often derived from papillary neoplasms arising from the ovary, thyroid gland, kidney, and lung. Intranuclear cytoplasmic inclusions can be seen in papillary carcinoma of the thyroid and bronchioloalveolar carcinoma of the lung. Rarely, well differentiated mesothelioma can have a papillary pattern.
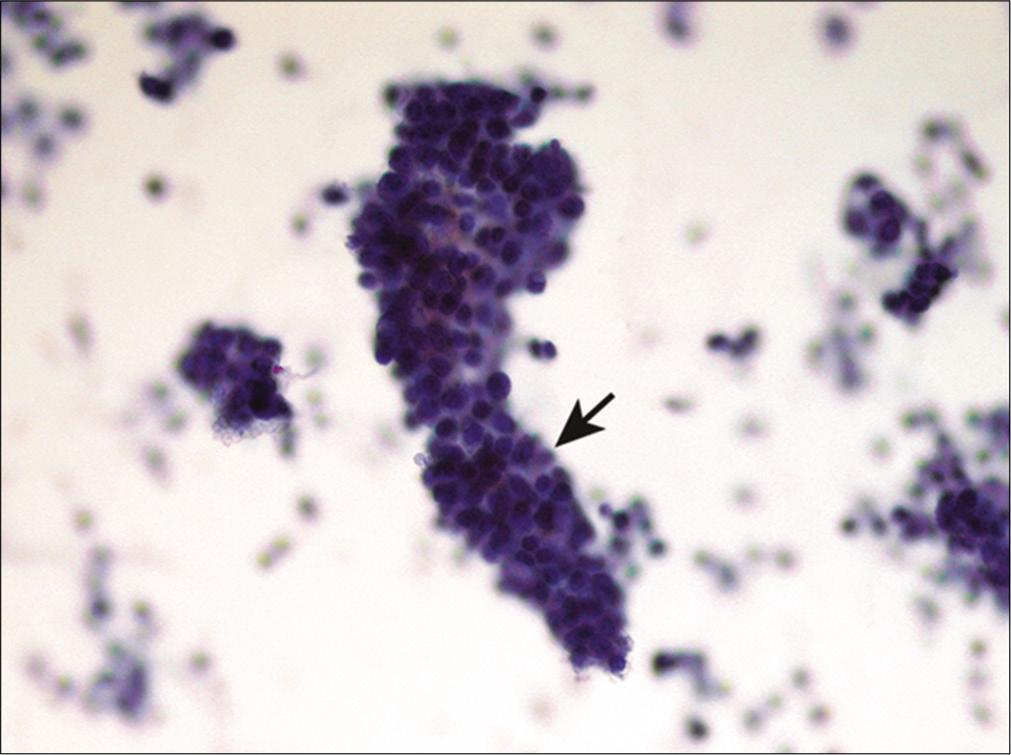
- Papillary fragment in metastatic ovarian carcinoma. The papillary fragment (arrow) shown is composed of moderately atypical cells. (Cytospin preparation, PAP stain, 400X.)
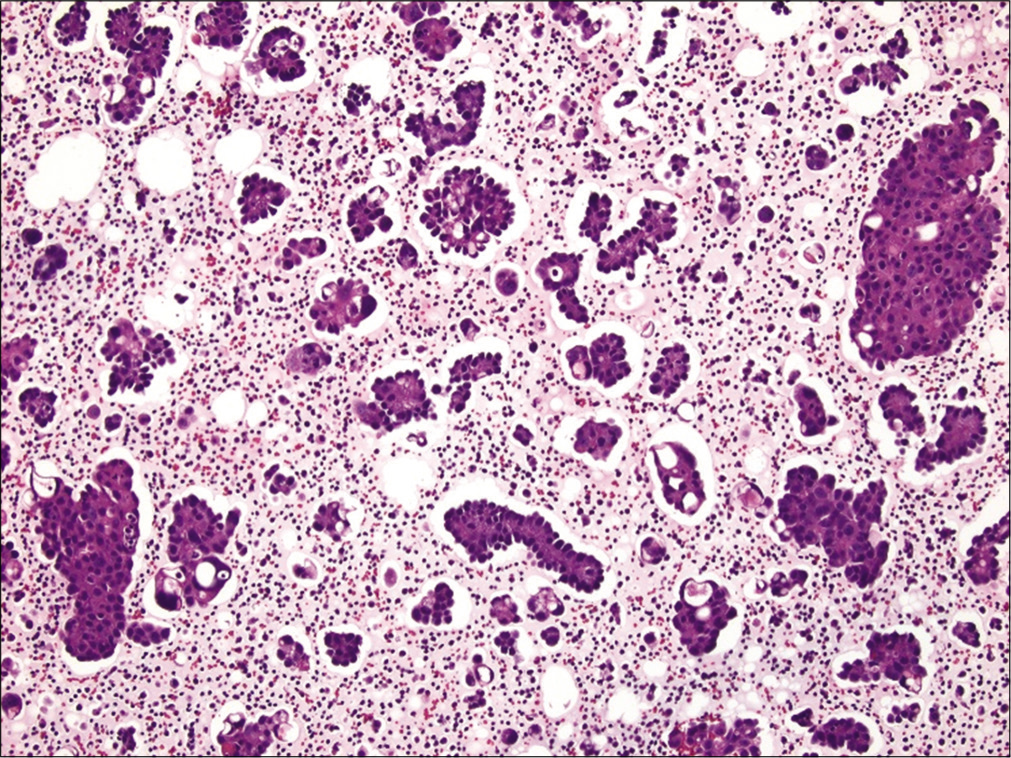
- Metastatic ovarian papillary serous carcinoma to pleural fluid showing many papillary fragments. Note the clear lacunae around most of the tumor cell groups, which some cytologists believe is supportive of metastatic carcinoma versus mesothelial proliferation. (Cell-block preparation, HE stain, 100X.)
Psammoma bodies are often noted in association with papillary clusters [Figure 7]. They are round to oval structures that consist of concentric layers of calcified material. Psammoma bodies are created by cells that have undergone degeneration and calcification. The presence of psammoma bodies with or without papillary formations in ascitic fluid from an elderly female patient is highly suggestive of a metastatic ovarian carcinoma. Other malignancies that can be accompanied by psammoma bodies include primary peritoneal serous tumor, papillary carcinoma of the thyroid, bronchogenic carcinoma, malignant mesothelioma, and infrequently metastases from breast and gastric carcinomas.[19] The finding of low-grade epithelial atypia in papillary cell groups accompanied by numerous psammoma bodies (usually > 80 bodies) is distinctive of psammocarcinoma.[20] One should be cautious not to over-interpret the presence of psammoma bodies and/or papillary formation in fluid specimens as unequivocal evidence of malignancy in ascitic fluids because patients with benign mesothelial hyperplasia may also shed papillary groups of mesothelial cells and/or psammoma bodies.[21-24]
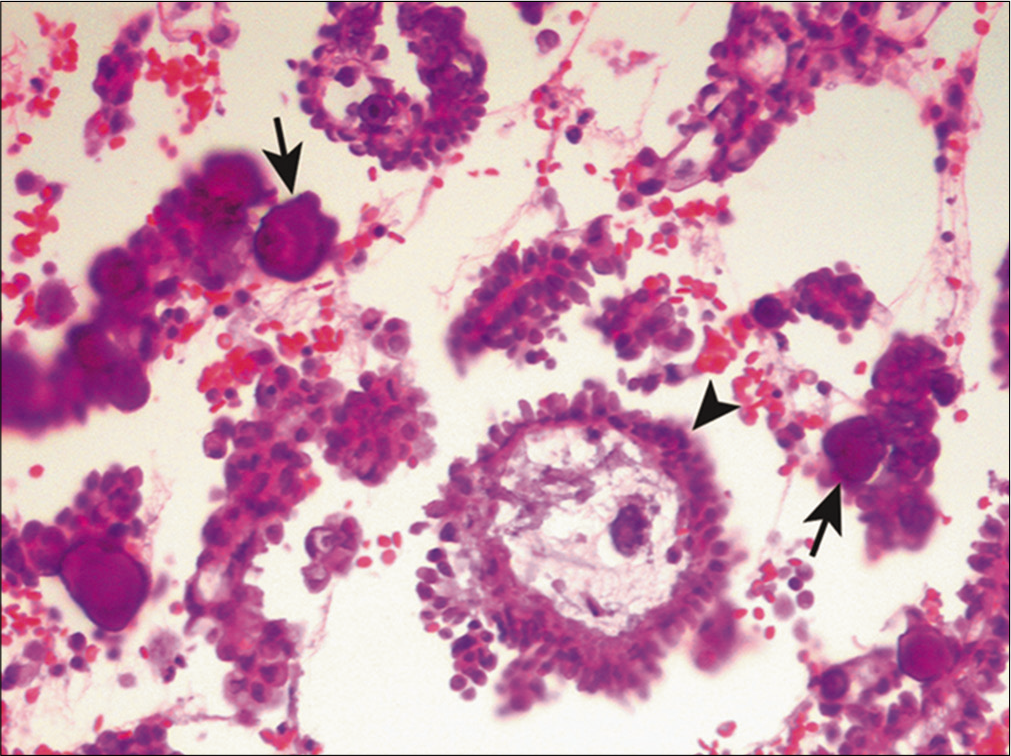
- Papillary fragments (arrowhead) and psammoma bodies (arrows) in metastatic ovarian carcinoma. Several cross sections are shown of papillary groups of neoplastic cells with a fibrovascular core. (Cell-block preparation, HE stain, 400X.)
Single file arrangement
Single file pattern refers to tumor cells aligning themselves in single rows. By electron microscopy, it has been shown that intercellular connections are present between the cells within these files. The presence of long chains of small, bland-appearing tumor cells is characteristic of infiltrating lobular breast carcinoma.[18] However, cells of infiltrating ductal carcinoma can occasionally also arrange in single file, but the individual cells are usually larger and more pleomorphic. Small cell carcinoma of the lung can also present with a single- file arrangement along with prominent nuclear molding [Figure 8]. This characteristic appearance has been described as a ‘stack of dishes,’ ‘pile of coins,’ or ‘vertebral column’.[25] Individual tumor cells have high nuclear/ cytoplasmic ratios and ‘salt and pepper’ chromatin. Small cell carcinoma can also present with other patterns including small or large cell clusters, as well as a single cell pattern.[26]
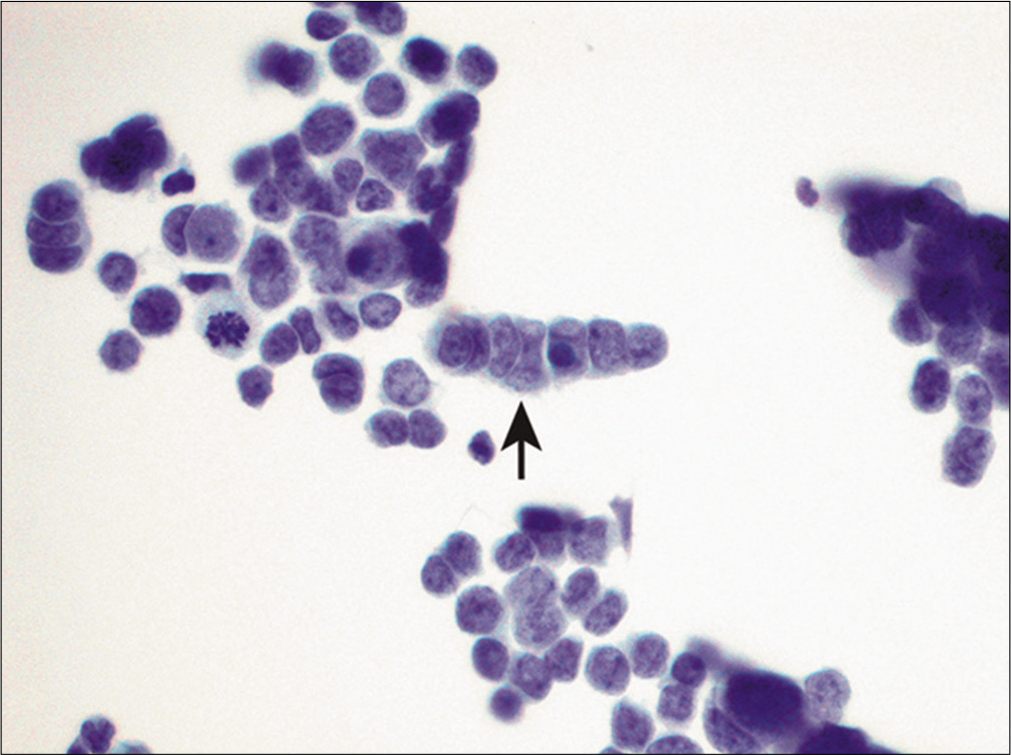
- Single-file pattern in metastatic small cell lung carcinoma. The center of the picture demonstrates a single file of neoplastic cells with nuclear molding (arrow). (Cytospin preparation, PAP stain, 400X.)
Pseudomyxoma peritonei
Pseudomyxoma peritonei (“jelly belly”) refers to the presence of mucin in ascitic fluid that is derived from a mucinous neoplasm.[27] Such mucin appears as light blue to magenta amorphous material with a modified Romanowsky stain. In such samples it is important to search carefully for neoplastic epithelial cells, singly or in small clusters, scattered within the mucin [Figure 9]. Individual cells often appear bland, with or without cytoplasmic mucinous vacuoles (i.e. mucinous differentiation). However, mucinous carcinoma peritonei can range from low-grade to high-grade cytology, and specimens may contain signet ring cells. In female patients, ovarian mucinous cystadenoma and cystadenocarcinoma are the most likely primary neoplasms that give rise to pseudomyxoma peritonei. The appendix is the most common primary site in male patients and the second most common primary site in female patients.[28] Other gastrointestinal neoplasms, pancreatic cancer, endocervical carcinoma, and mucinous carcinomas that occur in the breast, can also cause pseudomyxoma peritonei.
- Pseudomyxoma peritonei. A cluster of relatively bland epithelial tumor cells is shown within a mucinous background. (Smear preparation, PAP stain, 100X.)
CELLULAR FEATURES
Signet ring cells
Signet ring cells are neoplastic cells with a single, large cytoplasmic vacuole that pushes an atypical nucleus towards the periphery [Figures 10 and 11]. Mucin is the most likely content to be found in these vacuoles, followed by lipid, and occasionally immunoglobulin.[19,29-30] The nuclei of these individual cells often display moderate to marked atypia. Gastric adenocarcinoma is the most likely primary tumor site, followed by breast carcinoma, particularly infiltrating lobular carcinoma. The neoplastic cells of breast origin tend to be smaller than those of gastric cancer. Other malignancies that may give rise to signet ring cells include carcinomas of the gallbladder, colon, pancreas, and ovary. The differential diagnosis should also include degenerative changes in mesothelial cells and macrophages with large cytoplasmic vacuoles. The lack of nuclear atypia, and if needed immunophenotyping, should help rule out malignancy. There is also a rare variant of signet-ring mesothelioma.
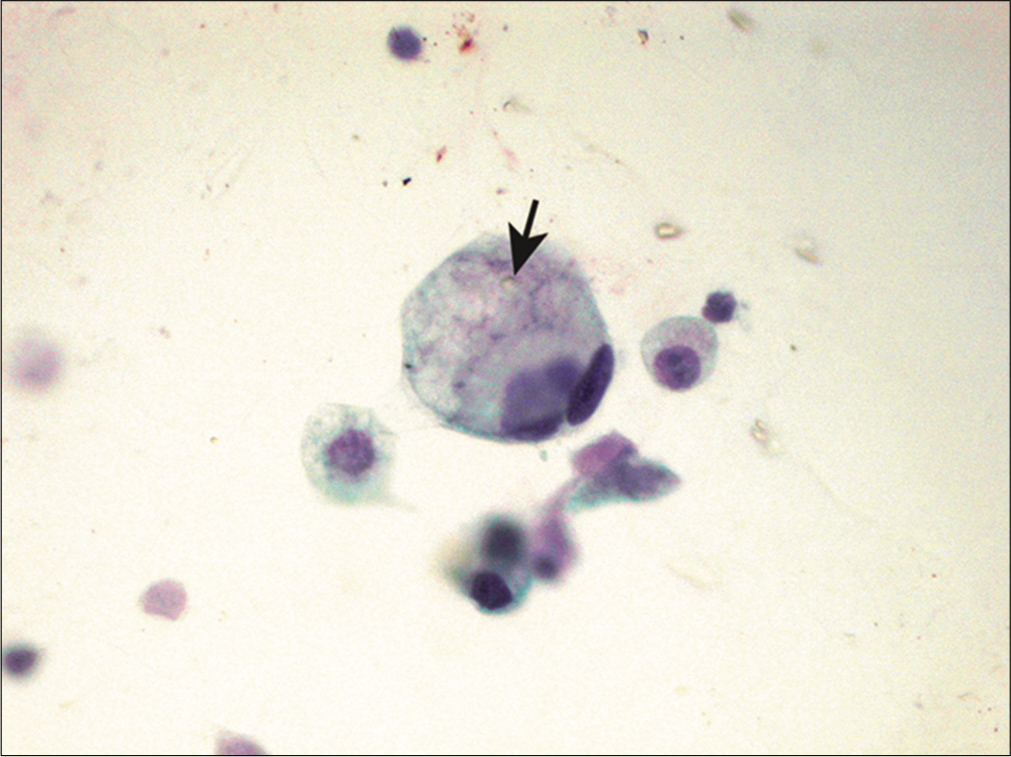
- Signet ring cell in metastatic gastric carcinoma. A single tumor cell is shown with abundant vacuolated cytoplasm (arrow) that pushes the atypical nucleus to the periphery, imparting a signet-ring appearance in histology sections. (Cytospin preparation, PAP stain, 400X.)
- Signet ring cells scattered within these tumor balls are shown in a case of metastatic gastric carcinoma involving peritoneal fluid. (Cell-block preparation, HE stain, 200X.)
Pigmented cells
When pigmented neoplastic cells are identified in fluid specimens, malignant melanoma should be considered in the differential diagnosis. In fact, the presence of melanin pigment in malignant cells is usually diagnostic of malignant melanoma [Figure 12]. However, not uncommonly, melanoma may be amelanotic, i.e. without obvious cytoplasmic pigment. Other features that are suggestive of a malignant melanoma include single cell pattern, eccentrically located nuclei (plasmacytoid appearance), intranuclear cytoplasmic inclusions, frequent binucleation and prominent nucleoli.[16-17] Other pigmented cells that can be found in fluid specimens include hepatocellular carcinoma and hemosiderin-laden macrophages. Rarely, one may encounter black anthracotic pigment in pleural fluid in patients with a history of crack abuse.[31]
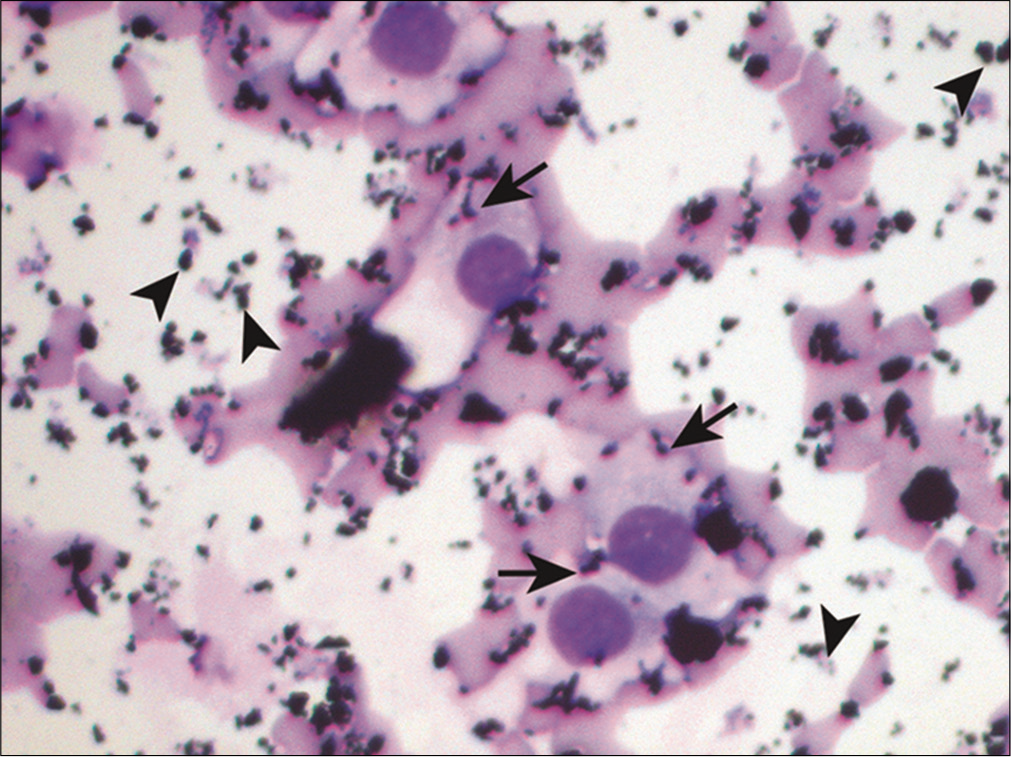
- Pigmented cells in metastatic melanoma. Scattered atypical cells are shown with intracytoplasmic pigment (arrows). Melanin pigment which stains gray-blue may also be noted extracellularly (arrowheads). (Cytospin preparation, modified Romanowsky stain, 400X.)
Small cells
Small cell tumors are composed of cells that are only slightly larger than a small round mature lymphocyte. The differential diagnoses includes both epithelial and nonepithelial malignancies. Among the epithelial malignancies, lobular breast carcinoma and small cell lung carcinoma are at the top of the list [Figure 13]. One should also consider low-grade non-Hodgkin lymphomas such as small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL), grade I follicular lymphoma, and mantle cell lymphoma. In children and young adults, malignant effusions may be attributed to a variety of SRBCTs of childhood including sarcomas (e.g. Ewing sarcoma, rhabdomyosarcoma, etc.), neuroblastoma, Wilms tumor, and medulloblastoma.[11,32]
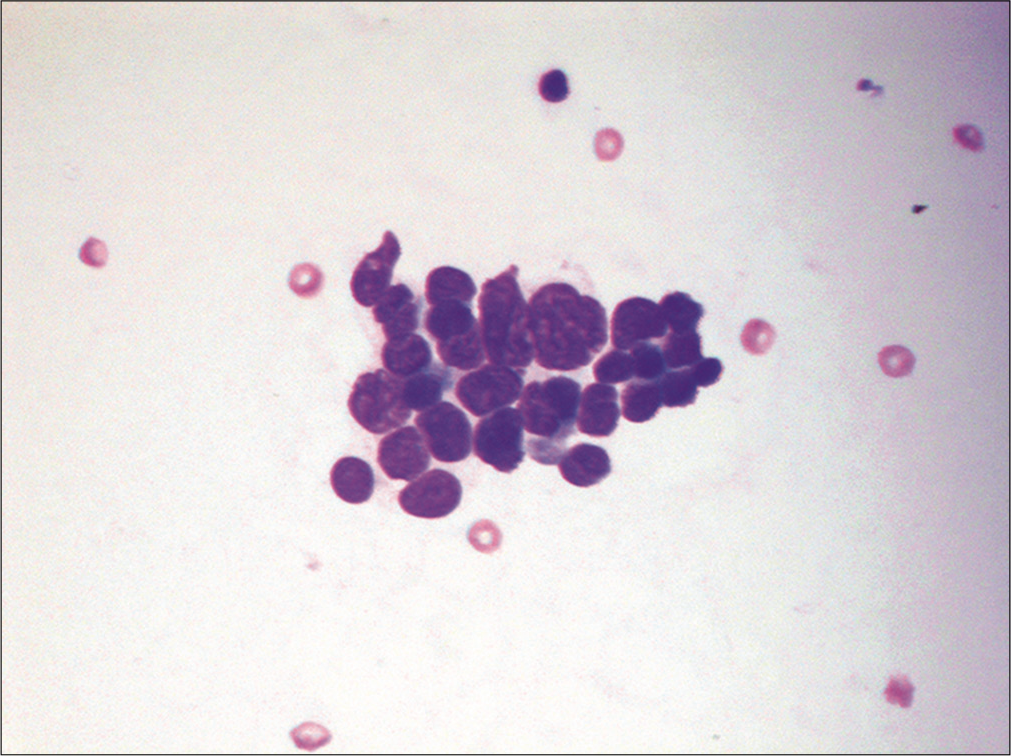
- Small tumor cells are shown in a case of metastatic small cell lung carcinoma. This effusion contains a loosely cohesive group of small cells (about 2–3 the size of red blood cells) with scant cytoplasm and nuclear molding. (Cytospin preparation, modified Romanowsky stain, 400X.)
Pleomorphic and multinucleated giant cells
The list of diagnostic possibilities for bizarre or giant cells is extensive [Figure 14]. Such tumor cells can be associated with carcinomas of the lung, pancreas, uterus, melanoma, sarcomas, lymphomas, and germ cell tumors, among others. Without a known history of the primary tumor or prior pathologic materials for review with the current sample, it may be impossible to identify the origin of these malignant cells in body fluids based on morphology alone. As a result, ancillary studies such as immunocytochemistry are often required to aid the differential diagnosis. Occasionally, cells of histiocytic and mesothelial origin, as well as megakaryocytes associated with extramedullary hematopoiesis, can be multinucleated and should not be mistaken for malignant multinucleated giant cells.[33]
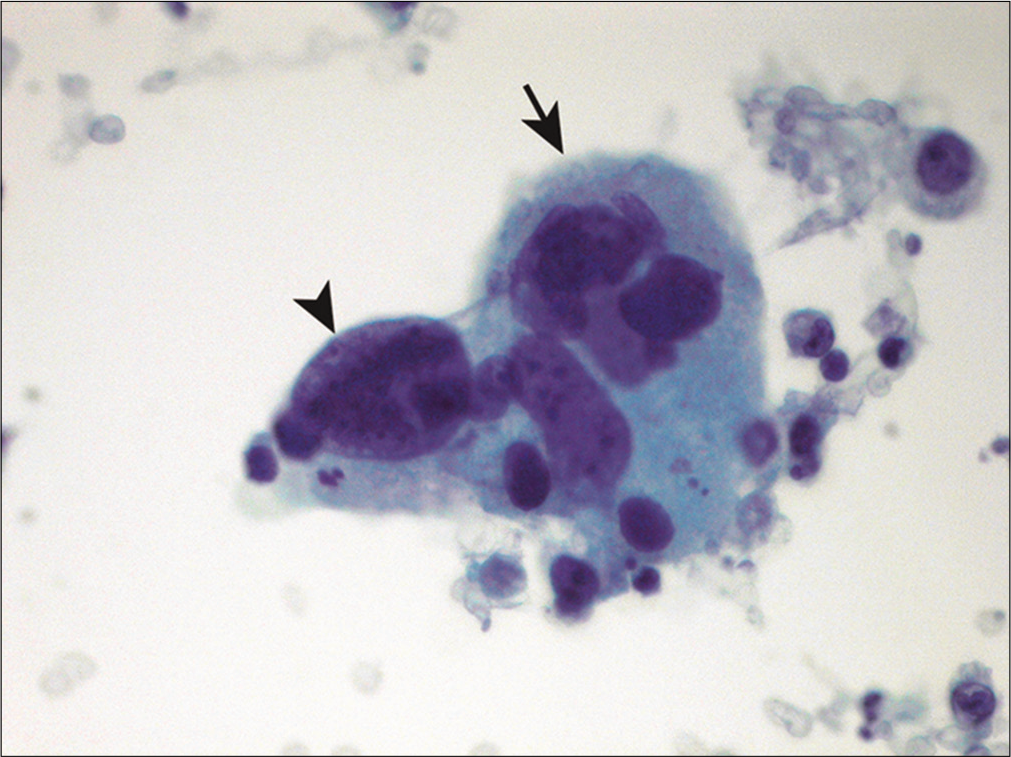
- Pleomorphic multinucleated giant cells are shown in a patient with metastatic non-small-cell carcinoma of the lung. A giant cell with multinucleation (arrow) and bizarre nuclei (arrowhead) is noted. (Cytospin preparation, PAP stain, 400X.)
Squamous cells
Squamous cell carcinoma rarely sheds neoplastic cells into body cavity fluids. Only about 5% of malignant pleural effusions and less than 2% of malignant ascites are due to squamous cell carcinoma.[34] The incidence is even less for pericardial effusions.[35] When evidence of keratinization such as abnormal cell shapes, squamous pearl formation, and dense orange cytoplasm is present, the diagnosis of keratinizing squamous cell carcinoma is usually straightforward [Figure 15]. The differential diagnosis includes a ruptured dermoid cyst[36] and incidental percutaneous contamination from skin. Metastatic urothelial carcinoma may occasionally demonstrate squamous cell differentiation, along with possible cercariform cells (nucleated cells with a globular body and non-tapering, fish tail-like cytoplasmic end) and Melamed-Wolinska (eosinophilic) intracytoplasmic bodies. Non-keratinizing squamous cell carcinoma is relatively more common in fluid specimens than its keratinizing counterpart. Since evidence of keratinization is usually lacking, these malignancies may be mistaken for adenocarcinoma or reactive mesothelial cells. Since squamous cell carcinomas from various primary sites resemble each other, it is difficult if not impossible to determine the primary site without knowledge of the primary tumor. Human Papillomavirus (HPV) testing, including p16 nuclear immunoreactivity, may be helpful in this regard, especially for those HPV-related squamous cell carcinomas that originate from the anogenital or tonsillar region. NUT (midline) carcinoma involving body fluids may show squamous differentiation in the form of sheets and single polygonal tumor cells.[37] Of note, malignant mesothelioma in pleural effusions has been reported to contain parakeratotic-like cells with orange cytoplasm and pyknotic nuclei that can mimic squamous cell carcinoma.[38]
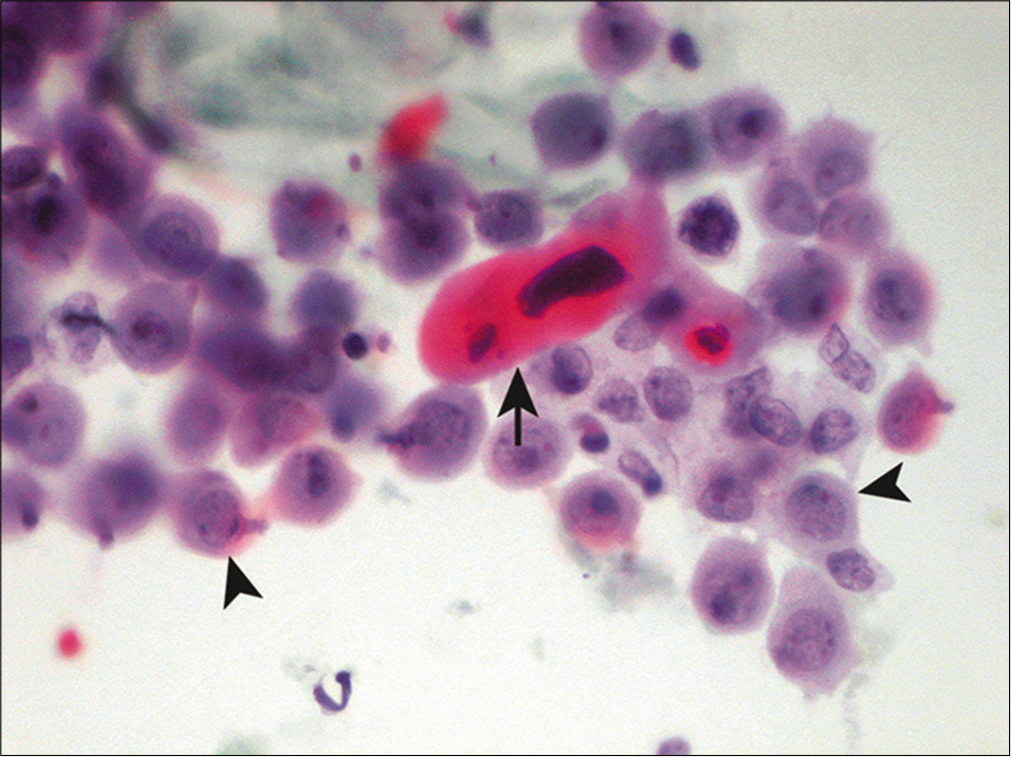
- Squamous cells in metastatic squamous cell carcinoma. A dyskeratotic squamous cell with a malignant-looking nucleus (arrow) is noted admixed with reactive mesothelial cells (arrowheads). (Cytospin preparation, PAP stain, 400X.)
Spindle cells
Spindle-shaped neoplastic cells are primarily associated with sarcomas [Figure 16]. Other features suggestive of metastatic sarcoma in fluid specimens include sparsely cellular specimens, loosely cohesive arrangement, binucleation and/or multinucleation, and irregular nuclear contours.[39] However, sarcomas rarely present in effusions without a history of a known primary tumor. In addition, sarcomatous spindle cells have a tendency to assume a more epithelioid configuration secondary to surface tension phenomenon when exfoliated in body cavity fluids.[39] Subclassification of sarcomas based on morphology is hence unreliable in fluids. Therefore, ancillary studies are often necessary to confirm the diagnosis and subtype these tumors when clinical data are not available. Other non-mesenchymal malignancies, such as spindle cell carcinoma, melanoma, and mesothelioma, can also demonstrate a spindle cell component in fluid specimens.
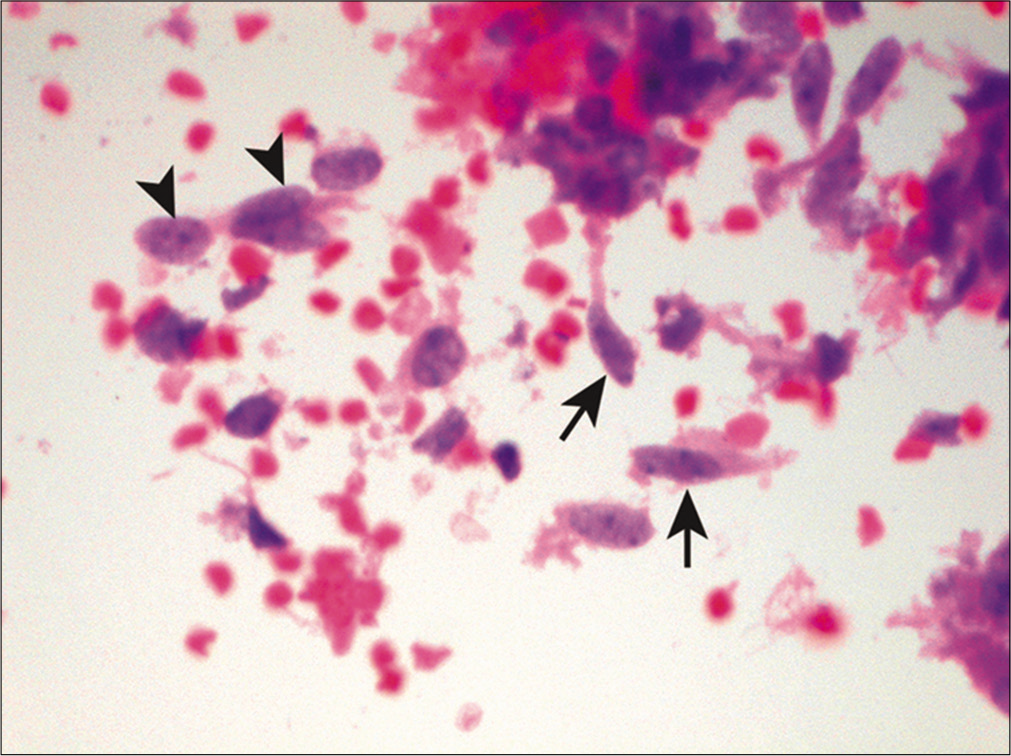
- Spindle cells in a case of metastatic high-grade undifferentiated sarcoma. This malignant effusion shows a mixture of atypical spindle (arrows) and epithelioid cells (arrowheads). (Cell-block preparation, HE stain, 400X.)
ANCILLARY STUDIES
Various ancillary studies are often used in evaluating fluid cytology. Such tests are extremely valuable in confirming malignancy, and are especially helpful in distinguishing between mesothelioma and metastatic adenocarcinoma.[40-41] They can also help identify the possible primary site of malignancy. Ancillary studies that have been applied to fluid specimens include cytochemistry, electron microscopy, immunocytochemistry, flow cytometry, fluorescence in situ hybridization (FISH) and molecular testing. Cell-blocks are not only complementary to smears in the detection of malignant effusions, but also provide an important opportunity for immunohistochemical testing and other ancillary studies.[42-44]
Cytochemistry is relatively inexpensive and easy to perform and can offer valuable information for tumor subclassification when used in the appropriate setting. For example, the presence of cytoplasmic melanin pigment in melanoma can be confirmed by the Fontana–Masson method, which relies upon melanin granules to reduce ammoniacal silver nitrate. In addition, demonstration of mucin production favors a diagnosis of adenocarcinoma.[45-47] Electron microscopy for investigation of effusions is rarely used today.
Immunocytochemistry is the most commonly employed ancillary technique in fluid cytology.[42,43] Running a panel of immunostains with SCIP (subtractive coordinate immunoreactivity pattern) approach[42] has proven to be very helpful in the differential diagnosis between mesothelioma and metastatic adenocarcinoma. Commonly used markers for adenocarcinoma include MOC-31, claudin-4, CEA, B72.3 and Ber-EP4.[48] They often demonstrate immunoreactivity with a wide variety of adenocarcinomas; therefore, they are useful in separating adenocarcinomas from mesothelioma.[49-51] Claudin-4 has been shown to perform superiorly compared to BerEP4 and B72.3 (out of two, BerEP4 is more sensitive as compared to B72.3) in distinguishing adenocarcinoma from mesothelial cells in pleural effusions.[52] However, none of these markers are sufficiently specific to classify malignant effusions according to the tumor origin.[53] Therefore, more specific markers are often necessary to further characterize malignant effusions [Table 2].
| Primary tumor | Positive immunomarkers |
|---|---|
| Prostate adenocarcinoma | Androgen receptor, NKX3.1, PSA, PSMA, PSAP |
| Lung adenocarcinoma | TTF-1, Napsin-A, surfactant protein A |
| Small cell carcinoma | Synaptophysin, chromogranin, CD56, TTF-1, INSM1, POUF2F3 |
| Breast carcinoma | Mammaglobin, GATA-3, GCDFP-15, TRPS1, ER |
| Papillary thyroid carcinoma | PAX-8, TTF-1, thyroglobulin |
| Ovarian carcinoma | PAX-8, CA-125, WT1, ER |
| Hepatocellular carcinoma | CAM5.2, HepPar-1, arginase-1, TTF-1 (cytoplasmic), AFP, pCEA |
| Renal cell carcinoma | PAX-8, CA-9, RCC, CD10 |
| Colorectal adenocarcinoma | CK20, CDX2, SATB-2 |
| Melanoma | S-100, SOX-10, Melan-A/Mart-1, HMB-45, tyrosinase, MITF, PRAME |
| Non-Hodgkin lymphoma | LCA (CD45), B-cell markers, T-cell markers |
| Mesothelioma | Calretinin, D2-40, WT1, CK5/6, GLUT-1, EMA, GATA3, IMP3, mesothelin |
AFP = alpha fetoprotein, CA-9 = carbonic anhydrase 9, pCEA = polyclonal carcinoembryonic antigen, EMA = epithelial membrane antigen, ER = estrogen receptor, GATA-3 = GATA binding protein 3, GCDFP-15 = gross cystic disease fluid protein 15, GLUT-1 = glucose transporter 1, IMP3 = insulin-like growth factor II mRNA binding protein 3, INSM1 = insulinoma-associated protein 1, PAX-8 = paired box gene 8, POUF2F3 = POU domain, class 2, transcription factor 3, PSA = prostate specific antigen, PSAP = prostate specific acidic phosphatase, PSMA = prostate specific membrane antigen, RCC= renal cell carcinoma antigen, SOX-10 = SRY-Box transcription factor 10, TRPS1 = transcriptional repressor GATA binding 1, TTF-1 = thyroid transcription factor-1, WT1 = Wilms tumor gene product 1
Owing to the restricted expression of certain cytokeratins (CKs): namely CK7 and CK20 [Figure 17] in various epithelia and their malignant counterparts, coordinate immunoreactivity pattern for CK7 and 20 is often used together as an aid in identifying broad categories of primary site of origin of metastatic carcinomas.[54-58] The combination of CK7 and CK20 results in four immunoprofiles: CK7+/CK20−, CK7−/CK20+, CK7+/CK20+, and CK7−/CK20 − carcinomas. Table 3 lists the most likely carcinomas that are associated with each of these CK7/CK20 immunophenotypes. Finally, it is important to keep in mind that no biomarker is absolutely specific for a particular tumor, and that any given tumor may occasionally exhibit aberrant immunoreactivity to certain immunomarker.
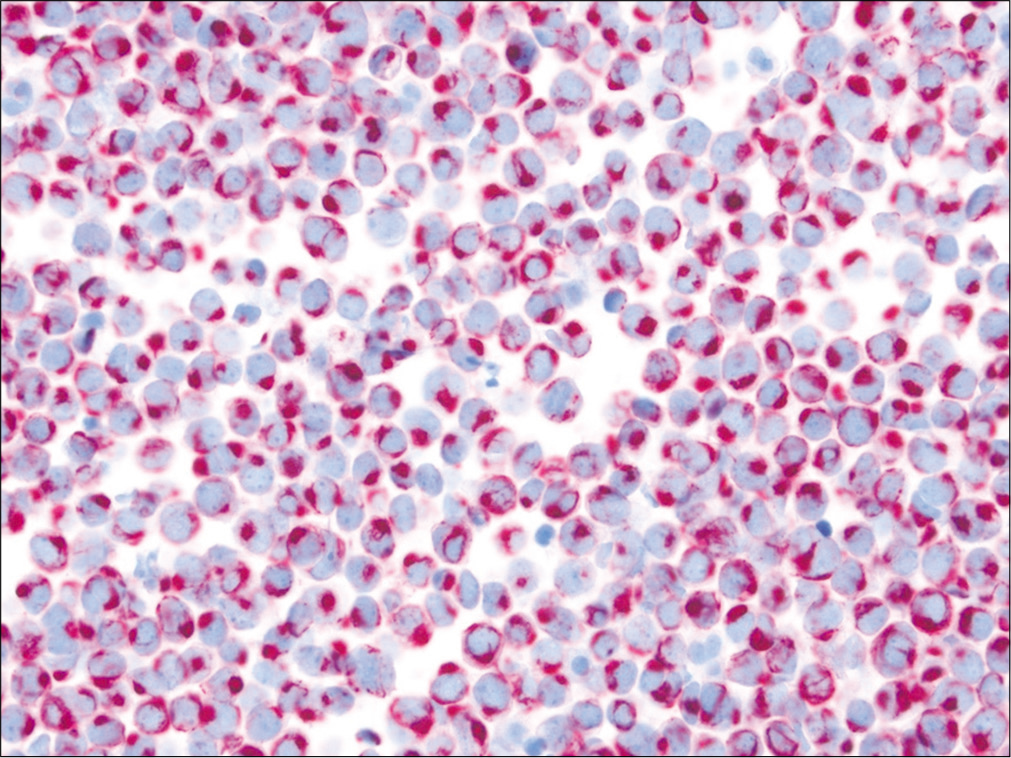
- Merkel cell carcinoma involving ascites. CK20 shows characteristic perinuclear dot-like immunoreactivity in these small tumor cells. (Cell-block preparation, IHC stain, 400X.)
| Immunophenotypic expression | Differential diagnosis |
|---|---|
| CK7+/ CK20− | Lung, breast, müllerian (endometrial), thyroid, bladder, upper gastrointestinal, ovarian (non-mucinous), & pancreatobiliary carcinoma; mesothelioma; thymoma |
| CK7−/ CK20+ | Colorectal, Merkel cell, & upper gastrointestinal carcinoma |
| CK7+/ CK20+ | Urothelial, upper gastrointestinal, pancreatobiliary, ovarian (mucinous), rectal, & occasional lung (mucinous) carcinoma |
| CK7−/ CK20− | Hepatocellular, renal, prostate, adrenal cortical, squamous cell, & neuroendocrine carcinoma; germ cell tumor |
SUMMARY
In a small number of patients presenting with a malignant effusion, a clinical history of a prior malignancy may be lacking and the effusion is thus the initial presentation. The search for a primary site of origin for the patient’s tumor can be a diagnostic challenge. Both clinical history, radiology imaging findings and cytomorphology can provide important clues regarding the possible primary site of origin. In addition, the judicious use of ancillary studies, particularly immunocytochemistry, can help not only to confirm the diagnosis of metastasis, but also help further narrow down the differential diagnosis of primary site.[42,43] It is important to keep in mind that, on rare occasions, the primary site of origin of a malignant effusion may remain unresolved despite an extensive work-up. For such tumors of unknown origin, identification of the occult primary site of origin may have little impact on the therapeutic choices and the patient’s outcome.
STUDY CASES
Case 1 [Figures 18-22]
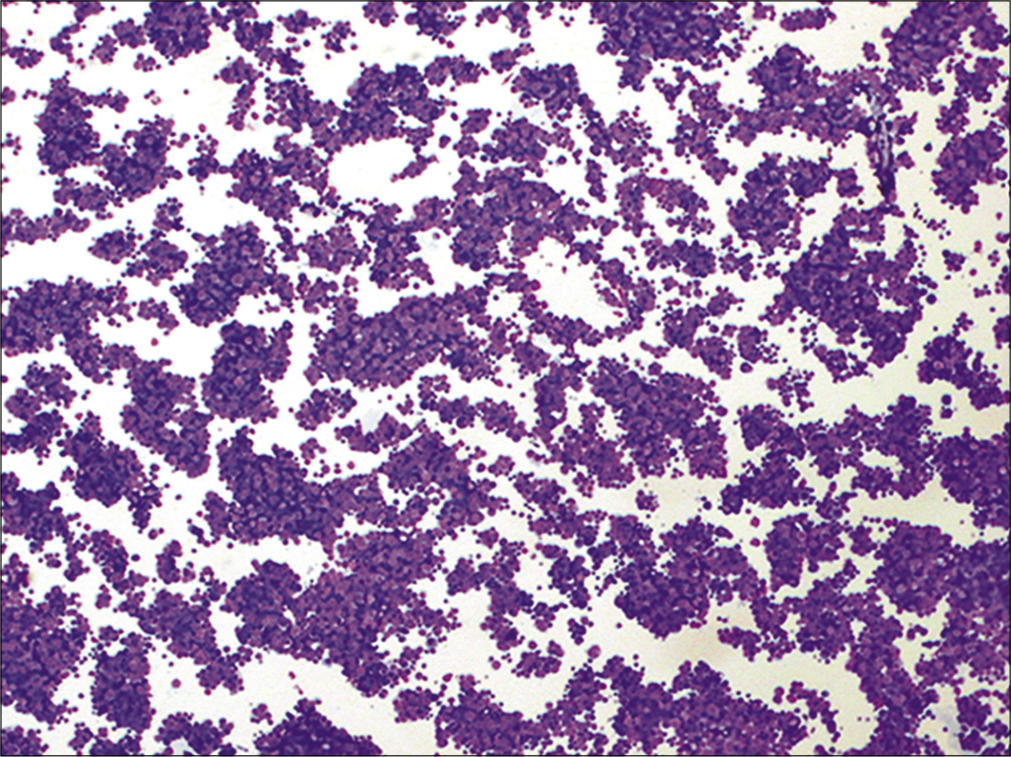
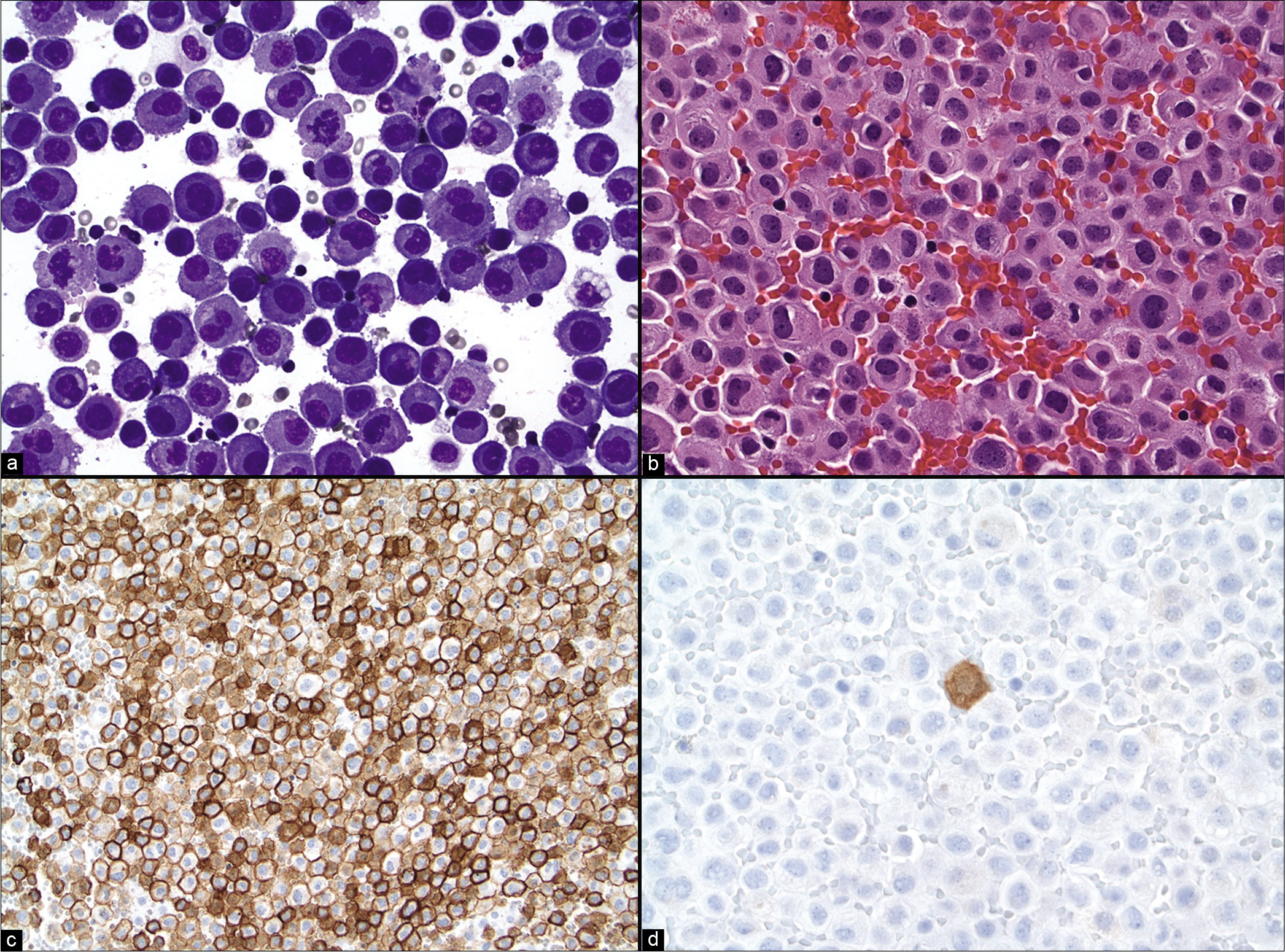
- Metastatic small cell carcinoma. This hypercellular specimen contains numerous cohesive groups of neoplastic cells. (Cytospin preparation, modified Romanowsky stain, 100X.
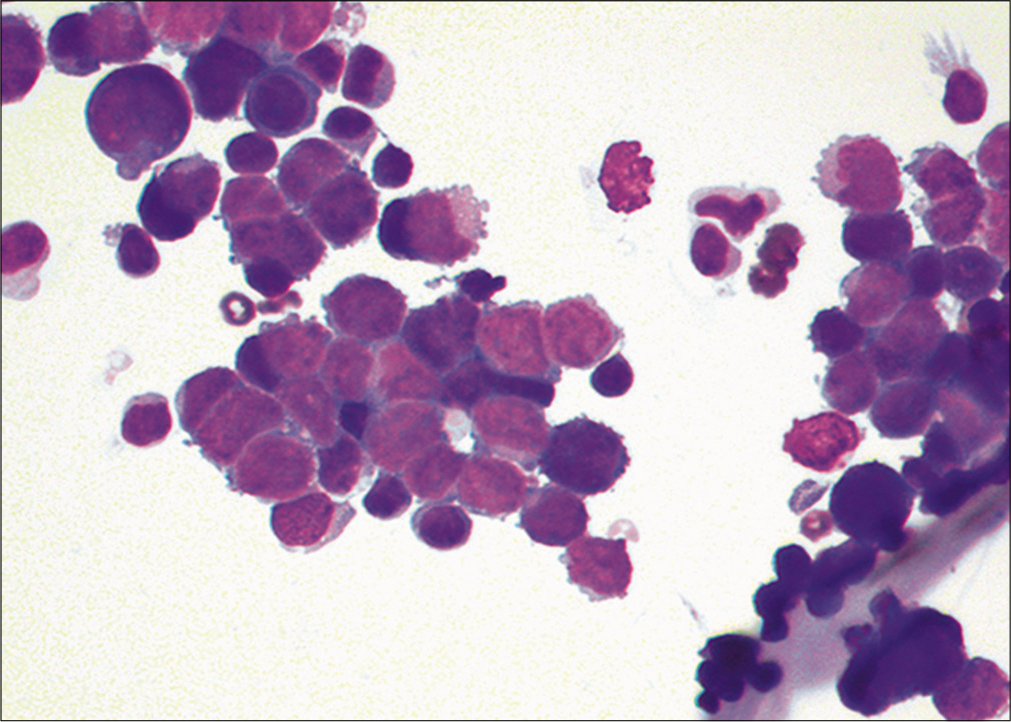
- Metastatic small cell carcinoma. Cohesive groups of neoplastic cells are shown with scant cytoplasm and high nuclear/ cytoplasmic ratios. (Cytospin preparation, modified Romanowsky stain, 400X.)
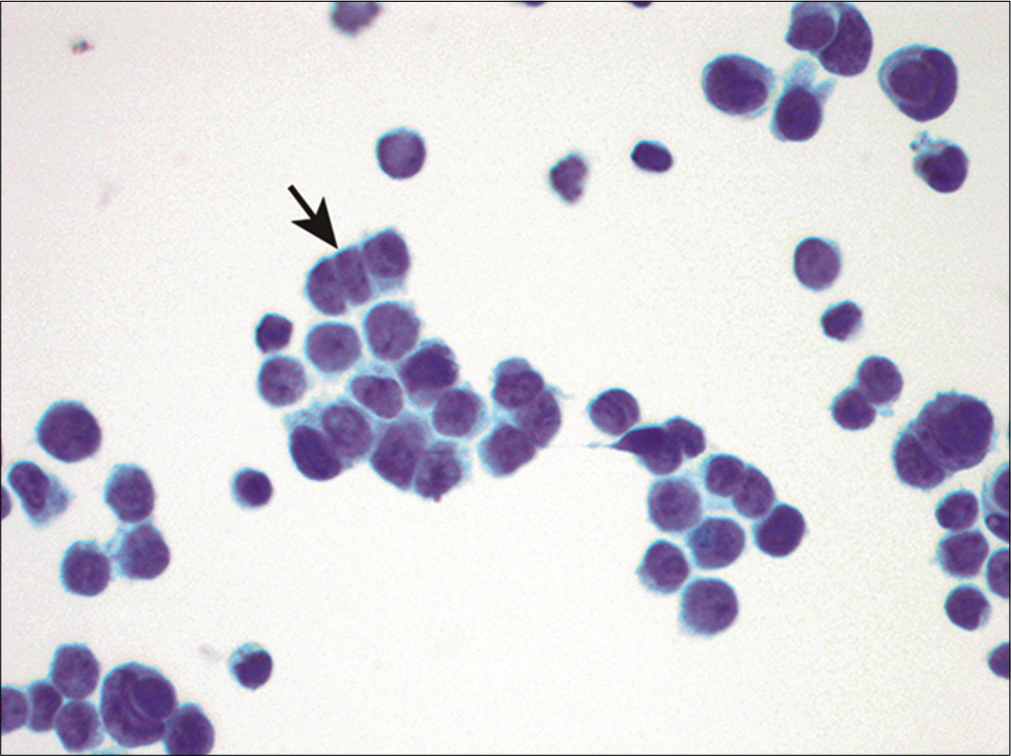
- Metastatic small cell carcinoma. Individual cells shave ‘salt-and-pepper’ chromatin without conspicuous nucleoli. Nuclear molding (arrow) is also apparent. (Cytospin preparation, Papanicolaou stain, 400X.)
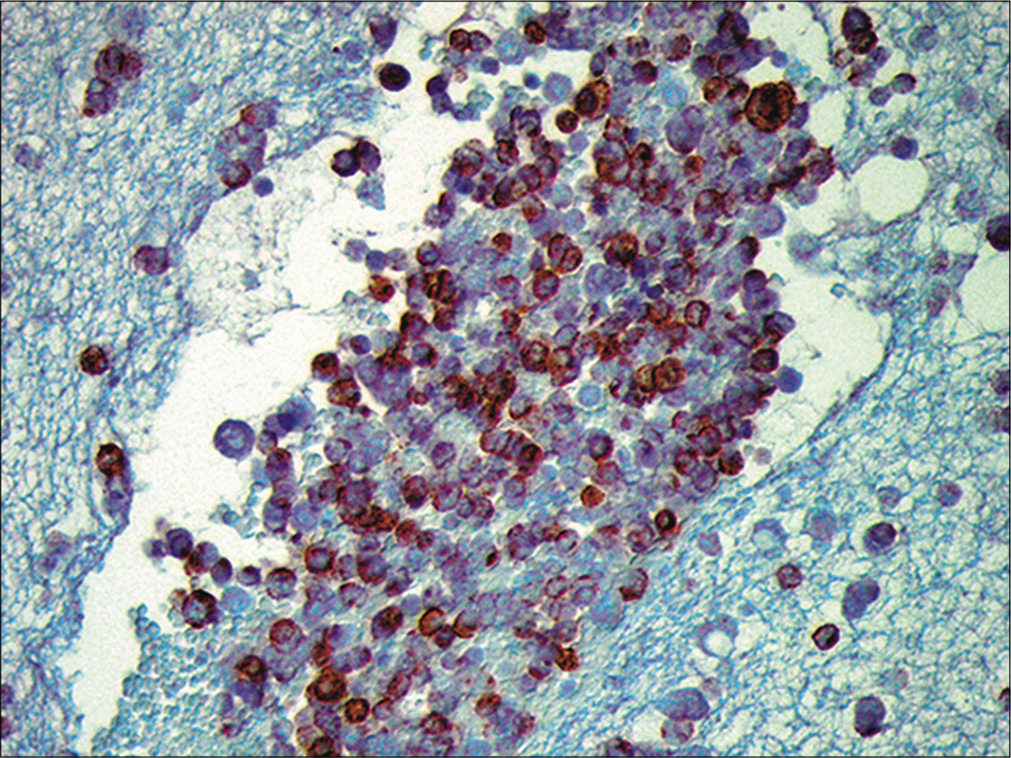
- Metastatic small cell carcinoma. The neoplastic cells are immunoreactive for chromogranin. (Cell-block preparation, chromogranin, 100X.)
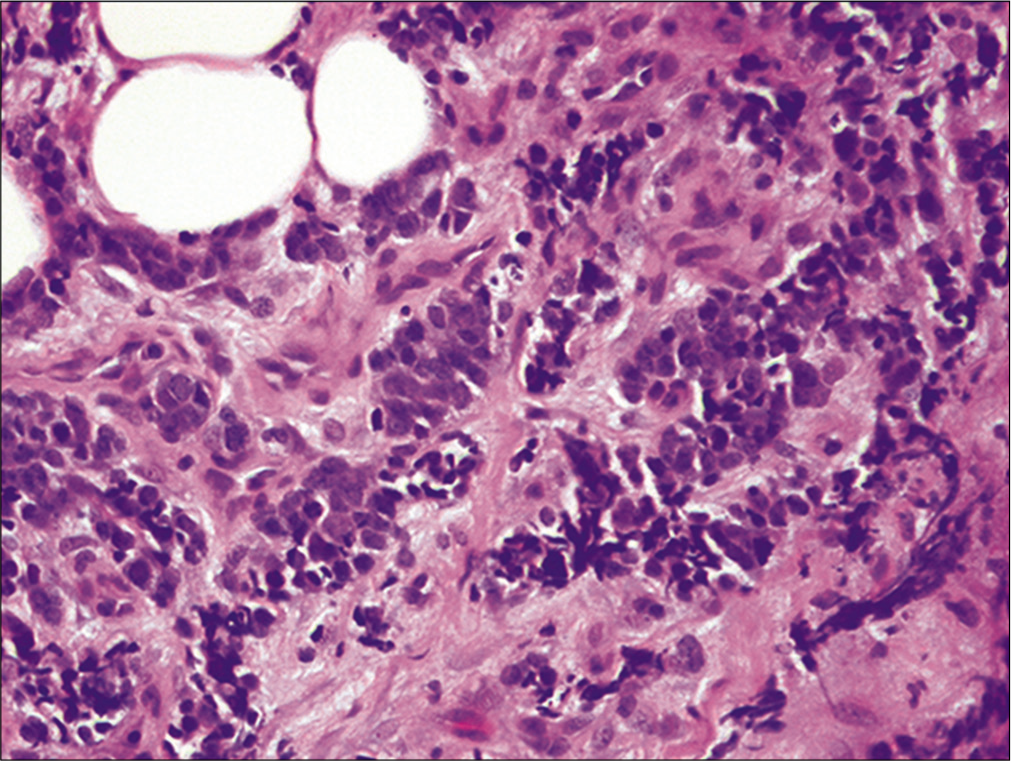
- Metastatic small cell carcinoma. Pleural biopsy showing infiltration of fibroadipose tissue by islands of neoplastic cells with high nuclear/cytoplasmic ratio and nuclear molding (the nuclear details are better discernible in cytology preparations: Figure 19 and 20) (Pleural biopsy, HE, 100X.)
History
A 55-year-old African–American woman presented to the emergency room with fever, chills, and cough. She had been a chronic smoker for over 30 years. Chest X-ray showed a large right pleural effusion and hilar lymphadenopathy. Thoracocentesis produced 1200 mL of straw-colored fluid and was sent for cytologic evaluation. Figures 18-21 show representative images of the malignant effusion.
Diagnosis
Metastatic small cell lung carcinoma
Discussion
The fluid was cellular and demonstrated tight cohesive groups and isolated cells with scant cytoplasm and high nuclear/cytoplasmic ratios. Individual cells were about 2–4 times the size of mature background lymphocytes. Nuclear molding was also apparent. The nuclei demonstrated ‘salt and pepper’ chromatin without distinct nucleoli. To confirm the diagnosis, immunohistochemistry was performed on a cell-block preparation. The atypical cells were immunoreactive for synaptophysin, chromogranin, and TTF-1 but were non-immunoreactive for calretinin and CK 5/6. These findings supported the diagnosis of small cell carcinoma, most likely of pulmonary origin.
Follow up
This patient also underwent a pleural biopsy at the time of thoracocentesis, which demonstrated a metastatic small cell carcinoma [Figure 22]. Further staging work up revealed multiple liver metastases. This patient was treated with chemotherapy.
Case 2 [Figures 23–25]
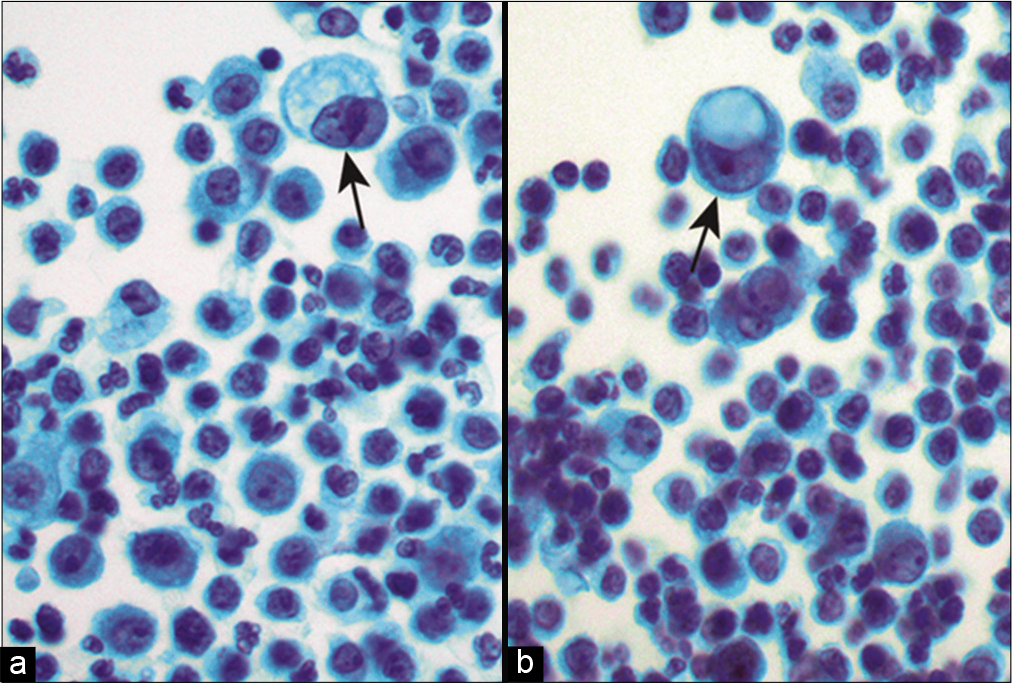
- a,b Metastatic gastric adenocarcinoma. Isolated atypical cells are shown (arrows) admixed with inflammatory cells. Large cytoplasmic vacuoles resulting in a signet ring cell appearance are noted. (Cytospin preparation, Papanicolaou stain, 400X)
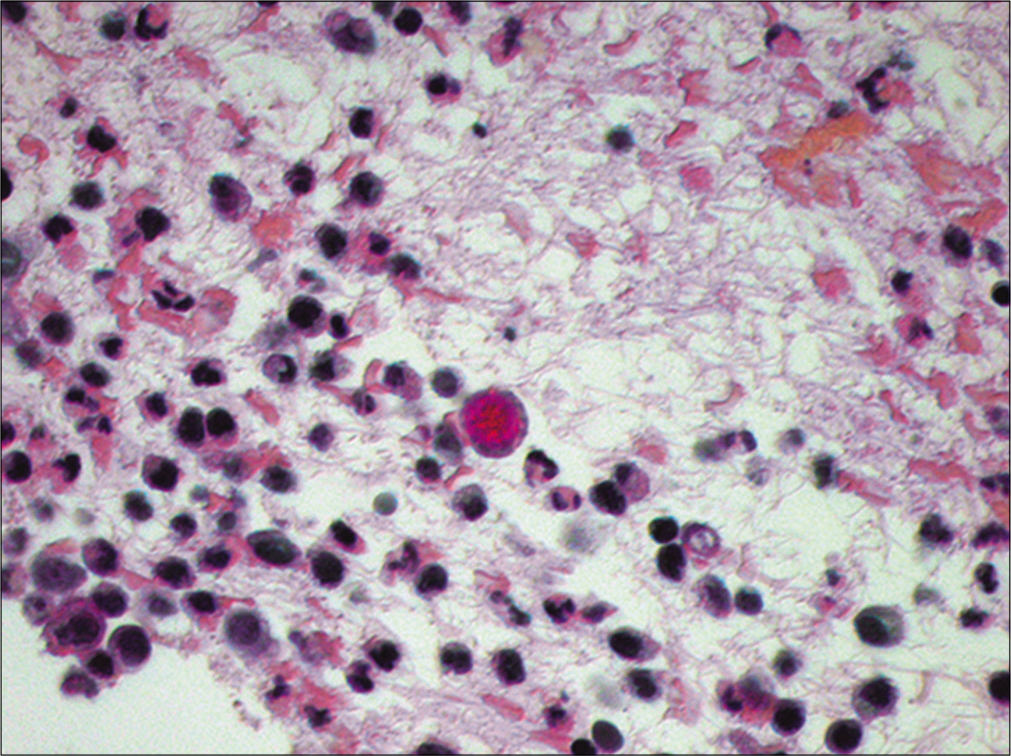
- Metastatic gastric adenocarcinoma. A central tumor cell is shown stained red (positive) for intracytoplasmic mucin (Cell-block preparation, mucicarmine stain, 400X)
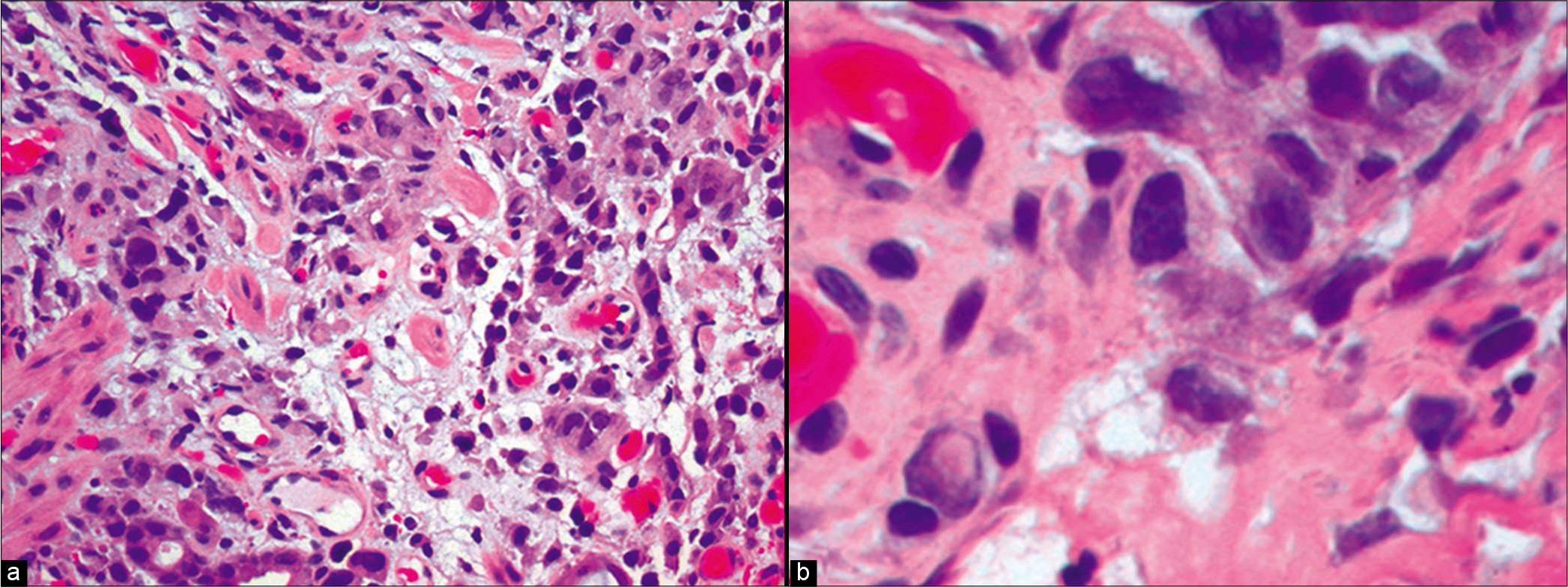
- Metastatic gastric adenocarcinoma. a, Gastric biopsy showing infiltration by a poorly differentiated adenocarcinoma. b, Pleomorphic malignant cells are shown at higher power, some with a signet ring cell appearance. (Gastric biopsy, a, HE, 100×; b, 400X)
History
A 50-year-old male with a history of heavy alcohol abuse and alcohol-induced gastritis with abdominal pain for 3 days, presented to the emergency room. Physical examination revealed a distended and slightly tender abdomen. A CT scan of his abdomen and pelvis showed a large amount of ascites and multiple peritoneal nodules, as well as thickening of the large and small bowel wall. A paracentesis was performed. Figures 23-24 show representative images of his ascitic fluid.
Diagnosis
Metastatic adenocarcinoma with signet-ring cell features. The likely primary site of this patient’s tumor is the upper gastrointestinal tract.
Discussion
The specimen showed numerous isolated atypical cells admixed with inflammatory cells. The atypical cells were large with an increased nuclear/cytoplasmic ratio. The nuclei displayed prominent nucleoli. There were also cells with large cytoplasmic vacuoles displacing the nuclei to the periphery, resulting in a signet-ring cell appearance. These atypical cells were positive for mucicarmine. Immunocytochemistry was performed showing that these tumor cells were immunoreactive for MOC-31 and CK20 with nuclear CDX2 but were non-immunoreactive for nuclear calretinin. The immunostaining pattern was supportive of a metastatic adenocarcinoma. Because of the presence of the signet-ring cells in a male patient, an upper gastrointestinal tract primary was favored.
Follow up
The patient underwent an upper endoscopic examination, which revealed a gastric mucosal irregularity. A gastric biopsy showed a poorly differentiated adenocarcinoma with signet ring cell features [Figure 25]. The patient was discharged to hospice care.
Case 3 [Figures 26–30]
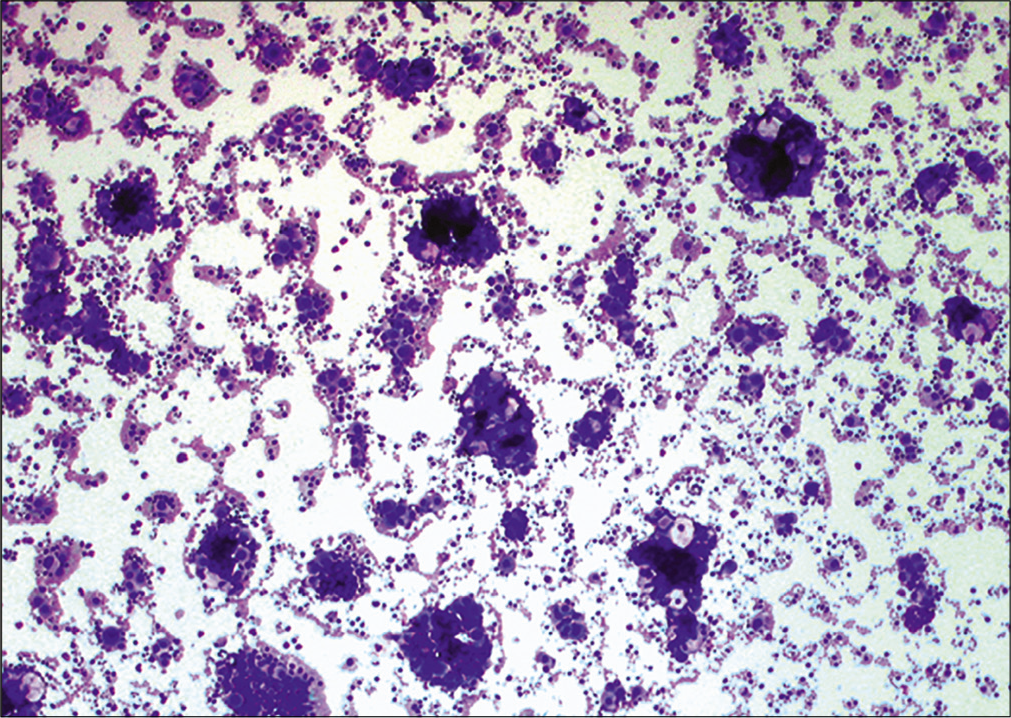
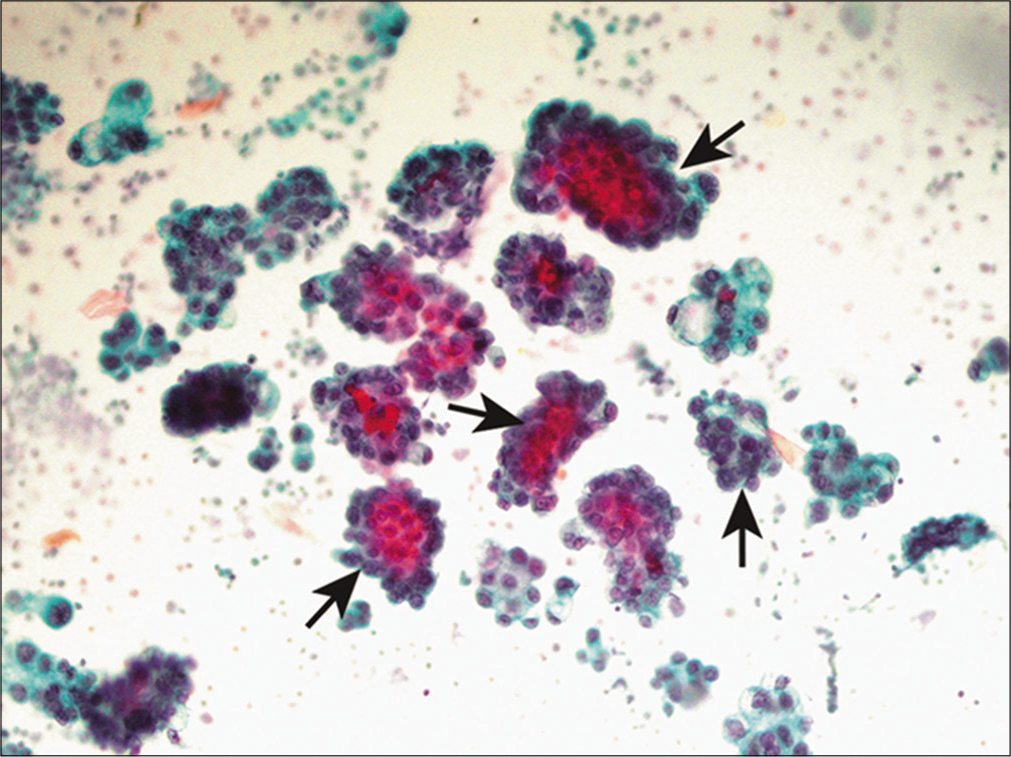
- Metastatic ovarian carcinoma. Low power showing numerous 3-dimensional cell groups, resulting in a ‘cannonball’ appearance. (Cytospin preparation, modified Romanowsky stain, 100X)
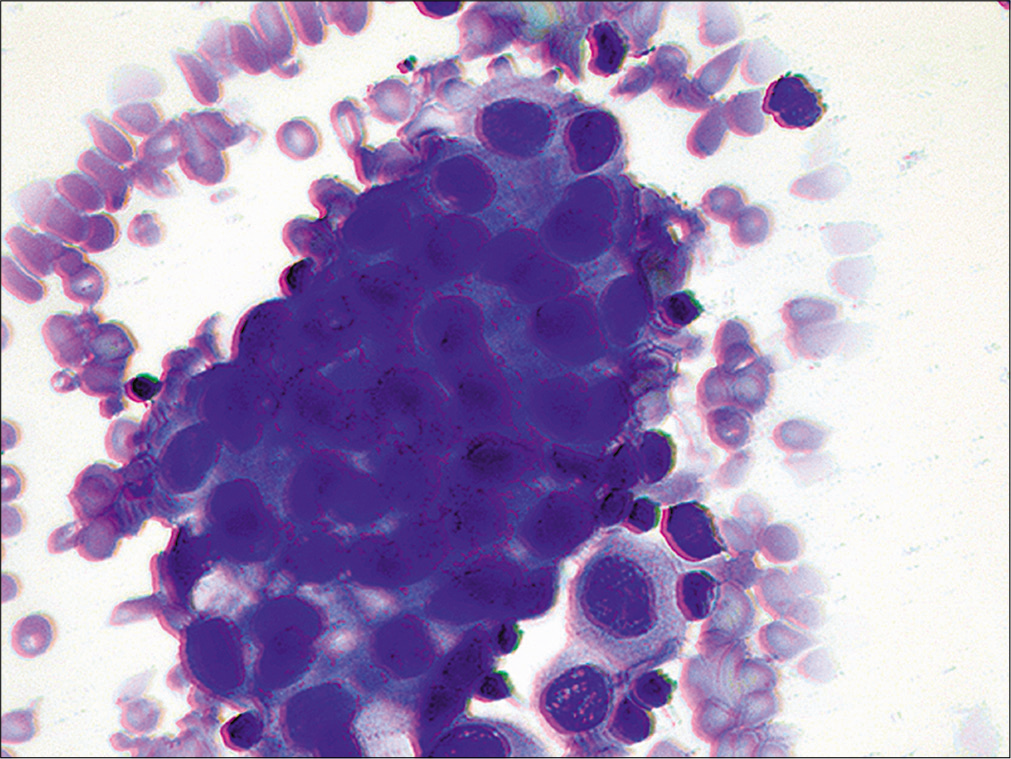
- Metastatic ovarian carcinoma. A cohesive, 3-dimensional group of cells with large nuclei and increased nuclear/cytoplasmic ratio. (Cytospin preparation, modified Romanowsky stain, 400X)
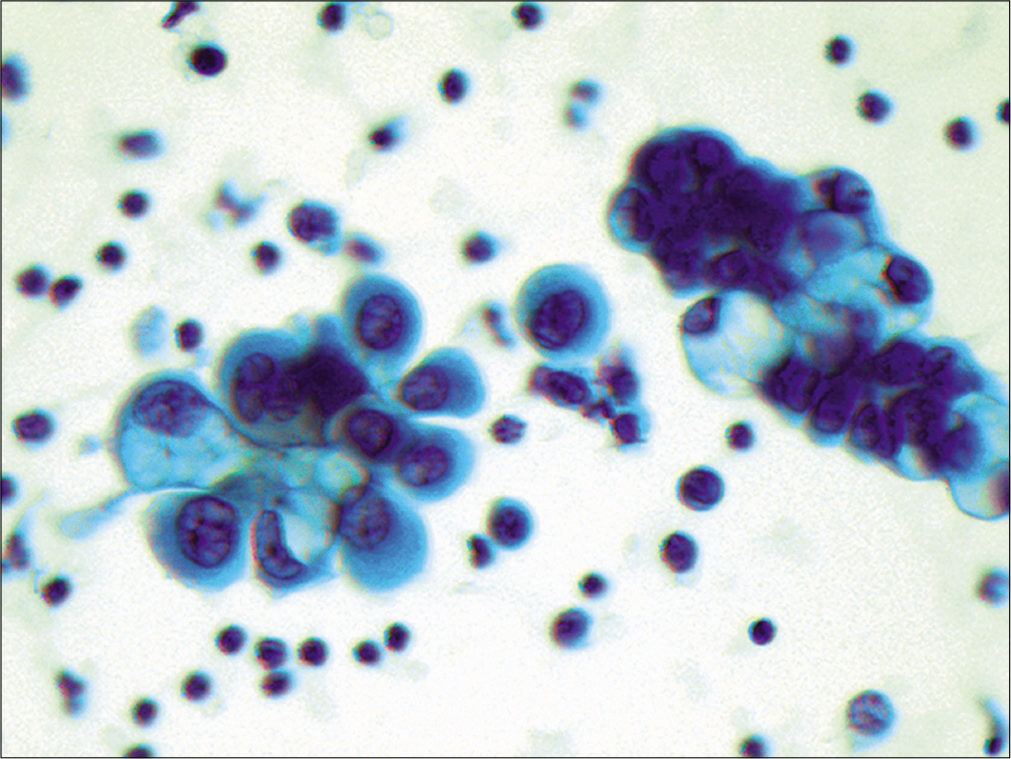
- Metastatic ovarian carcinoma. Papillary groups of tumor cells with large neoplastic cells. Some cells have vacuolated cytoplasm, which is characteristic of ovarian carcinoma present in body fluids. (Cytospin preparation, Papanicolaou stain, 400X)
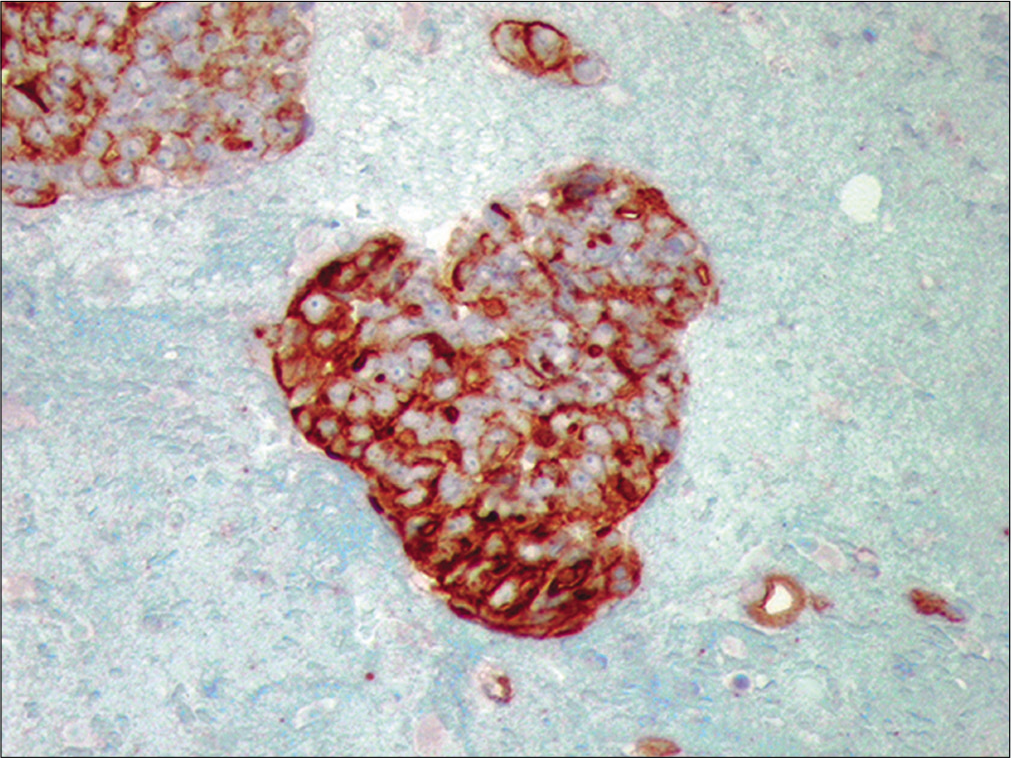
- Metastatic ovarian carcinoma. The neoplastic cells are positive for CA-125, supporting their ovarian origin (Cell-block preparation, Ca125, 100X)
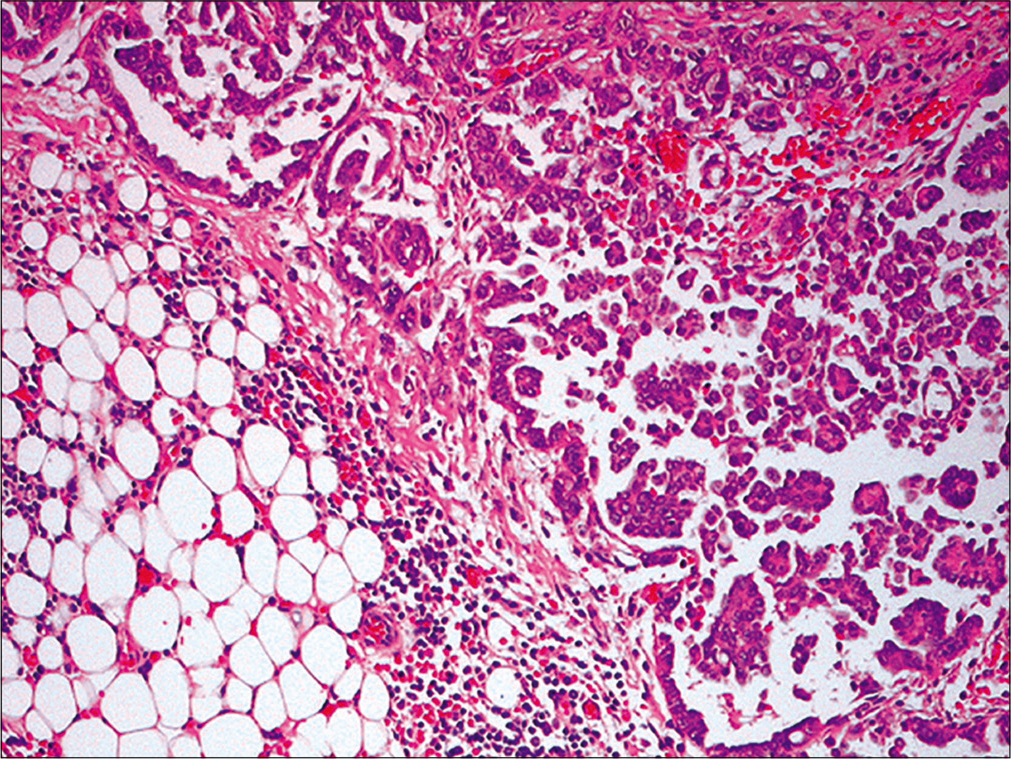
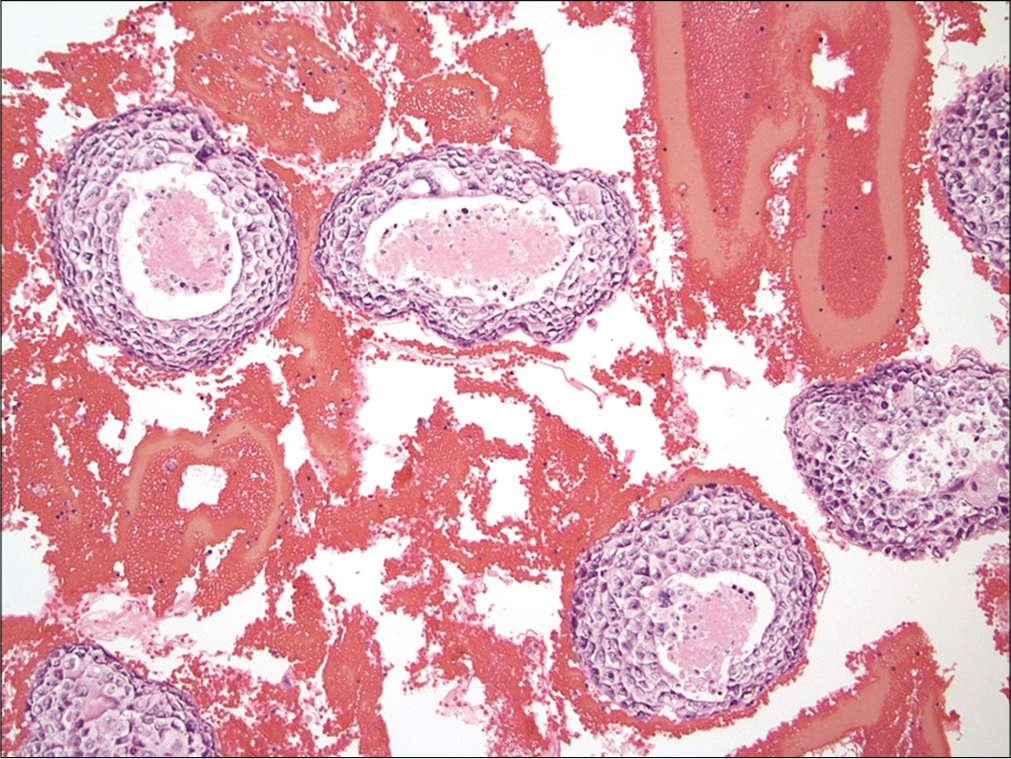
- Metastatic ovarian carcinoma. Section from omentum reveals metastatic ovarian papillary serous carcinoma (Omentectomy, HE, 100X)
History
A 52-year-old female presented to the emergency room with shortness of breath and increasing abdominal distention for 3 months. Ultrasound examination of the pelvic cavity demonstrated a large intraabdominal fluid collection and a right complex pelvic mass. A paracentesis was performed and 1500 mL of serosanguineous fluid was obtained. Figures 26-29 show representative images of the patient’s effusion.
Diagnosis
Metastatic carcinoma, most likely of ovarian origin.
Discussion
At low power, the cytologic findings consisted of multiple three-dimensional, tightly cohesive cell groups, giving rise to a cannonball appearance. Papillary groups were also noted. High magnification revealed large atypical cells with increased nuclear/cytoplasmic ratios and prominent nucleoli. Vacuolated cells were common at the periphery. Immunocytochemistry performed on cell-block material demonstrated positive immunostaining of the tumor cells with MOC-31 and CA-125 but negative immunoreactivity with calretinin and CK5/6. This staining pattern supported an ovarian primary tumor. Recently nuclear immunoreactivity for PAX-8 has shown to be promising for Mullerian primary including ovary [Table 2].
Follow up
The patient underwent a total hysterectomy and bilateral salpingo-oophorectomy with tumor debulking. The histology revealed a papillary serous carcinoma [Figure 30] involving the right ovary and fallopian tube, serosal surface of the uterus and urinary bladder with infiltration into the omentum.
Acknowledgment
Author of this review and editors of CMAS #2 series thank the initial authors (David C Chhieng, MD, PhD; Nirag C Jhala, MD) for all their efforts for the first edition material on which the current review (as chapter #11 in the final CMAS #2) is based.
The author thank Janavi Kolpekwar for copy-editing support.
ABBREVIATIONS (IN ALPHABETIC ORDER)
PEL - primary effusion lymphoma
SRBCTs - Small round blue cell tumors
HIV - Human immunodeficiency virus
SLL/CLL - Small lymphocytic lymphoma/chronic lymphocytic leukemia
HPV - Human Papillomavirus
FISH - Fluorescence in situ hybridization
References
- Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J. 1989;2:366-9.
- [Google Scholar]
- Diagnosis and management of malignant pleural effusions. Am J Surg. 1995;170:69-74.
- [CrossRef] [Google Scholar]
- The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56:905-9.
- [CrossRef] [Google Scholar]
- Positive effusion cytology as the initial presentation of malignancy. Acta Cytol. 1987;31:448-52.
- [Google Scholar]
- Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 1990;39:1990-2005.
- [CrossRef] [Google Scholar]
- Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364-7.
- [CrossRef] [Google Scholar]
- Malignancies in Pleural, Peritoneal, and Pericardial Effusions. Arch Pathol Lab Med. 2020;144:1086-91.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and categorization of malignant effusions: A 6-year review from a single academic institution. Diagn Cytopathol. 2021;49:615-21.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology of fluids from pleural, peritoneal and pericardial cavities in children. A comprehensive survey. Acta Cytol. 1994;38:209-17.
- [Google Scholar]
- Exfoliative cytology of nonlymphoreticular neoplasms in children. Acta Cytol. 1984;28:16-28.
- [Google Scholar]
- Burkitt's lymphoma: a pericardial presentation. Cytopathology. 1990;1:239-42.
- [CrossRef] [PubMed] [Google Scholar]
- Primary effusion lymphoma: cytopathologic diagnosis using in situ molecular genetic analysis for human herpesvirus 8. Mod Pathol. 2002;15:944-50.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant melanoma in effusions: a source of false negative cytodiagnoses. Diagn Cytopathol. 1986;2:150-3.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant Effusions: A Multimodal Approach to Cytologic Diagnosis. >New York: Igaku-Shoin; 1994.
- [CrossRef] [Google Scholar]
- Peritoneal washings in ovarian tumors. Potential sources of error in cytologic diagnosis. Acta Cytol. 1985;29:310-6.
- [Google Scholar]
- Cytologic findings of psammocarcinoma in peritoneal washings. Acta Cytol. 2009;53:263-7.
- [CrossRef] [PubMed] [Google Scholar]
- Benign papillary structures with psammoma bodies in culdocentesis fluid. Acta Cytol. 1969;13:178-80.
- [Google Scholar]
- Mesothelial papilloma: a case of mistaken identity in a pericardial effusion. Acta Cytol. 1976;20:266-8.
- [Google Scholar]
- Significance of psammoma bodies in serous cavity fluid: a cytopathologic analysis. Cancer. 2004;102:87-91.
- [CrossRef] [PubMed] [Google Scholar]
- The unique cytologic picture of oat cell carcinoma in effusions. Acta Cytol. 1976;20:298-302.
- [Google Scholar]
- Pseudomyxoma peritonei-nonoperative management and biochemical findings. A case report. Cancer. 1975;36:1834-7.
- [CrossRef] [Google Scholar]
- Pseudomyxoma peritonei and malignant mucocele of the appendix. A case report. Acta Cytol. 1986;30:169-72.
- [Google Scholar]
- Pleural fluid cytology in lymphoplasmacytoid lymphoma with numerous intracytoplasmic immunoglobulin inclusions. A case report with immunocytochemistry. Acta Cytol. 1984;28:419-24.
- [Google Scholar]
- The cytomorphologic spectrum of small-cell carcinoma and large-cell neuroendocrine carcinoma in body cavity effusions: A study of 68 cases. Cytojournal. 2011;8:18.
- [CrossRef] [PubMed] [Google Scholar]
- Intracellular mucous inclusions. A feature of malignant cells in effusions in the serous cavities, particularly due to carcinoma of the breast. J Clin Pathol. 1975;28:929-36.
- [CrossRef] [PubMed] [Google Scholar]
- Megakaryocytes in pleural and peritoneal fluids: prevalence, significance, morphology, and cytohistological correlation. J Clin Pathol. 1980;33:1153-9.
- [CrossRef] [PubMed] [Google Scholar]
- The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol. 1987;31:85-97.
- [Google Scholar]
- Metastatic squamous-cell carcinoma in pericardial effusion: report of four cases, two with cardiac tamponade. Diagn Cytopathol. 1998;18:422-4.
- [CrossRef] [Google Scholar]
- Anthracotic pigment in pleural fluid: a case report. Acta Cytol. 2009;53:306-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effusion cytomorphology of small round cell tumors. J Cytol. 2016;33:85-92.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic findings in an effusion caused by rupture of a benign cystic teratoma of the mediastinum into a serous cavity. Acta Cytol. 1015;29:1015-20.
- [Google Scholar]
- Cytomorphology of primary pulmonary NUT carcinoma in different cytology preparations. Cancer Cytopathol. 2021;129:53-61.
- [CrossRef] [PubMed] [Google Scholar]
- Parakeratotic-like cells in effusions-A clue to diagnosis of malignant mesothelioma. Cytojournal. 2012;9:18.
- [CrossRef] [PubMed] [Google Scholar]
- Ancillary studies in pleural, pericardial, and peritoneal effusion cytology. Cancer Cytopathol. 2018;126(Suppl 8):590-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of cell blocks from effusion specimens in Gynecologic Oncopathology: An experience of 220 cases, diagnosed at a Tertiary Cancer Referral Center. Indian J Pathol Microbiol. 2020;63:427-34.
- [CrossRef] [PubMed] [Google Scholar]
- Immunocytochemistry of effusions: Processing and commonly used immunomarkers. CytoJournal. 2022;19:6.
- [CrossRef] [Google Scholar]
- Immunocytochemistry of effusion fluids: Introduction to SCIP approach. CytoJournal. 2022;19:3.
- [CrossRef] [Google Scholar]
- Cell-blocks and other ancillary studies (including molecular pathology and proteomics) CytoJournal. 2021;18:4.
- [CrossRef] [PubMed] [Google Scholar]
- The distinction of adenocarcinoma from malignant mesothelioma in cell blocks of effusions: the role of routine mucin histochemistry and immunohistochemical assessment of carcinoembryonic antigen, keratin proteins, epithelial membrane antigen, and milk fat globule-derived antigen. Hum Pathol. 1987;18:67-74.
- [CrossRef] [Google Scholar]
- Histochemistry in the diagnosis of malignant mesothelioma. Ann Clin Lab Sci. 1973;3:207-11.
- [Google Scholar]
- The results of some cytochemical reactions in metastatic malignant tumor cells in pleural and peritoneal effusions. Acta Cytol. 1977;21:141-6.
- [Google Scholar]
- MOC31 Immunostaining in the Diagnosis of Metastatic Adenocarcinoma in Serous Fluid: Special Emphasis on Atypical Cytological Cases. Acta Cytol. 2021;65:242-9.
- [CrossRef] [PubMed] [Google Scholar]
- Use of a panel of markers in the differential diagnosis of adenocarcinoma and reactive mesothelial cells in fluid cytology. Am J Clin Pathol. 2001;116:709-15.
- [CrossRef] [PubMed] [Google Scholar]
- Immunocytochemical panel for the identification of malignant cells in serous effusions. Am J Clin Pathol. 1991;95:867-74.
- [CrossRef] [PubMed] [Google Scholar]
- Markers for metastatic adenocarcinoma in serous effusion specimens. Diagn Cytopathol. 1994;11:237-45.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of Claudin-4 versus BerEP4 and B72.3 in pleural fluids with metastatic lung adenocarcinoma. J Am Soc Cytopathol. 2020;9:146-51.
- [CrossRef] [PubMed] [Google Scholar]
- Ber-EP4 staining in effusion cytology: A potential source of false positives. Rev Esp Patol. 2021;54:114-22.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of cytokeratin 20 in identifying the origin of metastatic carcinomas in effusions. Diagn Cytopathol. 1995;12:303-8.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of cytokeratin 7 and 20 subset analysis as an aid in the identification of primary site of origin of malignancy in cytologic specimens. Diagn Cytopathol. 1999;20:63-6.
- [CrossRef] [Google Scholar]
- Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962-72.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokeratins 20 and 7 in the differential diagnosis of metastatic carcinoma in cytologic specimens. Diagn Cytopathol. 1997;16:132-6.
- [CrossRef] [Google Scholar]
- Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunohistochem. 1995;3:99-107.
- [Google Scholar]








