Translate this page into:
Stromal tissue as an adjunct tool in the diagnosis of follicular thyroid lesions by fine-needle aspiration biopsy
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The stroma in fine-needle aspiration biopsy (FNAB) of thyroid lesions has not been well investigated.
Design:
We studied 256 consecutive cases of thyroid FNAB prepared with traditional smear technique. The stroma was categorized: Type 1a consisted of long (more than 3 mm), broad bands composed of mesh containing collagen fibrils thickened by entrapped blood components and follicular cells. Type 1b consisted of dense strands/bands. Type 2 was similar to Type 1a but with shorter (<2 mm) and looser stromal strands.
Results:
Types 1a and b showed straight/curved/circular branching patterns suggestive of incomplete frameworks of nodular/papillary architectures or fragments of capsule. Type 1b stroma likely represented thick/collagenized fibrous septae. Incomplete or complete rings of small encapsulated tumor were occasionally identified. These frameworks of stroma were frequently associated with multinodular goiters (MNGs) which are often hypocellular and follicular neoplasms/papillary thyroid carcinoma with increased cellularity. Type 2 was associated with microfollicles in encapsulated neoplasms or with macrofollicles in MNG. Follicular lesions of unknown significance (n = 41) either negative (n = 26) or positive (n = 15) for carcinoma in subsequent follow-up were frequently associated with stroma characteristic of MNG and carcinoma, respectively.
Conclusion:
The preservation of the in vivo architecture of Type 1 is likely due to its elasticity. Recognition of the stromal architecture will likely facilitate the diagnosis.
Keywords
Biopsy
follicular
stroma
thyroid
INTRODUCTION
Fine-needle aspiration biopsy (FNAB) along with ultrasound examination of the thyroid is a cost-effective tool for the early diagnosis of thyroid carcinoma.[1] Computerized tomography, magnetic resonance imaging, core needle biopsy, and molecular characterization play a supplementary role in avoiding unnecessary surgical thyroid excision in a small number of cases.[123456] Due to the nature of thyroid lesions, a significant number of biopsies are classified as follicular lesions of undetermined significance (FLUS), which account for 10-20% of papillary thyroid carcinoma (PTC).[7891011] Cytological criteria distinguishing benign multinodular goiter (MNG) from PTC are well documented and include cellularity, nuclear changes (chromatin pattern, nuclear enlargement, grooves, and inclusions), nuclear overlapping, colloid material, macrophages, and lymphocytes. Cellular arrangements in microfollicles/papillary formations are also important in the diagnosis of benign follicular lesions versus PTC.
In addition, the mesenchymal tissue of the thyroid gland is prone to develop varying degrees of fibrosis related to both inflammation and neoplasia. Stromal fibrosis is commonly seen in Riedel's and Hashimoto's thyroiditis (HT), follicular adenoma (FA)/carcinoma (that are encapsulated), PTC (associated with sclerosing stroma), and even in degenerated MNG. Thyroid lesions with predominant stromal tissue such as fibrotic thyroid nodule, De Quervain's thyroiditis, PTC with nodular fasciitis-like stroma or Warthin-like stroma, hyalinizing adenoma, diffuse sclerosing PTC, and Hodgkin's disease were previously studied in a few reports.[121314151617] However, the stromal features of these thyroid lesions have not received much attention in most investigative studies and reviews. In this study, the stromal tissue in the smear was correlated with the cytologic findings on FNAB and on surgical resection to determine its significance. Since the purpose of this study was to characterize the fibrous stroma in common follicular thyroid lesions, other lesions such as medullary thyroid carcinoma, anaplastic carcinoma, sarcoma, and lymphoma were not studied.
MATERIALS AND METHODS
Cases of FNAB adequate for cytologic assessment with either surgical surgical pathology specimens or follow-up FNAB were reviewed at our institution. FNAB prepared with liquid-based technique was excluded from the study due to the alteration of blood cell component and stroma caused by the chemical lysis. Consecutive cases with or without Hurthle cell features identified consisted of encapsulated PTC-follicular variant (PTC-FV) (36 cases), nonencapsulated PTC frequently with papillary variant (PTC-PV) (73 cases), follicular carcinoma (FC) (7 cases), FA (27 cases), HT (33 cases), MNG (39 cases), FLUS-positive for carcinoma in subsequent follow-up (FLUS-CA) (15 cases), and FLUS-negative for carcinoma in subsequent follow-up (FLUS-N) (26 cases).
As per the protocol at our institution, commonly, 3–4 passes through suspicious lesions are performed by endocrinologists or radiologists, with the aspirate from the individual needle expelled onto respective slides and the smears made into a set of two slides. Each set is stained with Papanicolaou (Pap) stain and May–Grunwald–Giemsa (MGG) stain. Cytological assessment was then made based on common cytological criteria described in the Bethesda system in 2009.
Since thyroid lesions exhibited a range of stromal reaction in surgical specimens, the stromal tissue in the FNAB was categorized into three categories.
Type 1
-
Type 1a: Loose, long (more than 3 mm in length), broad bands composed of mesh containing tiny delicate or barely discernible collagen fibers often arranged haphazardly. The stroma frequently contained scattered fibroblasts and was thickened by entrapped single or groups of thyroid follicular cells/lymphocytes, and blood components commonly interpreted as simple blood clots [Figure 1b–d]
-
Type 1b: Dense strands/bands of varying sizes showing: (i) Collagen fibrils packed into bundles with sizes larger than 30 microns in width of varying length with varying degree of hyalinization often with a semi-translucent appearance with absent or rare fibroblasts or (ii) reactive change and fibroblastic proliferation. Follicular cells and blood components were attached to the surface rather than being entrapped [Figure 1c–e].
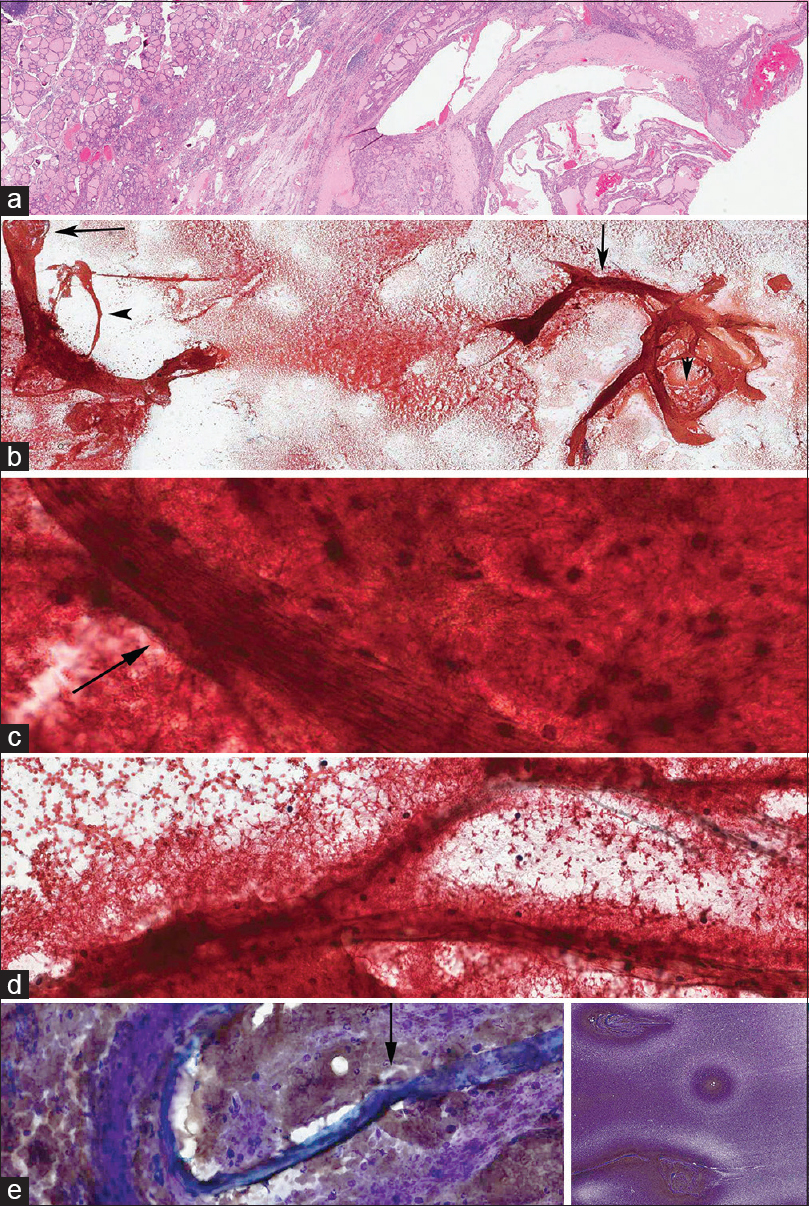
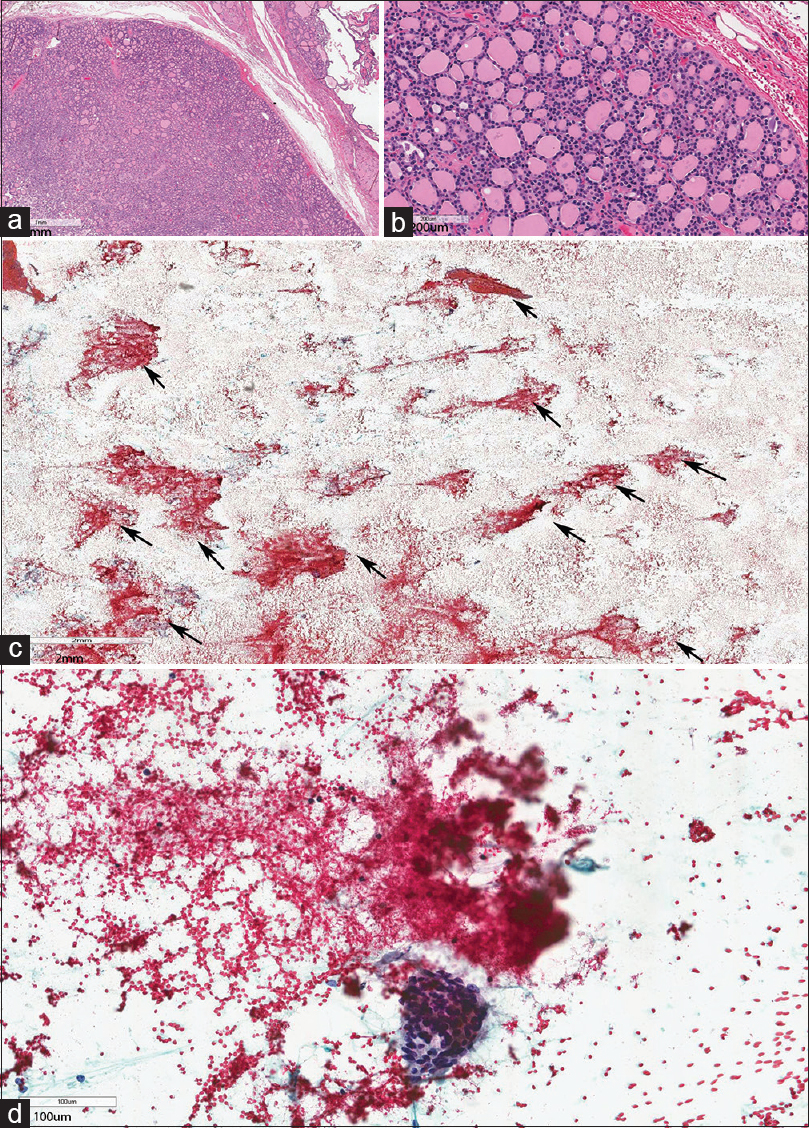
- Multinodular goiter with fine-needle aspiration biopsy predominantly consisting of Type 1a stroma and focally Type 1b. (a) Multinodular goiter. (b) Predominantly Type 1a stroma showing thick, long, and curved or circular bands with entrapped blood (arrow). Note the clearing background of blood around the stromal fragments. (c and d) High magnification views of Type 1a stroma entrapped with red blood cells, fibrin with focal areas of Type 1b stroma (arrow). (c) Predominantly Type 1a stroma with mesh-like architecture and with a bundle of parallel fibers forming Type 1b stroma (arrow). Scattered fibroblasts were seen in Type 1b stroma. (d) Semi-translucent bands of Type 1b. (e) MGG stain showing the background of Type 1a stroma, fragments of Type 1b with (arrow) and without hyalinization. Note the entrapped blood clots, readily identified fibroblasts in the nonhyalinized stroma. Inset: Low magnification view of the smear showing dark-stained areas with stromal fragments with a clear halo due to decreased number of red blood cells
- (For improved resolution with enlargement of all images, please refer to the digital / online version of the article.)
Type 2
Similar to Type 1a but with shorter (frequently <2 mm in greatest dimension) and looser mesh containing collagen fibrils with blood component entrapment; very occasionally, fibroblasts were seen [Figure 2c and d].
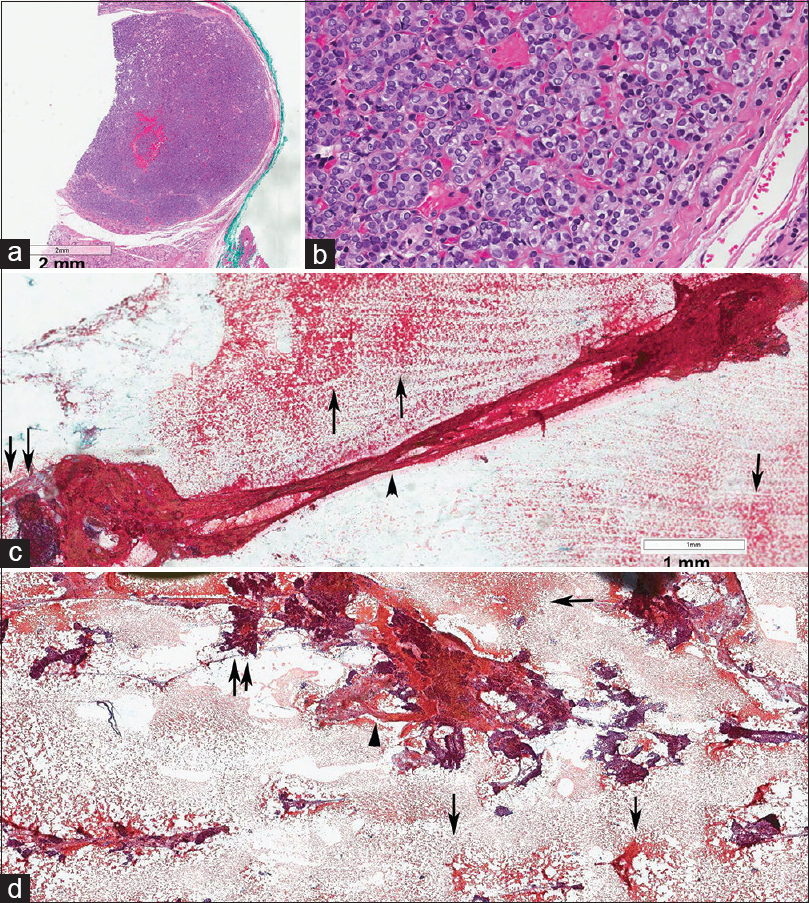
- (a and b) Encapsulated papillary thyroid carcinoma measuring 6 mm in diamter. (c and d) fine-needle aspiration biopsy showing Type 2 stroma (arrows) and fragment of Type 1b stroma with a flat and twisted ring of stroma in the shape of an “8” (arrowhead), likely from the capsule of the neoplasm. Note the entrapping and coating with blood clot and numerous microfollicles (double arrows)
- (For improved resolution with enlargement of all images, please refer to the digital / online version of the article.)
Cytologic smears were grouped into the original cytopathological diagnoses with observers blinded to the final surgical pathological diagnoses. The stroma was characterized into three types depending on the predominant type by one observer then re-examined by another observer. Discrepancies between the observers were reviewed with mutual agreement and reached on the final classification. The cases were subsequently matched with the respective surgical pathology diagnoses.
Statistical analysis was performed using Fisher's exact test (Sisa software, http://www.quantitativeskills.com/sisa/statistics/fisher.htm).
RESULTS
The stromal tissue was recognized by the presence of collagen fibers arranged in mesh (Types 1a and 2) [Figures 1 and 2] or in bundles of parallel fibers (Type 1b) [Figure 1c–e]. Blood components and follicular cells were seen entrapped within the mesh-like stroma or coating the Type 1b stromal bands [Figure 3d]. Fibroblasts were seen in the stroma with loose collagen fibers and were rarely seen in the stroma with scant fibers or with hyalinization. The fibroblasts were also seen in some cases of Type 1b [Figures 1d and 3d]. Areas of the smear surrounding the stromal bands with blood clot coating type 1b fragments showed a clearer background due to decreased number of red blood cells and fibrin [Figure 1c]. The colloid material was often not entrapped by the stroma or blood components. Stromal bands with features transitional between Type 1a and 1b were frequently seen [Figure 1c].
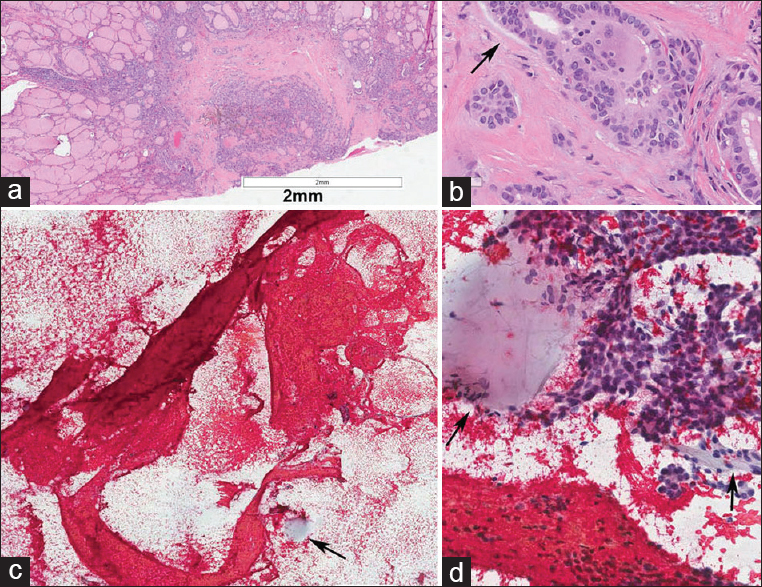
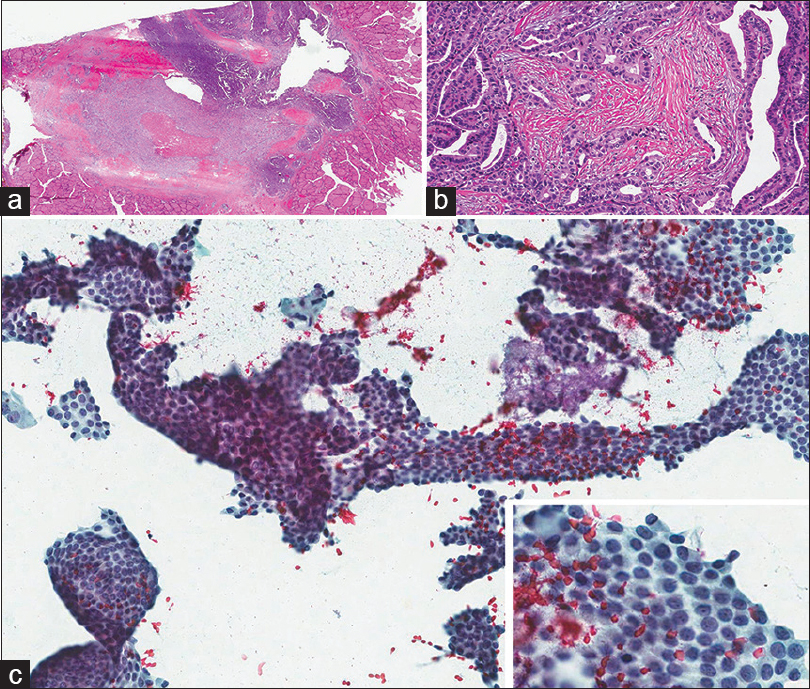
- (a and b) Microscopic sclerosing papillary thyroid carcinoma with desmoplasia of the stroma measuring 3mm in diameter, note the stroma showed focal mucoid change (arrow). (c) Type 1 stroma with ring shape and radiating strands consistent with the stroma of the microcarcinoma. Note the size and the stroma structure stretched in the smear measured 6 mm, twice the diameter of the tumor (postfixed in formalin). (d) Clusters of papillary thyroid carcinoma cells. Note the mucoid material indicated by arrow in c and d
- (For improved resolution with enlargement of all images, please refer to the digital / online version of the article.)
In the MGG stain, stromal fragments showed varying degrees of metachromasia. Semi-translucent fragments of Type 1b stroma often demonstrated decreased or absent metachromasia [Figure 1e]. Cellular components, particularly fibroblasts and inflammatory cells were more readily identified with the MGG stain than with the Pap stain. Delicate collagenous fibers were not visualized with the MGG stain [Figure 1e].
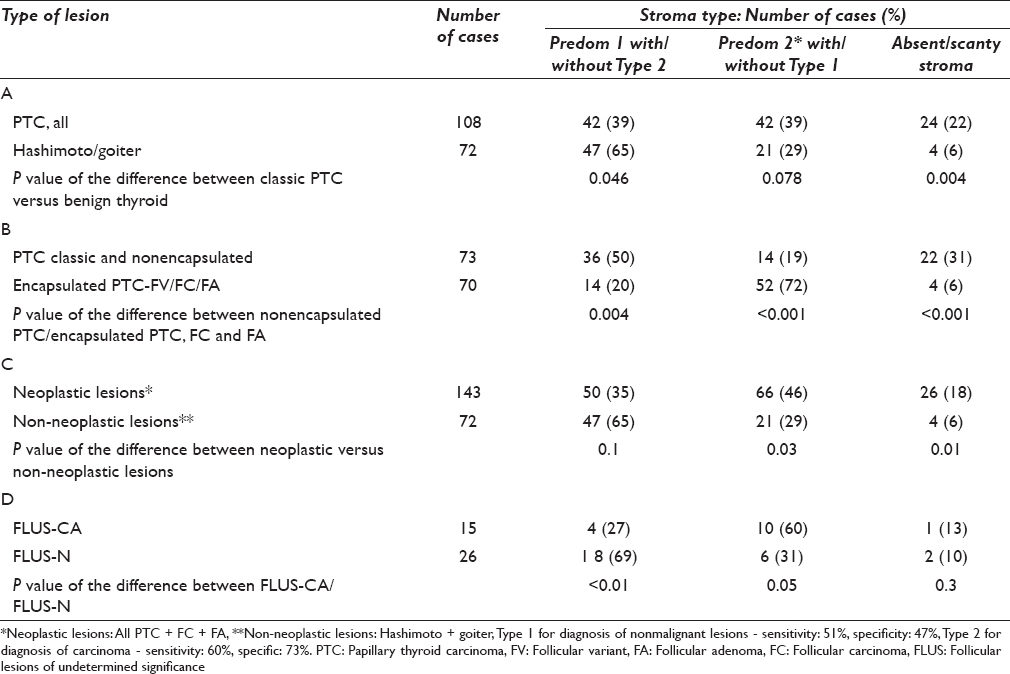
Type 1a/b bands showed curved/circular, branching patterns suggestive of incomplete frameworks of nodular/papillary architecture or fragments of capsule [Figures 1b, 2c, and 3c]. Within the large nodule framework, delicate fibrous septae were also evident [Figure 1a and b]. As summarized in Table 1, these frameworks of stroma were frequently associated with MNG which are often hypocellular (61/72) [Figure 1b] or acellular (hence “inadequate for cytologic assessment,” 6/72) and follicular neoplasms/PTC with moderate to marked cellularity (50/143) [Figures 2, 3 and 5]. Of note, cystic and macrofollicular lesions were often hypocellular.
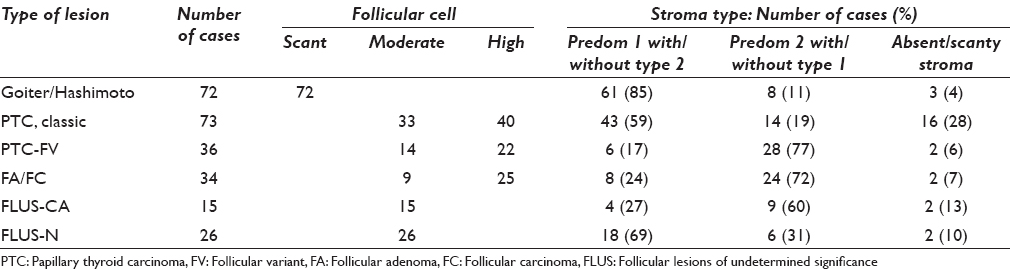
Type 2 bands were associated with micro- or macro-follicles in hyperplastic nodules of MNG or with microfollicles in encapsulated neoplasms (52/70) [Figure 4c and d]. Absent or inadequate stromal tissue was encountered from cases with large cysts, encapsulated follicular neoplasms with oncocytic change, and hypercellularity (n = 11) and PTC with sclerotic stroma (n = 6) [Figure 5]. Two small nodules of occult encapsulated or sclerosing PTC were characterized by the Type 1b curved/circular stroma and radiating stromal strands [Figures 2 and 3]. FLUS, either negative or positive for neoplasm in subsequent follow-up, was frequently associated with stromal characteristics of MNG and neoplasm, respectively.
- (a and b) Follicular adenoma. (c and d) fine-needle aspiration biopsy predominantly consisting of Type 2 stroma with entrapped blood cells in multiple clusters (arrows). Type 1 stroma was scant
- (For improved resolution with enlargement of all images, please refer to the digital / online version of the article.)
- (a and b) Papillary thyroid carcinoma with desmoplasia of the stroma. (c) Fine-needle aspiration biopsy showing almost only clusters of papillary thyroid carcinoma cells. Inset: High magnification showing nuclear changes of papillary thyroid carcinoma
- (For improved resolution with enlargement of all images, please refer to the digital / online version of the article.)
Table 2 shows statistical analysis of the differences between the different stromal features and their associations with different thyroid lesions. As seen in row A, all PTC versus MNG/HT, the differences were statistically significant for Type 1a/b (P = 0.046) and Type 2 (P = 0.078). As seen in row B, nonencapsulated or classic PTC versus all encapsulated follicular neoplasms (PTC-FV, FC, and FA), the differences were statistically significant for Type 1a/b (P = 0.004) and Type 2 (P < 0.001). As seen in row C, neoplastic follicular lesions (PTC + FC + FA) versus non-neoplastic lesions (MNG + HT), the differences were statistically significant for Type 2 (P = 0.03) and Type 1a/1b (P < 0.02). As seen in row D, FLUS-CA versus FLUS-N, the differences were statistically significant for Type 1a (P < 0.01) and Type 1b (P = 0.05).
DISCUSSION
FNAB plays a pivotal role in the management of nodular thyroid lesions. It is a cost-effective tool to diagnose thyroid carcinoma. Unlike other organs in the body, thyroid nodules display a range of cytological features from benign/reactive follicular cells to distinctly abnormal cells with typical nuclear changes of PTC. Thyroid FNAB is directed at a nodule intended for pathological diagnosis. Samples from thyroid tissue surrounding nodular lesions can contribute to the complexity of interpretation.
In our study, Type 1a and Type 2 stroma consisted of loosely packed collagenous filaments, visualized by haphazardly arranged filaments in a mesh-like pattern. As a result, this type of stroma commonly showed a significant entrapment of blood clot, inflammatory and follicular cells. Fibroblasts were rarely seen in Type 2 stroma due to the small amount of collagen fibrils. Blood component entrapment occurred in vitro as evidenced by the clear background surrounding the stromal fragments. Due to the viscous nature, colloid material was not entrapped in the collagenous tissue or blood clots.
The stroma seen in the smears, particularly Type 1a may be misinterpreted as simple blood clots. The semi-translucent stroma of Type 1b can be distinguished from “ropy” colloid due to the presence of occasional fibroblast, its association with Type 1a and Type 2 stroma, and/or the absence of thin colloid in the background. The size and architecture of Type 1 stroma (long, broad/thick, and commonly curved/circular) likely represents large bands of intervening stroma surrounding nodules or cysts. The preservation of the in vivo architecture of Type 1a/b is likely due its elasticity. This impression is supported by 2 cases of occult sclerosing PTC represented by Type 1b stroma which is almost identical to that seen in the histopathological section. Type 2 stroma likely originates from lesions with hypercellularity and macro- or micro-follicles, which are commonly seen in adenomatous or neoplastic nodular lesions.
With the exception of HT which is diagnosed by the increased number of lymphocytes in the stroma, most thyroid lesions exhibited characteristic features when examined along with cellular component:
-
Type 1 stroma with absent or scant follicular cells (often two-dimensional [2D] cell groups) is suggestive of MNG
-
Type 1 stroma + Type 2 stroma and significant amount of follicular cells (often three-dimensional (3D)cell groups) are suggestive of neoplastic thyroid nodule
-
Type 2 stroma with absent or scant follicular cells (often 2D cell groups) is suggestive of a macrofollicular lesion
-
Type 1 stroma + Type 2 stroma with a significant amount of follicular cells (often three-dimensional (3D)cell groups) is suggestive of a neoplastic thyroid nodule. Depending of the cytologic features and the papillary/circular of architecture of the stroma, the lesion can be diagnostic as PTC of PV or FV or FA/carcinoma
-
Type 2 stroma or absence of stroma with the presence of a significant amount of follicular cells (often 3D cell groups) is suggestive of a neoplastic thyroid nodule. Depending on the cytologic features, a diagnostic of PTC of FV or FA/carcinoma can be suggested.
In summary, the addition of the stromal component to the cellular component in the evaluation of thyroid aspirates can be valuable in the diagnosis of thyroid disease. Since follicular cells and lymphocytes are closely associated with the stromal fragments, integration of stromal tissue from the background in FNAB smears with the cellular component is helpful in correlating cytological changes with surgical histological slides. This correlation will likely facilitate the diagnosis of follicular thyroid lesions by FNAB. Due to the limitation of number of cases of thyroid FNAB with liquid-based preparation in our institution, the value of stroma in this type of preparation cannot be appreciated in this study.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Kien T Mai MD, Planning, conducting, and reporting the work. Kevin Hogan: Reporting the work and review of the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
Ethics approval from the investigational review board of our medical center was obtained and the study was conducted in a manner compliant with HIPAA (The Health Insurance Portability and Accountability Act of 19960). (The review board waived the need for informed consent for all patients).
LIST OF ABBREVIATIONS (In alphabetic order)
FA - Follicular Adenoma
FLUS - Follicular Lesions of Undetermined Significance
FNAB - Fine-Needle Aspiration Biopsy
HT - Hashimoto's Thyroiditis
MGG - May–Grunwald–Giemsa
MNG - Multinodular Goiter
Pap - Papanicolaou
PTC - Papillary Thyroid Carcinoma
PTC-FV - PTC-Follicular Variant.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-214.
- [Google Scholar]
- Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: A prospective multicentre study. Lancet Oncol. 2008;9:543-9.
- [Google Scholar]
- Beyond the suspicious thyroid fine needle aspirate. A review. Acta Cytol. 2003;47:709-22.
- [Google Scholar]
- Towards improving the utility of fine-needle aspiration biopsy for the diagnosis of thyroid tumours. Clin Endocrinol (Oxf). 2002;56:281-90.
- [Google Scholar]
- Detection of RET/PTC, TRK and BRAF mutations in preoperative diagnosis of thyroid nodules with indeterminate cytological findings. Clin Endocrinol (Oxf). 2007;66:678-83.
- [Google Scholar]
- Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092-8.
- [Google Scholar]
- The indeterminate thyroid fine-needle aspiration: Experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009;117:195-202.
- [Google Scholar]
- Implications of the proposed thyroid fine-needle aspiration category of “follicular lesion of undetermined significance”: A five-year multi-institutional analysis. Diagn Cytopathol. 2009;37:710-4.
- [Google Scholar]
- The Bethesda thyroid fine-needle aspiration classification system: Year 1 at an academic institution. Thyroid. 2009;19:1215-23.
- [Google Scholar]
- The impact of atypia/follicular lesion of undetermined significance on the rate of malignancy in thyroid fine-needle aspiration: Evaluation of the Bethesda system for reporting thyroid cytopathology. Surgery. 2011;150:1234-41.
- [Google Scholar]
- The Bethesda system for reporting thyroid cytopathology: A single-center experience over 5 years. Ann Surg Oncol. 2014;21:3522-7.
- [Google Scholar]
- Cytology of the thyroid gland: Pitfalls in aspiration of the fibrotic nodule. Diagn Cytopathol. 1996;14:362-6.
- [Google Scholar]
- Papillary thyroid carcinoma with nodular fasciitis-like stroma. Pitfalls in fine-needle aspiration cytology. Arch Pathol Lab Med. 1999;123:838-41.
- [Google Scholar]
- Fine-needle aspiration cytology of papillary Hurthle cell carcinoma with lymphocytic stroma “Warthin-like tumor” of the thyroid. Endocr Pathol. 1998;9:317-23.
- [Google Scholar]
- Hyalinizing trabecular tumor of the thyroid gland: A puzzling entity on fine needle aspiration cytology. Clin Cancer Investig J. 2014;3:108-11.
- [Google Scholar]
- Fine needle aspiration cytodiagnosis of subacute (de Quervain's) thyroiditis in an endemic goitre area. Cytopathology. 1994;5:33-40.
- [Google Scholar]
- Fine needle aspiration cytology in a case of diffuse sclerosing carcinoma of the thyroid. Acta Cytol. 1990;34:352-4.
- [Google Scholar]








