Translate this page into:
The physiological and pathogenic roles of yes-associated protein/transcriptional co-activator with PDZ-binding motif in bone or skeletal motor system-related cells

*Corresponding author: Yiguo Yan, Department of Spinal Surgery, Orthopaedic Research Center, The First Affiliated Hospital of the University of South China, Hengyang, China. CJ241120@163.com
-
Received: ,
Accepted: ,
How to cite this article: Huang Y, Ouyang X, Tan J, Meng Z. Ma X, Yan Y. The physiological and pathogenic roles of yes-associated protein/transcriptional co-activator with PDZ-binding motif in bone or skeletal motor system-related cells. CytoJournal. 2025;22:13. doi: 10.25259/Cytojournal_237_2024
Abstract
Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) are the primary downstream effectors of the Hippo signaling pathway. This pathway plays a crucial role in regulating organ size, maintaining tissue homeostasis, and controlling cellular processes such as fate determination and tissue development. This review provides an overview of the current understanding of how the transcriptional regulators YAP and TAZ contribute to the physiological and pathological processes in tissues and cells associated with the skeletal motor system. The underlying molecular mechanisms and mechanical transduction were reviewed.
Keywords
Bone
Cartilage
Heterotopic ossification
Mesenchymal stem cell
Osteoporosis
INTRODUCTION
Yes-associated protein (YAP), also known as YAP1, and transcriptional co-activator with PDZ-binding motif (TAZ), are closely related proteins that regulate transcription by interacting with the Tata-box Binding protein (TBP)-like Extra A domain transcription factor (TEAD) family of transcription factors.[1] They shuttle between the cytoplasm and nucleus and are key mediators of the Hippo pathway, which controls YAP/TAZ activity through inhibitory phosphorylation. The Hippo pathway, initially identified in Drosophila melanogaster,[2,3] is conserved across mammals and regulates cell proliferation, apoptosis, organ size, and cancer progression.[4,5]
Bone is a dynamic tissue that continuously remodels and adapts to changes in the mechanical environment, maintaining integrity through its composition, quality, and quantity.[6] Osteoblast differentiation, regulated by key factors such as Runt-related transcription factor 2 (Runx2), alkaline phosphatase, and Sex determining Region Y-box 9 (SOX9), is primarily controlled by the Wingless-related integration site (Wnt)/β-catenin signaling pathway. YAP/TAZ plays a considerable role in bone homeostasis by modulating these coordinators.[7] Recent studies have shown that YAP/TAZ regulates various processes in bone and related tissues, including cell development, differentiation, mechanical signal transduction, and disease progression involving bone marrow mesenchymal stem cells (BMSCs), cartilage, and nucleus pulposus.[8] YAP and TAZ have osteogenic-promoting and -inhibitory effects on osteogenesis, which may depend on experimental and cellular conditions.[9] Recent studies have highlighted the role of YAP and TAZ in regulating bone development and homeostasis by influencing osteoblast function, matrix quality, and osteoclast remodeling.[8,10]
Recently, growing evidence has highlighted the remarkable role of mechanical signals in determining cell fate. Cues from the cellular microenvironment are increasingly recognized as key regulators of cellular behavior.[11,12] Previous studies have shown that bone- and bone tissue-related cells are capable of perceiving changes in mechanical signals and that YAP/TAZ is essential in automatic signal transduction.[13] Despite some insights into YAP/TAZ’s role in bone homeostasis, their effects on osteoclasts and the mechanisms of osteogenic signal transduction remain poorly understood. In addition, the role of YAP/TAZ in bone-related diseases such as osteoporosis, heterotopic ossification (HO), and bone tumors has not been systematically reviewed. This article explores the involvement of YAP/TAZ in the physiological and pathological processes of skeletal muscle motor system-related tissues.
ROLE OF YAP/TAZ IN OSTEOGENESIS
Promotion of osteogenic differentiation in stem cells
Bone is a dynamic tissue that undergoes continuous remodeling during osteogenesis and healing, processes that involve the differentiation and migration of mesenchymal stem cells (MSCs) to form bone.[14,15] YAP/TAZ plays a critical role in regulating osteogenesis. In MSCs, Snail/Slug interacts with YAP/TAZ to activate Hippo-dependent signaling, which, in turn, activates downstream factors such as TEAD and Runx2 that control stem cell homeostasis and osteogenesis [Figure 1].[16,17] Previous studies have suggested that biological functions, such as migration and osteogenic abilities, are positively correlated with the expression of calcitonin gene-related peptide (CGRP) in BMSCs, and this phenomenon is related to enhanced expression of YAP, a crucial downstream effector of the Hippo pathway.[18]
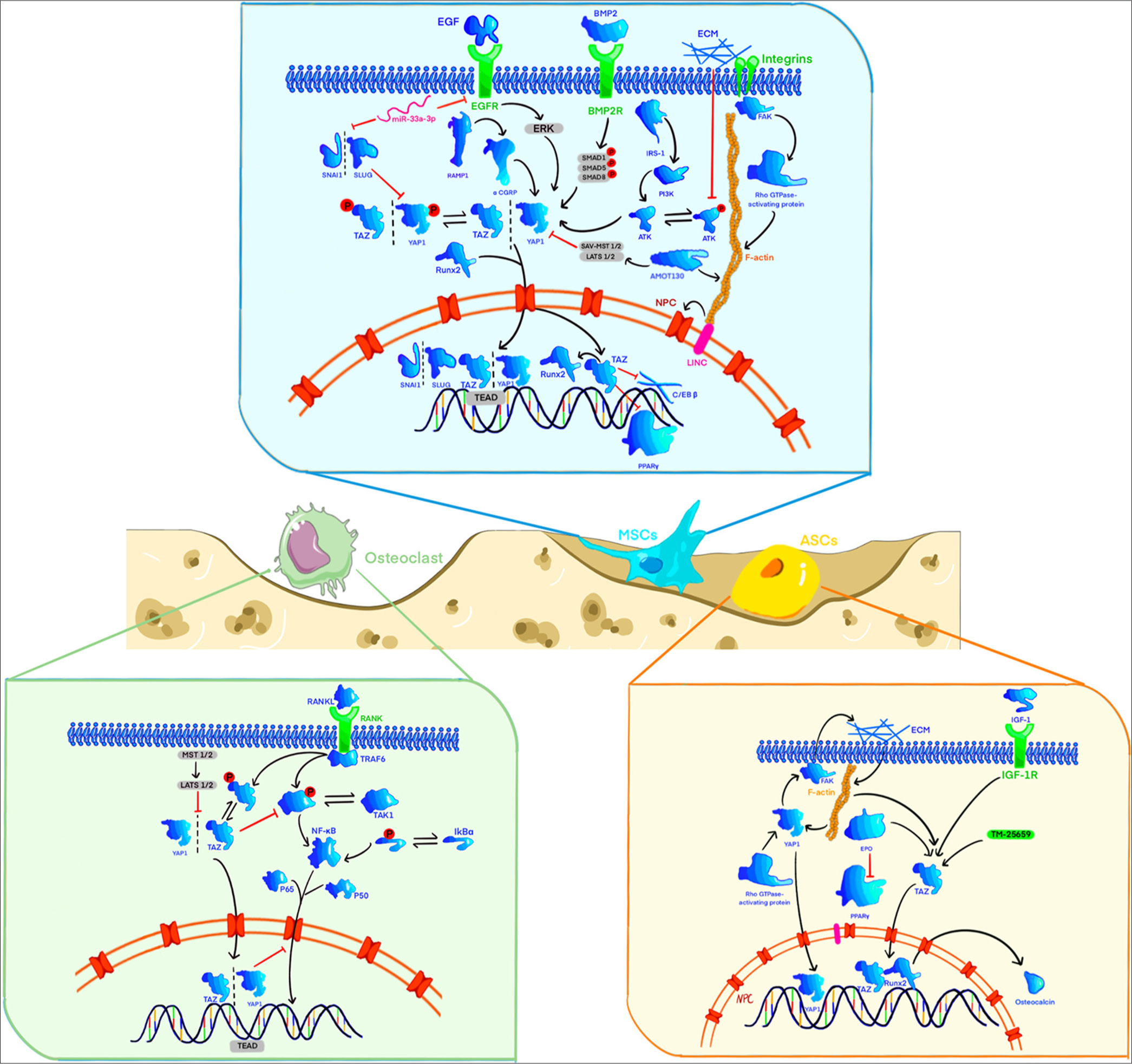
- When yes-associated protein (YAP) or transcriptional co-activator with PDZ-binding motif (TAZ) is activated, it can mediate multiple signaling pathways to induce osteogenic differentiation of mesenchymal stem cells (MSCs), inhibit adipogenic differentiation of MSCs, inhibit the biological activity of osteoclasts, and promote the differentiation of adipose-derived stem cells (ADSCs) into osteoblasts. YAP/TAZ can mediate extracellular signals such as extracellular matrix stiffness and mechanical signal stimulation (Figure created using Adobe Illustrator CS6 Adobe Systems, San Jose, USA). AMOT130: Angiomotin 130, ATK: Akt, also known as protein kinase B, BMP2: Bone morphogenetic protein 2, BMP2R: Bone morphogenetic protein 2 receptor, CGRP: Calcitonin gene-related peptide, ECM: Extracellular matrix, EGF: Epidermal growth factor, EGFR: Epidermal growth factor receptor, EPO: Erythropoietin, ERK: Extracellular signal-regulated kinase, FAK: Focal adhesion kinase, F-actin: Filamentous actin, IGF-1: Insulin-like growth factor 1, IGF-1R: Insulin-like growth factor 1 receptor, IkBα: Inhibitor of kappa B alpha, IRS-1: Insulin receptor substrate 1, LATS 1/2: Large tumor suppressor 1/2, LINC: Long intergenic noncoding RNA, MSCs: Mesenchymal stem cells, NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells, PI3K: Phosphoinositide 3-kinase, PPARγ: Peroxisome proliferator-activated receptor gamma, RAMP1: Receptor activity-modifying protein 1, RANKL: Receptor activator of nuclear factor kappa-B ligand, Runx2: Runt-related transcription factor 2, SAV-MST 1/2: Salvador/mammalian ste20-like kinase 1/2 signaling complex, SMAD: SMA and MAD related protein, SNAI1: Snail family transcriptional repressor 1, SNAI2: Snail family transcriptional repressor 2, TAK1: Transforming growth factor-beta-activated kinase 1, TAZ: Transcriptional co-activator with PDZ-binding motif, TEAD: Transcriptional enhanced associate domain, TRAF6: TNF receptor-associated factor 6, YAP: Yes-associated protein.
Mechanical forces play a key role in bone development and homeostasis by mediating YAP/TAZ-driven biological effects through mechanical signal transduction.[19,20] These effects are activated by forces that reduce mechanical constraints on nuclear pores, allowing YAP/TAZ nuclear translocation independent of the Hippo pathway.[21] YAP/TAZ can sense mechanical cues, such as shear stress and extracellular matrix (ECM) stiffness, and translate these into cellular responses.[22] The physical function of YAP/TAZ automatic signal transduction depends on cell tension, actomyosin-driven contractility, and Ras homolog (Rho) Guanosine Triphosphatase (GTPase) activity. F-actin assembly enhances the association between the Linker of Nucleoskeleton and Cytoskeleton complex and focal adhesions (FAs), which increases nuclear tension and pore permeability, allowing YAP/TAZ to enter the nucleus. Upon activation, YAP/TAZ interacts with transcription factors like TEAD to drive the expression levels of target genes [Figure 1].[21]
In experiments with human bone marrow MSCs (HBMMSCs), the angiomotin 130 (AMOT130) protein enhances F-actin formation, promoting YAP nuclear localization. This activation upregulates osteocyte genes and facilitates HBM-MSC differentiation into osteoblasts.[23] Orthodontic tooth movement-related experiments have shown that high levels of F-actin can bind to angiomotin, thereby inhibiting Hippo signaling pathways, including Salvador (SAV) family WW domain-containing protein 1 -mammalian sterile 20-like kinase 1/2 and large tumor suppressor 1/2 (LATS1/2) [Figure 1].[24] This phenomenon may be another mechanism by which F-actin promotes the functional effects of YAP/TAZ in HBM-MSCs.
Inhibiting adipogenic differentiation of stem cells
Adipose-derived stem cells (ADSCs) are a promising resource for skeletal tissue engineering, with substantial capacity for bone regeneration and recovery.[25] Previous studies have shown that low-amplitude and high-frequency treatments promote osteogenic differentiation of human ADSCs.[26] A study found that TAZ drives the differentiation of MSCs into osteoblasts and inhibits adipogenesis by binding to Smad4 to co-activate Runx2.[27] Previous studies have shown that inhibiting TAZ negates the osteogenic differentiation-promoting effects of erythropoietin and insulin-like growth factor 1 on ADSCs, highlighting TAZ’s critical role in regulating their osteogenic differentiation [Figure 1].[28] Subsequent studies demonstrated that the TAZ activator TM-25659 significantly boosted TAZ phosphorylation and facilitated its translocation to the nucleus, leading to the formation of the TAZ-Runx2 complex. This complex was then recruited to the osteocalcin promoter, enhancing its transcription and markedly improving bone regeneration in ADSCs loaded with porous β-tricalcium phosphate in vivo. Overactivation of TAZ promoted osteogenic differentiation of ADSCs while inhibiting their adipogenic differentiation potential [Figure 1].[29]
YAP/TAZ mediation of extracellular mechanical signal transduction to promote osteogenesis
ECM stiffness is a critical factor in regulating HBM-MSC differentiation. In adipose-derived MSCs, the RhoA GTPase activity controls the assembly of FAs through YAP, stabilizing the actin cytoskeleton’s anchoring to the membrane, which strengthens the mechanical link between the nucleus and the ECM.[30] Meanwhile, ECM stiffness alters cytoskeletal tension and cell geometry, enhancing YAP/TAZ nuclear translocation and promoting MSC osteogenesis.[31] Caveolin-1 mediates YAP’s response to substrate stiffness through an actin-dependent mechanism, where Rho activity is necessary but not sufficient on its own for full YAP activation. This regulation involves YAP’s interaction with the 14-3-3 protein Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein theta (YWHAH).[32] The study investigated how ECM stiffness regulates the differentiation of MSCs by activating intracellular signaling pathways, particularly highlighting the critical roles of the Extracellular Signal-Regulated Kinase (ERK) and c-Jun N-terminal kinase (JNK) pathways in this process, which function through the activation of TAZ.[33] In addition, the properties of ECM influence human mesenchymal stem cells (hMSC) adhesion and proliferation, with TAZ expression notably increasing in this process, highlighting YAP/TAZ’s role in mechanical signal transduction [Figure 1].[34]
Integrins play a key role in ECM mechanosensing. These proteins link ECM components to the cytoskeleton and mediate cell adhesion, converting mechanical stimuli into biochemical signals.[35] Integrin-induced mechanical stimuli activate cellular responses on the basis of the integrin type involved. Increased integrin expression can alter cell responses to ECM stiffness, partly due to RhoA’s role in stabilizing the ECM–integrin interaction.[36] Furthermore, integrin αv activation in osteoblasts induces Src kinase activity and p130Cas, leading to JNK phosphorylation and YAP/TAZ activation.[37] In another experiment, the researchers created a 3D osteocyte compression model using the murine osteoblast-like cell line murine lacrimal gland osteoblast-like cell line 4 (MLO-Y4). The knockdown of YAP/TAZ partially inhibited the increase in the levels of macrophage colony-stimulating factor and C-X-C motif chemokine ligand 3 during stress response in MLO-Y4 cells.[38]
Inhibition of osteoclast function by overexpression of YAP/TAZ
Osteoclasts are essential for bone resorption, and their dysregulation can result in osteolytic diseases.[39] Osteoclastogenesis, which is the process of osteoclast differentiation and activation, is influenced by various genetic, hormonal, and mechanical factors. Recent studies have focused on the role of YAP/TAZ signaling in regulating osteoclast function, revealing new mechanisms in bone resorption.
YAP1 regulates the differentiation and function of osteoclasts. Zhao et al.[1] found that silencing YAP1 or using the YAP inhibitor verteporfin inhibited osteoclast differentiation and function, resulting in reduced osteoclast formation and downregulation of marker gene expression. This study suggests that YAP1 plays a role in osteoclast formation and function by binding to the transcriptional enhancer domain. TAZ binds to transforming growth factor-beta-activated kinase 1 (TAK1) in chondrocytes, limiting TAK1’s activity and inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, thereby reducing osteoclast differentiation.[40] Further studies confirmed the interaction between TAZ and TAK1, which is critical for inhibiting osteoclast formation.[41] YAP1 can suppress tumor necrosis factor-alpha (TNF-α)-induced NF-κB activation by decreasing phosphorylated p65 expression and preventing its nuclear translocation, thereby inhibiting the production of osteoclast key mediators such as interleukin (IL)-6 and NF-κB.[42] Mechanical signals, such as ECM stiffness, influence YAP/TAZ nuclear translocation. In experiments with L929 cells on soft substrates, mechanical cues prevented YAP nuclear translocation, increasing the expression levels of pro-inflammatory mediators and proteases (e.g., prostaglandin-endoperoxide synthase 2 [Ptgs2], IL-1β, IL-6, matrix metalloproteinase [Mmp]-2, and Mmp9) and the production of prostaglandin E2 (PGE2), which enhance osteoclast induction and inflammatory osteolysis.[43] Previous experiments have shown that YAP/TAZ knockout mice exhibited increased osteoclast formation, indicating their critical role in suppressing osteoclastogenesis.[44,45] The findings above indicate that YAP/TAZ could serve as a promising therapeutic target for osteolytic diseases resulting from osteoclast dysfunction [Figure 1]. However, additional research is necessary to assess the safety of inhibiting TAZ.
Indirect effect of YAP/TAZ on the osteogenic process through affecting the function of other cells
Recent studies have shown that CGRP regulates osteogenesis by regulating the inflammatory activity of M2 macrophages through YAP1.[46] Bone tissue macrophages in the trabecular bone can regulate subchondral bone osteogenesis by secreting oncostatin M (OSM). The mechanism involves OSM-driven tyrosine phosphorylation, with signal transducer and activator of transcription 3 interacting with YAP1 to promote osteoblast differentiation and suppress osteoclast differentiation.[47] Macrophages play a key role in bone tissue osteogenesis and can effectively promote MSC osteogenic differentiation under specific mechanical stimuli. The mechanism involves macrophage activation by automatic signals such as cyclic stretching, which triggers YAP activation and nuclear translocation. This process regulates the expression of downstream bone morphogenetic protein 2 (BMP2), thereby enhancing MSC osteogenesis [Figure 2].[48]
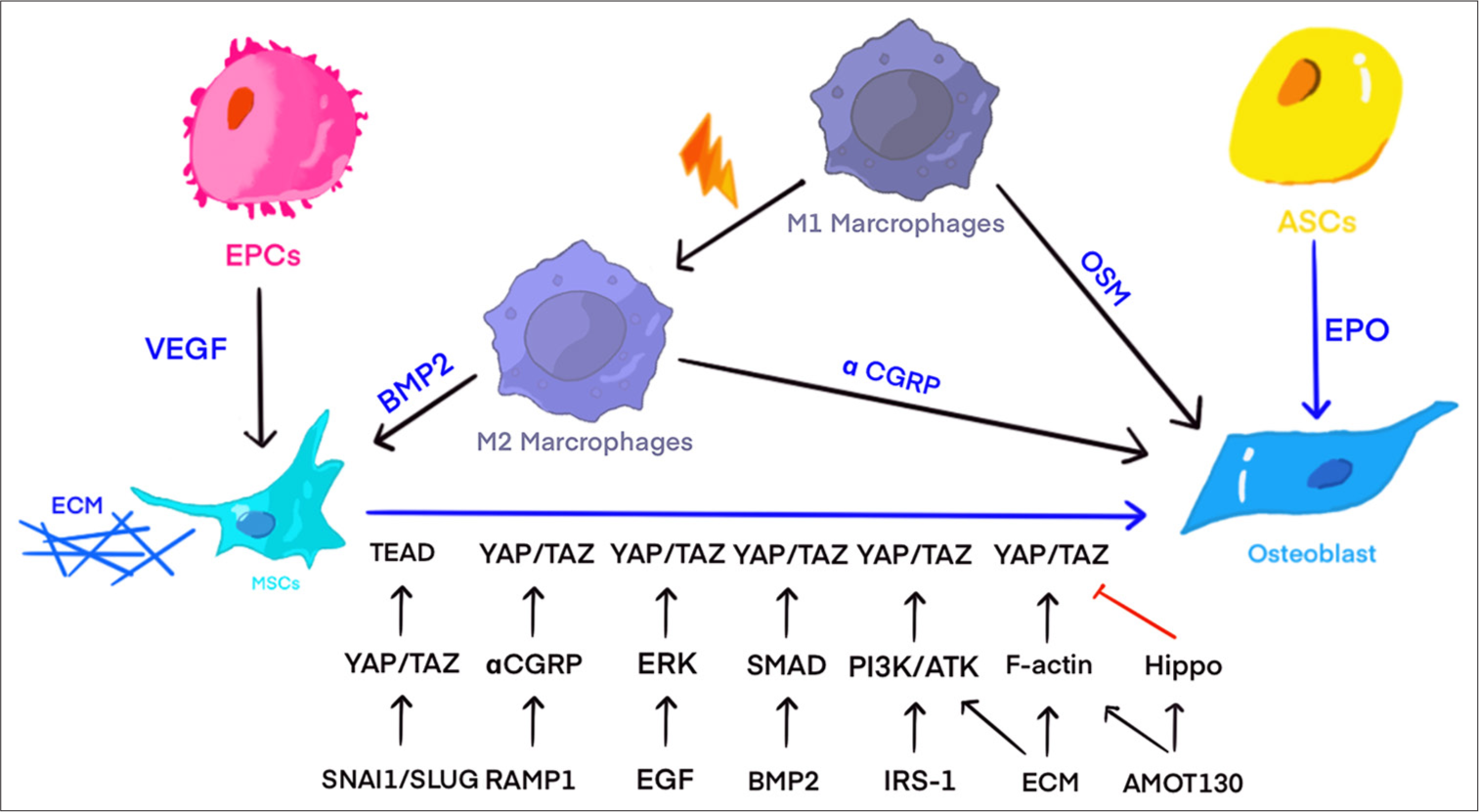
- Co-culture of various cells can promote the differentiation of mesenchymal stem cells into osteoblasts through cell-to-cell interactions. Under the co-culture of endothelial cells and mesenchymal stem cells, endothelial cells promote the differentiation of mesenchymal stem cells into osteoblasts by secreting more vascular endothelial growth factor. Under moderate mechanical signal stimulation, macrophages can be transformed into M2 phenotype, promoting osteogenic differentiation of mesenchymal stem cells by affecting local inflammatory factors and secreting more bone morphogenetic protein 2. Macrophages and M2 phenotype macrophages can promote osteogenesis by secreting more oncostatin M and alpha calcitonin gene-related peptide (Figure created using Adobe Illustrator CS6 Adobe Systems, San Jose, USA). BMP2: Bone morphogenetic protein 2, CGRP: Calcitonin gene-related peptide, ECM: Extracellular matrix, EPCs: Endothelial progenitor cells, ERK: Extracellular signal-regulated kinase, OSM: Oncostatin M, TAZ: Transcriptional co-activator with PDZ-binding motif, TEAD: Tata-box binding protein-like extra A domain transcription factor, VEGF: Vascular endothelial growth factor, YAP: Yes-associated protein, EGF: Epidermal growth factor, EPO: Erythropoietin, F-actin: Filamentous actin, IRS-1: Insulin receptor substrate 1, RAMP1: Receptor activity-modifying protein 1, PI3K: Phosphoinositide 3-kinase, SMAD: SMA and MAD related protein, SNAI1: Snail family transcriptional repressor 1.
Some experiments have shown that BMSCs co-cultured with Endothelial Progenitor Cells (EPCs) have increased vascular endothelial growth factor (VEGF) levels compared with other cell types. By contrast, VEGF-cultured cells exhibit a robust actin cytoskeleton, increased nuclei, and elevated total YAP levels. Co-culturing with EPCs can partially promote BMSC osteogenic differentiation through VEGF, potentially by VEGF receptor activation, which then suppresses large tumor suppressor (LATS) and YAP phosphorylation [Figure 2].[49] Subsequently, more YAP is translocated to the nucleus to activate downstream pathways.[50] The present review suggests a close relationship between F-actin and YAP, which will be discussed later. Recent research has indicated that co-culturing endothelial cells with sulfonated polyrotaxane surfaces, which allow molecular mobility, further boosts HBM-MSC mineralization. The accumulation of YAP-related proteins in the nucleus of HBM-MSCs, along with cell-to-cell signaling through cytokines or cadherins, appears to be key in the enhanced mineralization of HBMMSCs.[51] This finding suggests that stem cell therapy in a suitable co-culture system is better than simple stem cell transplantation [Figure 2].
Different views on the effect of YAP/TAZ on osteogenesis
Other researchers have proposed different views on the osteogenic processes involving YAP. First, YAP may inhibit the accumulation of bone progenitor cells by inhibiting the level of the BMP2a in surrounding mesenchymal cells and involuntarily preventing osteoblast differentiation.[52] Therefore, YAP/TAZ is beneficial for bone tissue osteogenesis. However, the exact role of YAP in osteogenesis remains debatable. Second, YAP can lead to the accumulation of osteoprogenitor cells and prevent osteoblast differentiation in a non-autonomous manner.[52] Osterix 1-crem mice were used to verify the effects of TAZ gene knockout on osteogenesis. The findings indicated that YAP and TAZ impeded the differentiation of osteoblast precursor cells into mature osteoblasts [Figure 2]. However, fully differentiated osteoblasts and osteocytes supported osteoblast formation and suppressed bone resorption without influencing well-established bone turnover regulators such as receptor activator of nuclear factor kappa-B ligand (RANKL), osteoprotegerin, and sclerostin [Figure 2].[44]
YAP/TAZ PLAYS A ROLE IN SIGNAL TRANSDUCTION OF OTHER MUSCULOSKELETAL SYSTEM-RELATED CELLS
Effects of YAP/TAZ on chondrogenesis
Increased levels of YAP/TAZ do not always promote cartilage tissue development, and YAP and TAZ do not consistently function in tandem. Overexpressing hYAP in mouse C3H10T1/2 MSCs suppressed chondrogenic differentiation, potentially due to a reduction in BMP-2-induced phosphorylation of Smad1, 5, and 8, along with decreased expression levels of BMP target genes that inhibit differentiation, such as Id1, Id2, and Id3.[53] Culturing L929 cells on soft substrates prevents YAP from entering the nucleus, boosting the expression of inflammatory mediators such as Ptgs2, Il1b, Il6, Mmp2, Mmp9, and PGE2. This process enhances osteoclast activation and promotes inflammatory osteolysis.[54] However, some experimental groups disagree on YAP promoting chondrocyte proliferation. They believe that excessive activation of YAP1/TAZ can damage the differentiation/maturation of chondrocytes and their proliferation [Figure 3]. Excessive activation of YAP1/TAZ affects the expression of the main regulatory factor-determining region-box nine protein (SOX9), which inhibits and negatively regulates cartilage formation.[55] Another experimental group confirmed a correlation between YAP and SOX9 expression in hypoxia-related experiments.[56] However, recent studies have indicated that the inhibitory effect of YAP on cartilage may be confined to in vitro models. Chondrocyte proliferation in vivo is dispensable, and its primary function is to regulate cartilage morphogenesis through the ECM.[57]
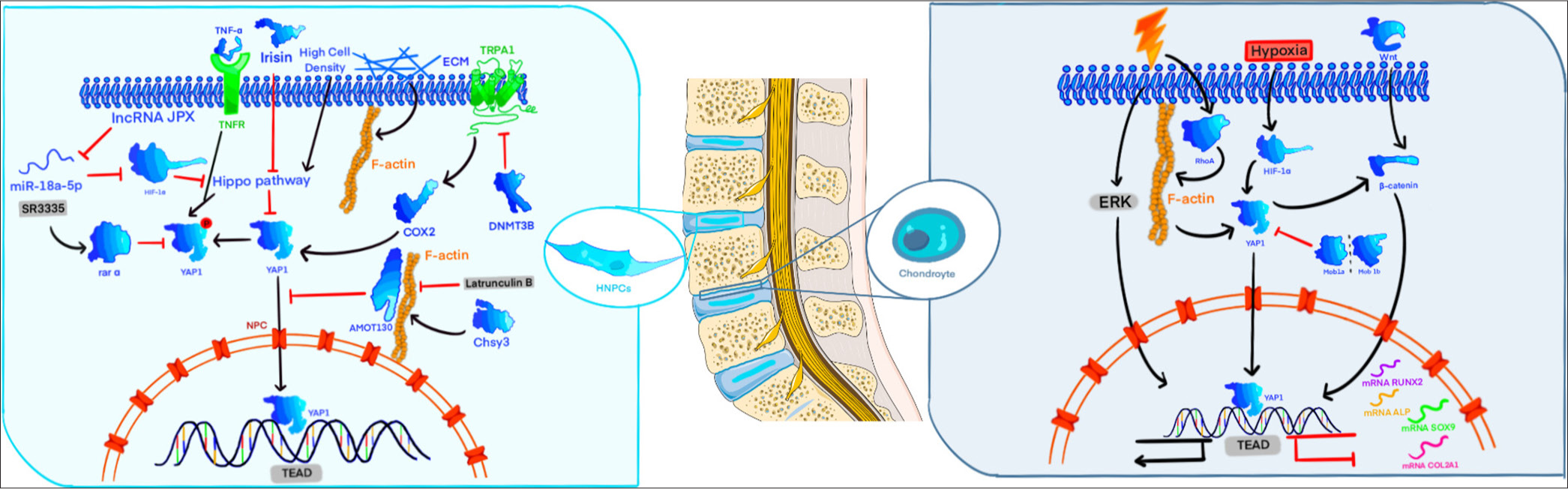
- Regulatory pathways of different epistatic regulators on yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) in human nucleus pulposus cells (HNPCs), ADSCs, and chondrocyte cells. The activation of YAP in HNPCs serves to decelerate the degeneration of these cells in lumbar intervertebral disc. The activation of YAP/TAZ in ADSCs can contribute to the differentiation of ADSCs into osteoblasts. The role of YAP/TAZ activation in chondrocytes remains unclear. The protein structures are based on models from SWISS-MODEL (https://swissmodel.expasy.org/) and the AlphaFold protein structure database (https://alphafold.com/). The illustration was created using procreate (https://procreate.com/) and Adobe Illustrator CS6 (Adobe Systems, San Jose, USA). ALP: Alkaline phosphatase, AMOT130: Angiomotin 130 kDa, COX2: Cyclooxygenase-2, DNMT3B: DNA methyltransferase 3 beta, ECM: Extracellular matrix, ERK: Extracellular signal-regulated kinase, F-actin: Filamentous actin, HIF-1α: Hypoxia-inducible factor 1 alpha, LncRNA JPX: Long non-coding RNA JPX, Mob1: Mps one binder 1, NPC: Nucleus pulposus cell, RhoA: Ras homolog family member A, RUNX2: Runt-related transcription factor 2, SOX9: Sex determining region Y-box 9, TNF-α: Tumor necrosis factor-alpha, TNFR: Tumor necrosis factor receptor, TRAP: Tartrate-resistant acid phosphatase, Wnt: Wingless-related integration site, YAP: Yes-associated protein, TEAD: Transcriptional enhanced associate domain.
The experimental findings suggest that overexpression of YAP suppresses cartilage differentiation in vitro. However, the effect of YAP/TAZ on chondrocyte proliferation remains a topic of debate. It is important to recognize that in vivo and in vitro results may not always align when studying the role of YAP/TAZ in cartilage.
Role of YAP/TAZ in nucleus pulposus cells (NPCs)
In an in vitro study of human NPCs (HNPCs), overexpression of long non-coding RNA (lncRNA) JPX stimulated HNPC proliferation and reduced apoptosis by upregulating Hypoxia-Inducible Factor 1 Alpha (HIF-1α), which inhibited miR-18a-5p and suppressed the Hippo-YAP pathway.[58] SR3335, a reverse agonist of retinoid-related orphan receptor alpha, counteracted TNF-α-induced apoptosis by inhibiting YAP phosphorylation, reversing the apoptotic trend.[59] Latrunculin B disrupted F-actin stress fibers, affecting YAP nuclear translocation. Deletion of LATS1/2 resulted in YAP retention in the cytoplasm, indicating that the actin cytoskeleton and LATS1/2 independently modulate YAP activity. AMOT130 anchors YAP in the cytoplasm through F-actin binding [Figure 3].[60]
YAP/TAZ mediates the interaction between the ECM and NPCs, delaying degeneration. ECM stiffness affects NPC morphology and enhances F-actin arrangement, weakening YAP/TAZ retention in the cytoplasm. Increased SRF and TEAD expression levels suggest that ECM stiffness may impact NPC phenotype and degeneration through the YAP/TAZ pathway.[61] Subsequent research has shown that ECM composition and cell density can modulate the Hippo pathway. Tubulin β and vimentin filaments contribute to cellular elasticity and resistance to mechanical forces.[62] Similarly, changes in ECM metabolism influence NPC degeneration. Chondroitin Sulfate Synthase 3 (Chsy3)-mediated Yap1 activation, which is dependent on Chondroitin Sulfate (CS)/actin tension, protects NPCs by enhancing ECM anabolic genes and suppressing catabolic ones.[63] Irisin promotes NPC anabolism and inhibits ECM catabolism through LATS/YAP/connective tissue growth factor signaling, thus delaying intervertebral disc degeneration (IDD).[64] While enhanced YAP activity seems to counteract IDD progression, some studies have reported that YAP1 knockdown can prevent IL-6-induced NPC degeneration, and using verteporfin to antagonize YAP signaling alleviated IDD in rat intervertebral discs.[65]
YAP/TAZ INVOLVEMENT IN THE EVOLUTION OF BONE AND BONE TISSUE DISEASE
Osteoporosis (OP)
OP is a common bone disorder, primarily affecting the elderly and postmenopausal women and characterized by progressive bone loss and increased fragility.[66] YAP/TAZ is crucial in regulating bone formation. TAZ mediates Wnt signaling and BMP2-driven osteoblast differentiation by interacting with Runx2 while collaborating with lymphoid enhancer-binding factor 1 to enhance the effects of Wnt3a and BMP2 on osteogenesis.[67] Conversely, dysregulation of YAP/TAZ is involved in osteoporosis. For instance, reduced TAZ activity impairs osteoblast differentiation by weakening Runx2 activity and enhancing adipocyte differentiation through the activation of peroxisome proliferator-activated receptor gamma.[68] In osteoporosis, alterations in the TAZ-mediated signaling pathway also disrupt the balance between osteoblast and adipocyte differentiation, leading to increased bone marrow adiposity and reduced bone formation.[68] Some studies have suggested that upregulation of YAP1 promotes osteogenesis in osteoporosis models. For example, circ_0024097, a YAP1-derived circular RNA (circRNA), enhanced YAP1 expression in BMSCs and Mus musculus 3T3 (MC3T3)-E1 cells, stimulating osteogenic differentiation.[69] Activation of YAP contributes to osteogenesis in disuse osteoporosis due to the lack or absence of mechanical load. Exosomal miR-1263 targets the 3’ untranslated region of Mob1, suppressing its expression in recipient cells and triggering the activation of YAP signaling. As a result, the apoptosis of BMSCs induced by high-level unloading in vitro was counteracted.[70] These results indicated that YAP/TAZ signaling could represent a novel target for osteoporosis intervention.
HO
Traumatic HO involves the abnormal differentiation of mesenchymal-like (MLin) cells, leading to bone formation in soft tissues after injury. YAP/TAZ is crucial in the development of HO by regulating the cellular processes involved in this aberrant bone formation. The transforming growth factor beta/BMP/Smad pathway has been identified as a potential target for HO treatment, with YAP acting as a coactivator that interacts with Smad proteins to influence HO progression.[71] Transforming growth factors upregulate TAZ expression, further implicating YAP/TAZ signaling in HO.[72] Recent findings suggest that discoidin domain receptor 2 alters FAs, influencing signaling pathways, such as FAK, YAP/TAZ, and ECM-MLin, receptor interactions. These pathways mediate the mechanical signaling that affects HO development.[73] In models of progressive ossifying fibrous dysplasia, abnormal mechanical signal transduction contributed to HO. In these models, RhoA and YAP1 regulated mechanical signaling, with mutant activin A receptor type 1 (ACVR1) increasing BMP pathway signaling and making cells more sensitive to mechanical stimuli.[74] These provide new ideas for preventing, reducing, and narrowing HO.
DISCUSSION
Significance of the research
The key roles of YAP/TAZ in maintaining the stability of bone, cartilage, and nucleus pulposus provide important insights for basic biology and potential clinical applications. This study emphasizes the multifaceted roles of YAP/TAZ in regulating key signaling pathways, such as Wnt/β-catenin, PI3K-AKT, and Hippo, which are crucial for cell proliferation, differentiation, and survival – processes essential for tissue homeostasis and repair [Table S1]. YAP/TAZ promotes osteoblastic differentiation while inhibiting adipogenesis, providing promising therapeutic directions for diseases like osteoporosis, which is characterized by an imbalance between bone formation and resorption. Furthermore, their central role in mechano-transduction is particularly critical in the dynamic mechanical environment of bone and cartilage. The regulation of chondrogenesis by YAP/TAZ offers new therapeutic avenues for degenerative joint diseases like osteoarthritis, especially in promoting cartilage repair and regeneration. In addition, their role in maintaining NPC homeostasis opens up new strategies for addressing IDD, which is crucial for improving spinal health.
The roles of YAP/TAZ in different bone cell types and their functional differences in various diseases further complement these findings. In osteoclasts, YAP1 has been identified as a key factor for maintaining their differentiation and function. Osteoclasts lacking YAP1 (e.g., through short hairpin RNA (shRNA) knockdown) exhibit severe defects in differentiation, with impaired expression of key markers, such as Nuclear Factor of Activated T-cells cytoplasmic 1 (NFATc1), Tartrate-Resistant Acid Phosphatase (TRAP), and Cathepsin K (CTSK), resulting in a significant reduction in bone resorption ability.[1] Specifically, when YAP1 is suppressed or absent, the RANKL-activated NF-κB signaling pathway is inhibited, further reducing bone resorption capacity.[75] By contrast, TAZ inhibits osteoclast differentiation and function.[76] In osteoblasts, YAP/TAZ plays a more complex role. YAP/TAZ promotes osteoblastic differentiation and bone matrix deposition by regulating osteogenic transcription factors such as Runx2 and osteocalcin.[77] However, during endochondral ossification, overexpression of YAP1 inhibits cartilage mineralization, thereby suppressing skeletal development,[78] indicating that YAP/TAZ have different roles depending on the bone cell type and differentiation stage.
YAP/TAZ expression and function vary significantly in different disease states. Studies suggest that their activity may be suppressed in osteoporosis, leading to reduced bone formation and decreased bone density.[79] Meanwhile, during fracture healing, activation of YAP/TAZ promotes osteogenesis and healing, although excessive activation may lead to abnormal bone remodeling, affecting the healing process.[80] Deng et al. found that in specimens of patients with osteoarthritis, YAP protein expression steadily decreased with higher grading according to the Osteoarthritis Research Society International scale. YAP activation can delay disease progression and reduce cartilage damage, whereas YAP knockout accelerates OA progression.[40] Moreover, aberrant activation of YAP/TAZ is closely associated with the onset and progression of bone cancers, such as osteosarcoma, indicating that they play an important role in bone tumors.[81] Taken together, YAP/TAZ’s roles in bone development and related diseases are complex and multifaceted, and a deeper understanding of their molecular mechanisms is crucial for the prevention and treatment of bone diseases and for intervening in bone tumors.
YAP/TAZ’s role in mechano-transduction within tissues not only reveals their potential in tissue engineering and regenerative medicine but also provides new perspectives for the evaluation and design of novel biomaterials. Stem cell self-renewal, proliferation, and differentiation can be influenced by modulating mechanical signals through biomaterials, thereby precisely controlling tissue repair and regeneration processes. For instance, variations in stiffness, stretch, and topography can activate or inhibit YAP/TAZ to regulate stem cell behavior, which is particularly critical in skin and bone tissue engineering.[82] Rigid substrates activate YAP/TAZ, promoting stem cell proliferation and self-renewal, and soft substrates favor differentiation.[83] Enhanced integration of stem cells with host tissues can be achieved by designing novel biomaterials that mimic these mechanical signals, optimizing regenerative outcomes. In conclusion, a deeper understanding of YAP/TAZ signaling mechanisms could not only accelerate the development of new biomaterials but also open new therapeutic avenues for diseases associated with biomechanical dysregulation.
Despite the enormous potential of YAP/TAZ in regenerative medicine and disease therapy, several challenges remain. First, the specific mechanisms of YAP/TAZ in different tissues and pathological conditions are not yet fully understood, and the heterogeneity of cell types and microenvironments complicates the development of unified therapeutic strategies. Preclinical studies, which mainly rely on animal models and in vitro experiments, may not accurately reflect the complexity of human diseases. The safety and specificity of targeted therapies pose challenges because excessive activation or inhibition of YAP/TAZ could lead to side effects. The translation of basic research into clinical applications faces significant technical hurdles, especially in integrating biomaterials and stem cell therapies with YAP/TAZ pathway regulation while ensuring clinical compatibility and long-term effectiveness. The regulation of YAP/TAZ activity may involve multiple approaches, including drugs, gene editing, or nanotechnology, thus requiring further advancements in technology and its translation to clinical use. Future research should focus on overcoming these barriers to facilitate the clinical application of YAP/TAZ-based therapies.
Future prospects of the research
The elucidation of YAP/TAZ roles in musculoskeletal biology and pathology opens numerous promising research avenues and potential clinical applications. Moving forward, a primary prospect is the exploration of YAP/TAZ as therapeutic targets in osteoporosis. Given their role in promoting osteoblast function and inhibiting adipogenesis, modulating YAP/TAZ activity could correct the imbalances in bone turnover seen in osteoporosis.[84] Targeted therapies that enhance YAP/TAZ activity in bone-forming cells could open new avenues for osteoporosis treatment, enhancing bone density and reducing the risk of fractures. In osteoarthritis, dysregulation of YAP/TAZ may provide early indicators of disease risk or early pathological changes.[85] By detecting alterations in YAP/TAZ activation levels or cellular localization, early screening could be conducted before clinical symptoms appear. In treatment monitoring, YAP/TAZ can serve as a biomarker to track therapeutic efficacy.[86] By assessing changes in YAP/TAZ activity, it is possible to monitor the effectiveness of treatment in real-time.
In the field of cartilage regeneration, further investigation into how YAP/TAZ regulates cartilage homeostasis could provide new therapeutic approaches for osteoarthritis. The application of YAP/TAZ in regenerative biomaterials holds great potential because modulating its signaling pathways in response to biomechanical properties could lead to the design of novel biomaterials that mimic the mechanical environment of bone and cartilage, thereby improving tissue engineering outcomes. YAP/TAZ’s role in NPCs offers a new direction for the treatment of degenerative intervertebral disc disease, and precise molecular mechanism studies could guide the development of targeted intervention strategies. Finally, considering the potential role of YAP/TAZ in HO, future research may explore its potential as a predictive biomarker or therapeutic target to reduce pathological calcification.
SUMMARY
YAP and TAZ are essential regulators of homeostasis, enabling structures and cells to adapt to the microenvironment of bone, cartilage, and nucleus pulposus. YAP/TAZ is crucial for bone, cartilage, and nucleus pulposus. They regulate Wnt/β-catenin, PI3K-AKT, Hippo, and other signaling pathways. YAP/TAZ substantially influences the physiological and pathological processes in the bone, cartilage, and nucleus pulposus by mediating biomechanical signal transduction. This function is pivotal in assessing the efficacy of new biomaterials.
ACKNOWLEDGMENT
The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
ACVR1: Activin A receptor type 1
ADSCs: Adipose-derived stem cells
AMOT130: Angiomotin 130
BMP2: Bone morphogenetic protein 2
BMSCs: Bone marrow mesenchymal stem cells
CGRP: Calcitonin gene-related peptide
Chsy3: Chondroitin sulfate synthase 3
circRNA: Circular RNA
CS: Chondroitin sulfate
CTSK: Cathepsin K
ECM: Extracellular matrix
EPCs: Endothelial progenitor cells
ERK: Extracellular signal-regulated kinase
FAs: Focal adhesions
GTPase: Guanosine Triphosphatase
HBM-MSCs: Human bone marrow MSCs
HIF-1α: Hypoxia-inducible factor 1 alpha
hMSC: Human Mesenchymal Stem Cells
HO: Heterotopic ossification
IDD: Intervertebral disc degeneration
IL-6: Interleukin-6
JNK: c-Jun N-terminal kinase
LATS: Large tumor suppressor
LATS1/2: Large tumor suppressor 1/2
lncRNA: Long non-coding RNA
MC3T3: Mus musculus 3T3
MLin: Mesenchymal-like
MLO-Y4: Murine lacrimal gland osteoblast-like cell line 4
Mmp: Matrix metalloproteinase
MSCs: Migration of mesenchymal stem cells
NFATc1: Nuclear factor of activated T-cells cytoplasmic 1
NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells
NPC: Nucleus pulposus cell
OP: Osteoporosis
OSM: Oncostatin M
PGE2: Prostaglandin E2
Ptgs2: Prostaglandin-endoperoxide synthase 2
RANKL: Receptor activator of nuclear factor kappa-B ligand
Rho: Ras homolog
Runx2: Runt-related transcription factor 2
SAV: Salvador
SOX9: Sex determining region Y-box 9
TAK1: Transforming growth factor-beta-activated kinase 1
TAZ: Transcriptional co-activator with PDZ-binding motif
TEAD: Transcriptional enhanced associate domain
TNF-α: Tumor necrosis factor-alpha
TRAP: Tartrate-resistant acid phosphatase
VEGF: Vascular endothelial growth factor
Wnt: Wingless-related integration site
YAP: Yes-associated protein
YWHAH: Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein eta
AUTHOR CONTRIBUTIONS
YH: Contributed to the study design and literature research, participated in manuscript preparation; XQ OY: Contributed to manuscript editing; JHT: Contributed to data acquisition; ZYM: Guarantor of the integrity of the entire study; XWM: Contributed to the study concepts; YGY: Defined the intellectual content and was involved in manuscript review.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This review was based on the previously published studies, thus ethical approval and patient consent are not required.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING: This work was supported by a grant from the National Natural Science Foundation of China (No. 82172505).
References
- YAP1 is essential for osteoclastogenesis through a TEADs-dependent mechanism. Bone. 2018;110:177-86.
- [CrossRef] [PubMed] [Google Scholar]
- Structural features and ligand binding properties of tandem WW domains from YAP and TAZ, nuclear effectors of the Hippo pathway. Biochemistry. 2011;50:3300-9.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of the hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42:862-72.
- [CrossRef] [PubMed] [Google Scholar]
- The role of extracellular biophysical cues in modulating the Hippo-YAP pathway. BMB Rep. 2017;50:71-8.
- [CrossRef] [PubMed] [Google Scholar]
- Yes-associated protein promotes bone healing after tooth extraction in mice. Biochem Biophys Res Commun. 2022;609:39-47.
- [CrossRef] [PubMed] [Google Scholar]
- Structural and metabolic changes in bone. Animals (Basel). 2022;12:1946.
- [CrossRef] [PubMed] [Google Scholar]
- Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024;10:71.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging role and function of Hippo-YAP/TAZ signaling pathway in musculoskeletal disorders. Stem Cell Res Ther. 2024;15:386.
- [CrossRef] [PubMed] [Google Scholar]
- Gone caving: roles of the transcriptional regulators YAP and TAZ in skeletal development. Curr Osteoporos Rep. 2020;18:526-40.
- [CrossRef] [PubMed] [Google Scholar]
- YAP and TAZ couple osteoblast precursor mobilization to angiogenesis and mechanoregulation in murine bone development. Dev Cell. 2024;59:211-27.e5.
- [CrossRef] [PubMed] [Google Scholar]
- Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in mechanically loaded human mesenchymal stem cells for bone tissue engineering. Int J Mol Sci. 2020;21:5816.
- [CrossRef] [PubMed] [Google Scholar]
- Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes. Stem Cell Res Ther. 2018;9:342.
- [CrossRef] [PubMed] [Google Scholar]
- Hierarchical structures of bone and bioinspired bone tissue engineering. Small. 2016;12:4611-32.
- [CrossRef] [PubMed] [Google Scholar]
- Natural compounds for bone remodeling: targeting osteoblasts and relevant signaling pathways. Biomed Pharmacother. 2024;180:117490.
- [CrossRef] [PubMed] [Google Scholar]
- Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle. 2017;16:399-405.
- [CrossRef] [PubMed] [Google Scholar]
- Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat Cell Biol. 2016;18:917-29.
- [CrossRef] [PubMed] [Google Scholar]
- αCGRP Affects bmscs' migration and osteogenesis via the hippo-YAP pathway. Cell Transplant. 2019;28:1420-31.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758-70.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical signals induces reprogramming of mature adipocytes through the YAP/TAZ-binding motif. Exp Cell Res. 2022;415:113109.
- [CrossRef] [PubMed] [Google Scholar]
- Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397-410.e14.
- [CrossRef] [PubMed] [Google Scholar]
- YAP and TAZ: A signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19:454-64.
- [CrossRef] [PubMed] [Google Scholar]
- AMOT130/YAP pathway in topography-induced BMSC osteoblastic differentiation. Colloids Surf B Biointerfaces. 2019;182:110332.
- [CrossRef] [PubMed] [Google Scholar]
- Roles and mechanisms of YAP/TAZ in orthodontic tooth movement. J Cell Physiol. 2021;236:7792-800.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive analysis of novel genes and pathways associated with osteogenic differentiation of adipose stem cells. Dis Markers. 2022;2022:4870981.
- [CrossRef] [PubMed] [Google Scholar]
- The differentiation of human adipose-derived stem cells (hADSCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone. 2011;49:295-303.
- [CrossRef] [PubMed] [Google Scholar]
- A reciprocal role of the Smad4-Taz axis in osteogenesis and adipogenesis of mesenchymal stem cells. Stem Cells. 2019;37:368-81.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibiting PPARγ by erythropoietin while upregulating TAZ by IGF1 synergistically promote osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2016;478:349-55.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological activation of TAZ enhances osteogenic differentiation and bone formation of adipose-derived stem cells. Stem Cell Res Ther. 2018;9:53.
- [CrossRef] [PubMed] [Google Scholar]
- YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 2017;8:15321.
- [CrossRef] [PubMed] [Google Scholar]
- Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179-83.
- [CrossRef] [PubMed] [Google Scholar]
- Caveolin-1 modulates mechanotransduction responses to substrate stiffness through actin-dependent control of YAP. Cell Rep. 2018;25:1622-35.e6.
- [CrossRef] [PubMed] [Google Scholar]
- Extracellular matrix stiffness regulates osteogenic differentiation through MAPK activation. PLoS One. 2015;10:e0135519.
- [CrossRef] [PubMed] [Google Scholar]
- Design of fibronectin type III domains fused to an elastin-like polypeptide for the osteogenic differentiation of human mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai). 2019;51:856-63.
- [CrossRef] [PubMed] [Google Scholar]
- Finding the weakest link: Exploring integrin-mediated mechanical molecular pathways. J Cell Sci. 2012;125:3025-38.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18:728-42.
- [CrossRef] [PubMed] [Google Scholar]
- Integrin αv in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun. 2014;447:352-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical stimulation prevents osteocyte apoptosis: Requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633-43.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325-41.
- [CrossRef] [PubMed] [Google Scholar]
- Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat Commun. 2018;9:4564.
- [CrossRef] [PubMed] [Google Scholar]
- Transforming growth factor β-activated kinase 1 regulates mesenchymal stem cell proliferation through stabilization of Yap1/Taz Proteins. Stem Cells. 2019;37:1595-605.
- [CrossRef] [PubMed] [Google Scholar]
- YAP1 inhibits the induction of TNF-α-stimulated bone-resorbing mediators by suppressing the NF-κB signaling pathway in MC3T3E1 cells. J Cell Physiol. 2020;235:4698-708.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanoregulation of osteoclastogenesis-inducing potentials of fibrosarcoma cell line by substrate stiffness. Int J Mol Sci. 2023;24
- [CrossRef] [PubMed] [Google Scholar]
- The YAP/TAZ transcriptional co-activators have opposing effects at different stages of osteoblast differentiation. Bone. 2018;112:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol. 2007;27:6383-95.
- [CrossRef] [PubMed] [Google Scholar]
- CGRP-modulated M2 macrophages regulate osteogenesis of MC3T3-E1 via Yap1. Arch Biochem Biophys. 2021;697:108697.
- [CrossRef] [PubMed] [Google Scholar]
- Osteal tissue macrophages are involved in endplate osteosclerosis through the OSM-STAT3/YAP1 signaling axis in modic changes. J Immunol. 2020;205:968-80.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol. 2021;236:6376-90.
- [CrossRef] [PubMed] [Google Scholar]
- Co-culture with endothelial progenitor cells promotes the osteogenesis of bone mesenchymal stem cells via the VEGF-YAP axis in high-glucose environments. Int J Med Sci. 2021;18:1628-38.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular endothelial growth factor enhances tendon-bone healing by activating Yes-associated protein for angiogenesis induction and rotator cuff reconstruction in rats. J Cell Biochem. 2020;121:2343-53.
- [CrossRef] [PubMed] [Google Scholar]
- Synergy of molecularly mobile polyrotaxane surfaces with endothelial cell co-culture for mesenchymal stem cell mineralization. RSC Adv. 2021;11:18685-92.
- [CrossRef] [PubMed] [Google Scholar]
- Yap induces osteoblast differentiation by modulating Bmp signalling during zebrafish caudal fin regeneration. J Cell Sci. 2019;132:jcs231993.
- [CrossRef] [PubMed] [Google Scholar]
- Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res Ther. 2015;17:147.
- [CrossRef] [PubMed] [Google Scholar]
- YAP1 negatively regulates chondrocyte differentiation partly by activating the β-catenin signaling pathway. Int J Biochem Cell Biol. 2017;87:104-13.
- [CrossRef] [PubMed] [Google Scholar]
- Loss of Mob1a/b in mice results in chondrodysplasia due to YAP1/TAZ-TEAD-dependent repression of SOX9. Development. 2018;145:dev159244.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia promotes maintenance of the chondrogenic phenotype in rat growth plate chondrocytes through the HIF-1α/YAP signaling pathway. Int J Mol Med. 2018;42:3181-92.
- [CrossRef] [PubMed] [Google Scholar]
- Control of skeletal morphogenesis by the Hippo-YAP/TAZ pathway. Development. 2020;147
- [CrossRef] [PubMed] [Google Scholar]
- LncRNA JPX regulates proliferation and apoptosis of nucleus pulposus cells by targeting the miR-18a-5p/HIF-1alpha/Hippo-YAP pathway. Biochem Biophys Res Commun. 2021;566:16-23.
- [CrossRef] [Google Scholar]
- Inverse agonist of retinoid-related orphan receptor-alpha prevents apoptosis and degeneration in nucleus pulposus cells via upregulation of YAP. Mediators Inflamm. 2021;2021:9954909.
- [CrossRef] [PubMed] [Google Scholar]
- AMOT130 linking F-actin to YAP is involved in intervertebral disc degeneration. Cell Prolif. 2018;51:e12492.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanosensitive transcriptional coactivators MRTF-A and YAP/TAZ regulate nucleus pulposus cell phenotype through cell shape. FASEB J. 2019;33:14022-35.
- [CrossRef] [PubMed] [Google Scholar]
- The hippo pathway orchestrates cytoskeletal organisation during intervertebral disc degeneration. Acta Histochem. 2021;123:151770.
- [CrossRef] [PubMed] [Google Scholar]
- Chondroitin synthase-3 regulates nucleus pulposus degeneration through actin-induced YAP signaling. FASEB J. 2020;34:16581-600.
- [CrossRef] [PubMed] [Google Scholar]
- Irisin ameliorates intervertebral disc degeneration by activating LATS/YAP/CTGF signaling. Oxid Med Cell Longev. 2022;2022:9684062.
- [CrossRef] [PubMed] [Google Scholar]
- IL-6/YAP1/β-catenin signaling is involved in intervertebral disc degeneration. J Cell Physiol. 2019;234:5964-71.
- [CrossRef] [PubMed] [Google Scholar]
- Osteoporosis and the ageing skeleton. Subcell Biochem. 2019;91:453-76.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction of LEF1 with TAZ is necessary for the osteoblastogenic activity of Wnt3a. Sci Rep. 2018;8:10375.
- [CrossRef] [PubMed] [Google Scholar]
- TAZ is required for the osteogenic and anti-adipogenic activities of kaempferol. Bone. 2012;50:364-72.
- [CrossRef] [PubMed] [Google Scholar]
- Circular RNA YAP1 attenuates osteoporosis through up-regulation of YAP1 and activation of Wnt/β-catenin pathway. Biomed Pharmacother. 2020;129:110365.
- [CrossRef] [PubMed] [Google Scholar]
- Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem Biophys Res Commun. 2020;524:883-9.
- [CrossRef] [PubMed] [Google Scholar]
- Rational design of YAP WW1 domain-binding peptides to target TGFbeta/BMP/Smad-YAP interaction in heterotopic ossification. J Pept Sci. 2015;21:826-32.
- [CrossRef] [PubMed] [Google Scholar]
- Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng Part A. 2010;16:725-33.
- [CrossRef] [PubMed] [Google Scholar]
- Discoidin domain receptor 2 regulates aberrant mesenchymal lineage cell fate and matrix organization. Sci Adv. 2022;8:eabq6152.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated BMP and mechanical signaling through YAP1/RhoA poises FOP mesenchymal progenitors for osteogenesis. J Bone Miner Res. 2019;34:1894-909.
- [CrossRef] [PubMed] [Google Scholar]
- TAZ inhibits osteoclastogenesis by attenuating TAK1/NF-κB signaling. Bone Res. 2021;9:33.
- [CrossRef] [PubMed] [Google Scholar]
- BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119-25.
- [CrossRef] [PubMed] [Google Scholar]
- Yap1 regulates multiple steps of chondrocyte differentiation during skeletal development and bone repair. Cell Rep. 2016;14:2224-37.
- [CrossRef] [PubMed] [Google Scholar]
- YAP/TAZ as molecular targets in skeletal muscle atrophy and osteoporosis. Aging Dis 2024
- [CrossRef] [Google Scholar]
- YAP and TAZ promote periosteal osteoblast precursor expansion and differentiation for fracture repair. J Bone Miner Res. 2021;36:143-57.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of the Hippo pathway transcriptional coactivators YAP/TAZ and β1-integrin in conventional osteosarcoma. Oncotarget. 2016;7:64702-10.
- [CrossRef] [PubMed] [Google Scholar]
- Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782-95.
- [CrossRef] [PubMed] [Google Scholar]
- Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat Mater. 2018;17:1063-75.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting sulfation-dependent mechanoreciprocity between matrix and osteoblasts to mitigate bone loss. Sci Transl Med. 2023;15:eadg3983.
- [CrossRef] [PubMed] [Google Scholar]
- The Hippo signalling pathway in bone homeostasis: Under the regulation of mechanics and aging. Cell Prolif. 2024;57:e13652.
- [CrossRef] [PubMed] [Google Scholar]
- Role of YAP/TAZ transcriptional regulators in resistance to anti-cancer therapies. Cell Mol Life Sci. 2017;74:1457-74.
- [CrossRef] [PubMed] [Google Scholar]









