Translate this page into:
Thyroid gland and adjacent lesions: Cytomorphological clues!
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 65-year-old female presented for evaluation of hypertension. Physical examination revealed a painless lump in the neck. Ultrasound examination of the thyroid showed a posteriorly located, hypervascular, single hypoechoic, solid lesion, measuring 1.4 cm, near the upper pole of the left thyroid gland. Figure 1a–d shows the cytomorphological features of the fine needle aspirate in Diff-Quik stained smears. Papanicolaou (Pap) stained smears were suboptimal with scant cellularity.
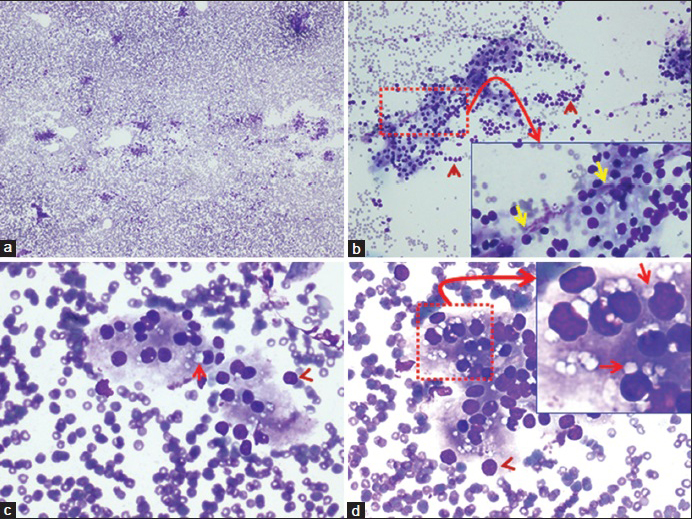
- Fine-needle aspiration of neck lesion (Diff-Quik stained preparation). (a) Mildly cellular smear without colloid (×4). (b) Showing small groups of cells forming syncitia, admixed with a few bare nuclei (arrowheads). Inset of “b” (×40 Zoomed) shows occasional fragment of capillary stroma with loosely attached cells (yellow arrows) (×10). (c and d) The cells showed solitary paranuclear intracytoplasmic vacuoles (arrows) without paravacuolar granules. A few bare nuclei are seen at the periphery of the groups (arrowheads). Inset of “d” (×40 Zoomed) highlights the paranuclear intracytoplasmic vacuoles indenting the nuclei (arrows) in many intact cells (×40)
WHAT IS YOUR INTERPRETATION?
-
Follicular lesion with Hurthle cell change
-
Hyperplastic thyroid nodule
-
Parathyroid gland tissue
-
Thyroiditis with Hurthle cell change.
ANSWER
The correct cytopathologic interpretation is:
· Parathyroid gland tissue
Fine-needle aspiration (FNA) biopsy is a simple and minimally invasive method for evaluating neck mass lesions. The identification of parathyroid gland tissue and its lesions may be challenging. The differential diagnoses include thyroid nodules, lymphoid tissue, adipose tissue, thymus, metastatic tumors, and paraganglioma. The algorithmic approach shown in Figure 2 would facilitate the cytomorphological interpretation.[1234]
Both thyroid and parathyroid gland lesions may show similar architecture consisting of tissue fragments or loose rounded clusters of epithelial cells. Parathyroid gland tissue may sometimes show follicle formation [Figure 1a–d] with colloid-like material in the background.
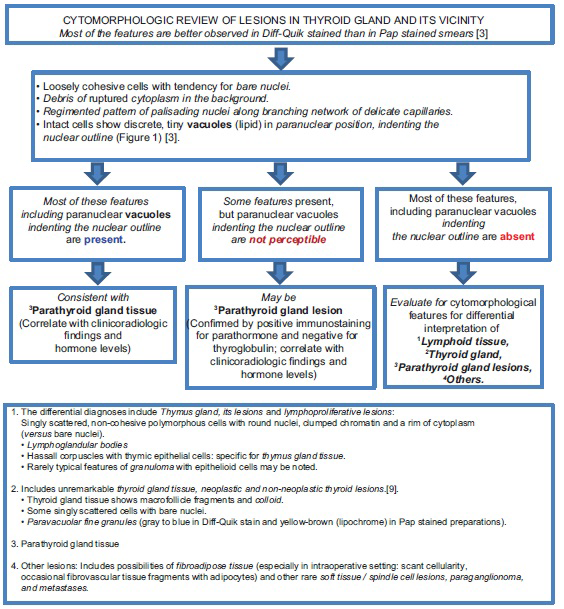
- Algorithm to guide broader cytomorphological interpretation of cytology preparations of the lesions in the vicinity of or in thyroid gland (with reference to parathyroid gland tissue/lesions)
Hyperplastic nodular goiter shows groups of follicular cells in honey-comb pattern with evenly spaced nuclei and colloid in the background. Follicular lesions show microfollicles and three-dimensional microfollicle complexes with relative lack of colloid. The nuclei are round to oval, relatively larger, that is, 7–9 μm in diameter, with smooth nuclear membrane, uniformly distributed granular to compact chromatin, and inconspicuous nucleoli.[3456]
Parathyroid lesions may also show oncocytic changes and may be clinically silent. The definitive distinction from thyroid follicular cells with oncocytic changes may be challenging, and ancillary support such as immunohistochemistry may be needed.[78]
Oncocytic change may be associated with hyperplastic nodular goiter, thyroiditis, and follicular lesions. The Hurthle cells are larger than follicular epithelial cells; possess well-defined cell borders and abundant finely granular cytoplasm. The nuclei are enlarged, eccentrically to centrally located, round to oval, with fine to coarsely granular chromatin, and prominent nucleoli.
Features favoring parathyroid over thyroid tissue:
-
Regimented pattern of palisading nuclei along branching network of delicate capillaries [Inset of Figure 1b].
-
Parathyroid cells are slightly smaller in size, measuring 6-7 μm in diameter. They possess central nuclei and abundant pale cytoplasm without paravacuolar lipochrome granules (seen as gray to blue granules in Diff-Quick stained and as brown granules in Pap stained smears in thyroid follicular cells)[3] [Figure 1b–d].
-
Stippled nuclear chromatin. Better seen in Pap stained smears.[3]
-
Intracytoplasmic vacuoles indenting the nucleus (seen distinctly in Diff-Quick stained smears) are present in parathyroid gland cells. Intra and intercellular lipid is depleted or absent in parathyroid hyperplasia and adenoma.[23] In such cases, other cytomorphological features and ancillary tests may be needed to confirm the nature of cells
-
Granular lacy background, owing to the ruptured cytoplasm.
-
Parathyroid cells are immunoreactive for parathormone with nonimmunoreactivity for thyroid transcription factor-1 (TTF-1) and thyroglobulin (thyroid follicular cells are immunoreactive for TTF-1 and thyroglobulin).[1234]
-
Surgical pathology sections (e.g. - frozen section) may show parathyroid follicles with colloid-like contents. Intraoperative cytopathology of imprint smears of tiny tissue fragments usually submitted for frozen sectioning in these situations is a dependable adjunct. Most of the cytomorphological features are better seen in Diff-Quik stained smears. However, Pap stained smears are also important for studying nuclear features including chromatin.[3]
Follow-up of present case
The serum chemistry showed elevated parathormone and serum calcium levels.
Parathyroid scan showed a parathyroid adenoma.
ADDITIONAL QUIZ QUESTIONS
Q1. Which of the following cytomorphological features is highly reproducible for parathyroid gland tissue in Diff-Quik stained preparations?
-
Intracytoplasmic lipid vacuoles, indenting the nucleus
-
Bare nuclei
-
Para vacuolar granules
-
Oncocytic cytoplasm
Q2. Which of the following cytomorphological features favor parathyroid neoplasms over thyroid follicular neoplasms?
-
Regimented pattern of palisading nuclei along branching network of delicate capillaries
-
Bare nuclei
-
Cytoplasmic vacuoles, indenting the nucleus
-
All of the above
Q3. Which of the following features does not favor parathyroid lesions?
-
Intracytoplasmic lipid vacuoles
-
Immunoreactivity for calcitonin
-
Oncocytic cytoplasm
-
Metachromatic neurosecretory granules
Answers to additional quiz questions
1.a; 2.d; 3.b
-
(a): [Figure 1c and d] the fat vacuoles appear as discrete, round to oval intracytoplasmic spaces with a sharp outline, and a tendency to indent a portion of nucleus, touching it, subtly. They are a hallmark of parathyroid gland cells and are most numerous in normal parathyroid glands.[3]
Both intercellular and intracellular lipid vacuoles decrease in parathyroid hyperplasia and adenoma. The intracytoplasmic fat vacuoles may also be seen in the setting of lipoadenoma and parathyroid hamartoma. Imprint smears preserve the cytoplasm of individual fragile cells better than scrape smears (and hypothetically conventional smears of FNA aspirates), permitting better visualization of intracytoplasmic fat vacuoles[356] [Figure 1c–d]. Some studies do not describe the vacuoles, but they are observed in their published Diff-Quik images of FNA of parathyroid lesions [Figure 2 pg. 409].[7] Rarely nonspecific vacuoles may be present in other neck lesions. However, they may not be solitary, paranuclear, and do not indent the nucleus.
Bare nuclei, devoid of cytoplasm may be dispersed singly [arrow heads in Figure 1b–d]. They may also be observed in aspirates from thyroid lesions, lymphoid neoplasms, and metastatic small cell carcinoma.
-
(d): [Figure 1b - Inset] regimented pattern of palisading nuclei along branching network of delicate capillaries is typically seen in parathyroid lesions and differentiates it from thyroid nodules.[1234] However, similar features may also be seen in carotid body tumors (paraganglioma), metastatic tumors and other neuroendocrine lesions.
-
(b): Chief cells of the parathyroid gland show cytoplasmic lipid, better observed in Romanowski stained preparations such as Diff-Quik stained smears [Figures 1d - Inset]. Hyperplastic and neoplastic glands tend to have less cytoplasmic lipid and smaller droplets than normal or atrophic parathyroid cells. Glycogen may be present in clear cells and stains with periodic acid-Schiff.
Neurosecretory granules may be seen as metachromatic inclusions in the cytoplasm, stained with Romanowsky stains.[2]
Immunoreactivity for calcitonin is a specific feature of parafollicular cells (C cells) and medullary carcinoma thyroid.
BRIEF REVIEW OF THE TOPIC
Awareness of cytomorphological features of parathyroid and other anatomical structures in the vicinity, inclusive of lymph nodes, thyroid, and branchial cleft remnants is important to make a definitive diagnosis.
Cytomorphological features suggesting parathyroid gland lesion may be encountered in the following clinical scenarios:
-
Suspected parathyroid lesion.
-
Intraoperative consultation: Frozen section evaluation of tissues in the vicinity of thyroid gland including thyroidectomies and parathyroid gland surgeries.
-
Evaluation of hyperparathyroidism.
-
Incidental finding on FNA, as in intrathyroidal parathyroid or ectopic parathyroid.
-
FNA of hypoechoic thyroid nodules.
-
FNA of parathyroid cyst.
-
FNA of ectopic parathyroid while evaluation of neck nodule.
-
FNA of thyroid bed, status post thyroidectomy.
Clinical associations
-
Hypercalcemia
-
Nephrolithiasis
-
Bone lesions (brown tumors)
Radiological correlation[17]
-
Normal parathyroid glands are not visualized by ultrasonography.
-
Parathyroid adenomas are usually hypervascular, hypoechoic, ovoid or lobulated lesions, associated with extrathyroidal feeding artery and one or more vascular pedicles.
-
They may show cysts and calcifications.
Fine-needle aspiration biopsy
Fine-needle aspiration biopsy of parathyroid lesions is a challenging procedure, with variable yield of diagnostic material. The inadequacy rates, range from 8.3% to 28.1%. The rate of contamination with thyroid follicular epithelial cells varies from 8.3% to 31.5%.[7]
FOCUSED DIFFERENTIAL DIAGNOSIS OF PARATHYROID GLAND LESIONS
Normal parathyroid glands
-
Architectural patterns: Solid sheets, branching anastomosing cords and acinar structures with rich vascularity.
-
Cellularity: Admixture of parenchyma and adipose tissue.
-
Cell types: Chief cells, oncocytic/oxyphilic cells, and water clear cells.
-
Cell size: Slightly smaller than follicular epithelial cells from thyroid.
-
Cytoplasm: Moderate amount of pale granular cytoplasm with small intracytoplasmic lipid vacuoles with a tendency to indent the nucleus [Figure 1c and d]
-
Oxyphil cells are slightly larger and have abundant oncocytic cytoplasm. The nuclei are round, central to eccentric, with dense chromatin, and prominent dark nucleoli.
-
Water clear cells are rarely seen in normal parathyroid glands. They have faintly eosinophilic to clear cytoplasm with abundant glycogen deposits and sharply defined cell membranes.[129]
-
Immunohistochemistry: Cytoplasmic immunoreactivity for keratin, chromogranin A, and parathormone. Lack of immunoreactivity for vimentin, glial fibrillary acidic protein, neurofilament, and chromogranin B.[10]
Parathyroid cysts
-
Derived from embryologic remnants, coalescence of microcysts or degeneration of an adenoma.
-
Contents of parathyroid cyst: Clear, watery; occasionally golden brown. The fluid is acellular or hypocellular.
-
Cytoarchitecture: Tissue fragments, honeycomb sheets or microfollicles.
-
Cells: Small, cuboidal, with round nuclei and granular to compact chromatin.
-
Background: Proteinaceous debris.
-
Differential diagnosis: Cystic degeneration of nodular goiter, branchial cleft cyst, thymic cyst, and thyroglossal duct cyst.
Parathyroid adenoma and hyperplasia
-
Cellularity: Moderate cellularity.
-
Architecture/cellular distribution: Two- or three-dimensional clusters, papillary fragments, complex branching, follicular pattern, dispersed single cell pattern. A branching network of capillaries and neoplastic cells arranged alongside capillaries, in a regimented pattern, is characteristic.
-
Cell size and shape: Monomorphous round to oval cells that exhibit stippled nuclear chromatin and high nucleo-cytoplasmic ratio. Endocrine atypia may be pronounced. Spindle-shaped cells may be seen.
-
Background: Inspissated colloid like material may be present, either mixed with the cells or distributed separately within the vicinity of cells. Macrophages, fat globules, delicate branching, vascularized stromal tissue fragments may be present.
-
Bare nuclei: Seen commonly in abundance.
-
Nuclear morphology: Round to oval, uniform, smooth membrane, coarsely granular/stippled chromatin, with micronucleoli. Intranuclear inclusions, nuclear pleomorphism/endocrine atypia, nuclear moulding, single vacuoles may be present. Mitosis and karyorrhexis should typically be absent in adenoma.
-
Distinction of different parathyroid lesions (hyperplasia versus adenoma) may not be possible on cytomorphologic evaluation alone.
Parathyromatosis
-
Multiple small nodules, containing bland cells are disseminated in the soft tissues of the neck.
-
They may be associated with Multiple Endocrine Neoplasia 1 (MEN 1) syndrome.
Parathyroid carcinoma
-
The lesions are extremely cellular
-
Cells: Small, medium, or large. Either monomorphic or pleomorphic in appearance. Cells with clear cytoplasm and tumor giant cells may be present. The cells are dyshesive with anaplasia, macronucleoli, typical and atypical mitoses, and necrosis.
-
High Ki-67 index, Cyclin D1 expression, and lower p27 indices indicate parathyroid carcinoma. Galectin-3 is expressed in parathyroid carcinomas and not in adenoma.[29]
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author.
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
As this is a quiz case without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent).
LIST OF ABBREVIATIONS
FNA = Fine-needle aspiration
TTF-1 = Thyroid Transcription Factor-1
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, all Quiz cases are reviewed by Quiz case section team prior to be accepted for publication.
REFERENCES
- Thyroid and parathyroid. Color Atlas of Differential Diagnosis in Exfoliative and Aspiration Cytopathology 2011:401-541.
- [Google Scholar]
- Intraoperative cytology increases the diagnostic accuracy of frozen sections for the confirmation of various tissues in the parathyroid region. Am J Clin Pathol. 2002;118:895-902.
- [Google Scholar]
- Tumors of the thyroid and parathyroid gland. Diagnostic Histopathology of Tumors 2013:1177-250.
- [Google Scholar]
- Intraoperative cytologic evaluation of lipid in the diagnosis of parathyroid adenoma. Am J Surg Pathol. 1988;12:282-6.
- [Google Scholar]
- Ultrasound guided fine needle aspiration biopsy of parathyroid gland and lesions. Cytojournal. 2006;3:6.
- [Google Scholar]
- Parathyroid fine-needle aspiration cytology in the evaluation of parathyroid adenoma: Cytologic findings from 53 patients. Diagn Cytopathol. 2009;37:407-10.
- [Google Scholar]
- Oncocytic parathyroid adenoma: Problem in cytological diagnosis. Diagn Cytopathol. 2004;31:276-80.
- [Google Scholar]
- Parathyroid glands. In: Rosai and Ackerman’s Surgical Pathology (10th ed). Philadelphia PA: Elsevier; 2011. p. :565-83.
- [Google Scholar]
- Follicular neoplasm, hurthle cell type/suspicious for a follicular neoplasm, hurthle cell type. In: Ali SZ, Cibas ES, eds. The Bethesda System for Reporting Thyroid Cytopathology. Definitions, Criteria, and Explanatory Notes. New York: Springer; 2010. p. :59-73. Based on NCI Thyroid Fine Needle Aspiration State of the Science Conference (Oct 22.23, 2007), Springer
- [Google Scholar]







