Translate this page into:
Tumor necrosis factor receptor-associated factor 5 enhances perianal fistulizing Crohn’s disease through epithelial–mesenchymal transition

Yunfei Gu

Youran Li
*Corresponding authors: Yunfei Gu, Department of Colorectal Surgery, The Affiliated Hospital of Nanjing University of Chinese Medicine, Jiangsu Province Hospital of Chinese Medicine, The First Clinical Medical College, Nanjing, Jiangsu, China. Guyunfei127@126.com
Youran Li Department of Colorectal Surgery, The Affiliated Hospital of Nanjing University of Chinese Medicine, Jiangsu Province Hospital of Chinese Medicine, The First Clinical Medical College, Nanjing, Jiangsu, China. magic8811@sina.com.
-
Received: ,
Accepted: ,
How to cite this article: Sun X, Gao H, Lu L, Wang Q, Li Y, Gu Y. Tumor necrosis factor receptor-associated factor 5 enhances perianal fistulizing Crohn’s disease through epithelial–mesenchymal transition. CytoJournal. 2024;21:82. doi: 10.25259/Cytojournal_148_2024
Abstract
Objective:
Crohn’s disease (CD) is a chronic inflammatory condition of the bowel that remarkably impairs a patient’s quality of life and often has a poor prognosis. Perianal fistulizing CD (PFCD) is one of the most common parenteral symptoms of CD and a huge challenge for the management of this illness. This study aimed to elucidate the molecular mechanisms underlying PFCD and identify potential biomarkers to advance our understanding and management of this condition.
Material and Methods:
Transcriptome sequencing was performed using the control and PFCD groups to investigate the mechanisms of PFCD development. The expression of tumor necrosis factor receptor-associated factor 5 (TRAF5), nuclear factor-kappa B (NF-κB), and interleukin 13 (IL-13) messenger ribonucleic acid (mRNAs) was detected by quantitative polymerase chain reaction (qPCR). Pathological morphology was observed using hematoxylin and eosin staining. The expression of TRAF5, Epithelial Cadherin (E-cadherin), Snail family transcriptional repressor 1 (SNAIL1), and vimentin protein was detected by immunohistochemistry. Following the knockdown of TRAF5 in human tumor-29 (HT-29) cells, the effects on cell proliferation and migration were assessed using the cell counting kit-8 and Transwell assays. The expression levels of crucial markers were analyzed by qPCR, Western blot, and immunohistochemistry.
Results:
Transcriptomic sequencing revealed a significant upregulation of TRAF5 in the PFCD group, accompanied by elevated mRNA levels of NF-κB and IL-13 compared with those in the control group. In addition, the PFCD group exhibited increased expression of TRAF5, SNAIL, and vimentin and marked reduction in E-cadherin levels, indicating that PFCD may facilitate epithelial–mesenchymal transition (EMT). Knocking down TRAF5 in HT-29 cells reduced cell proliferation and migration; inhibited NF-κB and IL-13 mRNAs, SNAIL1, and vimentin levels; and promoted E-cadherin levels.
Conclusions:
The development of PFCD was associated with EMT, and TRAF5 was a key gene of PFCD. Knocking down TRAF5 alleviated the EMT promotion of PFCD, indicating that TRAF5 drove the development of PFCD through EMT.
Keywords
Crohn’s disease
Epithelial–mesenchymal transition
Tumor necrosis factor receptor-associated factor 5
Nuclear factor-kappa B
Interleukin 13
INTRODUCTION
Crohn’s disease (CD) is a persistent inflammatory condition that often occurs in the right half of the colon and terminal ileum of human body, though it can influence any region of the digestive tract.[1] The prevalence of CD has continued to rise over the past few years,[2] highlighting the importance of in-depth studies on this disease. However, the cause of CD is not clear. A disorder of the innate and adaptive immune response resulting from an intricate interplay of genetic susceptibility, environmental factors, and intestinal flora changes[3] possibly triggers this disease. Statistical reports indicate that 25–80% of adults with CD experience perianal complications, including anal fistula, perianal abscess, anal fissure, and rectal stenosis.[4] Among them, perianal fistulizing CD (PFCD) is the most frequent parenteral symptom of CD.[5] Its fistula running is generally complex and difficult to treat. Medical or surgical treatment alone cannot provide satisfactory clinical results, and this condition often requires integral options for comprehensive treatment. Recommended drugs for PFCD management mostly include biological agents, immunosuppressants, antibiotics, and aminosalicylic acid and its precursor drugs.[6-8] In general, hanging drainage or catheter drainage is performed for patients with clinically acute fistula CD, and definitive surgery is feasible for perianal CD in remission or without concurrent infection. Despite clinical treatment, the wound often remains extensive and resistant to healing, exposing patients to a high recurrence rate and significantly impacting their well-being.[9] This phenomenon underscores the need to investigate the molecular mechanisms and new biomarkers of PFCD to prevent its development.
The epithelial–mesenchymal transition (EMT) is a complex biological phenomenon involving a multitude of steps and molecular mechanisms. In this process, epithelial cells lose connection to recombine their cytoskeleton, enhancing migration, invasion, and anti-apoptosis.[10] These cells are crucial in embryonic development, tissue repair, fibrosis, and cancer metastasis.[11,12] EMT may be associated with PFCD.[13] One of the characteristics of PFCD is the transformation of epithelial cells that have undergone EMT into mesenchymal cells. In the environment surrounding CD fistulas, EMT is induced by diverse signaling molecules, such as transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and interleukin-13 (IL-13). During this process, specialized epithelial cells are transformed into mesenchymal cells under the stimulation of endothelial cell transition inducers TGF-β, TNF-a, and IL-13.[14] Studies on the mechanism of CD fistula demonstrated that two-thirds of non-epithelial fistulas are lined by a layer of myofibroblast-like cells, that is, “transitional cells” (TCs), which undergo EMT.[15] Therefore, EMT may be crucial in the formation and development of CD fistula. Targeting this process could offer a new approach for treating PFCD, highlighting the potential for novel therapeutic strategies.
Transcriptomic sequencing is a widely applied technology for the transcriptomic investigation of cells and tissues. It can provide an overall perspective of gene expression in different physiological or pathological states. This technique has been applied in diseases including colorectal cancer,[16] breast cancer,[17] and gastric cancer.[18] When used for PFCD, transcriptomic sequencing can provide valuable information on the pathogenesis of PFCD. Therefore, this study investigated EMT and its role in PFCD through transcriptomic sequencing and performed validation through cell experiments to explore the developmental mechanism of PFCD and optimal treatment strategies, providing new ideas and methods for managing PFCD. The role of TRAF5 in the formation of CD-related fistula was clarified from the perspectives of transcriptomics, pathology, and molecular biology. The findings provided new insights into the pathogenesis of CD-related fistula and laid the foundation for formulating clinical treatment strategies.
MATERIAL AND METHODS
This study was approved by the Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine under the approval number 2023NL-062-01 and was guided by the Declaration of Helsinki. All the participants provided written informed consent.
Experimental grouping
Twelve clinical samples were collected and divided into two groups: Control (normal human colorectal tissue samples) and PFCD (post-operative patients with PFCD). Six patients in each group were subjected to transcriptomic sequencing, quantitative polymerase chain reaction (qPCR), hematoxylin and eosin (HE) staining, and immunohistochemical detection. The baseline characteristics of the patients are shown in Table 1.
| Groups | Number | Sex | Age | Past history | Family history | WBC (/L) | HGB (g/L) | PLT (/L) | AST (U/L) | ALT (μmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 | W | 36 | 0 | 0 | 5.63 | 74 | 276 | 11 | 8 |
| 2 | W | 27 | 0 | 0 | 6.7 | 103 | 303 | 14 | 11 | |
| 3 | W | 37 | 0 | 0 | 6.09 | 133 | 264 | 25 | 233 | |
| 4 | M | 32 | 0 | 0 | 10.21 | 149 | 292 | 31 | 57 | |
| 5 | M | 34 | 0 | 0 | 4.34 | 85 | 219 | 12 | 6 | |
| 6 | M | 38 | 0 | 0 | 9.22 | 143 | 304 | 14 | 15 | |
| PFCD | 1 | W | 22 | 0 | 0 | 8.15 | 98 | 347 | 14 | 6 |
| 2 | M | 37 | 0 | 0 | 5.57 | 144 | 286 | 23 | 30 | |
| 3 | M | 30 | 0 | 0 | 4.15 | 148 | 259 | 28 | 18 | |
| 4 | M | 33 | 0 | 0 | 5.79 | 143 | 291 | 28 | 25 | |
| 5 | M | 35 | 0 | 0 | 7.94 | 99 | 497 | 22 | 23 | |
| 6 | M | 31 | 0 | 0 | 5.74 | 146 | 193 | 27 | 58 |
M: Man, W: Woman, WBC: White blood cell, HGB: Hemoglobin, PLT: Platelet, AST: Glutamic oxaloacetic transaminase, ALT: Glutamic-pyruvic transaminase, PFCD: Perianal fistulizing Crohn’s disease
Transcriptomic sequencing analysis
Transcriptomic sequencing data were analyzed using a bioinformatic approach with R 4.3.2 (Lucent Technologies Inc., Union, NJ, USA, https://www.r-project.org/). Differentially expressed genes (DEGs) were identified by R programming, with statistical significance determined by controlling the false discovery rate. The DEGs were considered significant if P-value was below 0.05. Genes with a log2 fold change (log2FC) >1.5 were classified as upregulated, and those with a log2FC <−1.5 were classified as downregulated. Fold change was calculated using the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichments were analyzed with R, and the significance of the enrichment was assessed by adjusting p-values for multiple hypothesis testing.
Vector construction
Short hairpin ribonucleic acid (shRNA)-tumor necrosis factor receptor-associated factor 5 (TRAF5) (NM_001033910.3) vector was designed and established by Chongqing Biomedicine Biotechnology Co., Ltd. (Chongqing, China). In particular, short hairpin TRAF5 (shTRAF5) was constructed using the pLVX-shRNA2-puro vector (KL-12240VT, KALANG, Shanghai, China) and validated for sequencing. TRAF5-oligo1: GCTGGAGGGTACTTGCTATAA; top strand: 5'-GATCCGGCTGGAGGGTACTTGCTATAACTCG AGTTATAGCAAGTACCCTCCAGCTTTTTTG-3'; bottom strand: loop sequence of 5'-AATTCAAAAAAGCTGGAGG GTACTTGCTATAACTCGAGTTATAGCAAGTACCCTCC AGCCG-3' shRNA was CTCGAG.
Cell model establishment
Human tumor-29 (HT-29) cells (CL-0118, Procell, Wuhan, China) were planted in plates at a cell density of 2 × 105 cells/mL. After adherence, the cells were assigned to control group, TGF- β1 group, TGF- β1 + short hairpin negative control (shNC) group, and TGF- β1 + shTRAF5 group. The control group was given normal culture using complete Dulbecco’s modified eagle medium (DMEM) (C11995500BT, Gibco, Waltham, MA, USA). The TGF-β1 group was cultured using complete DMEM with a final concentration of 10 ng/mL TGF- β1 (P02279, Solarbio, Beijing, China). The TGF- β1 + shNC group was cultured with complete DMEM containing 10 ng/mL TGF- β1 after transfection with an empty vector. The TGF- β1 + shTRAF5 group was cultured with complete DMEM containing 10 ng/mL TGF- β1 at final concentration after transfection with TRAF5 interfering plasmids. Samples were collected after 48 h for subsequent testing. All cell lines were validated through short tandem repeat profiling and confirmed to be free of mycoplasma contamination.
Cell counting kit-8 (CCK-8)
For cell viability assessment, 10 μL of CCK-8 solution (C0038, Beyotime Biotechnology, Shanghai, China) was added to the treated cells in a 96-well plate. Wells without cells served as blank controls. After 1-h incubation, absorbance at 450 nm was measured using a microplate reader (CMax Plus, Molecular Devices Instruments Co., Ltd., USA).
Transwell assay
Well-grown cells were digested with trypsin-EDTA solution (0.25%) (S310KJ, BasalMedia, Shanghai, China), centrifuged, resuspended with serum-free medium, and adjusted to a density of 5 × 105 cells/mL. In Transwell chambers with 8.0 μm pore size (3421, Corning, NY, USA), 100 μL of cell suspension was introduced to each well and incubated for 24 h. Following two cycles of washing with calcium-free phosphate-buffered saline (PBS) (G0002, Servicebio Biotechnology, Wuhan, China), the cells were fixed with 4% paraformaldehyde (prepared using PBS) (DF0135, Leagene Biotechnology, Beijing, China), stained with 0.1% crystal violet (1425163, Leagene, Beijing, China), and counted under a ×100 microscope (CKX3-SLP, OLYMPUS, Tokyo, Japan).
HE staining
The samples were rinsed with PBS (G0002, Servicebio, Wuhan, China), fixed with 4% paraformaldehyde (59– 104, Labcoms, Beijing, China), dehydrated with ethanol (Chuandong Chemical, Chongqin, China), and treated with xylene (Chuandong Chemical, Chongqin, China) for transparency. The treated tissues were soaked in melted paraffin (39601006, Leica, German) for 2 h, prepared into 2.5 μm slices, tiled on superfrost slides, and baked at 55°C. The paraffin sections were treated with xylene for dewaxing, ethanol for rehydration, and double distilled water for cleaning and then subjected to hematoxylin staining (G1004, Servicebio, Wuhan, China) for 5 min. The sections were then treated with 1% ethanol hydrochloride for discoloration and eosin solution (G1002, Servicebio, Wuhan, China) for staining for 2 min, rinsed with tap water, dehydrated with ethanol, dewaxed with xylene, and finally embedded in neutral resin (10004160, Sinopharm, Beijing, China). Pathological changes were observed under a microscope (MF53, Guangzhou MSHOT Optoelectronic Technology, Guangzhou, China).
qPCR
Total ribonucleic acid (RNA) of each cell group was isolated utilizing the Trizol reagent (B511311-0500, Sangong Biotech, Shanghai, China), and 1 μL of RNA extract from each sample was added to the spectrophotometer (NanoDrop One/One C, Thermofisher, Waltham, MA, USA) for concentration and purity. complementary deoxyribonucleic acid (cDNA) synthesis was performed following the kit instructions (TSK302M, Tsingke Biotechnology, Beijing, China), and the qPCR reaction system (TSE002, Tsingke Biotechnology, Beijing, China) was prepared. The reaction was conducted in a real-time fluorescence qPCR instrument (IQ5, BioRad, Hercules, CA, USA). As an internal parameter, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression levels were calculated by 2 −ΔΔCT. The sequences of experimental primers are provided in Table 2.
| Primer name | Sequences |
|---|---|
| TRAF5-F | GTTCTGAAGCGGAATGGCTT |
| TRAF5-R | TTCCAACCGCTCCACAAACT |
| NF-κB-F | CCGCTTAGGAGGGAGAGC |
| NF-κB-R | CTGCCATTCTGAAGCTGGTG |
| IL-13-F | TGGAGGACTTCTAGGAAAACG |
| IL-13-R | TCTGGTTCTGGGTGATGTTGA |
| h-GAPDH-F | TCAAGGCTGAGAACGGGAAG |
| h-GAPDH-R | CTCCTCCTCCTCCTGCTTCT |
TRAF5: Tumor necrosis factor receptor-associated factor 5, NF-κB: Nuclear factor-kappa B, IL-13: Interleukin 13, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, A: Adenine, C: Cytosine, G: Guanine, T: Thymine
Immunohistochemistry
The paraffin slices were dewaxed with xylene, rehydrated with ethanol, and washed with double distilled water. The antigen was boiled for 30 min, treated with 3% Hydrogen peroxide for 15 min, and sealed using goat serum (C0265, Beyotime Biotechnology, Shanghai, China) at room temperature for 30 min after PBS washing. The primary antibody was added for incubation overnight at 4°C. After PBS cleaning, secondary antibody was added for incubation 1.5 h. After another PBS cleaning, 3,3'-diaminobenzidine chromogenic solution (ZLI-9019, ZSGB-BIO, Beijing, China) was added for color development. The samples were stained with hemp semen dyeing solution (G1004, Servicebio, Wuhan, China) for 1 min. Differentiation was performed using Acid Alcohol Fast Differentiation Solution (C0163M, Beyotime Biotechnology, Shanghai, China), rinsed with tap water, dehydrated, dewaxed, sealed with neutral gum, and observed under a microscope (ICX41, Sunny Optical Technology (Group) Co Ltd., China). The antibodies included TRAF5 (1:200, bs-16563R, Bioss, Beijing, China), Epithelial cadherin (E-cadherin) (1:500, A22850, ABclonal, Wuhan, China), SNAIL1 (1:200, bs-219581R, bioss, Beijing, China), vimentin (1:200, R22775, ZEN- bioscience, Chengdu, China), and HRP-labeled goat anti-rabbit immunoglobulin G (IgG) (H + L) (1:50, A0208, Beyotime, Shanghai, China). Images were quantified using ImageJ (version 1.53, Wayne Rasband, National Institutes of Health, https://imagej.nih.gov/ij/) (the percentage of positive cell stained area to total stained area is optical density).
Western blot (WB)
WB assay was employed to evaluate protein levels of TRAF5, E-cadherin, SNAIL1, and vimentin in each cell group. The cell samples were transferred to a 1.5 mL centrifuge tube and precipitated by centrifugation at 3000 rpm. Radio immunoprecipitation Assay (RIPA) lysis buffer (p0013B, Beyotime, Shanghai, China) was added to extract the proteins. Protein concentration was quantified through BCA method. The protein samples were then combined with ×5 SDS loading buffer (8015011, Dakewei, Beijing, China), heated in a boiling water bath to denature, and separated by 10% SDS-PAGE gel electrophoresis. The gel was transferred onto polyvinylidene difluoride (10600023, Amersham, Germany) membrane, which was then placed in TBST buffer and blocked with 5% skim milk at room temperature for 1 h. Incubation with the primary antibodies, including TRAF5 (1:1,000), (41658T, univ-bio, Shanghai, China), E-cadherin (1:2,000), (A20798, ABclonal, Wuhan, China), SNAIL1 (1:10,000), (A24806, ABclonal, Wuhan, China), Vimentin (1:1,000), (A2584, ABclonal, Wuhan, China), and GAPDH (1:100,000), (A19056, ABclonal, Wuhan, China), was performed overnight on a shaker at 4°C. The secondary antibody, horseradish peroxidase (HRP) goat anti-Rabbit IgG (H + L) (AS014, ABclonal, Wuhan, China), was diluted at a ratio of 1:2000 in TBST and incubated at room temperature for 1 h. Immunoreactivity was detected using the enhanced chemiluminescence (ECL) reagent (34580, Thermo, Massachusetts, USA) and imaged with a nucleic acid and protein gel imaging system (Universal Hood II, Bio-Rad, CA, USA). The grayscale values of the bands were analyzed with ImageJ (version 1.53, Wayne Rasband, National Institutes of Health, https://imagej.nih.gov/ij/) for the determination of relative protein expression by calculating the ratio of the target protein’s grayscale value to that of GAPDH.
Data statistics and analysis
R 4.3.2 version (Lucent Technologies Inc., Union, NJ, USA, https://www.r-project.org/) was used for the bioinformatics analysis of transcriptomic sequencing results, and GraphPad 8.0 software (GraphPad Company, San Diego, CA, USA) was employed for one-way analysis of variance and plotting. Duncan’s multiple comparisons were conducted to determine the differences between groups. Data are presented as mean ± standard deviation (x ± s), with statistical significance defined as P < 0.05.
RESULTS
Transcriptomic sequencing reveals a key role of TRAF5 in PFCD
The volcano map revealed 1942 DEGs between the two groups, of which 1103 were up-regulated in the PFCD group and 839 were down-regulated in the PFCD group [Figure 1a]. These DEGs were subjected to GO analyses, covering biological process (BP), cellular component (CC), and molecular function (MF). Significant enrichment was observed in BP terms such as immune response, inflammatory response, and regulation of immune response; CC terms such as external side of plasma membrane, cell periphery, and extracellular space; and MF terms such as transmembrane signaling receptor activity, TNF receptor binding, and chemokine activity [Figure 1b]. KEGG analyses revealed the significant enrichment of DEGs in several pathways, including cytokine– cytokine receptor interaction, viral protein interaction with cytokine and cytokine receptor, chemokine signaling pathway, toxoplasmosis, and TNF signaling pathway [Figure 1c]. KEGG enrichment results revealed that the TNF signaling pathway was significantly enriched and involved in the development of PFCD. Therefore, we focused on the DEGs in the TNF signaling pathway. TRAF5, the DEG of this pathway, was significantly up-regulated in the disease group, contributing to disease progression. Li et al.[19] suggested that mice with TRAF5 deficient are highly susceptible to colitis, although the exact underlying mechanism remains unclear. On the basis of present and previous findings, TRAF5 was selected for subsequent exploration.
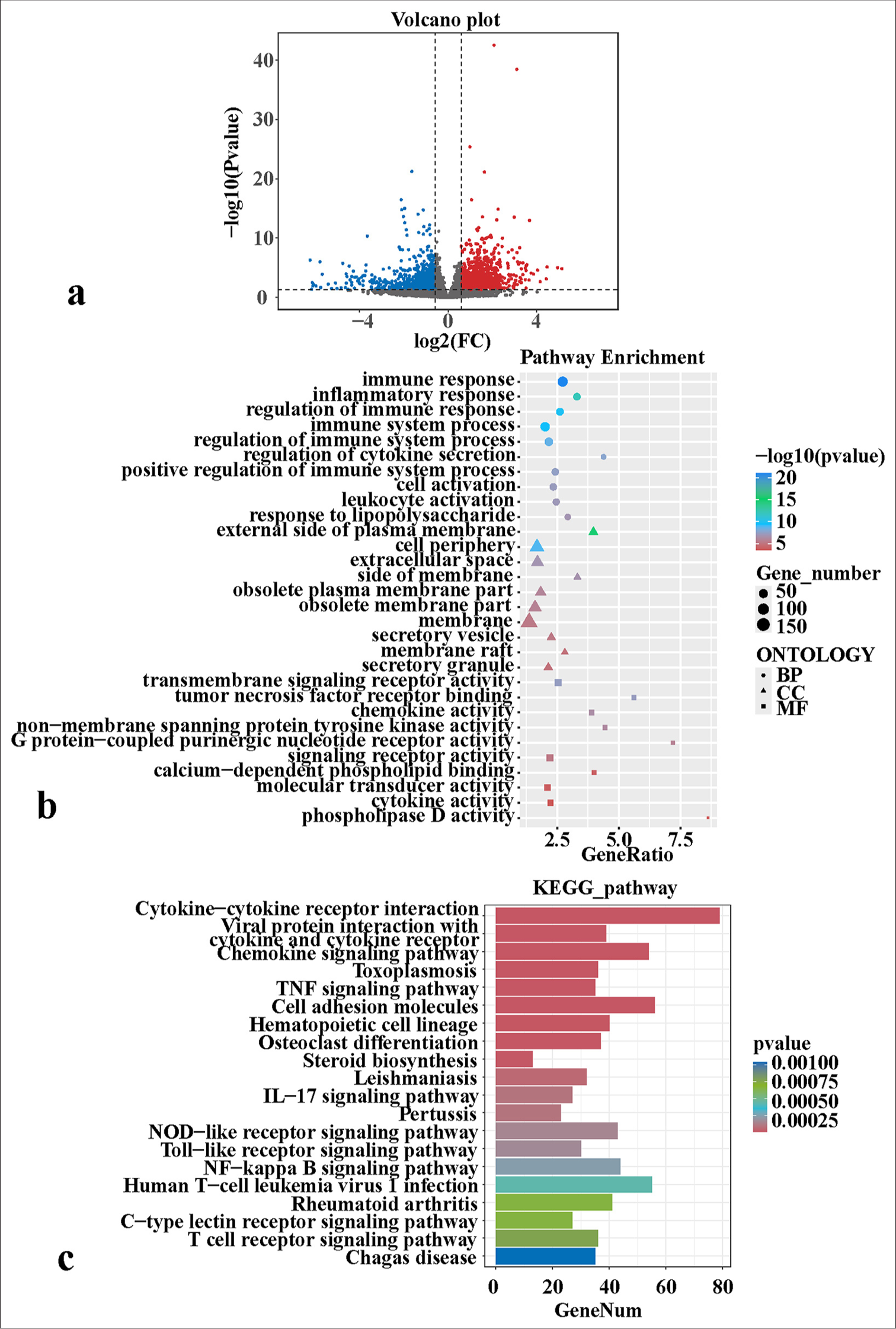
- Transcriptomic sequencing reveals the molecular mechanisms of PFCD development. (a) Differential expression volcano map. (b) GO enrichment bubble charts of differentially expressed genes. (c) KEGG enrichment bar charts of differentially expressed genes. FC: Fold change, BP: Biological process, CC: Cellular component, MF: Molecular function, TNF: Tumor necrosis factor, PFCD: Perianal fistulizing Crohn’s disease, GO: Gene ontology, KEGG: Kyoto encyclopedia of genes and genomes, IL: Interleukin, NF-kappa B: Nuclear factor kappa B.
Increased levels of TRAF5 and EMT in PFCD
Compared with the control group, the PFCD group exhibited significantly elevated mRNA expression levels of TRAF5, nuclear factor-kappa B (NF- κB), and IL-13 [Figure 2a-c] (P < 0.001). HE staining revealed that the control group displayed normal tissue architecture, mature and evenly distributed fibroblasts, minimal tissue hemorrhage, and mild inflammatory cell infiltration [Figure 2d]. The PFCD group exhibited disordered tissue architecture, decreased fibroblast numbers, prominent tissue hemorrhage, extensive inflammatory cell infiltration, and visible fat vacuoles [Figure 2e].
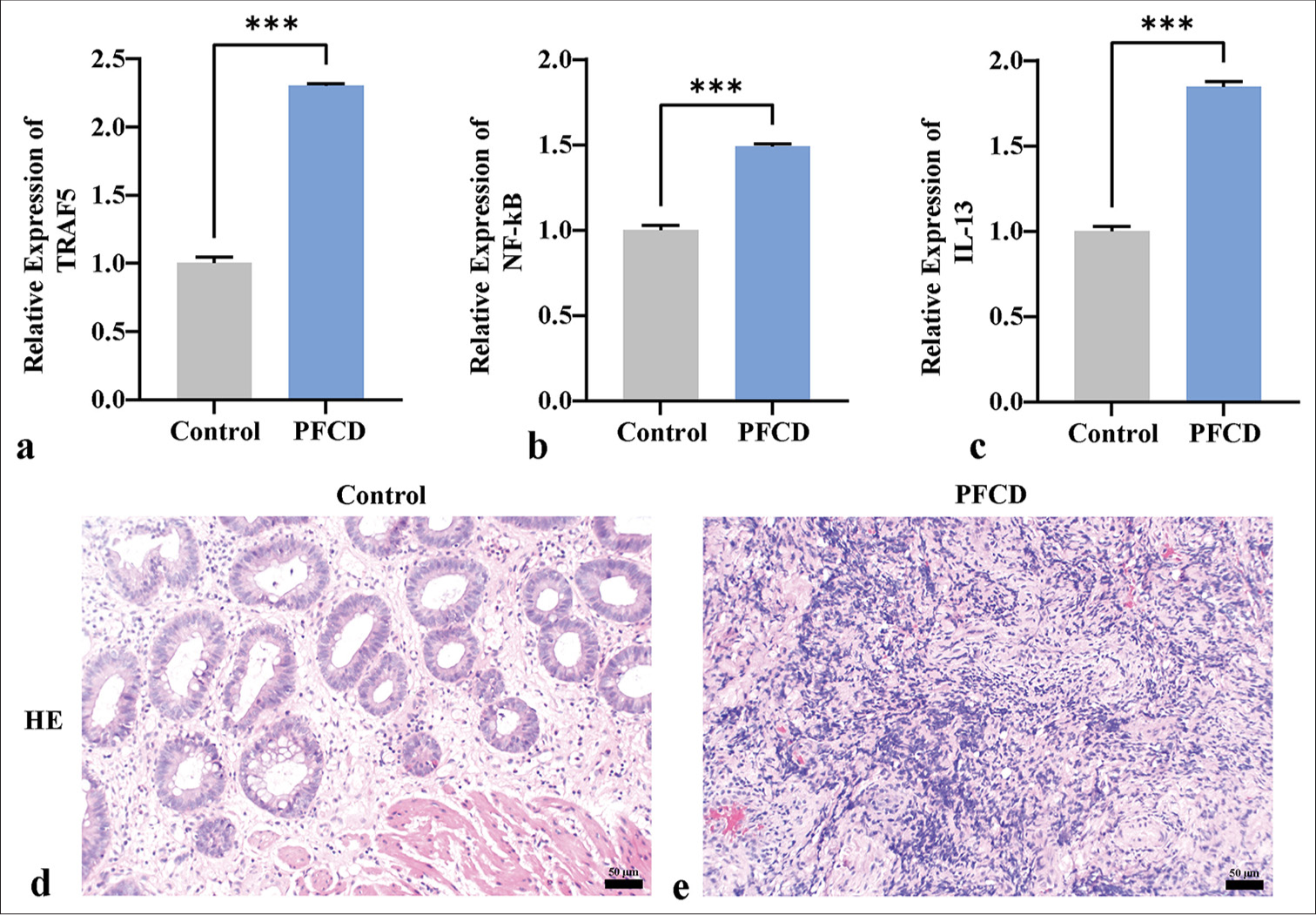
- qPCR and HE staining reveal the expression of TRAF5 in PFCD and EMT. (a-c) qPCR detection of TRAF5, NF-κB, and IL-13 mRNA expression levels. (d and e) HE staining depicting the pathological morphology of each group (×200) (scale bar = 50 μm). ✶✶✶P < 0.001, n = 3. TRAF5: Tumor necrosis factor receptor-associated factor 5, NF-κB: Nuclear factor kappa B, IL-13: Interleukin 13, PFCD: Perianal fistulizing Crohn’s disease, HE: Hematoxylin and eosin, qPCR: Quantitative polymerase chain reaction, EMT: Epithelial–mesenchymal transition.
Immunohistochemical staining revealed cytoplasmic positivity for TRAF5, which was more pronounced in the PFCD group than in the control group [Figure 3a-c]. E-cadherin was positively expressed at the cell membrane, and e-calmodulin expression was significantly lower in the PFCD group than in the control group [Figure 3d-f]. SNAIL1 was positively expressed in the nucleus and cytoplasm, with greater levels observed in the PFCD group [Figure 3g-i]. Vimentin exhibited cytoplasmic positivity and a modest increase in the PFCD group relative to that in the control group [Figure 3j-l].
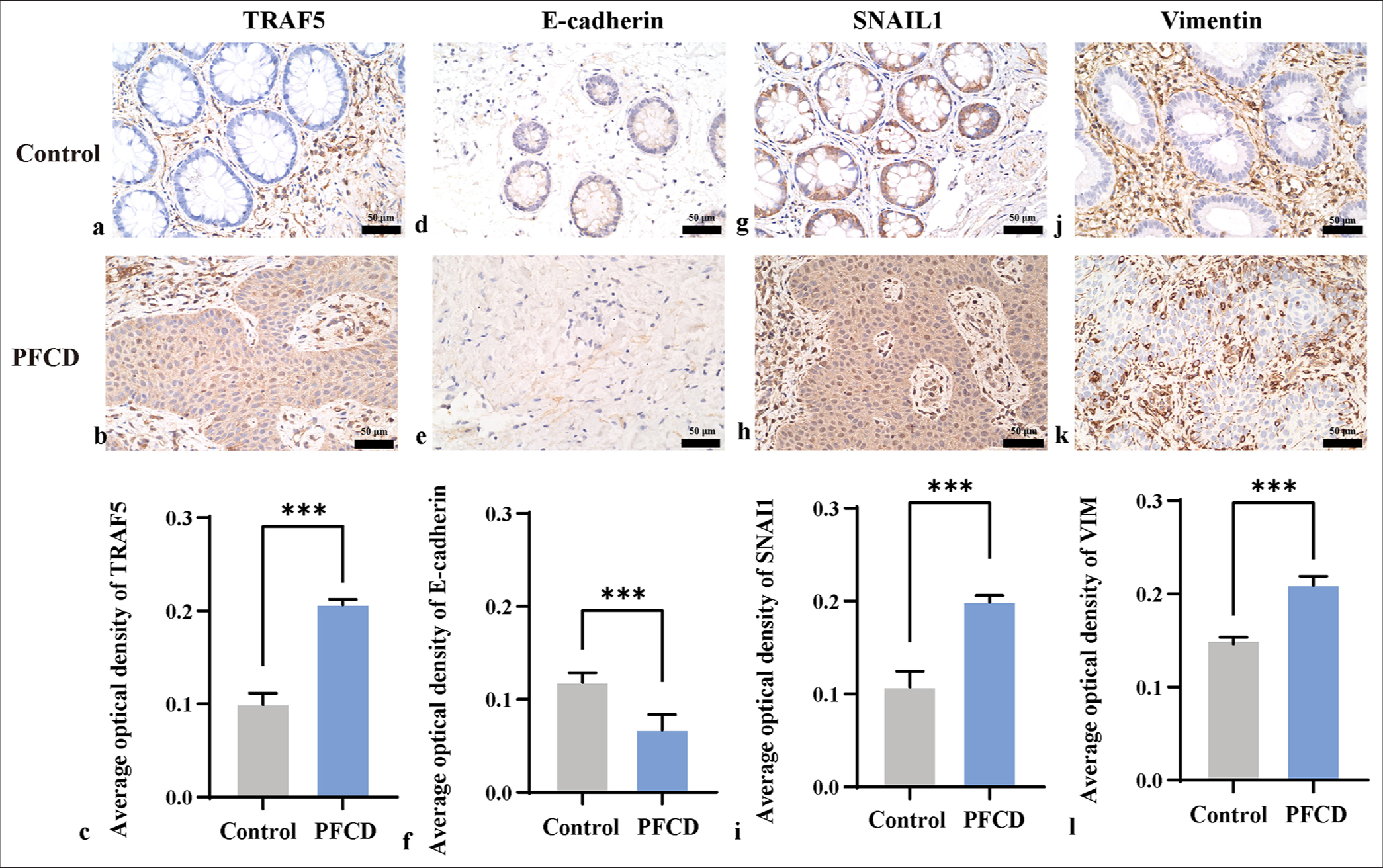
- Immunohistochemistry was used to detect the expression levels of TRAF5, E-cadherin, SNAIL1, and vimentin (×400) in each group (scale bar = 50 μm). (a-b, d-e, g-h, and j-k) Immunohistochemical staining of the intestine; (c, f, i, and l) TRAF5, E-cadherin, SNAIL1 and vimentin protein expression levels, ✶✶✶P < 0.001. E-cadherin: Epithelial cadherin, SNAIL1: Snail family transcriptional repressor 1, VIM: Vimentin, TRAF5: Tumor necrosis factor receptor-associated factor 5.
Knockdown of TRAF5 may inhibit EMT
Transcriptomic sequencing revealed that TRAF5 was present in the TNF signaling pathway and was highly expressed in PFCD. Therefore, we interfered with TRAF5 to explore its molecular mechanism. CCK-8 results showed a significant increase in proliferative capacity in the TGF- β1 and TGF- β1 + shNC groups compared with that in the control group (P < 0.001). Meanwhile, the proliferative capacity in the TGF- β1 + shTRAF5 group was lower than those in the TGF- β1 and TGF- β1 + shNC groups [Figure 4a] (P < 0.001). Transwell assays were conducted to detect cell migration, revealing a similar trend [Figure 4b and c] (P < 0.001). qPCR analysis showed a significant upregulation of TRAF5, NF- κB, and IL-13 mRNA expression levels in the TGF- β1 and TGF- β1 + shNC groups (P < 0.001) compared with those in the control group. However, the TGF- β1 + shTRAF5 group exhibited significantly decreased mRNA expression levels of TRAF5, NF- κB, and IL-13 compared with the TGF- β1 and TGF- β1 + shNC groups [Figure 4d-f] (P < 0.001).
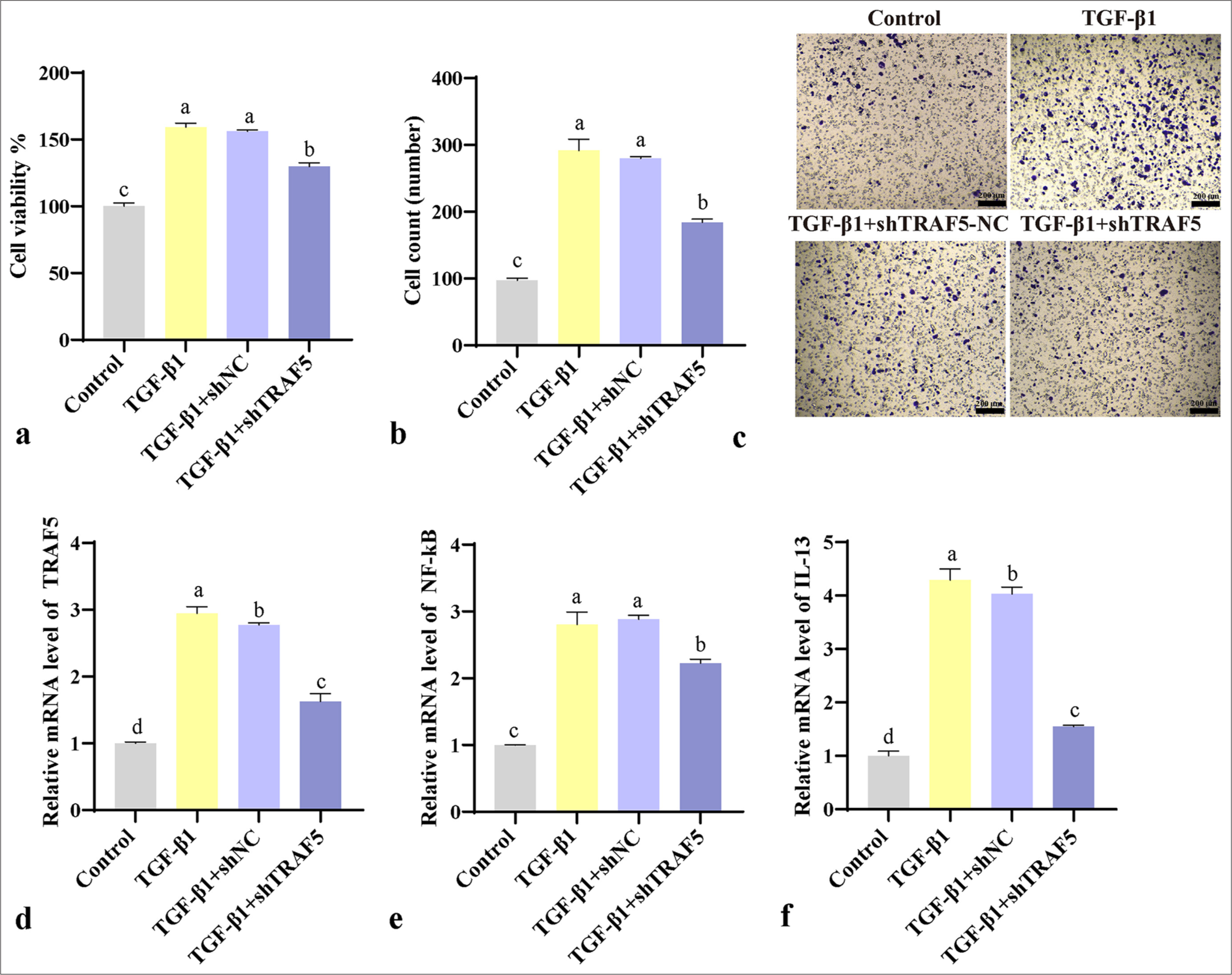
- Cellular experiments validate the effect of TRAF5 on PFCD. (a) CCK-8 detects cell proliferation. (b and c) Transwell detects cell migration (scale bar = 200 μm). (d-f) qPCR detection of TRAF5, NF-κB, and IL-13 mRNA expression levels. The different letters represent the significance level (P < 0.05), (n = 3). TGF-β1: Transforming growth factor-beta 1, TRAF5: Tumor necrosis factor receptor-associated factor 5, PFCD: Perianal fistulizing Crohn’s disease, CCK-8: Cell counting kit-8, qPCR: Quantitative polymerase chain reaction, NF-κB: Nuclear factor-kappa B, IL-13: Interleukin 13, shNC: Short hairpin negative control.
Immunohistochemical analysis revealed a notable upregulation of TRAF5, SNAIL1, and vimentin level in the TGF- β1 and TGF- β1 + shNC groups compared with those in the control group (P < 0.01). Conversely, the TGF- β1 + shTRAF5 group exhibited significantly reduced expression of these proteins compared with the TGF- β1 and TGF- β1 + shNC groups [Figures 5a-j; 6a-e]. The trend of E-cadherin was the opposite [Figure 6f-j]. WB results were consistent with the immunohistochemistry results [Figure 7a-e]. Interfering with TRAF5 could significantly inhibit the increase in proliferation and migration caused by PFCD and inhibit EMT.
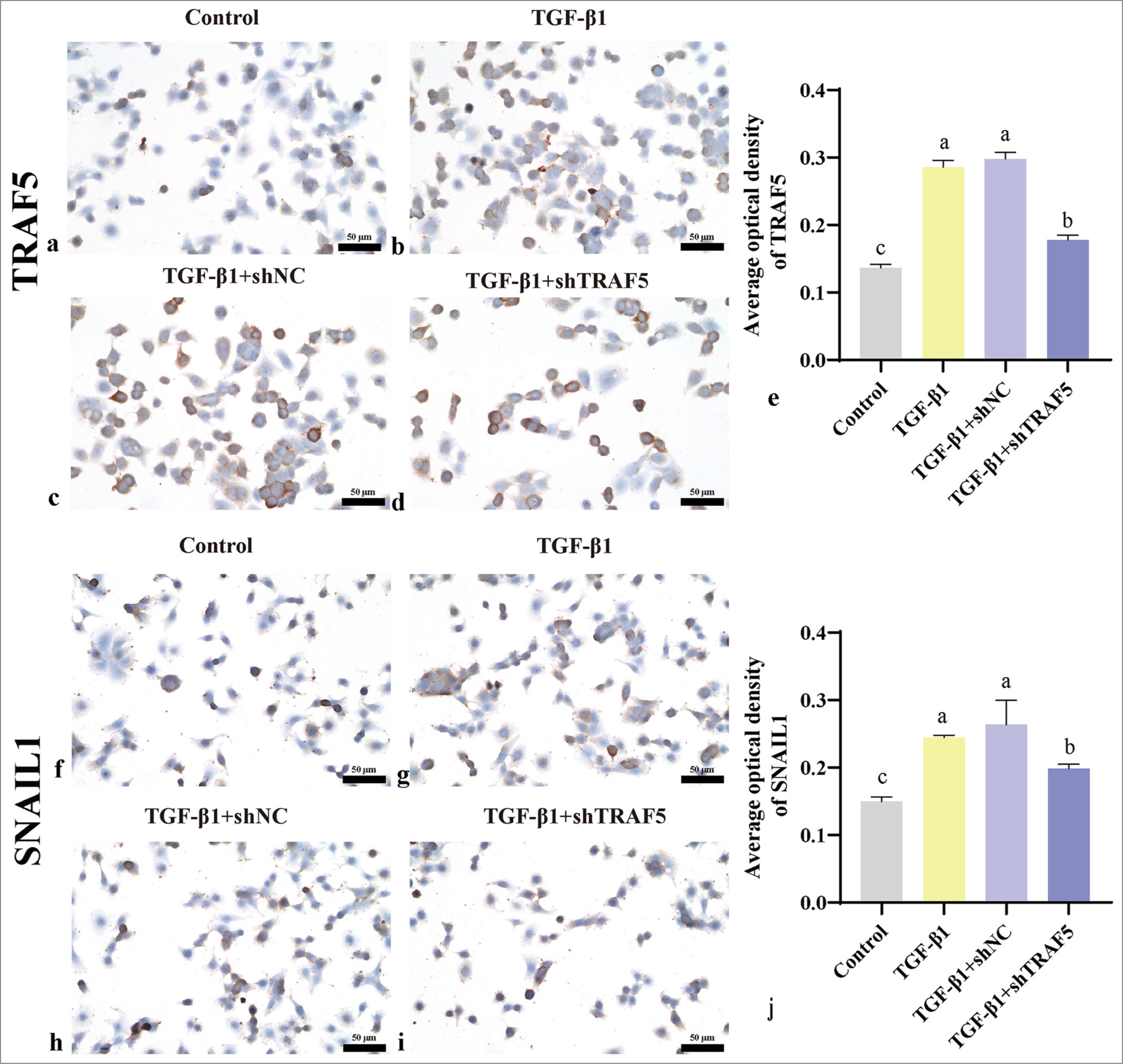
- TRAF5 and SNAIL1 protein expression levels (scale bar = 50 μm). (a-e) Immunohistochemistry was used to detect the protein levels of TRAF5 (×400); (f-j) Immunohistochemistry was used to detect the protein levels of SNAIL1 (×400). The different letters represent the significance level (P < 0.05), n = 3. TGF-β1: Transforming growth factor-beta 1, TRAF5: Tumor necrosis factor receptor-associated factor 5, SNAIL1: Snail family transcriptional repressor 1, shNC: short hairpin negative control.
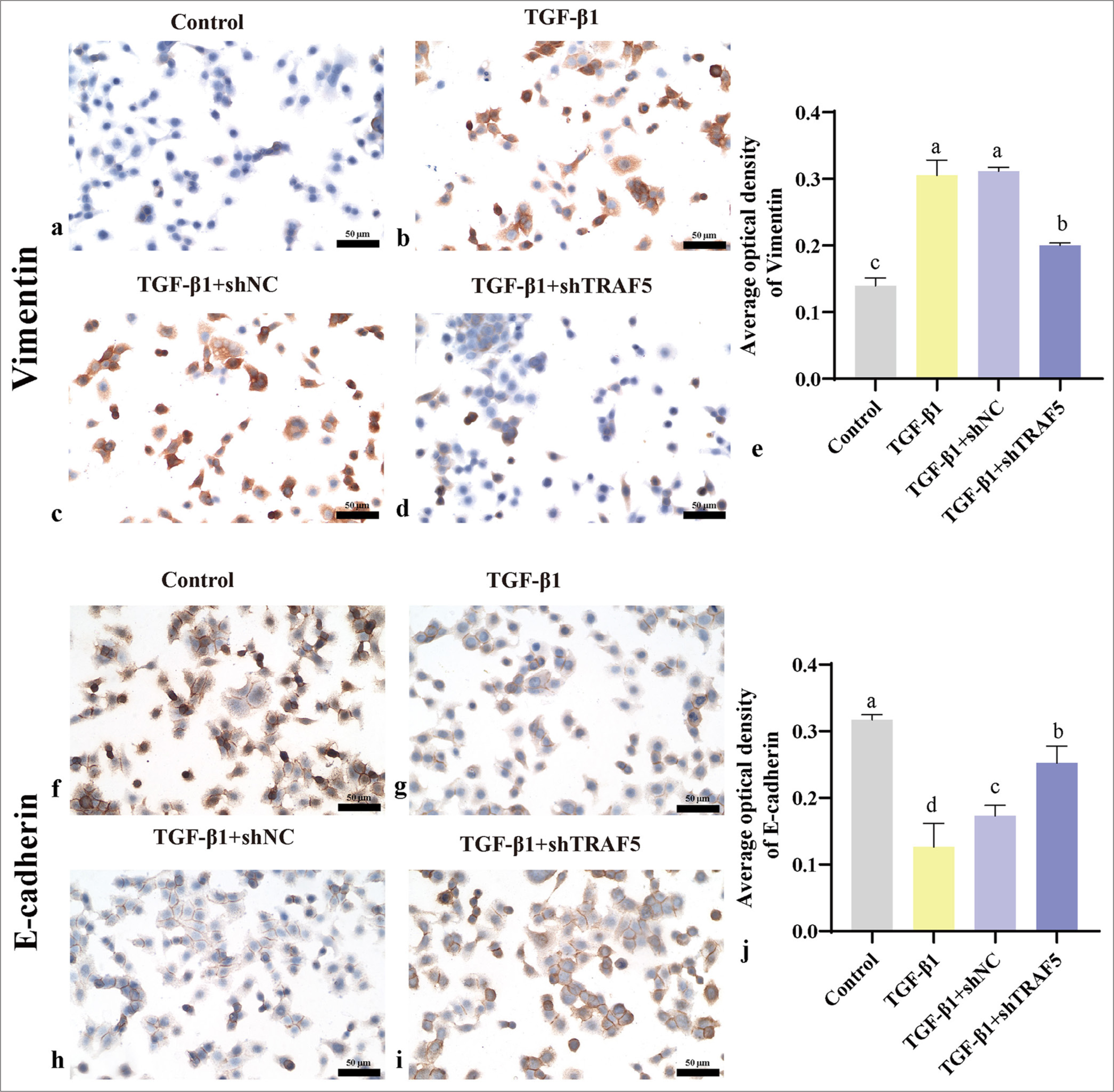
- Vimentin and E-cadherin protein expression levels (scale bar = 50 μm). (a-e) Immunohistochemistry was used to detect the protein levels of vimentin (×400); (f-j) Immunohistochemistry was used to detect the protein levels of E-cadherin (×400). The different letters represent the significance level (P < 0.05), n = 3. E-cadherin: Epithelial cadherin, TGF-β1: Transforming growth factor-beta 1, TRAF5: Tumor necrosis factor receptor-associated factor 5, shNC: short hairpin negative control.
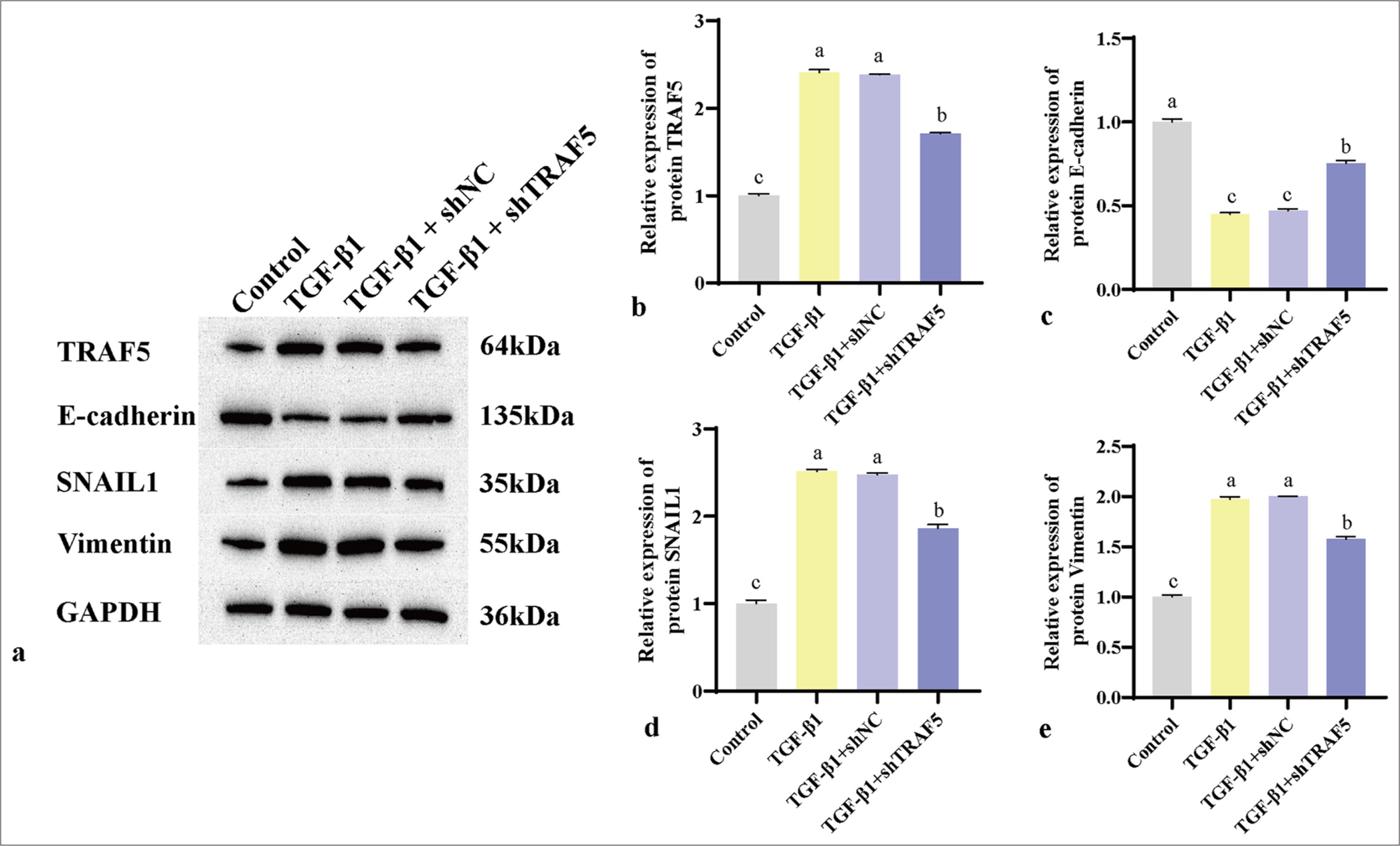
- (a-e) WB was used to detect the protein levels of TRAF5, E-cadherin, SNAIL1, and vimentin. The different letters represent the significance level (P < 0.05), n = 3. SNAIL1: Snail family transcriptional repressor 1, TRAF5: Tumor necrosis factor receptor-associated factor 5, WB: Western blot, TGF-β1: Transforming growth factor-beta 1, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, shNC: Short hairpin negative control.
DISCUSSION
Perianal fistula formation is a prevalent benign colorectal condition characterized by the development of abnormal connections between two epithelial surfaces, typically linking the rectal lining to the perianal skin.[20] Individuals with PFCD frequently experience perianal discomfort and discharge of pus or fecal matter. Damage to the anal sphincter and perianal scarring can result in fecal incontinence,[21] significantly influencing the patient’s overall well-being. This study sought to clarify the molecular mechanisms of PFCD and identify potential biomarkers by performing transcriptomic sequencing analysis on fistula tissue samples obtained from healthy individuals and patients with PFCD post-surgery. The findings offered a theoretical foundation for creating novel therapeutic strategies against PFCD.
TRAF proteins are crucial components and mediators of adaptive and innate immune signaling pathways,[22] which often involve the activation of NF- κB.[23] TRAF5, a member of the TRAF family, is involved in modulating the NF- κB pathway.[24] Its overexpression suppresses apoptosis and inflammation in cardiomyocytes.[25] Although TRAF5 is likely involved in immune and inflammatory processes as a key signal transduction protein, its specific association with PFCD remains unclear. Further research is necessary to explore the potential role of TRAF5 in PFCD development. In the present work, transcriptomic sequencing revealed a significant enrichment of the TNF signaling pathway, with TRAF5 identified as a DEG in this pathway. In addition, TRAF5 was markedly upregulated in the PFCD group, suggesting its potential role in promoting disease progression.
The transcription factor NF- κB is a complex composed of a group of similar transcriptional proteins.[26] In general, NF- κB dimers survive in the cytoplasm of most cells as inactive complexes by binding to one of the three inhibitors of the IκB family.[27] Abnormally activated NF- κB regulates tight junctional function by increasing the level of myosin light chain kinase in intestinal epithelial cells, resulting in impaired intestinal mucosal barrier and increased permeability.[28] Liu et al.[29] found that abnormally activated NF- κB in CD4+ T-cells promoted the progression of colitis in mice.[30] IL-13, a cytokine mainly produced by Th2 cells, contributes to immune-mediated diseases when its expression or function is dysregulated.[31] IL-13 is elevated in late colitis, stimulating macrophages to secrete TGF- β1. TGF- β1 subsequently leads to collagen deposition and fibrosis.[32,33] IL-13 marks the transition from acute inflammation to chronic inflammation, as its level gradually increases from the early to later stages of the disease.[34] Its expression is increased in the intestinal smooth muscles of patients with CD.[35] IL-13 is also involved in promoting fibrosis and tissue remodeling, which can lead to stenosis and fistula formation common in CD. In the present study, the mRNA levels of NF- κB and IL-13 were elevated in the PFCD group compared with those in the control group. Knocking down TRAF5 restored the PFCD-induced increase in the mRNA levels of NF- κB and IL-13.
EMT refers to the process where epithelial cells separate from adjacent cells, penetrate the basement membrane, and migrate to different tissue locations through the extracellular matrix.[36] During EMT, epithelial cells exhibit morphological changes, characterized by a decrease in epithelial markers, such as E-cadherin, and an increase in mesenchymal markers, such as vimentin.[37] E-cadherin, a crucial cell adhesion molecule, is essential for preserving the integrity of epithelial cells and the overall structure of tissues.[38] In patients with CD and ulcerative colitis, E-cadherin levels are frequently diminished, which impacts the maintenance of the intestinal epithelial lining’s mechanical stability.[39] SNAIL1 is a transcription factor that inhibits E-cadherin expression and induces EMT[40] and is notably upregulated in transitional cells associated with perianal fistulas in CD.[41] Recent studies have highlighted the notch1/Snail1/E-cadherin pathway as a key regulator of EMT in various diseases.[42] Vimentin, an intermediate fibrin, is a marker of interstitial cells and a protein associated with various pathophysiological disorders.[43] The expression of vimentin is elevated in CD,[44] indicating the presence of an active EMT. This study observed significant increases in TRAF5, SNAIL, and vimentin levels and a notable decrease in E-cadherin in the PFCD group, suggesting enhanced EMT. However, TRAF5 knockdown effectively mitigated the EMT induced by PFCD.
Although this study has provided insights into the molecular mechanisms of PFCD through transcriptomic sequencing, it still has limitations. First, the clinical sample size is small. Additional clinical samples must be included for future analysis to improve the accuracy of the experiment. Meanwhile, the verification experiments were carried out at the cellular level. Further verification in animals or clinics is required in the future.
This study demonstrated that the development of PFCD was related to EMT. TRAF5, the key gene of PFCD, was identified through transcriptomic analysis. Knocking down TRAF5 suppressed cell proliferation and migration, reduced NF- κB and IL-13 levels, and inhibited EMT, suggesting that TRAF5 may regulate the development of PFCD through EMT. These insights into the molecular mechanisms deepened our understanding of PFCD and provided supportive evidence for potential therapeutic targets. Studies focusing on EMT might help improve clinical outcomes in patients with CD.
SUMMARY
This study identified TRAF5 as a critical regulator in the pathogenesis of PFCD, with transcriptomic analysis highlighting its significant upregulation in PFCD tissues. TRAF5’s role in promoting EMT was demonstrated through its impact on cell proliferation and migration and the expression of key inflammatory markers NF- κB and IL-13. TRAF5 contributed to disease progression by enhancing EMT, which is implicated in PFCD development. Knocking down TRAF5 effectively mitigated these effects, suggesting TRAF5 as a potential therapeutic target. These insights advanced our understanding of PFCD mechanisms and opened avenues for novel therapeutic strategies targeting TRAF5 to improve patient outcomes. Further validation in large clinical samples and animal models is needed to confirm these findings and explore their clinical applicability.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
ABBREVIATIONS
CD: Crohn’s disease
PFCD: Perianal fistulizing crohn’s disease
TRAF5: Tumor necrosis factor receptor-associated factor 5
NF-κB: Nuclear factor-kappa B
IL-13: Interleukin 13
qPCR: Quantitative polymerase chain reaction
HE: Hematoxylin and eosin
SNAIL1: Snail family transcriptional repressor 1
HT-29: Human tumor-29
EMT: Epithelial–mesenchymal transition
TGF-β: Transforming growth factor-β
TNF-α: Tumor necrosis factor-α
TCs: Transitional cells
DEGs: Differentially expressed genes
FDR: False discovery rate
log2FC: Log2 fold change
GO: Gene ontology
KEGG: Kyoto Encyclopedia of Genes and Genomes
DMEM: Dulbecco’s modified eagle medium
CCK-8: Cell counting kit-8 PBS: Phosphate-buffered saline
DAB: 3,3'-diaminobenzidine
WB: Western blot
PVDF: Polyvinylidene difluoride
BP: Biological process
CC: Cellular component
MF: Molecular function
M: Man
W: Woman
WBC: White blood cell
HGB: Hemoglobin
PLT: Platelet
AST: Glutamic oxaloacetic transaminase
ALT: Glutamic-pyruvic transaminase.
GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase Cadherin: Epithelial Cadherin
VIM: Vimentin
mRNA: messenger ribonucleic acid
FPKM: Fragments Per Kilobase of transcript per Million mapped reads
shRNA: short hairpin ribonucleic acid
shTRAF5: short hairpin TRAF5
shNC: short hairpin negative control
RNA: ribonucleic acid
cDNA: complementary deoxyribonucleic acid
RIPA: Radio immunoprecipitation Assay
HRP: Horseradish Peroxidase
ECL: Enhanced Chemiluminescence
ACKNOWLEDGMENT
Not applicable.
AUTHOR CONTRIBUTIONS
XMS and HRG: Methodology, investigation, data curation, formal analysis, and writing-original draft; LL and QQW: Formal analysis, validation, and visualization; YFG and YRL: Conceptualization, supervision, and writing-review and editing.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine under the approval number 2023NL-062-01, dated 2023.03.29, guided by the Declaration of Helsinki, and all participants provided written informed consent.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57.
- [CrossRef] [PubMed] [Google Scholar]
- Ethnicity influences phenotype and outcomes in inflammatory bowel disease: A systematic review and meta-analysis of population-based studies. Clin Gastroenterol Hepatol. 2018;16:190-7.e111.
- [CrossRef] [PubMed] [Google Scholar]
- The intersection of human and veterinary medicine-a possible direction towards the improvement of cell therapy protocols in the treatment of perianal fistulas. Int J Mol Sci. 2022;23:13917.
- [CrossRef] [PubMed] [Google Scholar]
- AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn's disease. Gastroenterology. 2021;160:2496-508.
- [CrossRef] [PubMed] [Google Scholar]
- Trends of 5-aminosalicylate medication use in patients with Crohn disease. Inflamm Bowel Dis. 2021;27:516-21.
- [CrossRef] [PubMed] [Google Scholar]
- Role of natural products in combating cancer. Cancer Insight. 2022;1:35-52.
- [CrossRef] [Google Scholar]
- High risk of anal and rectal cancer in patients with anal and/or perianal Crohn's disease. Clin Gastroenterol Hepatol. 2018;16:892-9.
- [CrossRef] [PubMed] [Google Scholar]
- Non-coding RNAs regulating epithelial-mesenchymal transition: Research progress in liver disease. Biomed Pharmacother. 2022;150:112972.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-54.
- [CrossRef] [PubMed] [Google Scholar]
- EMT promoting transcription factors as prognostic markers in human breast cancer. Arch Gynecol Obstet. 2017;295:817-25.
- [CrossRef] [PubMed] [Google Scholar]
- 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 2: Surgical management and special situations. J Crohns Colitis. 2017;11:135-49.
- [CrossRef] [PubMed] [Google Scholar]
- Remodeling of bronchial epithelium caused by asthmatic inflammation affects its response to rhinovirus infection. Sci Rep. 2021;11:12821.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn's disease. Inflamm Bowel Dis. 2008;14:1514-27.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of colorectal cancer-associated alternative splicing based on transcriptome. DNA Cell Biol. 2020;39:16-24.
- [CrossRef] [PubMed] [Google Scholar]
- A systems pharmacology approach based on oncogenic signalling pathways to determine the mechanisms of action of natural products in breast cancer from transcriptome data. BMC Complement Med Ther. 2021;21:181.
- [CrossRef] [PubMed] [Google Scholar]
- Parallel single-cell and bulk transcriptome analyses reveal key features of the gastric tumor microenvironment. Genome Biol. 2022;23:265.
- [CrossRef] [PubMed] [Google Scholar]
- TRAF5 regulates intestinal mucosal Th1/Th17 cell immune responses via Runx1 in colitis mice. Immunology. 2023;170:495-509.
- [CrossRef] [PubMed] [Google Scholar]
- Fistulectomy versus fistulotomy with marsupialisation in the treatment of low fistula-in-ano: A prospective randomized controlled trial. Tanzan J Health Res. 2013;15:193-8.
- [CrossRef] [Google Scholar]
- Long-term outcomes of perianal fistulizing Crohn's disease in the biologic era. JGH Open. 2021;5:235-41.
- [CrossRef] [PubMed] [Google Scholar]
- All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci. 2002;115:679-88.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern recognition scavenger receptor CD204 attenuates Toll-like receptor 4-induced NF-kappaB activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J Biol Chem. 2011;286:18795-806.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of TRAFs in NF-κB signaling pathways mediated by BAFF. Immunol Lett. 2018;196:113-8.
- [CrossRef] [PubMed] [Google Scholar]
- TRAF5 protects against myocardial ischemia reperfusion injury via AKT signaling. Eur J Pharmacol. 2020;878:173092.
- [CrossRef] [PubMed] [Google Scholar]
- Plumbagin is a NF-κB-inducing kinase inhibitor with dual anabolic and antiresorptive effects that prevents menopausal-related osteoporosis in mice. J Biol Chem. 2022;298:101767.
- [CrossRef] [PubMed] [Google Scholar]
- Tranilast treatment attenuates cerebral ischemia-reperfusion injury in rats through the inhibition of inflammatory responses mediated by NF-κB and PPARs. Clin Transl Sci. 2019;12:196-202.
- [CrossRef] [PubMed] [Google Scholar]
- Matrix metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-κB activation. PLoS One. 2021;16:e0249544.
- [CrossRef] [PubMed] [Google Scholar]
- Oridonin derivative ameliorates experimental colitis by inhibiting activated T-cells and translocation of nuclear factor-kappa B. J Dig Dis. 2016;17:104-12.
- [CrossRef] [PubMed] [Google Scholar]
- Sulfasalazine inhibits activation of nuclear factor-kappaB in patients with ulcerative colitis. J Gastroenterol Hepatol. 2005;20:1016-24.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-IL-13 in inflammatory bowel disease: From the bench to the bedside. Curr Drug Targets. 2013;14:1444-52.
- [CrossRef] [PubMed] [Google Scholar]
- IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003-13. 2013.e2001-7
- [CrossRef] [PubMed] [Google Scholar]
- Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859-70.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of resolution pathways to prevent and fight chronic inflammation: Lessons from asthma and inflammatory bowel disease. Front Immunol. 2019;10:1699.
- [CrossRef] [PubMed] [Google Scholar]
- Medical therapy of fibrostenotic Crohn's disease. Viszeralmedizin. 2015;31:259-64.
- [CrossRef] [PubMed] [Google Scholar]
- The evolving concept of cancer stem-like cells in thyroid cancer and other solid tumors. Lab Invest. 2017;97:1142-51.
- [CrossRef] [PubMed] [Google Scholar]
- The CD44high subpopulation of multifraction irradiation-surviving NSCLC cells exhibits partial EMT-program activation and DNA damage response depending on their p53 status. Int J Mol Sci. 2021;22:2369.
- [CrossRef] [PubMed] [Google Scholar]
- Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199-214.
- [CrossRef] [PubMed] [Google Scholar]
- A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325.
- [CrossRef] [PubMed] [Google Scholar]
- Snail1-dependent cancer-associated fibroblasts induce epithelialmesenchymal transition in lung cancer cells via exosomes. QJM. 2019;112:581-90.
- [CrossRef] [PubMed] [Google Scholar]
- Potential role for SNAIL family transcription factors in the etiology of Crohn's disease-associated fistulae. Inflamm Bowel Dis. 2011;17:1907-16.
- [CrossRef] [PubMed] [Google Scholar]
- Aberrant expression of Notch1/numb/snail signaling, an epithelial mesenchymal transition related pathway, in adenomyosis. Reprod Biol Endocrinol. 2015;13:96.
- [CrossRef] [PubMed] [Google Scholar]
- SIRT1 stabilizes β-TrCP1 to inhibit snail1 expression in maintaining intestinal epithelial integrity to alleviate colitis. Cell Mol Gastroenterol Hepatol. 2024;18:101354.
- [CrossRef] [PubMed] [Google Scholar]








