Translate this page into:
Ultrasound-guided fine-needle aspiration of hyperenhancing lesion suspicious for pancreatic neuroendocrine tumor in the tail of pancreas-potential pitfalls
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Magnetic resonance imaging (MRI) showed a 1 cm hyperenhancing lesion in the tail of the pancreas in a 37-year-old female with past medical history significant for interstitial lung disease due to mixed connective tissue disease (rheumatoid arthritis/systemic lupus erythematosus). Partial pancreatectomy was recommended due to high suspicion of pancreatic neuroendocrine tumor (PanNET), but the patient was reluctant. An upper endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was ordered.
QUESTION
What is your interpretation?
-
Peripancreatic lymph node
-
Intrapancreatic accessory spleen (IPAS)
-
PanNET
-
Lymphoepithelial cyst.
ANSWER
B. Intrapancreatic accessory spleen (IPAS).
MRI showed hyperenhancing lesion in the tail of the pancreas. Moreover, the upper EUS showed mild chronic pancreatitis changes and unremarkable pancreatic duct along with the 1 cm tail of pancreas lesion. Considering the clinical information and the results of the EUS-FNA, the main differential diagnosis included IPAS, PanNET (islet cell tumor), lymphoepithelial cyst, solid pseudopapillary neoplasm, chronic pancreatitis, and lymphoma.
Features favoring IPAS over other differential diagnosis are as follow:
-
Presence of abundant mature small lymphocytes admixed with chronic inflammatory cell infiltrate including plasma cells and neutrophils
-
Presence of lymphocyte tangles with crush artifact
-
Presence of large aggregate of platelets
-
Absence of epithelial squamous cells
-
Absence of malignant cells and papillary structures
-
Clinical history in conjunction with imaging findings.
Unfortunately, the cell block section was negative for diagnostic materials precluding further workup studies such as immunohistochemistry staining to further confirm the diagnosis.
The patient is being followed up regularly for her interstitial lung disease, and 5 months later, she has no significant gastrointestinal symptoms.
ADDITIONAL QUIZ QUESTIONS
-
Which immunohistochemistry stain helps confirm the diagnosis of IPAS?
-
CD56
-
CD8
-
Beta-catenin
-
CD45.
-
-
All of the following are cytologic features of IPAS except
-
Lymphocytes tangles
-
Tangible body macrophages and germinal center fragments
-
Platelet clusters
-
Clusters and singly scattered spindle cells.
-
-
The most common site of accessory spleen
-
Head of the pancreas
-
Tail of the pancreas
-
Hilum of the spleen
-
Omentum.
-
ANSWERS FOR ADDITIONAL QUIZ QUESTIONS
-
B: Immunostains for CD8 highlights the traversing vessels confirming the presence of sinusoidal endothelial cells of IPAS. The cells of pancreatic endocrine tumor are usually reactive for CD56. CD45 highlights lymphocytes. Beta-catenin is positive in solid pseudopapillary tumor of the pancreas.
-
(B): IPAS show lymphocytic tangles with platelets aggregates. Bland-looking spindle cells are also present. Tingible body macrophages are not a feature of IPAS.
-
(C): The most common location of IPAS is the hilum of the spleen. The most common location in pancreas is in the tail. All others are relatively uncommon locations for IPAS.
BRIEF REVIEW OF THE TOPIC
The most common site for accessory spleen is the splenic hilum and the second most common location is the pancreas.[1] IPAS is a benign congenital lesion of the pancreas that results from entrapment of portion of the spleen in the dorsal pancreatic bud during embryologic development.[23] IPAS affects approximately 10% of the population with 15%–20% of the cases present in the tail of the pancreas.[23] IPASs are usually incidentally discovered due to increased use of sophisticated imaging studies. IPAS does not require surgical intervention unless it is involved by a secondary process, such as benign disease or malignancy.[23] Radiologically, the IPAS mimics PanNET.[13] To clarify the nature of an incidentally discovered pancreatic mass, EUS-FNA is the method of choice due to its high sensitivity.[23]
Cytologic features of intrapancreatic accessory spleen
Our case, similar to the majority of cases, showed:
-
Numerous small mature lymphocytes admixed with other chronic inflammatory cells [Figure 1a]
-
Lymphocytes tangles with crush artifact [Figure 1a]
-
Tingible body macrophages and germinal center fragments were absent
-
Scattered and loose aggregate of bland spindle cells and endothelial cells [Figure 1b]
-
Background of blood and lymphoglandular bodies
-
The most striking feature is the presence of variably sized platelet aggregates, especially on the Diff-Quik-stained air-dried smears which appear as sky-blue fluffy, acellular “cotton candy-like” material [Figure 1c and d].[3]
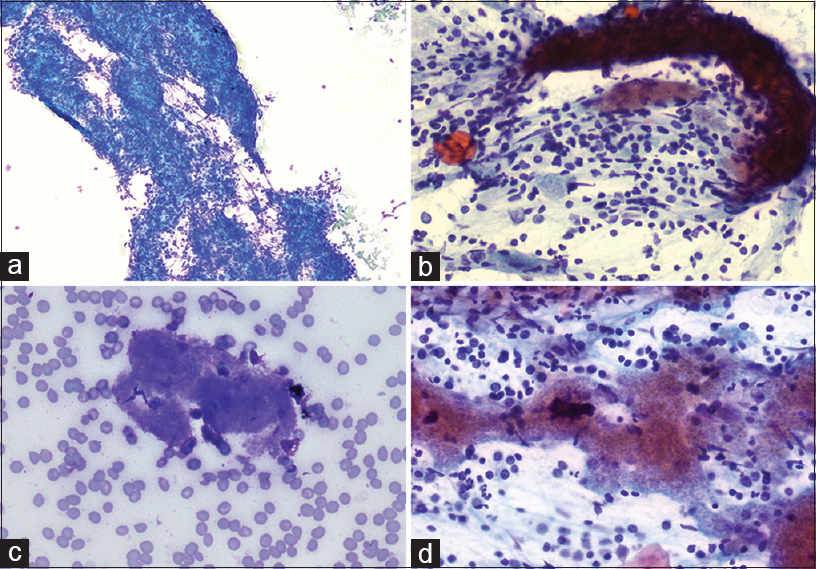
- Endoscopic ultrasound-guided fine-needle aspiration of pancreatic tail lesion. (a) Diff-Quik air-dried smear preparation (×10) shows many lymphocytes tangles with crushing artifact. (b) Loose aggregates of bland spindle cells (Rapid Papanicolaou, ×40). (c) Diff-Quik air-dried smear preparation (×40) highlights Blue fluffy, acellular, “cotton candy” materials. (d) Aggregates of the same fluffy materials (Rapid Papanicolaou, ×40)
Differential diagnosis
-
PanNET
-
Pancreatic pseudopapillary neoplasm
-
Peripancreatic lymph node
-
Chronic pancreatitis
-
Lymphoepithelial cyst.
PanNET usually appears predominantly as dispersed single cells exhibiting eccentric nuclei (plasmacytoid appearance) with finely stippled (salt and pepper) chromatin and moderate amount of cytoplasm.[4]
The aspirate smears of pancreatic pseudopapillary neoplasm are usually highly cellular with vascular stalks lined by stratified neoplastic cells. The neoplastic cells have round to oval shaped nuclei with intranuclear grooves and inconspicuous nucleoli. Blood, debris, and macrophages are seen in the background.[4]
Peripancreatic lymph node is a possibility; however, germinal center fragments and tangible body macrophages are readily recognized.
Chronic pancreatitis may form mass lesion due to fibrosis. Cytologically, the aspirate is of low cellularity with benign appearing pancreatic ducts and acini in a background of fat necrosis and inflammation.[4]
Lymphoepithelial cysts are rare benign cyst, usually multiloculated and may cause abdominal pain. Histologically, they are composed of epithelial component of benign keratinized squamous cells and subepithelial lymphoid tissue.[4]
ANCILLARY STUDIES
Immunoperoxidase stain for CD8 highlights the traversing vessels confirming the presence of sinusoidal endothelial cells of IPAS.[56]
OTHER HELPFUL IMAGING
Technetium-99m (99mTC)-labeled sulfur colloid or 99mTC-labeled heat-damaged red blood cell scintigraphy (highly sensitive and specific) has been used to detect accessory spleen.[17]
SUMMARY
In summary, upper EUS is a helpful tool in evaluation of IPAS. IPAS should be considered in the differential diagnosis of any small lesion in the tail of the pancreas along with any neoplastic process during interpretation of EUS--FNA of lesions in this setting with the application of appropriate ancillary studies as indicated.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article are qualified for authorship and each has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
This is a quiz without identifiers, our institution does not require approval from Institutional Review Board (IRB).
LIST OF ABBREVIATIONS (In alphabetic order)
EUS-FNA - Endoscopic UltraSound guided Fine Needle Aspiration
IPAS - IntraPancreatic Accessory Spleen
PanNET - Pancreatic Neuroendocrine Tumor.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Intrapancreatic accessory spleen: CT appearance and differential diagnosis. Abdom Imaging. 2012;37:812-27.
- [Google Scholar]
- Fine-needle aspiration of intrapancreatic accessory spleen: Cytomorphologic features and differential diagnosis. Cancer Cytopathol. 2012;120:261-8.
- [Google Scholar]
- Intrapancreatic accessory spleen: Utilization of fine needle aspiration for diagnosis of a potential mimic of a pancreatic neoplasm. J Gastrointest Oncol. 2016;7(Suppl 1):S62-5.
- [Google Scholar]
- Pancreas and biliary tree. In: Cibas ES, Ducatman BS, eds. Cytology: Diagnostic Principles and Clinical Correlates (4th ed). Philadelphia, PA: Elsevier; 2014. p. :399-421.
- [Google Scholar]
- Large platelet aggregates in endoscopic ultrasound-guided fine-needle aspiration of the pancreas and peripancreatic region: A clue for the diagnosis of intrapancreatic or accessory spleen. Diagn Cytopathol. 2013;41:661-72.
- [Google Scholar]
- Intrapancreatic accessory spleen: Mimic of pancreatic endocrine tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol. 2008;36:262-5.
- [Google Scholar]







