Translate this page into:
Utility of BRAF V600E mutation detection in cytologically indeterminate thyroid nodules
Address for correspondence:Joel Bentz, Institute for Clinical and Experimental Pathology, Associated Regional and University Pathologists (ARUP) Laboratories; Department of Pathology, University of Utah, Salt Lake City, UT, USA joel.bentz@hsc.utah.edu
-
Received: ,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Fine needle aspiration (FNA) is widely utilized for evaluation of patients with thyroid nodules. However, approximately 30% are indeterminate for malignancy. Recently, a mutation in the BRAF gene has been reported to be the most common genetic event in papillary thyroid carcinoma (PTC). In this retrospective study, we assessed the utility of BRAF V600E mutation detection for refining indeterminate preoperative cytologic diagnoses in patients with PTC.
Methods:
Archival indeterminate thyroid FNAs and corresponding formalin-fixed, paraffin-embedded (FFPE) surgical samples with PTC were identified in our patient files. DNA extracted from slide scape lysates and 5 μm FFPE sections were evaluated for the BRAF V600E mutation using LightCycler PCR and fluorescent melting curve analysis (LCPCR). Amplification products that showed deviation from the wild-type genomic DNA melting peak, discordant FNA and FFPE matched pairs, and all benign control samples, underwent direct DNA sequencing.
Results:
A total of 19 indeterminate thyroid FNAs demonstrating PTC on FFPE surgical samples were included in the study. Using BRAF mutation analysis, the preoperative diagnosis of PTC was confirmed in 3/19 (15.8%) FNA samples that could not be conclusively diagnosed on cytology alone. However, 9/19 (47.4%) FFPE tissue samples were positive for the V600E mutation. Of the discordant pairs, 5/6 FNAs contained less than 50% tumor cells.
Conclusion:
When used with indeterminate FNA samples, BRAF mutation analysis may be a useful adjunct technique for confirming the diagnosis of malignancy in an otherwise equivocal case. However, overall tumor cell content of some archival FNA smear slides is a limiting factor for mutation detection.
Background
While the frequency of thyroid cancer in the general population is relatively low, thyroid nodules are a very common clinical problem, and palpable thyroid nodules can be identified in 4-7% of all adults in the United States 1. The prevalence of malignancy, however, in a solitary thyroid nodule is only approximately 5% in normal adults [2-4]. Consequently, the primary clinical challenge is to sort out the vast majority of nodules that are benign, which can generally be followed with surveillance, from those requiring surgical intervention.
In 2003, approximately 75-80% of all thyroid cancers were papillary thyroid carcinoma (PTC) 5. Among the most curable of cancers, PTC tends to remain localized in the thyroid gland, but in time it may metastasize to regional lymph nodes and, less commonly, to the lungs. At the time of initial assessment, most patients with PTC present with a painless, palpable, solitary thyroid nodule.
As early as the1930′s, studies reported on the use of fine needle aspiration (FNA) cytology for the diagnosis of thyroid carcinoma 67]. However, as often as 30% of the time, FNA-based evaluation of solitary thyroid nodules displays limited ability to discriminate between benign and malignant lesions and an indeterminate cytologic diagnosis is rendered 8. Although surgical intervention is generally recommended following an indeterminate finding on FNA cytology, malignancy within indeterminate thyroid nodules varies between 3-52% [9-16]. Consequently, planning optimal surgical management in patients with an uncertain preoperative diagnosis is challenging.
In view of the increasing number of thyroid nodules that require FNA evaluation, there is a clear need for the development of adjunctive diagnostic assays that would help refine indeterminate diagnoses on thyroid cytology. Recently, a single hotspot mutation at nucleotide 1799 of the BRAF gene has been identified as the most common genetic event in 29-83% of all cases of PTC [17-27]. This thymine (T) to adenine (A) transversion mutation results in the substitution of valine with glutamate (V600E) and converts BRAF into a dominant transforming protein that causes constitutive activation of the MAPK pathway, independent of RAS activation 28. Additionally, this mutation appears to be fairly specific for PTC.
In early polymerase chain reaction (PCR) testing platforms, sample DNA or RNA was amplified first and then detected in a separate step, using a technique such as gel electrophoresis to assess the size and purity of the products. Recently developed instrumentation combines PCR amplification and target nucleic acid characterization in the same closed reaction vessel 29. Using LightCycler PCR with fluorescent melting curve analysis (LCPCR), the difference in melting profiles between mismatched probe/target and perfectly matched probe/target can be used to characterize amplification products and indicate the presence of a mutation 30.
The primary objective of this study was to identify the BRAF V600E mutation in thyroid FNA samples using LCPCR for the purpose of refining indeterminate preoperative cytologic diagnoses in patients with PTC.
Materials and methods
Case and sample selection
This retrospective study was approved by the University of Utah Institutional Review Board (#13005). Study samples were identified from a database established by the University of Utah Department of Surgery (University of Utah Institutional Review Board #11565) of all patients having undergone treatment for cancer of the thyroid gland at the University of Utah Hospitals and Clinics from 1994-2004. This dataset of patients was reviewed to identify only individuals with both an archival, preoperative FNA demonstrating cytologic findings indeterminate for malignancy and follow-up archival surgical pathology formalin-fixed, paraffin-embedded (FFPE) tissue demonstrating PTC. The “indeterminate” cytologic category encompassed those samples demonstrating hypercellularity suggestive of a follicular or papillary neoplasm and/or atypical cytomorphologic features suggestive of, but not diagnostic for, malignancy Figure 1. The diagnostic correlation was restricted to cases in which the cytology was reported as indeterminate within a 6-month period preceding the histology report.
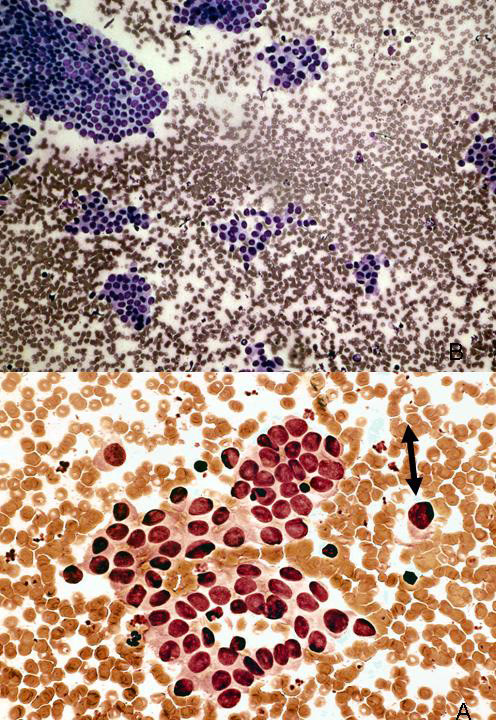
- Example of indeterminate thyroid fine needle aspiration (FNA). Fine needle aspirate sample from 28 year-old female with thyroid nodule. Case was interpreted as “fragments of atypical epithelial cells in a background of reactive lymphocytes. A follicular neoplasm or papillary carcinoma cannot be excluded.” The thyroidectomy specimen showed a classical papillary thyroid carcinoma in the corresponding lobe of the thyroid.
The archival cytologic slides were retrieved from the cytopathology laboratory slide archives. Only previously-coverslipped and Diff-Quik stained direct smear slides were pulled for DNA extraction and mutation analysis. A case was selected for study inclusion if: age greater than 18; indeterminate cytology report; cytology slide appeared to contain more than 15% cellularity; follow-up surgical pathology tissue block was available; and the use of a single archival cytology slide did not compromise the patient′s permanent slide record. Once identified as available for mutation analysis, the patient′s cytopathology report was reviewed in order to establish the final cytologic diagnosis and document the area of the thyroid having undergone FNA.
The surgical pathology tissue blocks were retrieved from the surgical pathology laboratory tissue block archives. A cytopathologist (JSB) reviewed the patient histopathology reports in order to select only the tissue block that corresponded to the nodule that underwent FNA. If the cytopathology report identified the FNA site as "thyroid-not otherwise specified, " the tissue block for analysis were selected from nodules demonstrating PTC.
Finally, we identified matched pairs of preoperative thyroid FNA and follow-up FFPE thyroid tissue samples demonstrating benign findings (e.g. nodular goiter, thyroiditis). This set of samples served as negative controls for the LCPCR assay.
DNA isolation from FFPE tissue sections
Five 5 μm sections were cut from the FFPE tissues. One tissue section was hematoxylin and eosin (H/E) stained and coverslipped for review by a cytopathologist (JSB). Using a pen, the area of the tissue that contained the tumor was marked. The remaining unstained sections were deparaffinized by immersion in 3 changes of xylene for 5 minutes each. The tissue sections were then hydrated in a graded series of ethanol, followed by immersion in dH 2O for 1 minute. The slides were allowed to air-dry completely. The unstained tissue section slide was then inverted over the H/E-stained slide and the area identified by the cytopathologist was marked on the underside of the unstained tissue section slide. Using the circled area of interest on the unstained tissue section slide as a guide, a scalpel blade was used to manually scrape the areas of the tissue containing the tumor cells of interest. Following manual microdissection, the scalpel blade was inserted into a clean microcentrifuge tube. A 25.0 μl aliquot of Proteinase K (3 mg/ml) digestion solution (50 mM Tris, 1 mM EDTA, pH8.0, 1% Tween 20) was pipetted onto the scraped area of the slide to pick up any remaining cells. The digestion solution was then pipetted from the slide and used to rinse the scalpel blade that was positioned inside the labeled microcentrifuge tube.
DNA isolation from FNA samples
A cytopathologist (JSB) assigned each FNA smear slide a score on based on overall cellularity and atypical cell content. Overall cellularity scores were as follows:
1+: Unsatisfactory (≤ 1 or 2 clusters of epithelial cells); 2+: Scant (3-10 clusters of epithelial cells) 3+: Adequate (10-20 clusters of epithelial cells) 4+: Abundant (> 20 clusters of epithelial cells with most fields of cells touching). Atypical cell content scores were as follows: 1+: < 25% atypical cells; 2+: 25-50% atypical cells; 3+: 50-75% atypical cells; 4+: >75% atypical cells.
Using a pen, the area of the archival FNA slide containing area of atypical cells of interest was marked. A diamond-tipped pencil was then used to mark the underside of the slide indicated by the cytopathologist. Slide coverslips were detached in xylene, and the slides were hydrated in a graded series of alcohol, followed by soaking in distilled water for 2 minutes. The FNA slides were then hydrated in a graded series of ethanol, followed by immersion in dH 2O for 1 minute. The slides were allowed to air-dry completely. Using the area of interest indicated by the diamond-tipped pencil marking on the FNA slide as a guide, slide scrape lysates (SLL) were prepared by using a single-edged razor blade to scrape the areas of the slide containing the atypical cells of interest. Following manual microdissection, a 50.0 μl aliquot of Proteinase K (3 mg/ml) digestion solution (50 mM Tris, 1 mM EDTA, pH 8.0, 1% Tween 20) was pipetted onto the scraped area of the slide to pick up any remaining cells. Using the same pipette tip, the digestion solution was then pipetted from the slide and used to rinse the scalpel blade that was positioned inside the labeled microcentrifuge tube.
DNA extraction
The samples were incubated in the digestion solution at 55°C for 12-16 hours. Following centrifugation at 12,000 rpm for 5 minutes, the supernatant was transferred into a newly labeled microcentrifuge tube. The samples were then placed into a 95°C heat block for 10 minutes to inactivate the proteinase K. All FFPE tissue sample DNA was diluted to a working concentration of 50 ng/μl prior to amplification. A MicroSpin G25 (Amersham Bioscience, Sweden) sephadex column was routinely used for all FNA samples following DNA extraction. Fine needle aspiration sample DNA then underwent PCR without additional dilution. Following DNA extraction, all samples were stored at -20°C prior to analysis.
Characterization of control material
To assess the sensitivity and specificity of the LCPCR method for detection of the BRAF V600E mutation, three cell lines were analyzed. A human PTC-derived cell line (NPA) was characterized for use as a positive control, while one follicular thyroid carcinoma (ROW-1), and one colorectal carcinoma (HCT116) cell lines were characterized for use as negative controls. All cell lines were grown to a concentration of 3 x 10 6cells per ml, trypsinized, and transferred to a 1.5 ml microcentrifuge tube. Cell line DNA was isolated and purified using the QIAamp DNA Mini Kit (QIAGEN Inc., Valencia, CA). DNA concentration and purity was determined and all cell lines were diluted to a working concentration of 50 ng/μl and stored at 4°C.
Polymerase chain reaction and fluorescent melting curve analysis
For this study, a pair of oligonucleotide primers were designed to amplify a 250 base-pair region of exon 15 in the BRAF gene:
forward: 5′CTCTTCATAATGCTTGCTCTGATAGG-3 and
reverse: 5′TAGTAACTCAGCAGCATCTCAGG-3′ (Integrated DNA Technologies, Inc, Coralville, IA).
Two fluorescent hybridization probes were designed to detect the BRAF V600E mutation: 23sensor: 5′-AGCTACAGTGAAATCTCGATGGAG-Fluoroscein-3′ and
anchor: 5′-LCRed640-GGTCCCATCAGTTTGAACAGTTGTCTGGA-Phosphate-3′
with the sensor probe spanning nucleotide position 1799 (Idaho Technologies, Salt Lake City, UT). Amplification was performed in glass capillaries using 50 ng of tissue or FNA (range 12.0 to 110.0 ng) sample DNA in a 10 μl volume containing 1 ul of 10x LightCycler DNA Master Hybridization Probes (Roche Molecular Biochemicals, Mannheim), 0.8 μl of 25 mM MgCl 2, 1 μl (5 μM) forward and reverse primer, and 1 μl (2 μM) anchor and sensor hybridization probe. The reaction mixture underwent 45 cycles of rapid PCR. Post amplification fluorescent melting curve analysis was performed by gradual heating of the samples at a rate of 0.1°C per second from 45°C to 95°C. Fluorescent melting peaks were determined by plotting of the negative derivative of fluorescence (F) with respect to temperature (T), or -dF/dT.
Limit of detection experiment
A limit of detection experiment was first conducted to determine the percent of tumor with normal cell contamination in which abnormal melts were detectable by LCPCR. The NPA cell line was diluted with human genomic wild-type (WT) DNA to 99% tumor, 95% tumor, 90% tumor, 75% tumor, 50% tumor, 25% tumor, 10% tumor, and 5% tumor. In addition, 100% NPA and 100% WT samples were tested. Each dilution was run in duplicate.
DNA sequencing analysis
All PCR products that showed deviation from the WT genomic DNA melting peak, as well as any discordant FNA and FFPE matched pairs, and benign control samples were confirmed by direct sequencing of exon 15. Ten ul of amplified sample plus 1.0 ul of Biotracker 6x tracking dye (Bioventures, San Francisco) was loaded into the sample wells of a 2% agarose DNA gel and electrophoresed at 70 volts. To isolate the DNA from the agarose gel, the desired ethidium-stained band was viewed with a UV transilluminator and excised using a razor blade. Sample DNA was then extracted from the agarose gel using a nebulizer (Millipore, Bellirica, MA). Bidirectional DNA sequence data was generated for each sample using fluorescently labeled terminator sequencing chemistry and sequencing primers (5′ primer and 3′ primer). DNA sequencing data files from the purified sequencing reaction products were generated using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems).
Results
A total of 24 archival FNA (indeterminate n = 19; benign n = 5) and FFPE matched pair samples were evaluated for the BRAF V600E point mutation using LCPCR.
Characterization of control material
A homozygous T→A mutation was identified in the BRAF gene (exon 15) at nucleotide 1799 in the NPA cell line, which is derived from PTC. The NPA cell line served as a positive control for BRAF V600E mutation analysis by LCPCR. To assess the specificity of the LCPCR method for detection of the BRAF V600E mutation, cell lines ROW-1 (follicular thyroid carcinoma) and HCT116 (colorectal carcinoma) underwent mutation analysis. Both ROW-1 and HCT115 cell lines showed a WT BRAF sequence. These findings were confirmed by direct DNA sequencing.
LCPCR limit of detection
Results of the limit of detection experiments confirmed that the 1 base pair change in the BRAF mutation was detectable down to the level of 25% tumor when a homozygous mutant cell line was used as a control. Consequently, it was determined that the results of LCPCR for detection of the heterozygous BRAF V600E mutation in FNA or FFPE samples containing less than 50% tumor cells may not be accurate.
Prevalence of BRAF V600E mutation in indeterminate thyroid fine needle aspirates
Using LCPCR and DNA sequencing, BRAF mutation analysis confirmed the preoperative diagnosis of PTC in 3/19 (15.8%) of the cases in the indeterminate group. Nine of 19 (47.4%) corresponding FFPE surgical samples collected from the same patients were positive for the BRAF mutation Table 1.
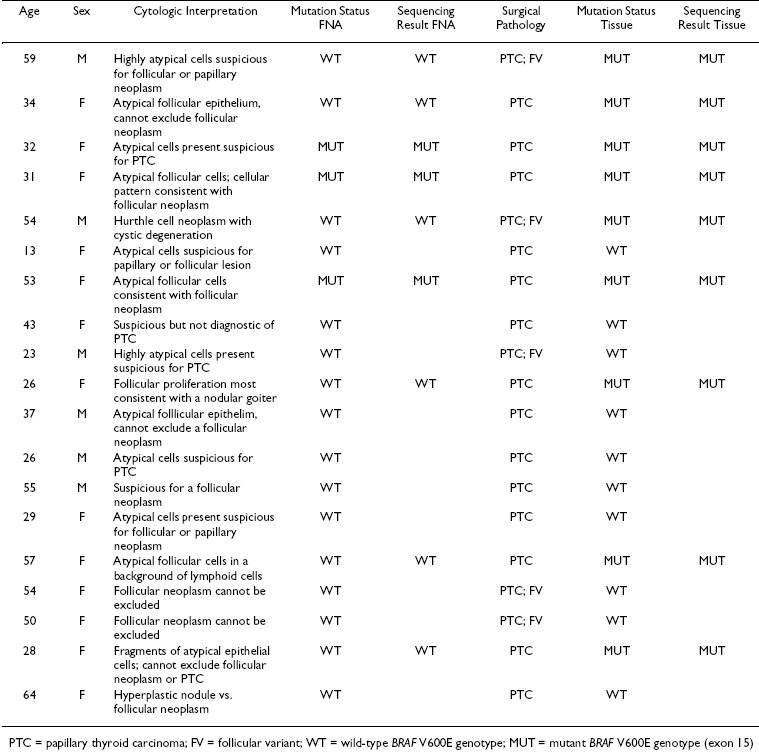
There was a 69% rate of concordance for BRAF mutation status between paired indeterminate FNA and malignant FFPE tissue specimens. Six FNA samples were negative for the BRAF V600E mutation, while the corresponding FFPE tissue samples were positive. Of these, 5/6 FNA samples contained less than 50% tumor cells. Conversely, an indeterminate FNA containing >75% atypical cells failed to demonstrate the mutation, while the resected tumor was positive for the mutation. The overall cellularity of this sample was scored at a 2+.
Melting curve analysis revealed that for the FNA samples, the WT sequence (GTG) Tm was 65.34°C ± 0.37°C, the GTG→GAG mutation at nucleotide 1799, resulted in a shift of Tm to 60.23°C ± 0.53°C Figure 1. For the FFPE samples, the WT Tm was 64.92°C ± 0.35°C, while the mutant Tm was 60.11°C ± 0.46°C Figure 2.
Bidirectional DNA sequencing revealed that all BRAF mutations were heterozygous and involved a T→A substitution at nucleotide 1799. The LCPCR assay demonstrated 100% concordance between melting curve and DNA sequence results. 0/5 benign thyroid FNA and matching FFPE samples were also found to be negative for the mutation.
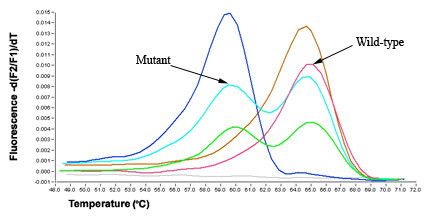
- Melting curve analysis of BRAF mutations in thyroid FNA samples. Overlapping fluorescein-labeled oligonucleotide probes were used to scan extracted DNA for mutations in exon 15 of BRAF. Multiple probes complementary to the wild-type (WT) sequences were placed within the same reaction, and the different sites were identified by their specific probe/target duplex melting temperatures. The position of each probe/target melting temperature and the relative ratio of the melting peak areas determined WT profiles. After amplification in a LightCycler, the instrument begins a melting program where the reactions are cooled to anneal the probes and then slowly heated (0.1°C/s) while fluorescence is continuously monitored. Somatic mutations are identified by changes from a characteristic WT melting curve profile. When melting curves from non-mutated and mutated samples are compared, additional melting peaks or changes in peak-area ratios indicate a sequence alteration (nucleotide mismatch) under the probe. Melting curve analysis revealed that for the WT BRAF sequence (GTG) Tm was 65.34°C ± 0.37°C, the GTG→GAG mutation at nucleotide 1799, resulted in a shift to 60.23°C ± 0.53°C.
Discussion
As often as 30% of the time, FNA cytology displays limited ability to discriminate between benign and malignant thyroid lesions, and an indeterminate diagnosis is rendered 31. Some clinicians feel that total thyroidectomy is appropriate for patients with an indeterminate FNA cytology result 910, 32. Proponents of this approach argue that it eliminates the probability of thyroid cancer recurrence [33-36]. Alternatively, if the suspect nodule is small, some clinicians opt to perform a hemi-thyroidectomy procedure following an indeterminate cytology result 37. Considered the minimum extent of surgery for a thyroid nodule, this procedure reduces the risk of postoperative complications associated with total thyroidectomy, such as hypoparathyroidism and laryngeal nerve injury 38. However, depending on patient/tumor risk stratification, postoperative confirmation of malignancy usually results in a second-stage completion thyroidectomy, which is associated with higher morbidity than initial total thyroidectomy 39. Regardless of the choice of surgical procedure, the incidence of malignancy in patients with indeterminate cytology findings varies greatly. Consequently, a large percentage of these patients would benefit from a method that improves the diagnosis of preoperative thyroid aspirate material.
A number of molecular markers have been evaluated as possible adjunct tests for refining the diagnosis of PTC on FNA. However, the predictive value of these markers has been limited to date due to a lack of specificity or sensitivity 40. Expressed by malignant thyrocytes, galectin 3 is a β-galactoside-binding protein that was initially believed to be a marker specific for PTC 41. Further analysis has revealed, however, that identification of this protein may be less reliable in conditions involving lymphocytic infiltration, such as Hashimoto′s thyroiditis 42. HBME-1 is a monoclonal antibody that recognizes an epitope expressed in malignant mesothelioma and other adenocarcinomas, as well as PTC and follicular thyroid tumors 4344]. Although benign thyroid lesions do not express immunoreactivity for HBME-1 44, positive staining has been found in malignant thyroid tumors besides those of papillary differentiation 45. Telomerase is a specialized reverse transcriptase enzyme that maintains chromosome ends. Detection of telomerase expression by reverse-transcriptase PCR originally showed promising sensitivity and specificity for PTC diagnosis 46, but telomerase repeat amplification (TRAP) has identified high expression of this molecule in FNA specimens from benign nodules 47. Up to 95% of PTC demonstrate strong immunostaining with cytokeratin 19 (CK19) 48. However, CK19 immunoreactivity is not specific for PTC, as positive immunoreactivity has been identified in benign follicular adenomas 49. Aberrant expression of the RET proto-oncogene results from chromosomal rearrangements in which the tyrosine kinase domain of RET is fused to the 5′-terminal region of an unrelated gene, leading to the generation of fusion proteins known as ret rearrangements in PTC (RET/PTC). Although RET/PTC rearrangements have been identified in a large percentage of PTC in individuals exposed to external radiation 50, a relatively high frequency of RET/PTC rearrangements have also been found in benign nodular thyroid diseases of patients exposed to nuclear fallout and in benign conditions such as trabecular adenomas and Hashimoto′s thyroiditis 51.
The mutation at V600E in the BRAF kinase gene appears to be an attractive molecular marker for thyroid cancer diagnosis as it has been found to be the most common genetic event in PTC, while being highly specific for this tumor Table 2. The goal of this study was to identify the BRAF V600E mutation in thyroid FNA samples in an attempt to determine if BRAF mutation analysis can serve as a useful adjunct technique in indeterminate cytologic diagnoses. The use of LCPCR was chosen for SNP detection because both gene amplification and allele analysis could be performed in a homogeneous, closed-tube system on the same instrument. Increased specificity for mutation detection is realized due to the hybridization of two independent probes and the fact that the probe melting temperature is sequence specific. Of the cases evaluated in this study, which included 24 matched pairs of FNA and FFPE surgical tissues, there were no false positive BRAF mutation results by LCPCR and LCPCR demonstrated 100% concordance with DNA sequencing results.

Previous investigators have also reported on use of the LCPCR method for detecting the V600E activating point mutation in the BRAF gene. In contrast to the present study, which analyzed archival FNA and FFPE surgical material for mutation detection, these groups utilized primarily cell lines or a combination of cell lines and FFPE surgical material. Nikiforova et al. evaluated thyroid tumors and anaplastic carcinoma cell lines to demonstrate that BRAF mutations, which in thyroid tumors were originally thought to be restricted to papillary carcinomas, also occur in poorly differentiated and anaplastic carcinomas 23. Among 259 thyroid tumor samples screened, this group demonstrated a 100% correlation in BRAF V600E detection rate between LCPCR and single strand conformational polymorphism. This finding is in agreement with the results of the present study, in which LCPCR assay demonstrated 100% concordance between melting curve and DNA sequencing results. In contrast to the present study, however, Nikiforova used laser capture microdissection (LCM) to obtain DNA either from a small focus of papillary microcarcinoma or to study well-differentiated and poorly differentiated or anaplastic areas within the same tumor. Ikenoue et al. analyzed 12 colon and 9 gastric cancer cell lines by LCPCR for presence of the BRAF V600E mutation 52. Using a mixture of standard DNA, Ikenoue determined that as little as 10% V600E mutant DNA could be identified in a background of WT DNA. In the present study, results of the limit of detection experiments confirmed that the 1 base pair change in the BRAF mutation was detectable down to the level of 25% tumor when a homozygous mutant cell line was used as a control. Consequently, it was determined that the results of LCPCR for detection of the heterozygous BRAF V600E mutation in FNA or FFPE samples containing less than 50% tumor cells may not be accurate. It is s possible that the level of sensitivity might be increased through the use of a technique such as LCM of the archival FNA slide material. As demonstrated by Nikiforova, et al, using LCM can significantly enhance the sensitivity for identifying mutant DNA in the presence of WT DNA, as the captured sample contains almost exclusively tumor cells.
In the present study, the preoperative diagnosis of PTC was confirmed in 3/19 (15.8%) indeterminate FNA samples that could not be conclusively diagnosed by cytology alone. This finding is consistent with reported mutation prevalence rates in indeterminate thyroid FNA cases Table 3 2753, 54. However, 9/19 (47.4%) corresponding FFPE surgical samples collected from the same patients were positive for the mutation, for a 69% rate of concordance between the sample types. Of the 6 discordant FNA cases in this study, 5/6 (83%) contained < 50% atypical cells. Because tumor DNA from FNA samples is invariably contaminated with the WT allele of the gene in question, the somatically mutated allele can be difficult to distinguish. This results in reduced sensitivity for identifying mutant DNA in the presence of WT DNA. Results of the limit of detection experiments confirmed that the one base pair change in the BRAF mutation was detectable by LCPCR down to the level of 25% tumor when a homozygous mutant cell line was used as a control. Because the BRAF V600E mutation in PTC is heterozygous, a detection limit of 50% atypical cells was established for the LCPCR assay, below which the results of LCPCR might not be accurate. Because LCPCR and DNA sequencing results were in 100% agreement for all samples, it is likely that the percentage of BRAF mutant DNA in the discordant FNA samples was below the limit of detection for both methods.
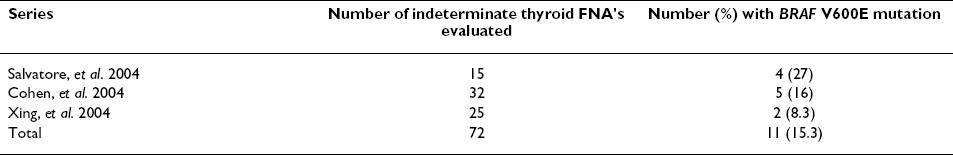
Other investigators have also experienced varying degrees of concordance for BRAF mutation status between matched FNA and FFPE samples. Cohen et al. noted discordant results in 3/49 matched pair samples for a 94% rate of concordance between the sample types 53. Of the three discordant FNA samples in Cohen′s study, the mutation was not detected in 2 FNAs while the resected tumors harbored the mutation. In these 2 cases, the FNA material was found to be sparsely cellular. These previous findings, combined with those of the present study, confirm that overall tumor cell content of the FNA sample is critical for mutation detection, whether by LCPCR or another method.
If the results of the limit of detection experiments in the present study were used to select samples for mutation analysis, thereby excluding from analysis any FNA sample that contained < 50% atypical cells, 2/8 indeterminate FNA samples would have been found to harbor the BRAF mutation. The resulting BRAF positivity rate in the indeterminate FNA samples would, therefore, be 25% rather than 15.8%. In actual clinical practice, the pathologist would control pre-analytic probability by carefully selecting which indeterminate thyroid FNAs would be referred for mutation analysis, based upon the cellular composition of the smears and/or the needle rinse pellet.
It is also possible that the discordant aspirate material in the present study contained only the BRAF WT genotype, and that the mutant DNA identified in the resected tumor samples was the result of an early tumorigenic event. However, this is an unlikely scenario, as inclusion criteria allowed cases to be selected for mutation analysis only if the cytology was reported as abnormal within a six-month period preceding the histology report. This restriction was instituted in an attempt to reduce mutation status discordance between the sample types. Five of the six discordant FNA cases were collected within one month prior to the FFPE surgical tissue.
Because PTC is frequently multifocal, there has been speculation regarding whether noncontiguous tumor foci arise from intraglandular metastases from a single primary tumor or originate as unrelated clones derived from independent tumors. A recent study by Shattuck et al. 55used PCR to evaluate the patterns of X chromosome inactivation of multiple distinct foci of PTC from 17 women. Discordant patterns indicative of independent origins were identified in tumors from 5 patients, leading to the conclusion that individual tumor foci in patients with multifocal PTC often arise as independent tumors. This finding could explain why an indeterminate FNA containing >75% atypical cells in the present study failed to demonstrate the mutation, while the resected tumor was positive for the mutation. The possibility exists that the tumor identified in the resected tissue, and in the nodule sampled by FNA, did not share the same clonal origin.
While the hotspot mutation at V600E is the most common genetic event in PTC, recent studies have demonstrated that up to 9% of follicular variant of PTC (FVPTC) cases demonstrate a mutation in codon 601 of the BRAF gene, resulting in the substitution of lysine with glutamate (K601E) 2556]. Although five of the indeterminate thyroid FNA cases in the present study were classified histologically as FVPTC, none were found to contain the K601E mutation. However, because the hybridization probes were designed as a perfect match to the BRAF WT genotype, other mutations covered by the probe would likely lead to a different temperature profile and probable detection of the K601E mutation.
Because the number of samples analyzed in the present study was limited, and the tumor cell content of some of the archival samples was scant, we were unable to determine the full diagnostic utility of LCPCR detection of the BRAF activating point mutation on indeterminate thyroid aspirates. Although molecular techniques such as LCPCR may be useful for refining a diagnosis of PTC, the absence of a BRAF mutation does not exclude the possibility of a malignant condition. In view of the large number of palpable thyroid nodules that require evaluation by FNA, a search for molecular markers such as BRAF may have clinical utility.
The results suggest that detection of BRAF mutation in thyroid aspirates may enhance the accuracy of FNA and refine preoperative diagnosis of PTC.
The authors would like to thank Dr. Joseph Holden, Dr. Elaine Lyon, Dr. Genevieve Pont-Kingdon, Alison Millson and Carlynn Willmore-Payne for their technical assistance on this study. The authors declare that they have no competing interests. This work was supported by the ARUP Institute for Clinical and Experimental Pathology.
References
- Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med :537-40.
- [Google Scholar]
- : High frequency of cancer in cold thyroid nodules occurring at young age. Acta Endocrinol (Copenh) :197-202.
- [Google Scholar]
- : Follicular lesions of thyroid: a 5-year fine-needle aspiration experience. Cancer :335-41.
- [Google Scholar]
- : Factors that predict malignant thyroid lesions when fine-needle aspiration is "suspicious for follicular neoplasm" Mayo Clin Proc :913-6.
- [Google Scholar]
- : BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene :4578-80.
- [Google Scholar]
- : Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab :4393-7.
- [Google Scholar]
- : BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab :5399-404.
- [Google Scholar]
- : BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol :247-51.
- [Google Scholar]
- : BRAF T1796A transversion mutation in various thyroid neoplasms. J Clin Endocrinol Metab :1365-8.
- [Google Scholar]
- : Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab :2867-72.
- [Google Scholar]
- : Contralateral papillary thyroid cancer is frequent at completion thyroidectomy with no difference in low- and high-risk patients. Thyroid :877-81.
- [Google Scholar]
- Evaluation of the results after a conservative surgical approach. Am J Surg :349-54.
- [Google Scholar]
- An adjunct to fine-needle aspiration diagnosis of papillary thyroid carcinoma. Arch Pathol Lab Med :579-83.
- [Google Scholar]
- : RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab :3211-6.
- [Google Scholar]
- : Rapid detection of mutations in the BRAF gene using real-time polymerase chain reaction and melting curve analysis. Cancer Genet Cytogenet :68-71.
- [Google Scholar]
- : Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res :2761-5.
- [Google Scholar]
- : Analysis of BRAF point mutation and RET/PTC rearrangement refines the fine-needle aspiration diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab :5175-80.
- [Google Scholar]
- : Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients′ age but not with tumour aggressiveness. Virchows Arch :.
- [Google Scholar]







