Translate this page into:
Utility of fine-needle aspiration cytology combined with flow cytometry in extramedullary hematolymphoid lesions – A cross-sectional study

*Corresponding author: Debasis Gochhait, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. debasis.go@gmail.com
-
Received: ,
Accepted: ,
Abstract
Objective:
Flow cytometry (FC) can be an adjunct to fine-needle aspiration cytology (FNAC) in the diagnosis and subclassification of hematolymphoid lesions. This study evaluates the utility of FC in diagnosing extramedullary hematolymphoid lesions in fine-needle aspirate samples.
Material and Methods:
This cross-sectional study enrolled patients who presented to the FNAC clinic and suspected to have hematolymphoid lesions (nodal and extra nodal lesions) from August 2020 to June 2022. Sixty-seven cases of hematolymphoid malignancies and 67 cases without hematolymphoid malignancies were included. The combined FNAC/FC diagnosis was compared with the gold standard: Biopsy, cell block, or peripheral blood/bone marrow aspirate FC.
Results:
Of 67 lymphoma cases, 63 were of the primary type and 4 were diagnosed with suspected recurrence/residual disease. Moreover, 57 cases were nodal, and 10 were extranodal. Four of the patients had discordant findings between FNAC and FC. The gold standard was available only in 56 cases, of which 5 had discordant findings between combined FNAC/FC and the gold standard. We subclassified 34 cases based on combined FNAC/FC. The five cases included anaplastic large cell lymphoma (1/5), classic Hodgkin lymphoma (3/5), and one case of atypical lymphoid hyperplasia. Non-contributory FC was attributed to the presence of large cells/necrosis/nodular lymphocyte predominant Hodgkin lymphoma/metastasis/Castleman disease/technical problem.
Conclusion:
Combining FC with FNAC enhances diagnostic accuracy and helps subclassify lymphoma. Future works should explore whether it can replace excision biopsy, especially in recurrent cases.
Keywords
Flow cytometry
Rapid onsite evaluation
Cytology
Lymphoma
INTRODUCTION
Lymphoma diagnosis is usually based on tissue biopsy and needs a detailed morphologic and immunohistochemical evaluation. Categorization of lymphomas is difficult in fine-needle aspiration cytology (FNAC). However, with the help of flow cytometry (FC) and substantial advances in sophisticated methods and equipment, the FNAC diagnosis of lymphoma has become accessible.[1]
Biopsy is not feasible in sites, such as the thyroid, salivary glands, and vital visceral organs (gastrointestinal tract, mediastinum), where FNAC may be the only tissue diagnosis and categorization modality. In suspected recurrent/progression of disease at the same site or a new site, FNAC and FC may help confirm and categorize lymphoma. The disadvantage of using FNAC or FC only can be addressed by combining them. Morphology cannot be analyzed in FC, and subclassification of lymphoma is not possible in FNAC because of a limited number of materials for ancillary studies. The combination of FC with FNAC has led to the diagnosis and subclassification of non-Hodgkin lymphoma in accordance with the World Health Organization (WHO) classification, especially in low-grade lymphomas and in recurrent cases.
In this research, we will analyze the role of flow cytometric immunophenotyping as an adjunct to FNAC for diagnosis and sub-classification of hematolymphoid lesions compared with biopsy. Our objective is to assess the diagnostic accuracy of FNAC to confirm and categorize hematolymphoid lesions in comparison with the gold standard.
MATERIAL AND METHODS
This cross-sectional study was conducted in the Department of Pathology from August 2020 to June 2022. The study was conducted after approval from the Postgraduate Research Monitoring Committee, and clearance from the Institute Ethics Committee (IEC) was obtained (JIP/IEC/2020/118 dated June 19, 2020). All patients who presented to the FNAC clinic suspected of having hematolymphoid lesions and reactive lesions (nodal and extra nodal) were enrolled. Informed consent was obtained in the regional language from each patient during the procedure.
Sample size was estimated with an expected sensitivity/specificity of FNAC combined with FC for the diagnosis of hematolymphoid malignancies as 85% at 5% level of significance and 10% relative precision. Nodal and extranodal cases were also considered in estimating the sample size for any sub-group analysis. Calculation using Cochran’s formula revealed 67 cases of hematolymphoid malignancies and 67 cases without hematolymphoid malignancies.
Study procedure
A detailed clinical evaluation was done. Relevant clinical information and provisional diagnosis were collected from the case sheet. After explaining the procedure and risks, informed consent was obtained from adult participants. For participants aged <18 years and those who cannot consent, informed consent was obtained from their parents/legally authorized/acceptable representative. Assent was also taken for children above 12 years and below 18 years. The study complied with Declaration of Helsinki.[2] FNAC was performed on palpable lymph node lesions and extranodal lesions. In the case of lesions that are inaccessible by blind FNAC, an image-guided FNAC was done under the radiologist’s supervision. In all patients, the first pass was used to prepare two traditional smears; the first was stained immediately with toluidine blue after fixation in 95% ethanol (rapid onsite evaluation [ROSE]) and evaluated to select patients for immunophenotyping. Other slides were air-dried and fixed in 95% ethanol at a ratio of 30:70. Slides were subjected to alcohol fixation because it could be used for immunocytochemistry (ICC) after morphological evaluation. ICC was routinely performed on alcohol-fixed smears (with control) in our center.
May–Grünwald–Giemsa (MGG) and Papanicolaou (PAP) stains were done on air-dried and alcohol-fixed smears. On ROSE, if it turns out to be a lymphoma/reactive, then a second pass was made. The sample was flushed with phosphate-buffered saline (PBS) in an ethylenediamine tetraacetic acid tube and processed for FC. Whenever possible, the third pass was made, and the aspirate was left in the slide to clot and then collected for cell block in 10% buffered formalin to compare the results with FC immunophenotyping in case subsequent tissue biopsy is unavailable. Cell block preparations were stained with routine hematoxylin–eosin and immunohistochemical stains (as needed if smears for ICC are inadequate).
On ROSE, if it turned out to be metastasis/carcinoma/infective lesions, a second pass was not made, or if needed, samples were collected for cell block. The samples collected for FC were processed as soon as possible (within 2 h). The reason for the urgency was that clotted/degenerated sample reduces the yield of cells for FC. All reagents and consumables were procured from Beckman Coulter, France and BD biosciences, USA. The instrument used was acquired from Beckman Coulter, France.
A basic panel of antibodies was used, which was then extended according to the number of cells available, the predicted subtype from the preliminary examination of the slides, the patient’s previously diagnosed lymphoma, and the clinical situation. Table 1 depicts the antibodies used. In our study, an extended panel of antibodies was not used in some instances because of the expected delay in processing and low cellularity. The cytologic findings and FC data were interpreted together to diagnose and subclassify hematolymphoid malignancy according to the WHO.
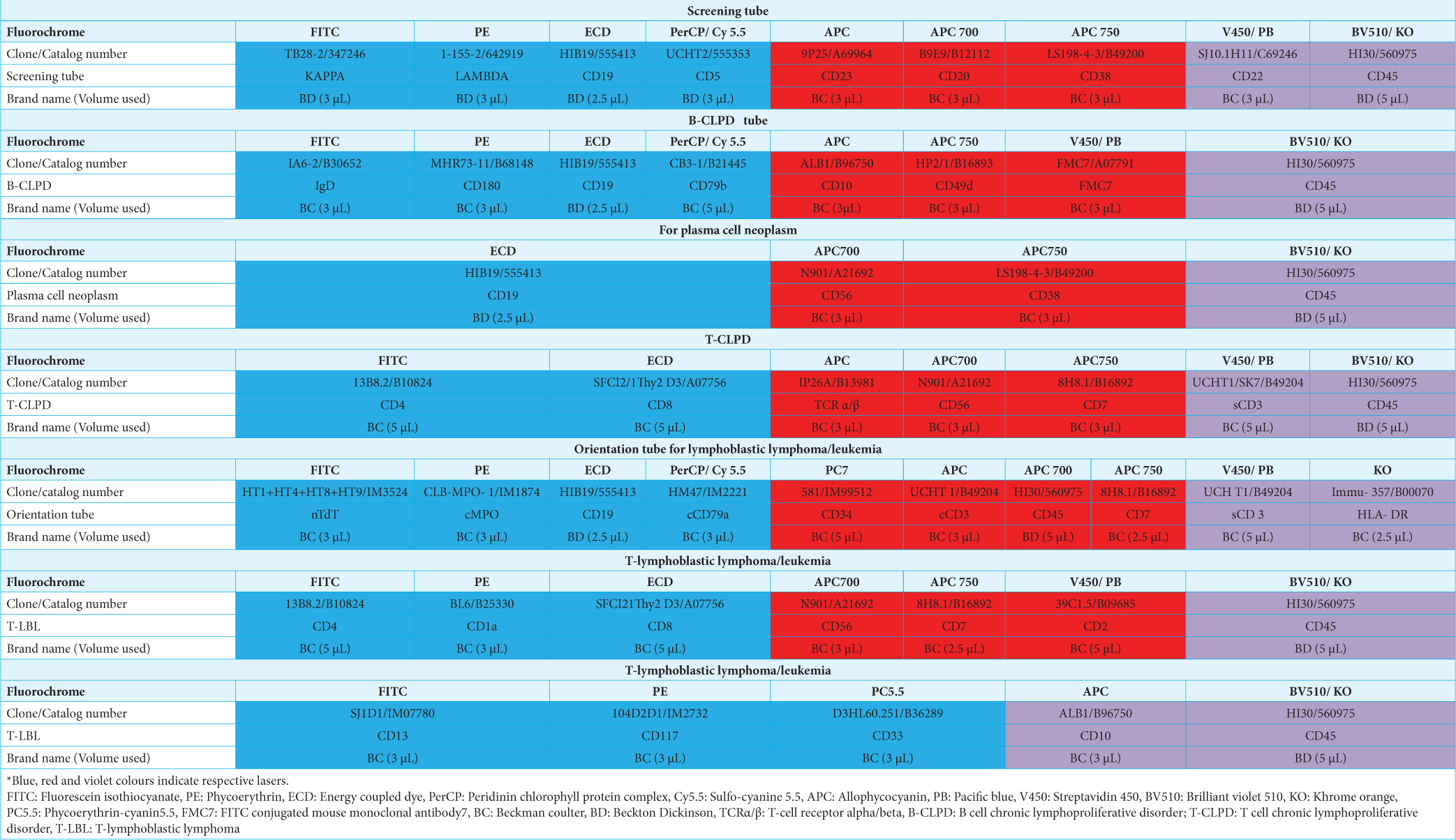 |
In biopsy/surgical follow-up patients, histopathology with immunohistochemistry was considered the “gold standard.” By contrast, cell block sections with ICC were considered the “gold standard” for cases not subjected to biopsy. In patients lacking biopsy/cell block, FC data of peripheral blood/bone marrow were taken as the “gold standard” (when available). The cell block provides similar results to biopsy, and FC of peripheral blood/bone marrow aspirate was standardized in our laboratory, so they were used as the gold standard in cases where biopsy is unavailable.
Sample processing for FC
The sample was immediately transferred to the FC laboratory. Stain–lyse–wash technique was used for sample processing. The sample was washed in PBS 3 times for 5 min at 2500 revolution per minute (RPM). About 100 µL of the sample was taken, added with a pre-titrated volume of surface antibodies, vortexed, and incubated in the dark for 20 min. The sample was added with 2 mL of PBS, vortexed, and centrifuged at 2500 RPM for 5 min to wash excess unbound antibodies. The supernatant was decanted, and 100 µL of formaldehyde (fixative agent) was added, vortexed, and incubated in the dark for 15 min. This was followed by washing with excess PBS and addition of 100 µL of saponin (permeabilizer). The sample was left undisturbed for 5 min, added with intracytoplasmic or nuclear antibodies, incubated, and washed. Optilyse was used to lyse red cells after staining. The unstained tube was processed along with each panel to check for autofluorescence.
Samples were acquired on a three-laser and 10-color flow cytometer comprised of two fluorescent channels from the violet (405 nm) laser, five channels from the blue (488 nm) laser, and three channels from the red (638 nm) laser (Navios, Beckman CoulterTM). At least 10,000–1,00,000 events per tube were acquired for the diagnostic immunophenotyping.
Data were interpreted using Kaluza software version 2.1 (Beckman Coulter, USA). Initial gating was conducted with a side scatter versus CD45 gating strategy. Granulocytes were excluded using side scatter versus CD38 gating. B cells were gated with CD19/CD20 versus side scatter gating. Aberrant expression of CD5 and CD23 was detected in B cells. T cells were used as a control for CD5. The percentage of B and T cells was recorded. The clonality of B cells was studied with CD19/CD20 versus kappa/lambda gating. Finally, the kappa: lambda ratio was noted for kappa against lambda gating. Antigen expression of blast/atypical cells was reported according to Associazione Italiana di Ematologia e Oncologia Pediatrica and Berlin-Frankfurt-Münster consensus guidelines and the WHO-style tripartite consensus reporting as negative, weak and strong based on median fluorescent intensity.[3]
RESULTS
Sixty-seven cases of suspected hematolymphoid malignancies and 67 cases of reactive lesions were recruited. In addition, 29 cases with suspected hematolymphoid malignancies/reactive lesions were excluded from the sample size as they yielded non-contributory results.
Lymphoma cases
In 67 cases with hematolymphoid malignancies, 63 (94.0%) were primary cases, and 4 (6.0%) were already diagnosed cases with suspected relapse/residual disease. Fifty-seven (85.1%) cases were nodal, and 10 (15.0%) were extranodal. Extranodal sites include parotid, thyroid, tonsil, scalp, cutaneous lesions, orbital mass, retroperitoneal mass, and mandibular swelling.
The final diagnosis was made based on the gold standard, which is available in 56/67 cases. The gold standard was either biopsy (lymph node/bone marrow) or FC of peripheral blood/bone marrow or cell block, whichever is available because biopsy is not available for all cases. Our study’s most common gold standard method was biopsy (40/56).
A morphological diagnosis was made in FNAC. The distribution of FNAC diagnosis is depicted in Table 2. Then, FC was interpreted separately, and a diagnosis was made. The distribution of FC diagnosis is depicted in Table 3. Finally, morphological and FC diagnoses were correlated, and a combined diagnosis was made. The distribution of combined diagnosis is shown in Table 4. The final diagnosis was the diagnosis of the gold standard. The distribution of the final diagnosis based on the available gold standard is depicted in Table 5.
| FNAC diagnosis | Number (n=67) | Percentage |
|---|---|---|
| Small cell lymphoma | 18 | 30.0 |
| Intermediate cell lymphoma | 5 | 8.0 |
| Large cell lymphoma | 19 | 28.4 |
| Suspicious of NHL | 3 | 5.0 |
| Suspicious of Burkitt lymphoma | 3 | 5.0 |
| Possibility of ALCL/CHL | 3 | 5.0 |
| CHL | 3 | 5.0 |
| Hematolymphoid malignancy -lymphoma/leukemia | 8 | 12.0 |
| Plasma cell neoplasm | 3 | 5.0 |
| Equivocal | 1 | 2.0 |
| Reactive | 1 | 2.0 |
NHL: Non-Hodgkin lymphoma, ALCL: Anaplastic large cell lymphoma, CHL: Classic Hodgkin lymphoma, FNAC: Fine-needle aspiration cytology
| FC diagnosis | Number (n=67) | Percentage |
|---|---|---|
| CLL | 7 | 10.4 |
| CD5+CD23- B-NHL | 7 | 10.4 |
| CD5-CD23- B-NHL | 11 | 16.4 |
| CD5-CD23-CD10+ B-NHL | 1 | 2.0 |
| CD5-CD23-CD10- B-NHL | 12 | 18.0 |
| Suspicious of Burkitt lymphoma | 5 | 8.0 |
| Lymphoplasmacytic lymphoma | 1 | 2.0 |
| Plasma cell neoplasm | 3 | 5.0 |
| Suspicious of T-NHL | 4 | 6.0 |
| T-NHL | 2 | 3.0 |
| T-LBL | 1 | 2.0 |
| Near ETP-ALL | 2 | 3.0 |
| Sezary syndrome | 1 | 2.0 |
| Blasts present | 4 | 6.0 |
| CD45- events with reactive events | 4 | 6.0 |
| Reactive | 2 | 3.0 |
CLL: Chronic lymphocytic lymphoma, T-LBL: T-lymphoblastic lymphoma, ETP-ALL: Early T-cell precursor acute lymphoblastic lymphoma, T-NHL: T-cell non-Hodgkin lymphoma, B-NHL: B cell non Hodgkin lymphoma, FC: Flow cytometry
| Combined diagnosis | Number (n=67) | Percentage |
|---|---|---|
| CLL | 7 | 10.4 |
| MCL | 4 | 6.0 |
| MZL | 3 | 5.0 |
| FL | 1 | 2.0 |
| MZL/FL | 5 | 8.0 |
| Lymphoplasmacytic lymphoma | 1 | 2.0 |
| DLBCL | 15 | 22.4 |
| DLBCL/blastoid MCL | 3 | 5.0 |
| Plasma cell neoplasm | 3 | 5.0 |
| Burkitt lymphoma | 5 | 8.0 |
| T-NHL | 2 | 3.0 |
| Suspicious of T-NHL | 2 | 3.0 |
| Suspicious of ALCL | 2 | 3.0 |
| Suspicious of CHL | 3 | 5.0 |
| Extramedullary blast crisis | 2 | 3.0 |
| T-LBL | 2 | 3.0 |
| Sezary syndrome | 1 | 2.0 |
| Leukemic infiltration | 3 | 5.0 |
| Inconclusive | 3 | 5.0 |
CLL: Chronic lymphocytic lymphoma, MCL: Mantle cell lymphoma, FL: Follicular lymphoma, DLBCL: Diffuse large B cell lymphoma, NHL: Non-Hodgkin lymphoma, ALCL: Anaplastic large cell lymphoma, CHL: Classic Hodgkin lymphoma, T-LBL: T-cell lymphoblastic lymphoma, FNAC: Fine-needle aspiration cytology
| Final diagnosis (gold standard) | Number (n=56) | Percentage |
|---|---|---|
| CLL | 4 | 7.1 |
| MCL | 1 | 2.0 |
| MZL | 2 | 4.0 |
| FL | 2 | 4.0 |
| LPL | 1 | 2.0 |
| DLBCL | 12 | 21.4 |
| Blastoid MCL | 4 | 7.1 |
| Burkitt lymphoma | 4 | 7.1 |
| HG B-NHL with double expression | 1 | 2.0 |
| Plasma cell neoplasm | 3 | 5.4 |
| Small cell lymphoma to Richter’s transformation | 4 | 7.1 |
| CHL | 5 | 9.0 |
| ALCL | 1 | 2.0 |
| EM blast crisis | 1 | 2.0 |
| PTCL-NOS/AITL | 4 | 7.1 |
| T-LBL | 4 | 7.1 |
| Sezary syndrome | 1 | 2.0 |
| Leukemic infiltration | 1 | 2.0 |
| Tuberculosis | 1 | 2.0 |
CLL: Chronic lymphocytic lymphoma, MCL: Mantle cell lymphoma, MZL: Marginal zone lymphoma, FL: Follicular lymphoma, LPL: Lymphoplasmacytic lymphoma, DLBCL: Diffuse large B cell lymphoma, HG B-NHL: High grade B cell non Hodgkin lymphoma, CHL: Classic Hodgkin lymphoma, ALCL: Anaplastic large cell lymphoma, EM: Extramedullary, PTCL: Peripheral T cell lymphoma, NOS: Not otherwise specified, AITL: Angioimmunoblastic T cell lymphoma, T–LBL: T cell lymphoblastic lymphoma, FNAC: Fine-needle aspiration cytology
Of 67 cases, for 4 (6.0%), the diagnosis made based on FNAC is discordant with FC. Of 56 cases for which the gold standard was available, in 5 (9.0%) cases, the diagnosis made based on combined FNAC and FC was discordant with the final diagnosis and 2 (4.0%) cases were indeterminate.
Reactive cases
Of 67 cases, 62 (93.0%) presented with localized lymphadenopathy, and one (2.0%) presented with generalized lymphadenopathy. Two (3.0%) cases presented with tonsillar enlargement. One case (2.0%) presented with parotid swelling, and one (2.0%) presented with thyroid swelling.
A morphological diagnosis was made on FNAC smears. The distribution of FNAC and biopsy diagnoses is depicted in Tables 6 and 7, respectively. Of 67 reactive cases, in 62 (93.0%) cases, the diagnosis made on FNAC is concordant with the final diagnosis. In 5 (8.0%) cases, diagnosis based on FNAC is discordant with FC.
| FNAC diagnosis | Number (n=67) | Percentage |
|---|---|---|
| Reactive lymphoid hyperplasia | 46 | 69.0 |
| Non-necrotizing granulomatous lymphadenitis | 7 | 10.4 |
| Necrotizing granulomatous lymphadenitis | 5 | 8.0 |
| Possibility of Kikuchi-Fujimoto disease | 1 | 2.0 |
| Kimura disease | 1 | 2.0 |
| Benign lymphoid aspirate | 1 | 2.0 |
| Benign lymphocytic thyroiditis | 1 | 2.0 |
| Atypical lymphoid hyperplasia | 1 | 2.0 |
| Suspicious of ALCL | 1 | 2.0 |
| Classic Hodgkin lymphoma | 3 | 5.0 |
FNAC: Fine-needle aspiration cytology, ALCL: Anaplastic large cell lymphoma
| Biopsy diagnosis | Number (n=10) | Percentage |
|---|---|---|
| Reactive | 2 | 20.0 |
| Granulomatous lymphadenitis | 1 | 10.0 |
| Caseating granulomatous Lymphadenitis | 2 | 20.0 |
| Kimura disease | 1 | 10.0 |
| FTCL transformed to AITL | 1 | 10.0 |
| ALCL | 1 | 10.0 |
| CHL | 2 | 20.0 |
FTCL: Follicular T cell lymphoma, AITL: Angiommunoblastic T cell lymphoma, CHL: Classic Hodgkin lymphoma
Non-contributory cases
Of 29 cases, the gold-standard method and final diagnosis were unavailable for 5 (17.2%) cases. The reason for non-contributory FC included the following: Presence of large cells in 6 (21.0%) cases, presence of necrosis in 7 (24.1%) cases, metastasis in 10 (35.0%) cases, Castleman disease in 2 (7.0%) cases, nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) in 1 (3.4%) case, and technical problems in 3 (10.3%) cases. The diagnosis of metastasis was made on FNAC based on ICC done on the smears. Castleman disease was suspected based on morphology in FNAC.[4]
DISCUSSION
FNAC has been widely used to diagnose metastasis and infective and reactive lesions. Few studies evaluate its role in diagnosing lymphomas when FC is complemented.[5-11] In the present study, we evaluated the utility of FNAC combined with FC in extramedullary hematolymphoid lesions.
Lymphoma cases
A morphological diagnosis was made based on FNAC smears, and the results were then correlated with FC findings, and a combined diagnosis was made. Diagnosis based on combined FNAC and FC was then compared with the gold standard. Four of the 67 cases had discordant findings between FNAC and FC. Of the four cases, one was an already diagnosed case of DLBCL of tonsil with suspected relapse. It was diagnosed as reactive on FNAC, but 39% (CD20+) cells were monoclonal on FC. Another case was diagnosed as high-grade lymphoma on FNAC. However, on FC, 94% of the events were CD45 negative, and it turned out to be classic Hodgkin lymphoma-lymphocyte depleted (CHL-LD) on biopsy [Figures 1 and 2]. The third case was diagnosed as intermediate to high-grade lymphoma on FNAC. FC was reactive in this case, and it eventually turned out to be angioimmunoblastic T cell lymphoma (AITL). The fourth case was found to have equivocal findings between reactive process and plasma cell dyscrasia because of the increase in plasma cells. However, on FC, it looked reactive, and it was finally diagnosed as AITL on biopsy.
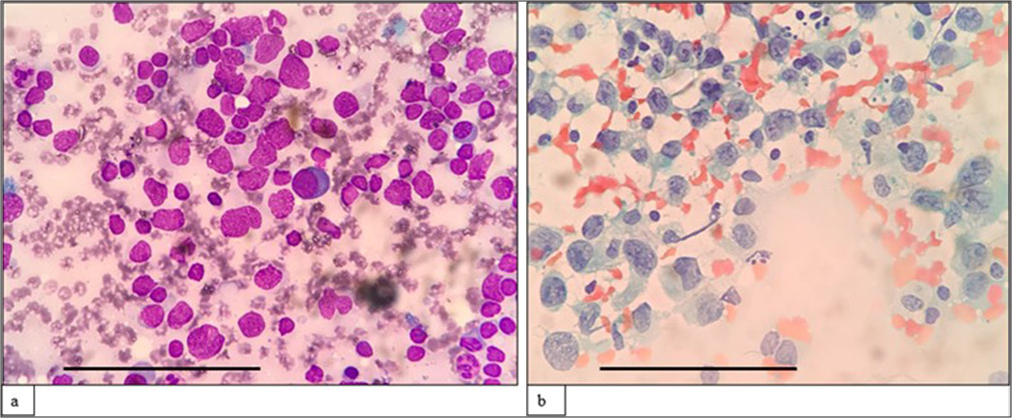
- (a) (May–Grünwald–Giemsa; ×1000) and (b) (Papanicolaou; ×1000) fine-needle aspiration cytology: Classic Hodgkin lymphoma- lymphocyte depleted mimicking high-grade lymphoma showing scattered large-sized cells with irregular nuclear membrane, vesicular nuclei, multiple prominent inclusion like nucleoli, and scant to moderate cytoplasm. The scale bar (black line) represents 100 μm.
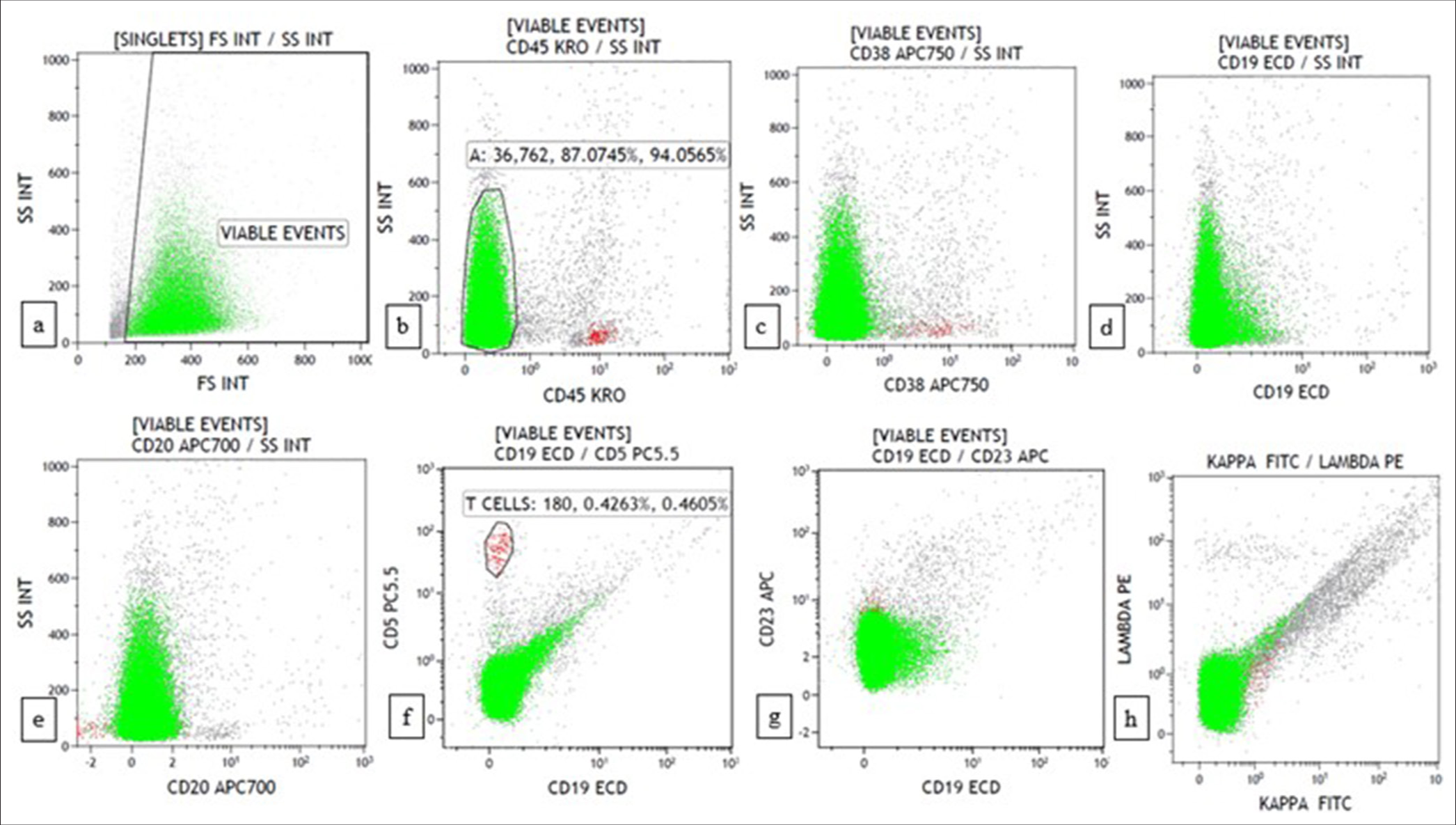
- Flow cytometry plots of classic Hodgkin lymphoma-lymphocyte depleted show 94% events in the CD45 negative region with a background minor population of reactive cells. (a) forward scatter (FSC) versus side scatter (SSC) (b) CD45 versus SSC (c) CD38 versus SSC (d) CD19 versus SSC (e) CD20 versus SSC (f) CD19 versus CD5 (g) CD19 versus CD23 (h) Kappa versus lambda.
Of 56 cases for which the gold standard was available, 5 had discordant findings between combined FNAC/FC and the gold standard. Of the five discordant cases, one was diagnosed as suspicious of lymphoma on FNAC. 80% of the events on FC were CD5+, and 18% were CD20+ with polyclonality. In reactive lymph nodes, T cells comprise 27–79% (average 54%).[12] Thus, it was suspicious of T cell lymphoma. The further extended panels did not show a skewed CD4:CD8 ratio or loss of antigen expression. On biopsy, it turned out to be caseating granulomatous lymphadenitis which explains the predominance of T cells in the basic panel as T cells play a significant role in granuloma formation.[13] Other cases were CHL-LD, as discussed above. The third case was diagnosed as a possibility of ALCL/Classic Hodgkin lymphoma (CHL) on FNAC. FC was suspicious of T cell lymphoma as the predominant population was CD5+. However, on biopsy, it turned out to be CHL. It was mentioned in previous studies[14-17] that FC will be negative in Hodgkin lymphoma, with few cases showing increased CD4+ T cells and decreased CD19+ B cells compared with reactive lymphoid hyperplasia. This finding is similar to our case, where T cells were increased, and B cells were decreased. However, the further panel was not extended in this case to comment on the increased CD4 to CD8 ratio. The maximum possible diagnosis was made on combined FNAC/FC for the other two cases, even though an exact diagnosis cannot be made. This phenomenon is due to the unavailability of further markers on FC. Hence, they were labelled as indeterminate. Of the two cases, one was diagnosed as marginal zone lymphoma based on combined FNAC/FC. However, on biopsy, it turned out to be CD10 negative follicular lymphoma with additional B-cell lymphoma 6 (BCL6) and B-cell lymphoma 2 (BCL2) positivity on immunohistochemistry. Another case was diagnosed as Burkitt lymphoma on combined FNAC/FC, and on biopsy, it turned out to be high-grade B-cell lymphoma with myelocytomatosis oncogene (MYC) and BCL2 expression.
We adopted a light chain ratio for assessing clonality from previous studies.[6,11] Kappa:Lambda ratio of >4:1 or 1:2 was considered evidence of monoclonality. Unlike B cell lymphomas, clonality has not been studied much in T cell lymphomas. However, a diagnosis of T cell lymphoma is considered if there is predominant expression of CD4 or CD8, double negative or double-positive T cells, aberrant expression of T cell markers like loss of T cell antigens or additional antigen expression (CD16, CD25) or aberrant expression of T cell receptor (weak expression of T-cell receptor alpha/beta [TCRαβ] or positive expression of TCRγδ or lack of expression of both TCRαβ and Tγδ in significant T cell).[12]
In some instances, FC contributed significantly to arriving at a diagnosis. One was a young pregnant female with a known case of chronic myeloid leukemia (CML) in chronic phase and diagnosed to have extramedullary blast crisis with near Early T-cell precursor acute lymphoblastic lymphoma (ETP-ALL) phenotype. This patient did not undergo an excision biopsy, and further treatment was implemented based on combined FNAC and FC findings. Thus, FC helped in early management in this case. Only in 20–30% of CML cases can lymphoid blast crisis be seen, which is most commonly of B cell lineage.[18] T lymphoid blast crisis with near ETP-ALL phenotype is exceedingly rare, with only a few reported cases.[19]
Another case where FC helped in arriving at a diagnosis was a 23-year-old female who was misdiagnosed with acute leukemia based on peripheral blood and bone marrow and was diagnosed with DLBCL with peripheral blood involvement. On further evaluation with fluorescence in situ hybridization (FISH), it turned out to be high-grade B cell lymphoma with MYC and BCL2 rearrangement (double hit lymphoma). Although lymphomas can present with peripheral blood involvement, there were only a few case reports of high-grade B cell lymphoma presenting as acute leukemia.[20-22]
The third case was a 69-year-old man who presented with multiple cutaneous swellings with suspected neurofibromatosis/multiple lipomatosis. The combined FNAC/FC diagnosis was mantle cell lymphoma (MCL) [Figures 3 and 4]. Subsequent biopsy was reported as diffuse large B-cell lymphoma (DLBCL) as it was BCL6 positive with high Ki67. As the combined FNAC/FC findings did not correlate, biopsy slides were reviewed, and a final diagnosis of blastoid MCL was made, which was confirmed by FISH with the presence of t (11;14). Aberrant phenotypes were described in MCL, especially in the blastoid variant and pleomorphic variant, which includes the absence of CD5 and expression of CD10 and BCL6, similar to our case.[18]
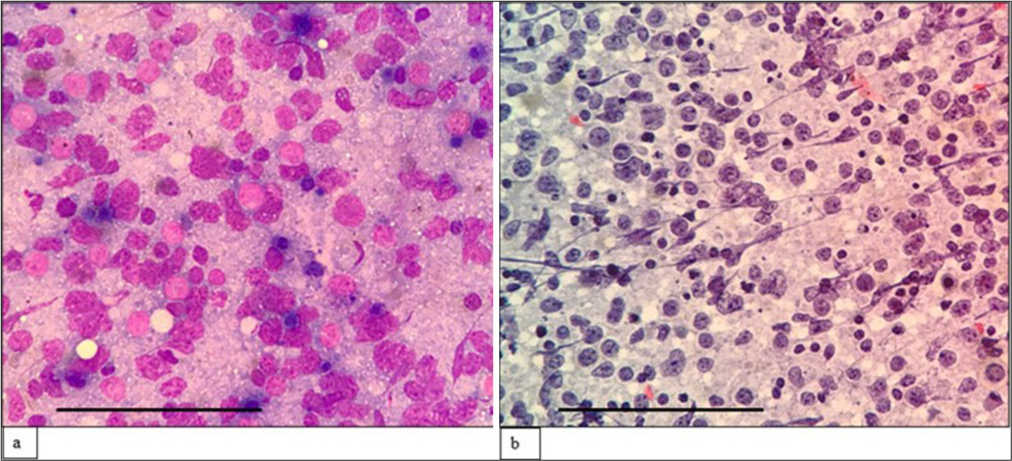
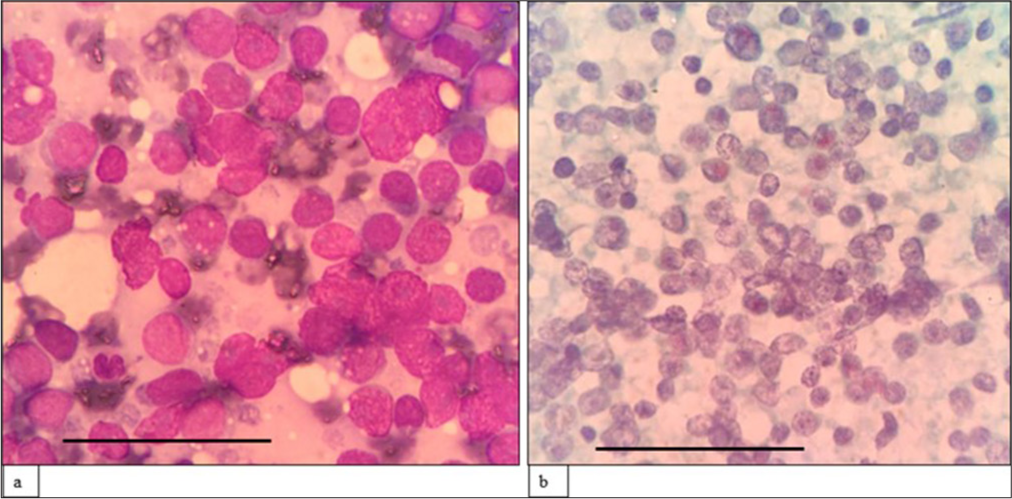
- (a) (May–Grünwald–Giemsa; ×400) and (b) (Papanicolaou; ×400) fine-needle aspiration cytology: Blastoid Mantle cell lymphoma of cutaneous swelling showing sheets of monomorphic intermediatesized cells with dispersed chromatin, inconspicuous nucleoli and scant cytoplasm. The scale bar (black line) represents 100 μm.
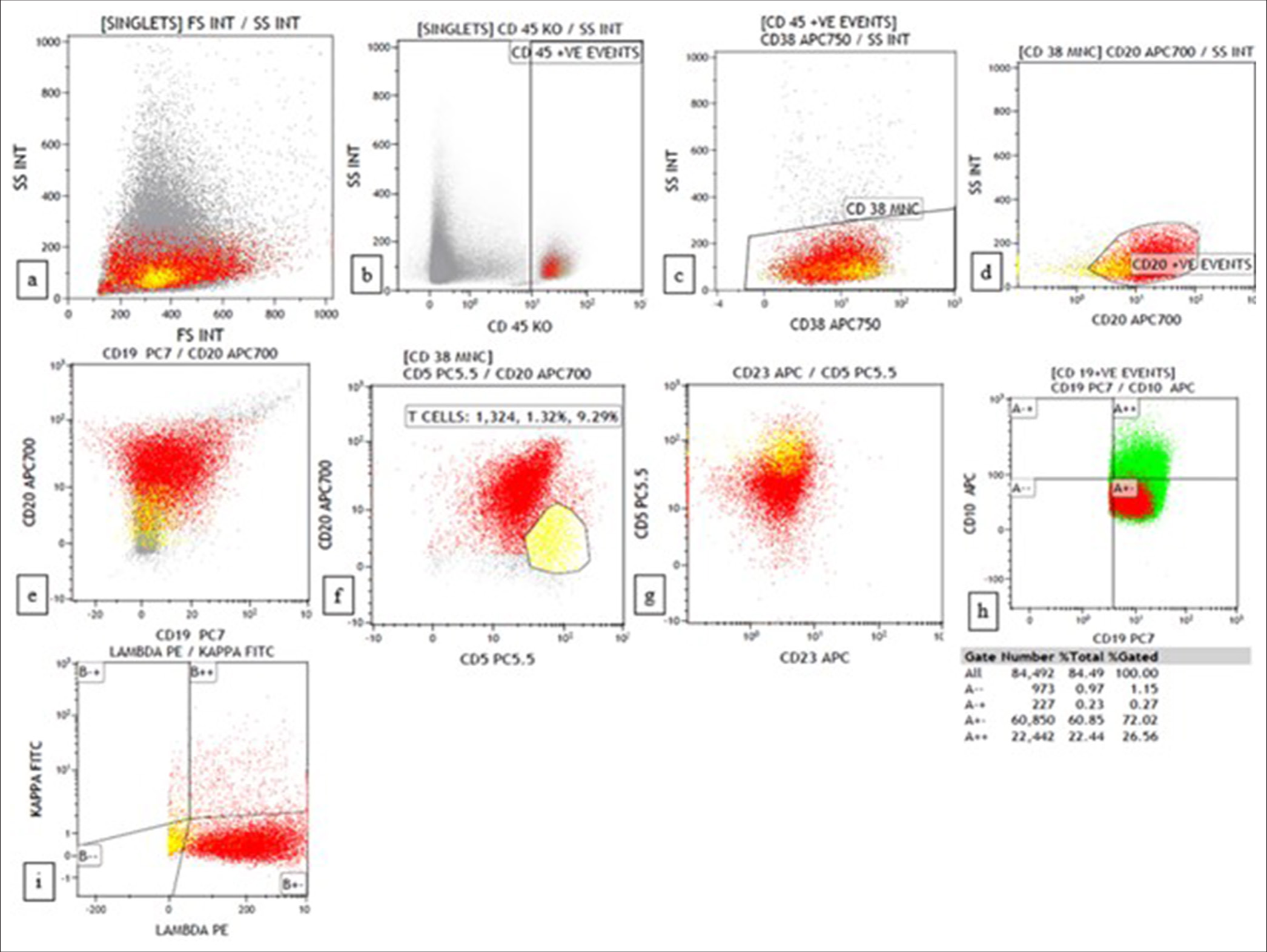
- Flow cytometry plots of blastoid mantle cell lymphoma show CD20+, CD19+, CD5+, CD23-, CD10+ (weak), and lambda restricted. (a) forward scatter (FSC) versus side scatter (SSC) (b) CD45 versus SSC (c) CD38 versus SSC (d) CD20 versus SSC (e) CD19 versus CD20 (f) CD20 versus CD5 (g) CD5 versus CD23 (h) CD19 versus CD10 (i) Kappa versus lambda.
Two cases of T cell lymphoma were diagnosed on combined FNAC/FC based on the predominant expression of CD4 with skewed CD4:CD8 ratio (>10:1) and loss of T cell antigen (CD7). Although TCRαβ was also used in the panel, it did not contribute to the diagnosis. One of the cases was confirmed as peripheral T cell lymphoma (PTCL)-not otherwise specified on cell block [Figures 5 and 6]. The other case did not have a gold standard for comparison. Sezary syndrome was diagnosed on combined FNAC/FC in an 81-year-old woman who presented with erythroderma involving 90% of the body surface with generalized lymphadenopathy.
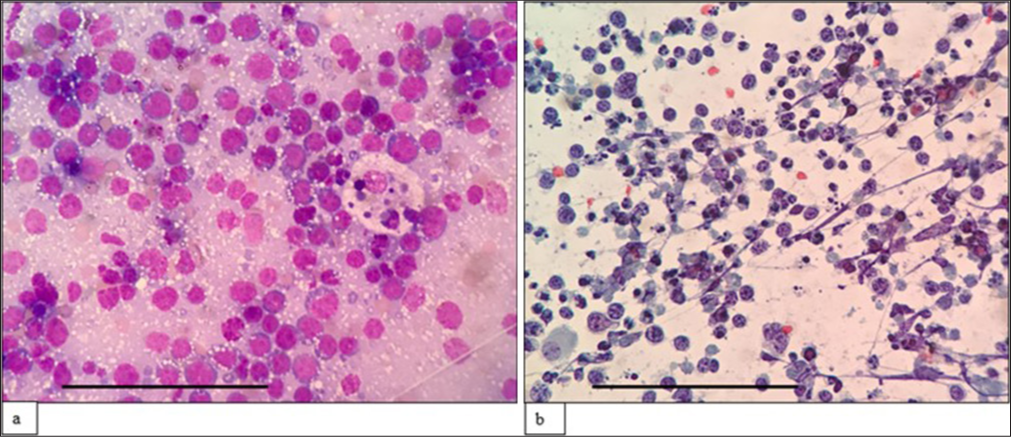
- (a) (May–Grünwald–Giemsa; ×400) and (b) (Papanicolaou; ×400) fine-needle aspiration cytology: Peripheral T cell lymphoma shows sheets of small to medium-sized cells with irregular nuclear membranes, coarse chromatin, inconspicuous nucleoli, and scant cytoplasm. The scale bar (black line) represents 100 μm.
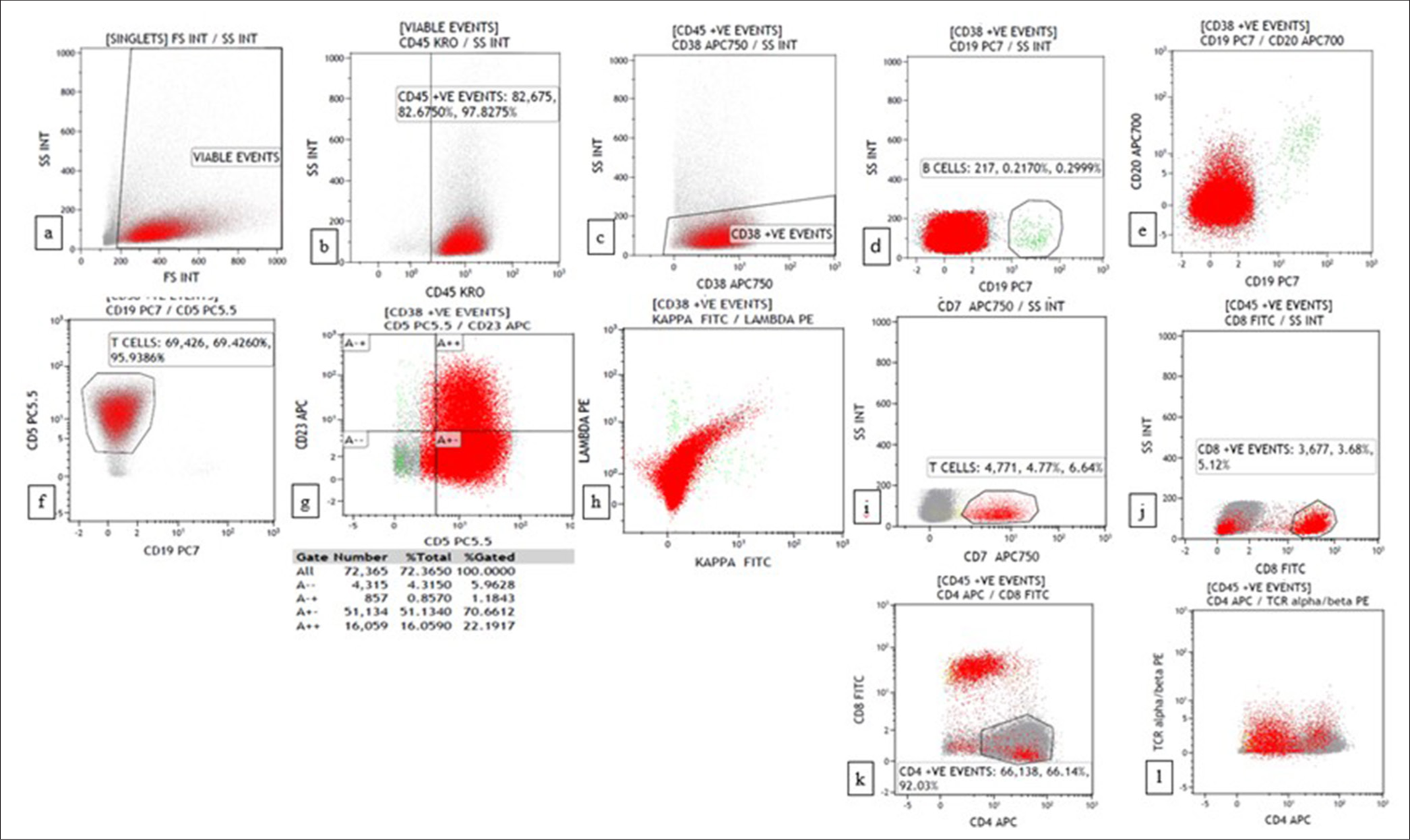
- Flow cytometry plots of Peripheral T cell lymphoma showing CD45+, CD19-, CD20-, CD5+ (bright), CD23 (dim+), CD7-, CD8-, CD4+ (92%), T-cell receptor alpha/beta (TCRα/β) (dim+). (a) forward scatter (FSC) versus side scatter (SSC) (b) CD45 versus SSC (c) CD38 versus SSC (d) CD19 versus SSC (e) CD19 versus CD20 (f) CD19 versus CD5 (g) CD5 versus CD23 (h) Kappa versus lambda (i) CD7 versus SSC (j) CD8 versus SSC (k) CD4 versus CD8 (l) CD4 versus TCRα/β.
Three cases of plasma cell neoplasm were diagnosed on combined FNAC/FC based on CD38 positivity, dim CD45 expression, CD56 positivity, CD19 and CD20 negativity with light chain restriction. However, as mentioned above, one of these three cases did not show light chain restriction, even though they fulfilled the other criteria for neoplastic plasma cells. This can be explained since we used only surface light chain markers on FC.
Four cases were suspected of Burkitt lymphoma on combined FNAC/FC [Figures 7 and 8]. It was then diagnosed as suspicious because cellular-myelocytomatosis oncogene (C-MYC) and BCL2 were unavailable in FC. Three of these cases turned out to be Burkitt lymphoma on biopsy, with one of the cases being high-grade B cell lymphoma with double expression as per the WHO 2017.[18]
- (a) (May–Grünwald–Giemsa; ×400) and (b) (Papanicolaou; ×400) fine-needle aspiration cytology: Burkitt lymphoma showing monomorphic medium-sized cells with round nuclei, coarse chromatin, multiple tiny nucleoli with deep basophilic cytoplasm showing cytoplasmic vacuoles and tingible body macrophage. The scale bar (black line) represents 100 μm.
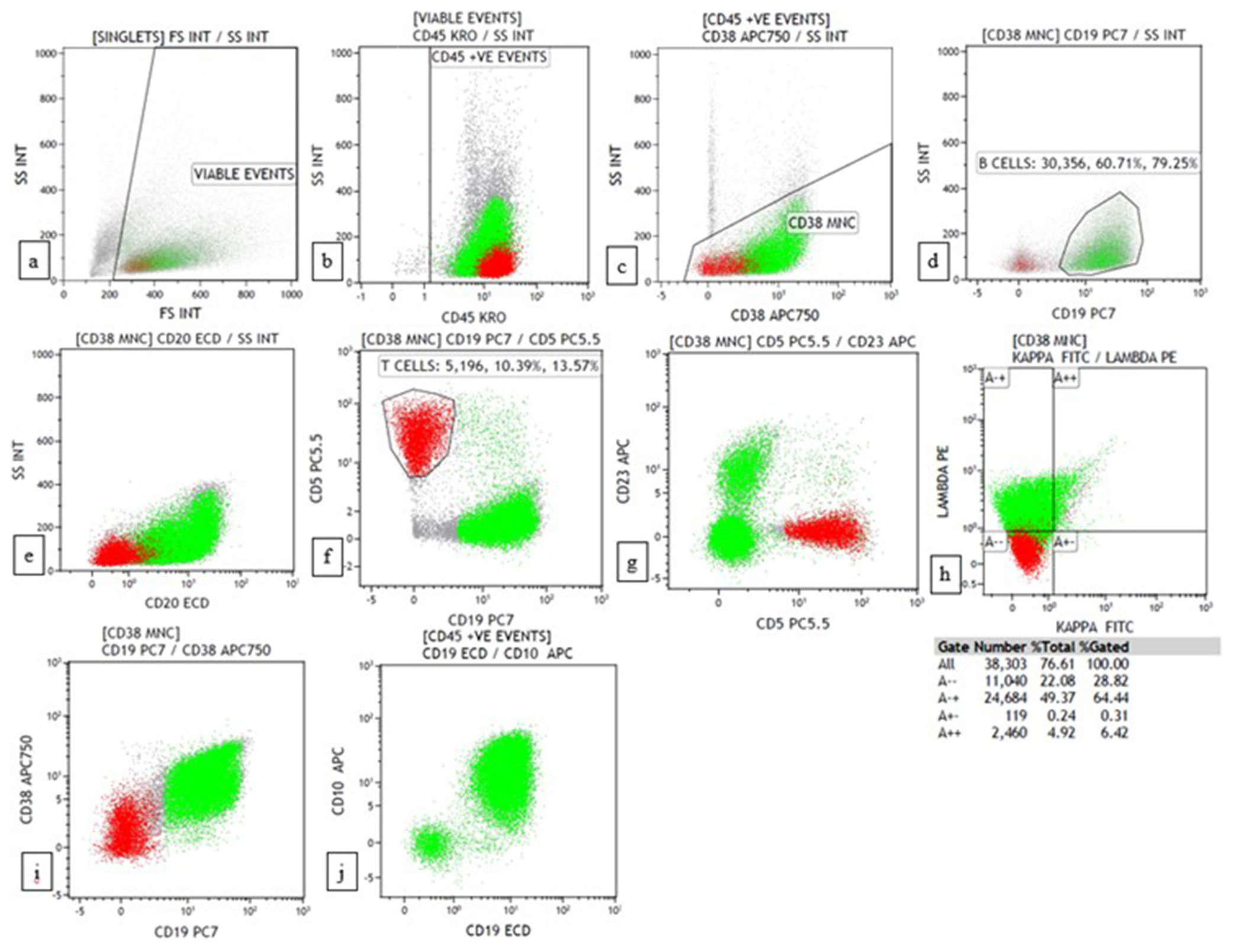
- Flow cytometry plots of Burkitt lymphoma showing CD45+ with high SSC, CD19+, CD20+, CD5-, CD23-, CD38+, lambda restricted, CD10+. (a) forward scatter (FSC) versus side scatter (SSC) (b) CD45 versus SSC (c) CD38 versus SSC (d) CD19 versus SSC (e) CD20 versus SSC (f) CD19 versus CD5 (g) CD5 versus CD23 (h) Kappa versus lambda (i) CD19 versus CD38 (j) CD19 versus CD10.
Fourteen cases were diagnosed as DLBCL based on combined FNAC/FC. Three cases were suspected of blastoid MCL on FC, as the atypical cells were positive for CD5 and negative for CD23. One of these cases was blastoid MCL, but the other two cases were reported as DLBCL. These two cases were CD5 negative on biopsy. On FC, CD5 was strongly positive in a case diagnosed as blastoid MCL. However, CD5 was weakly positive in both cases of DLBCL. CD5 positivity can be seen in 5–10% of DLBCL.[18] CD5+ DLBCL has to be differentiated from blastoid MCL by the absence of cyclin D1 and/or sex determining region Y-box transcription factor 11 (SOX 11) expression. Large or blastoid B cell neoplasms that are negative for cyclin D1 and positive for SOX11 are classified as cyclin D1 negative blastoid/pleomorphic MCL rather than DLBCL or blastoid high-grade B cell lymphoma.[23]
Five cases of leukemic infiltration were selected and diagnosed on combined FNAC/FC. Although leukemia cases were an exclusion criterion of our study, the sample was taken for FC because these cases presented initially to the FNAC clinic with lymphadenopathy. FC helps in the identification of blasts that were CD45 dim positive, whereas atypical lymphoid cells will be CD45 bright positive. This helps exclude leukemic infiltration, which can mimic lymphoma.
Eleven cases did not have a gold standard diagnosis, even though aspirated material was also taken for cell block in all the cases. This is because, in these cases, the material in the cell block was inadequate for ICC.
We could subclassify the lesions according to the WHO 2017 based on morphology alone (FNAC) in 11/67 cases. However, we were able to subclassify 34/67 cases based on combined FNAC/FC, which is higher than morphology alone. In other cases, FC could be diagnosed as B cell lymphoma but failed to subclassify it further.
Reactive cases
This group included 67 cases that were suspected to be reactive on ROSE. On FC, 63/67 were reactive, and 4/67 cases, in addition to the reactive population, showed a significant amount of debris. These four cases were diagnosed as necrotizing granulomatous lymphadenitis on MGG- and PAP-stained smears. Apart from these four cases, one case also had debris with predominant reactive population. The sample was processed after 24 h, which led to the death of the cells. The sample should be processed within 12 h.[24] Fixation in paraformaldehyde allows sample storage for a couple of days so repeat evaluation can be done in equivocal cases or sample processing can be delayed in unexpected situations. All of these cases had a kappa: lambda ratio of <4:1.
Of 67 cases, five had discordant findings between FNAC and FC. These cases include ALCL (1/5), CHL (3/5) and one case of atypical lymphoid hyperplasia. All these five cases were reactive on FC. Four of these cases had a biopsy for confirmation concordant with findings on morphology (FNAC smear). However, one case of atypical lymphoid hyperplasia diagnosed based on morphology in a 15-year-old male did not have a follow-up biopsy for confirmation. Apart from these five cases, there was one case, a 40-year-old man with a known case of PTCL, who presented with cervical lymphadenopathy. On FNAC smear and FC, it was diagnosed as reactive. However, given increased clinical suspicion, a follow-up biopsy was done, which showed AITL transformation from follicular T cell lymphoma. Thus, polyclonality in clinically and cytologically suspicious cases does not exclude hematolymphoid neoplasms like Hodgkin lymphoma, T cell lymphoma or partial involvement by B cell lymphoma. In such cases with a high index of suspicion, where cytologic and FC diagnosis did not explain clinical presentation, close follow-up with repeat FNAC or excision biopsy permits proper diagnosis and timely management.
In 57 out of 67 cases diagnosed as reactive on FC, no corresponding tissue biopsy was available. A review of clinical information in these cases showed no suspicion of lymphoma or no evidence of development of lymphoma. Ten out of 67 cases had follow-up biopsy because of a high index of clinical suspicion, two of which were reactive, one case was diagnosed as granulomatous lymphadenitis, one case as caseating granulomatous lymphadenitis, one case as Kimura disease, which is concordant with FNA, one case as AITL transformed from follicular T cell lymphoma, one case as AITL and two cases as CHL.
Non-contributory cases in FC
On FC, 12/29 cases had low cellularity [Figure 9]. The reason for low cellularity was either because of large cells or necrosis. False-negative results can occur in large cell lymphoma, either because of selective loss of tumor cells or because the cells are too large to be detected by FC.[25,26] One of these cases had polyclonality which on biopsy was reported as high-grade B cell lymphoma. Thus, polyclonality in FC with low cellularity should not be used to exclude lymphoma, especially in clinically and cytologically suspicious cases. In such cases, a follow-up biopsy can help with the diagnosis.
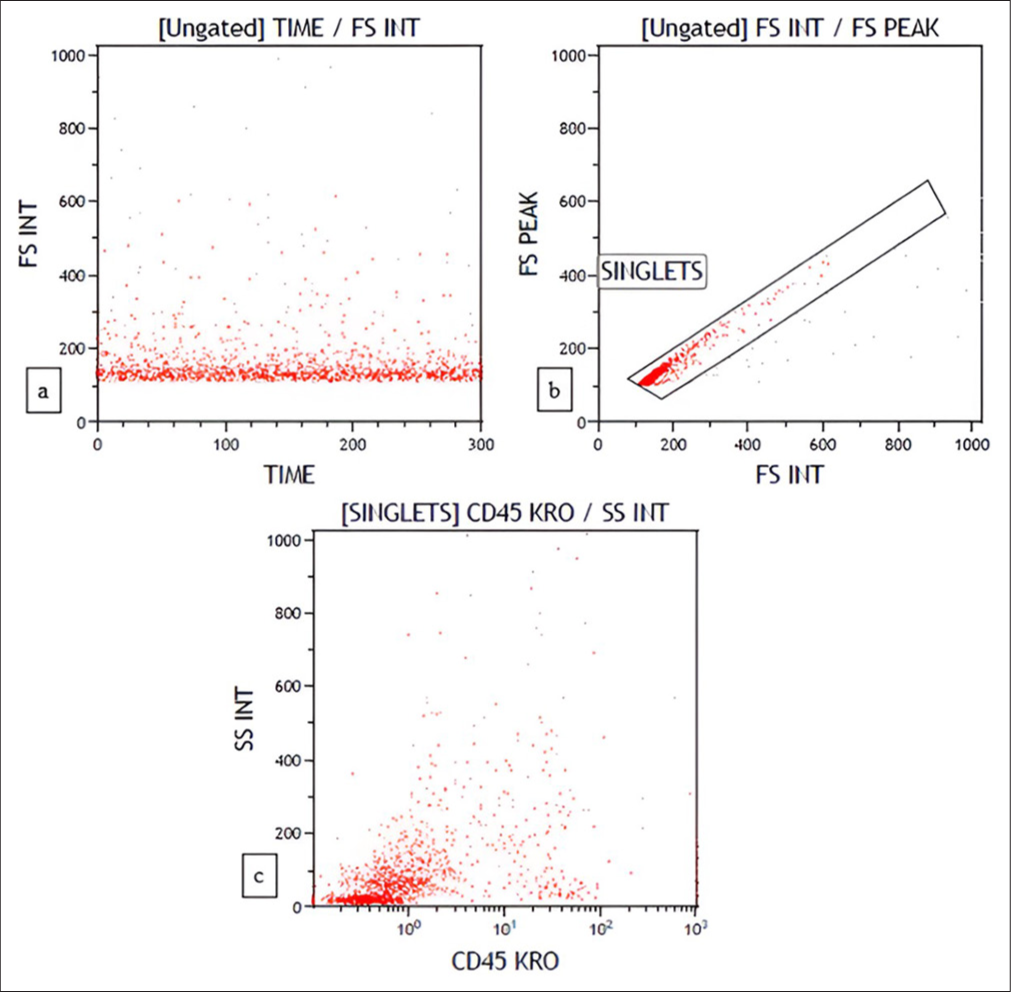
- Flow cytometry plots depicting low cellularity (a) Time versus forward scatter (b) FS INT versus FS Peak (c) CD45 versus side scatter.
Although there was low cellularity in two cases, the cells were monoclonal. These two cases turned out to be DLBCL. Thus, FC can help suspect lymphoma even if there is low cellularity, although subclassification and confirmation are difficult. In two other cases, most cells were in the debris region with non-specific markers positivity. This was because of necrosis/blood contamination.
In a study by Peng et al.[27] to determine the factors responsible for the failure to detect DLBCL by FC, they found that neither the sample collection site, necrosis, fibrosis, nor apoptosis reduced the detection rate by FC. They found that in cases where DLBCL was not detected, lymphocytes were a lower percentage of total events, whereas there was a higher percentage of non-lymphocyte and non-granulocyte events, which is noted in (3/6) of our DLBCL cases in which FC did not detect. The other reasons reported for the failure of detection of DLBCL cases were elevation of T cells, Epstein Barr virus-positive DLBCL cases, and absence of C-MYC expression. However, this finding is not noted in our study.
In 10 cases, there were separate clusters of cells, with all markers being negative [Figure 10]. These cases turned out to be metastasis/poorly differentiated malignancy on biopsy. All these cases mimicked lymphoma on ROSE because of the discohesion of cells. Thus, in such cases, FC can help exclude lymphoma so that ICC can be used judiciously for identifying the primary site of the tumor, especially in cases with less FNAC smears or the absence of cell block.
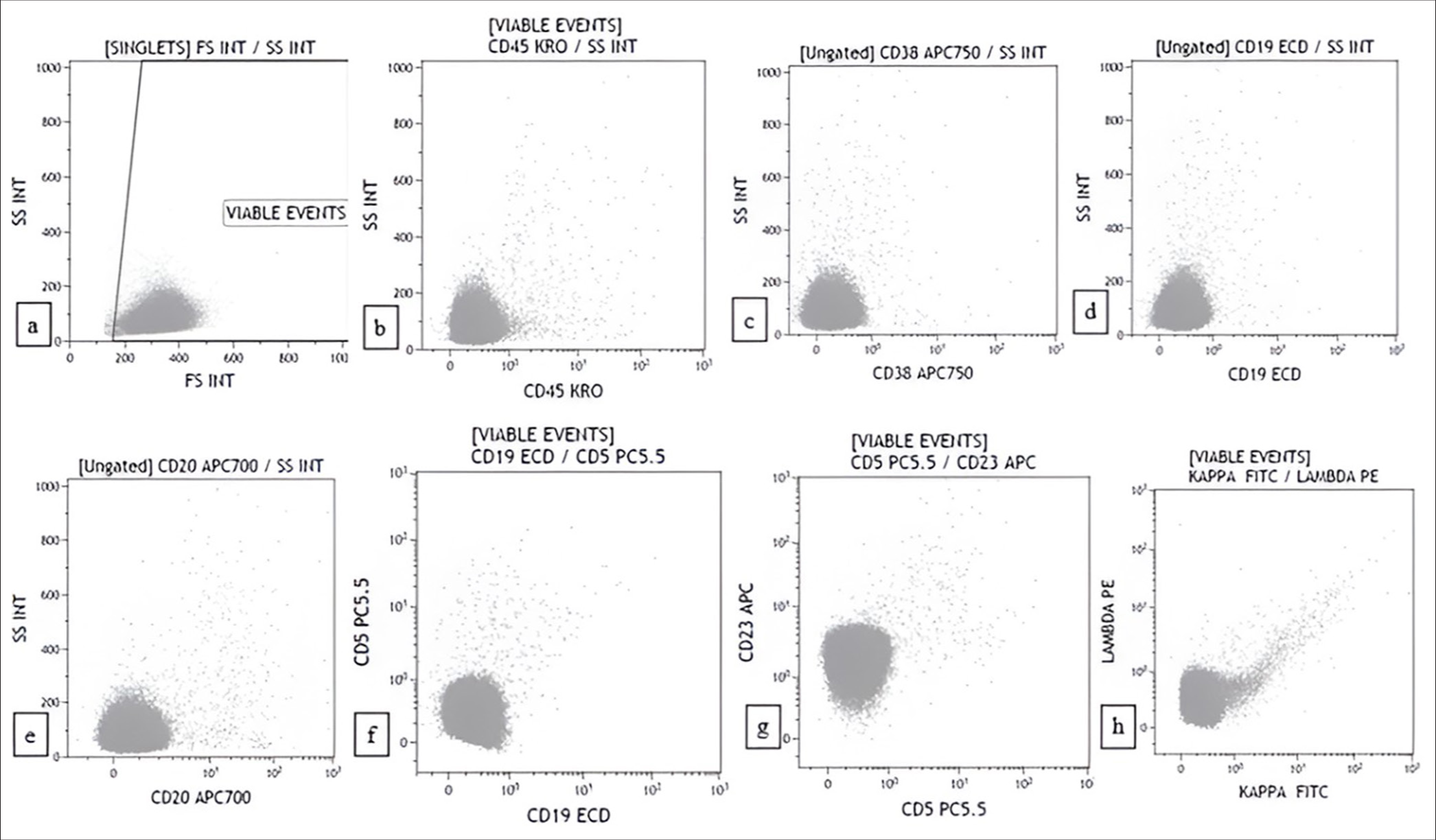
- Flow cytometry plots of poorly differentiated malignancy mimicking lymphoma showing discrete clusters of cells that are negative for all CD markers. (a) forward scatter (FSC) versus side scatter (SSC) (b) CD45 versus SSC (c) CD38 versus SSC (d) CD19 versus SSC (e) CD20 versus SSC (f) CD19 versus CD5 (g) CD5 versus CD23 (h) Kappa versus lambda.
Two cases of suspected Castleman disease and one case of NLPHL were highlighted showed non-specific findings on FC. In three cases, the reason for non-contributory FC can be attributed to technical reasons. Even though a check smear was made in all cases to assess cellularity, sometimes most of the cells will be pushed in a check smear with samples taken for FC having low cellularity. This may lead to a false interpretation of adequate cellularity and wastage of markers.
In our study, as expected, cases in which FC was not contributory were Hodgkin lymphoma, anaplastic large B cell lymphoma, DLBCL and metastases. In these cases, ROSE generally orientates the diagnosis and, in most cases, helps avoid using FC in favor of ICC. One of the cases of Hodgkin lymphoma, which yielded non-contributory results in FC and was diagnosed using ICC [Figure 11].
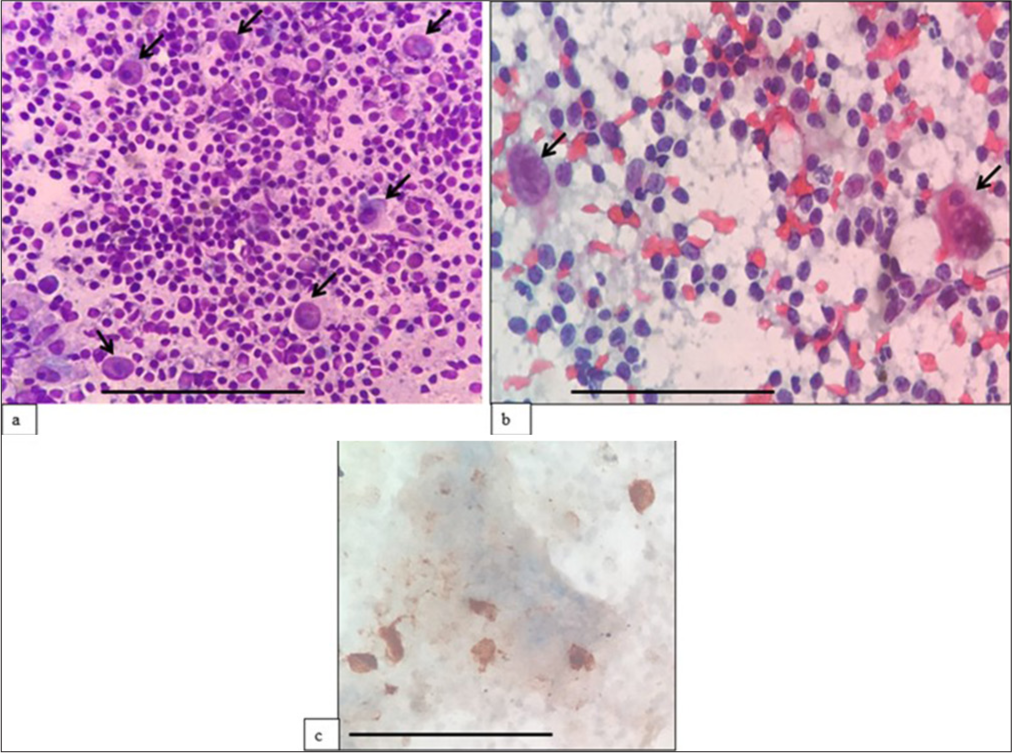
- (a) (May–Grünwald–Giemsa; ×400) and (b) (Papanicolaou; ×400) fine-needle aspiration cytology: Classic Hodgkin lymphoma showing scattered mononuclear Reed Sternberg cells (black arrows) in a background of the polymorphous population of lymphocytes. (c) (Diaminobenzidine; ×400) CD30 immunocytochemistry done on alcohol-fixed smear highlights R-S cells. The scale bar (black line) represents 100 μm.
Diagnostic accuracy of combined FNA/FC in diagnosing hematolymphoid lesions
The diagnostic accuracy of FNAC, FC, and combined FNAC/FC is depicted in Table 8. In our study, the sensitivity and negative predictive value increase when FNAC is combined with FC compared to FNAC or FC alone. The sensitivity of FNAC alone is high compared to FC. This could be attributed to Hodgkin lymphoma and anaplastic large cell lymphoma cases, identified on morphology, while in FC, it was interpreted as reactive. There was almost perfect agreement between the combined FNAC/FC and gold standard with a kappa value of 0.86.
| Diagnostic accuracy | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| FNAC | 95.0 | 99.0 | 98.1 | 96.0 |
| Flow cytometry | 90.0 | 98.4 | 98.4 | 91.1 |
| Combined FNAC/FC | 96.4 | 98.4 | 98.1 | 97.0 |
PPV: Positive predictive value, NPV: Negative predictive value, FNAC: Fine-needle aspiration cytology, FC: Flow cytometry
Combined FNAC/FC helps clinicians make early and appropriate therapeutic decisions in many cases, especially in recurrent cases. In selective cases, patients will be spared from an unnecessary invasive biopsy and unnecessary surgery. Diagnosis can be made easily in non-biopsiable sites like the thyroid, visceral organs, retroperitoneum, mediastinum, salivary glands, etc. Additional use of ploidy analysis/cytogenetics/molecular studies helps accurately subclassify lymphoma according to the WHO classification, especially Hodgkin lymphoma and T cell lymphomas. It helps predict prognosis by providing insight into associated biology.[28]
Limitations
Despite the advantages of combining FC with FNAC, we still faced certain difficulties, as discussed below:
Insufficient material for FC - This can lead to wastage of antibodies. Apart from the technical problem, low cellularity is also attributed to the presence of necrosis and large cells.
Sampling error - This can be a problem, especially in cases with partial involvement of lymph nodes or focal transformation to high-grade lymphoma. We had three cases with transformation to high-grade lymphoma from low-grade lymphoma, which was falsely interpreted as low-grade lymphoma on combined FNAC/FC. Thus, even on combined FNAC and FC, if the diagnosis did not explain the clinical situation like its rapidly progressive nature, then close follow-up with either repeat FNAC or excision biopsy can help in proper diagnosis and timely management.
Diagnosing T cell lymphoma- Two cases of T cell lymphoma were suspected based on combined FNAC/FC, which was confirmed in one case. Two other cases of biopsy-proven T-cell lymphoma were also diagnosed as reactive on FC. Hence, T cell lymphoma cannot be diagnosed confidently based on combined FNAC/FC. A diagnosis of reactive on FC does not exclude T cell lymphoma.
Hodgkin lymphoma and ALCL: We faced difficulty diagnosing Hodgkin lymphoma and ALCL. Three cases were suspected to be Hodgkin lymphoma based on morphology and FC due to the presence of a significant number of R-S cells on morphology with a predominant CD45 negative population on FC. CD45 negativity cannot be explained otherwise in these cases since debris can also be seen in the CD45 negative region, but necrosis occurs uncommonly in Hodgkin lymphoma. There was also a case of Hodgkin lymphoma, which was suspected as T cell lymphoma (ALCL) based on morphology and FC due to the presence of a predominant population of T cells and polyclonality of B cells. Similarly, a case of ALCL was diagnosed as reactive on FC. Therefore, polyclonality and reactive diagnosis on FC do not exclude Hodgkin lymphoma or ALCL. Further antibody markers like CD30 in correlation with morphology can help in the diagnosis of ALCL.
Absent immunoglobulin expression - Two cases of large cell lymphoma showed no immunoglobulin light chain expression. However, because of predominance of B cells in correlation with morphology, B cell lymphoma was suspected, which was further confirmed on biopsy demonstrating the importance of correlation with morphology. Lack of surface immunoglobulin light chain expression is unusual in B cell lymphoma. However, other authors noted this as well.[11,29] They suggested that if CD20+ cells were >85% or CD19/CD10 coexpression seen in 18% of cells or CD19 or CD20 with CD5 coexpression seen in 35% of cells, then it can be diagnostic of B-cell lymphoma, especially in cytologically suspicious cases.
SUMMARY
Combining FNAC with FC enhances the accuracy of cytological diagnosis and helps subclassify lymphoma. It is beneficial in recurrent cases and in sites where biopsy is inaccessible. A biopsy may be needed in clinically suspicious cases when there is an absence of monoclonality or when lymphoma grade and subtype cannot be determined. FC alone may not be helpful in Hodgkin lymphoma, ALCL and T-cell lymphoma. Combining FC with FNAC can help suspect but not exclude these lymphomas. Future studies with a multiparametric approach, help improve diagnostic accuracy with early diagnosis.
AVAILABILITY OF DATA AND MATERIALS
Data and Material of the study are available with us. There is no sharing of any patient details/data in the manuscript.
ABBREVIATIONS
AITL: Angioimmunoblastic T cell lymphoma
ALCL: Anaplastic large cell lymphoma
BCL2: B-cell lymphoma 2
BCL6: B-cell lymphoma 6
CHL: Classic Hodgkin lymphoma
CHL-LD: Classic Hodgkin lymphoma-lymphocyte depleted
CLL: Chronic lymphocytic leukemia
CLPD: Chronic lymphoproliferative disorder
CML: Chronic myeloid leukemia
C-MYC: Cellular-myelocytomatosis oncogene
DLBCL: Diffuse large B cell lymphoma
EBV: Epstein Barr virus
EDTA: Ethylenediamine tetraacetic acid
EM: Extramedullary
ETP-ALL: Early T-cell precursor acute lymphoblastic lymphoma
FC: Flow cytometry
FISH: Fluorescence in situ hybridization
FL: Follicular lymphoma
FNAC: Fine-needle aspiration cytology
FSC: Forward scatter
FTCL: Follicular T cell lymphoma
ICC: Immunocytochemistry
IEC: Institute Ethics Committee
LBL: Lymphoblastic lymphoma
LPL: Lymphoplasmacytic lymphoma
MCL: Mantle cell lymphoma
MGG: May–Grünwald–Giemsa
MYC: Myelocytomatosis oncogene
MZL: Marginal zone lymphoma
NHL: Non Hodgkin lymphoma
NLPHL: Nodular lymphocyte predominant Hodgkin lymphoma
NOS: Not otherwise specified
NPV: Negative predictive value
PAP: Papanicolaou
PBS: phosphate-buffered saline
PPV: Positive predictive value
PTCL: Peripheral T cell lymphoma
ROSE: Rapid onsite Evaluation
RPM: Revolutions per minute
SOX11: Sex determining region Y-box transcription factor 11
SSC: Side scatter
WHO: World
ACKNOWLEDGMENT
We acknowledge the Dept. of Medical Oncology for their contribution in the management of the cases. We also acknowledge all other clinical departments which referred patients to our clinic for FNAC.
AUTHOR CONTRIBUTIONS
RM: Manuscript preparation and diagnosis of cases; PM: Hematological work up and manuscript correction; DG: Concept, cytodiagnosis and manuscript preparation; RK: Hematological work up and manuscript correction; NS: Cytodiagnosis; SS: Standardization and processing of the FC samples.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research/study was approved by the Institutional Review Board at JIPMER, Puducherry, number JIP/IEC/2020/118, dated June 15, 2020. The authors certify that they have obtained all appropriate patient consent.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Lymphoma diagnosis: Lessons learned from the comparison of histology and cytology associated with flow cytometry. Ann Biol Clin (Paris). 2022;80:157-68.
- [CrossRef] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-4.
- [CrossRef] [PubMed] [Google Scholar]
- AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of Pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2018;94:82-93.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic picture of Castleman's disease: A report of two cases. J Cytol. 2010;27:152-4.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of fine-needle aspiration cytology in cervical lymphadenopathies at a tertiary care center in the Kingdom of Bahrain. Cureus. 2024;16:e62150.
- [CrossRef] [Google Scholar]
- The kappa/lambda ratio of surface immunoglobulin light chain as a valuable parameter for MRD assessment in CLL with atypical immunophenotype. Sci Rep. 2024;14:13452.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of fine-needle biopsy (FNB) versus fine-needle aspiration (FNA) combined with flow cytometry in the diagnosis of deep-seated lymphoma. Diagnostics (Basel). 2023;13:2777.
- [CrossRef] [PubMed] [Google Scholar]
- THE role of flow cytometry for the timely diagnosis of lymphoma in the head and neck district. Oral Oncol Reports. 2023;6:100045.
- [CrossRef] [Google Scholar]
- Fine needle aspiration cytology in the evaluation of lymphoid lesions: A retrospective study of the utility of flow cytometry in conjunction with morphology. Ann Saudi Med. 2012;32:137-42.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of fine needle aspiration cytology to diagnose intraoral lymphoma: 7 years study from a tertiary care center. Diagn Cytopathol. 2021;49:487-93.
- [CrossRef] [PubMed] [Google Scholar]
- Lymph node fine-needle cytology of non-Hodgkin lymphoma: Diagnosis and classification by flow cytometry. Acta Cytol. 2016;60:302-14.
- [CrossRef] [PubMed] [Google Scholar]
- Flow cytometry in neoplastic hematology: Morphologic--immunophenotypic correlation. (3rd ed). New Jersey, USA: CRC Press; 2017. p. :350.
- [CrossRef] [Google Scholar]
- Robbins and Cotran pathologic basis of disease (10th ed). Philadelphia, PA: Elsevier/Saunders; 2021.
- [Google Scholar]
- Role of flow cytometric immunophenotyping for classic Hodgkin lymphoma in small biopsy and cytology specimens. Arch Pathol Lab Med. 2022;146:462-8.
- [CrossRef] [PubMed] [Google Scholar]
- Reactive T cells by flow cytometry distinguish Hodgkin lymphomas from T cell/histiocyte-rich large B cell lymphoma. Cytometry B Clin Cytom. 2016;90:424-32.
- [CrossRef] [PubMed] [Google Scholar]
- A new approach to the study of Hodgkin lymphoma by flow cytometry. Pathology. 2023;55:86-93.
- [CrossRef] [PubMed] [Google Scholar]
- Implicit flow cytometric diagnosis of classic Hodgkin lymphoma using CD3(+)CD4(+) CD26(-) T-cells. J Clin Lab Anal. 2024;38:e25096.
- [CrossRef] [PubMed] [Google Scholar]
- WHO classification of tumours of haematopoietic and lymphoid tissues In: Revised (4th ed). Lyon: IARC; 2017.
- [Google Scholar]
- Blast phase of chronic myeloid leukemia presenting as early T-cell precursor lymphoblastic leukemia. EJHaem. 2021;2:895-6.
- [CrossRef] [PubMed] [Google Scholar]
- Non-Hodgkin lymphoma mimicking acute leukemia: A report of six cases and review of the literature. J Hematop. 2022;15:63-73.
- [CrossRef] [PubMed] [Google Scholar]
- Leukemic presentation with discordant morphology in triple-hit lymphoma-A diagnostic pitfall during COVID-19 pandemic. Int J Lab Hematol. 2021;43:e300-2.
- [CrossRef] [PubMed] [Google Scholar]
- American Society of Hematology. Available from: https://ashpublications.org/blood/article/123/8/1126/32817/Aleukemic-presentation-of-a-triple-hit-lymphoma [Last accessed on 2022 Jul 22]
- [CrossRef] [PubMed] [Google Scholar]
- SOX11+ Large B-cell neoplasms: Cyclin D1-negative blastoid/pleomorphic mantle cell lymphoma or large B-cell lymphoma? Mod Pathol. 2024;37:100405.
- [CrossRef] [PubMed] [Google Scholar]
- Cytological features of diffuse large B-cell lymphoma can mimic metastatic carcinoma on fine needle aspiration cytology. Cytopathology. 2013;24:340-2.
- [CrossRef] [PubMed] [Google Scholar]
- A basic approach to lymph node and flow cytometry fine-needle cytology. Acta Cytol. 2016;60:284-301.
- [CrossRef] [PubMed] [Google Scholar]
- Lymph node cytopathology: Essential ancillary studies as applied to lymphoproliferative neoplasms. Cancer Cytopathol. 2018;126(Suppl 8):615-26.
- [CrossRef] [PubMed] [Google Scholar]
- Factors determining whether diffuse large B-cell lymphoma samples are detected by flow cytometry. Int J Lab Hematol. 2023;45:927-34.
- [CrossRef] [PubMed] [Google Scholar]
- Morphology and multiparameter flow cytometry combined for integrated lymphoma diagnosis on small volume samples: Possibilities and limitations. Virchows Arch. 2024;485:591-604.
- [CrossRef] [PubMed] [Google Scholar]
- The patterns and diagnostic significance of the lack of surface immunoglobulin light chain on mature B cells in clinical samples for lymphoma workup. Cytometry B Clin Cytom. 2023;104:263-70.
- [CrossRef] [PubMed] [Google Scholar]








