Translate this page into:
Diagnostic rescue of a silent scalp swelling by fine-needle aspiration
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
A 71-year-old male presented with large soft fluctuant 5 cm × 6 cm swelling on the scalp 2 years back. The swelling was present for 2–3 months, and clinical diagnosis of abscess or adnexal skin tumor was considered. The patient had no other swelling or discomfort in the body, and there was no history of any other symptoms. Fine-needle aspiration cytology (FNAC) was advised which yielded hemorrhagic aspirate, and multiple air-dried and wet-fixed smears were prepared which were stained by May–Grunwald–Giemsa and Papanicolaou stains, respectively. The cytological picture is as shown in Figure 1.
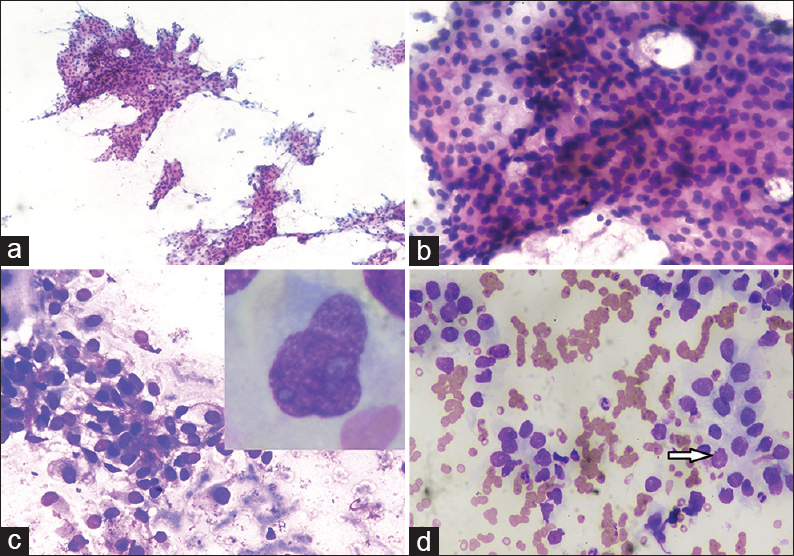
- Fine-needle aspiration cytology scalp swelling showing (a) smears showing cellular sheets and papillaroid fragments of tumor cells (Papanicolaou, ×10); (b) smears showing cellular fragment comprised of round to oval mildly pleomorphic cells with low nucleocytoplasmic ratio, and pale to clear cytoplasm (PAP, ×40); (c) smears showing round to oval cells adherent to endothelial lining with pale cytoplasm in hemorrhagic background (May–Grunwald–Giemsa, ×40); (d) smears showing cells forming acini with nuclei showing fine chromatin, small nucleoli, clear cytoplasm and occasional atypical mitotic figure (arrow) (May–Grunwald–Giemsa, ×40). Inset: Cell showing moderate pale cytoplasm with nuclei having irregular nuclear membrane, coarse chromatin and prominent nucleoli (May–Grunwald–Giemsa, ×100)
QUESTION
What is your interpretation
-
Abscess
-
Metastatic renal cell carcinoma (RCC)
-
Sebaceous carcinoma
-
Hidradenoma.
ANSWER
The correct cytological interpretation is:
B. Metastatic RCC.
EXPLANATION
Cytological smears were cellular with cells arranged in sheets, acini, and few papillaroid structures in hemorrhagic background [Figure 1a]. The cells showed mild pleomorphism, abundant pale cytoplasm with vacuolation and nuclei having coarse chromatin, and a few with prominent nuclei. Occasional mitotic figures were seen [Figure 1b and d]. At places, the cells were adherent to endothelial lining [Figure 1c]. A cytological diagnosis of clear cell adenocarcinoma was given with probable metastases from RCC. The absence of inflammatory cells clearly excluded the possibility of abscess (option A). The skin adnexal tumors with clear cells which constituted the differential diagnosis include sebaceous carcinoma and clear cell hidradenomas. However, sebaceous carcinoma was excluded due to the absence of cells with bubbly cytoplasm along with the absence of admixed basaloid cells (option C). Clear cell hidradenoma is a rare tumor usually seen in the females and mostly shows the presence of dual population of clear cells and cells with moderate eosinophilic cytoplasm with no atypical mitosis (option D).
This was followed by contrast-enhanced computed tomography of the abdomen which showed right renal mass showing an ill-defined lobulated heterogeneously enhancing lesion involving the upper pole measuring 5.4 cm × 4.9 cm × 4.6 cm. Ultrasonography (USG) revealed an enlarged right kidney with a hypoechoic mass measuring 5.4 cm × 4.9 cm × 4.6 cm [Figure 2]. USG-guided FNAC of the renal mass was performed, which revealed the cytological features as described above [Figure 3], and the final diagnosis of RCC was rendered. The patient refused for any surgery and received palliative external beam radiation therapy and was on a continuous follow-up. However, after about 1 year and 9 months, the patient presented with another cutaneous nodule on the nose, which was also subjected to FNAC, and the cytological diagnosis of cutaneous metastatic RCC was made. The condition of the patient deteriorated now, and he succumbed to his illness within 15 days.
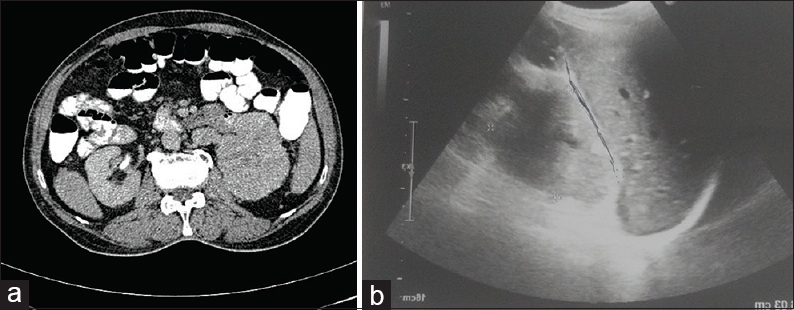
- (a) Contrast-enhanced computed tomography abdomen showing right renal mass with lobulated heterogeneously enhancing lesion involving upper pole; (b) Ultrasonography abdomen showed enlarged right kidney with hypoechoic mass
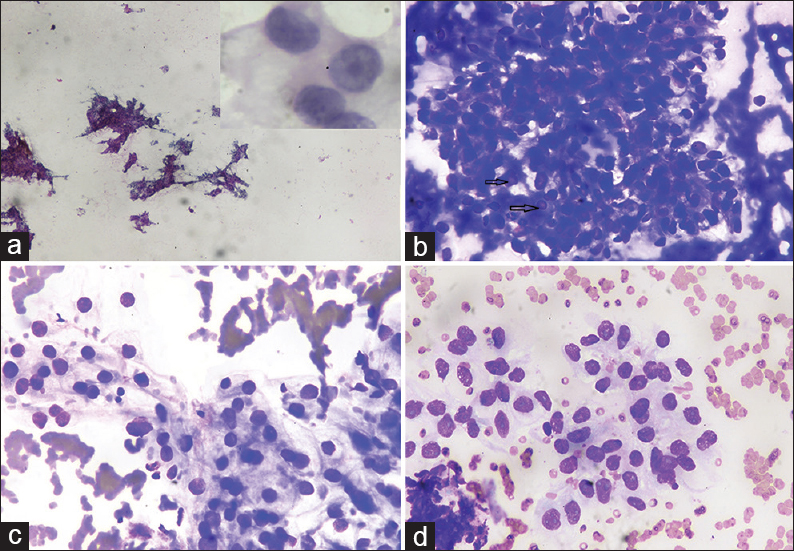
- Ultrasound-guided fine-needle aspiration cytology renal mass showing (a) Smears are hypercellular showing cells arranged in papillaroid structures and sheets (Papanicolaou, ×4); (b) smears showing cellular fragment with few nuclei showing intranuclear inclusions (arrow) in thick hemorrhagic background (May–Grunwald–Giemsa, ×40); (c and d) smears show mildly pleomorphic cells arranged in vague acini having pale vacuolated cytoplasm and nuclei with fine chromatin and small nucleoli (May–Grunwald–Giemsa, ×40). Inset: Cells showing moderate pale cytoplasm with nuclei having coarse chromatin and prominent nucleoli (Papanicolaou, ×100)
DISCUSSION
RCC commonly metastasizes to lung, lymph nodes, bone, adrenals, and brain.[1] The cutaneous metastases are considered to be rare and present in only 1%–3.3% of RCC cases.[2] It is even more uncommon to find the cutaneous metastases as the primary manifestation of silent RCC. The common sites of RCC cutaneous metastases which have been reported by handful of cases reports in the literature are toe, head and neck, and scrotum.[345] RCC develops metastasis in 25% of cases at the time of diagnosis or during follow-up.[6] Our case presented as cutaneous metastasis in the scalp with no associated abdominal signs or symptoms of RCC such as abdominal lump, hematuria, or other urinary symptoms. Thus, it is important to evaluate any cutaneous swelling of the body in the light of any occult primary. FNAC may be utilized as an important diagnostic tool to evaluate such lesions and suspecting the primary malignancy. The present case which was clinically suspected to be an abscess or adnexal tumor scalp turned out to be metastatic RCC on FNAC. This lays the importance of aspiration cytology and careful examination of cytological smears for precise diagnosis. The cytological features which may be helpful in the diagnosis of the RCC are the presence of tumor cells adherent to the endothelial lining or basement membrane with cells having pale vacuolated cytoplasm and few nuclei with intranuclear inclusions. In addition, blood in the background with hemosiderin-laden macrophages may also be helpful in clinching the diagnosis. Literature reports only few cases in which the silent RCC first presented as cutaneous metastasis diagnosed on cytology.[6789] CD10 and EMA may be helpful in precise diagnosis of RCC. However, it was not performed in our case due to financial constraints and patient's reluctance.
The skin metastasis of RCC is usually solitary, which may present as a nodule varying from 0.5 cm to 7 cm. However, in the present case, the cutaneous manifestation was present at different times at two different sites with size of 5 cm × 6 cm on the scalp and 2 cm × 2 cm on the nasal bridge. It has been postulated that increased vascularity of RCC facilitates hematogenous distant metastasis of this tumor.[10]
The cutaneous metastasis of RCC is associated with poor prognosis, and life expectancy is usually about 6 months.[1011] In our case, however, the patient survived for 2 years after the initial cutaneous manifestation and was subjected to palliative radiotherapy. This may be possible due to early diagnosis followed by appropriate treatment. However, later, when he presented with nasal cutaneous deposits, he succumbed to his illness.
Summary
The silent RCC may primarily present as cutaneous metastasis, and therefore, every skin swelling should also be evaluated in light of the presence of any occult primary. FNAC may prove to be an important diagnostic tool for evaluating such cutaneous metastasis. Vigilant examination of cytological smears will be helpful in clinging precise diagnosis even in unsuspected cases. The early diagnosis will thus prevent delay in treatment and further complications.
ADDITIONAL CME QUESTIONS
Cutaneous metastatic renal cell carcinoma may be differentiated from skin adnexal tumor by presence of
-
Bubbly cytoplasm
-
Presence of basaloid cells
-
Cells with pale vacuolated cytoplasm adherent to endothelial lining
-
Dual population of clear and eosinophilic cells.
Answer: C
Common metastatic sites of renal cell carcinoma include all except
-
Lung
-
Bone
-
Skin
-
Brain.
Answer: C
Hemosiderin laden macrophages are usually seen in
-
Clear cell carcinoma of salivary gland
-
RCC
-
Sebaceous carcinoma
-
Urothelial carcinoma.
Answer: B
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare no conflicts of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors have contributed significantly and agree with the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
The authors have taken permission from institutional review board for publication of this manuscript.
LIST OF ABBREVIATIONS (In alphabetic order)
CD - Cluster of Differentiation
FNAC - Fine Needle Aspiration Cytology
RCC - Renal cell carcinoma
USG - Ultrasonography.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Current management of advanced and metastatic renal cell carcinoma. Urol J. 2010;7:1-9.
- [Google Scholar]
- Isolated metachronous cutaneous metastases from renal cell carcinoma. Indian J Cancer. 2010;47:482-3.
- [Google Scholar]
- Scrotal skin metastases of renal cell carcinoma: A case report. Case Rep Clin Med. 2015;4:93-6.
- [Google Scholar]
- Cutaneous metastasis of renal cell carcinoma: A report of two cases. Int J Clin Exp Pathol. 2012;5:175-8.
- [Google Scholar]
- Multiple cutaneous metastasis of renal cell carcinoma: A rare presentation. Clin Cancer Investig J. 2016;5:504-6.
- [Google Scholar]
- Renal cell carcinoma presenting as a solitary cutaneous facial metastasis: Case report and review of the literature. Int Semin Surg Oncol. 2006;3:27.
- [Google Scholar]
- Renal cell carcinoma presenting with cutaneous metastasis: A case report. Case Rep Med 2010 2010 pii: 913734
- [Google Scholar]
- Effect of sunitinib on renal cell carcinoma cutaneous metastasis. Int J Dermatol. 2009;48:1269-70.
- [Google Scholar]







