Translate this page into:
Cytomorphologic spectrum of Hashimoto's thyroiditis and its clinical correlation: A retrospective study of 52 patients
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Hashimoto's thyroiditis (HT) is an autoimmune disease and it is more prevalent in Asians. The incidence of HT seems to be increasing in the recent times. It is one of the most common cause of hypothyroidism. The purpose of this study is to review the cytomorphologic spectrum of HT and correlate it with clinical findings including thyroid function and antibody profile.
Materials and Methods:
We retrospectively analyzed the fine-needle aspiration (FNA) features of 52 HT patients. Based on cytomorphologic features patients were categorized into three groups. Clinical findings including thyroid function and thyroid peroxidase (TPO) antibody profile were correlated with cytomorphologic features in all three groups.
Results:
Majority of the patients were females and in 2nd, 3rd and 4th decades. Diffuse goiter and thyroid hypofunction were the common findings. Significant number of patients had thyroid hyperfunction. Increased lymphocytes on the background and lymphocytic infiltration of thyroid follicular cell clusters in cytology smears were diagnostic of HT. The 32 patients showed elevated titers of TPO antibodies. In the early stages and mild form of the disease, results of thyroid function and anti TPO antibodies are quite variable.
Conclusions:
HT is a disease of young and middle age and mostly occur in females. Clinical findings alone may not be adequate for definitive diagnosis. FNA is the gold standard for diagnosis. In the presence of abundant colloid, follicular hyperplasia or co-existing neoplasm, careful interpretation of cytology smears should be done. Aspiration from more than one site minimizes the diagnostic pitfalls.
Keywords
Fine-needle aspiration
Hashimoto
lymphocytes
thyroiditis
thyroid function
INTRODUCTION
Hashimoto, in 1912, described four patients with a chronic disorder of the thyroid gland characterized by diffuse lymphocytic infiltration, fibrosis, parenchymal atrophy and eosinophilic changes in some of the acinar cells, which he described as strauma lymphomatosa and hence bears the name Hashimoto's thyroiditis (HT).[12] HT may usually be considered a synonym of chronic lymphocytic thyroiditis or autoimmune thyroiditis including atrophic and non-goitrous thyroiditis.[3] It is the most common form of thyroiditis and the second most common thyroid lesion next to goiter, diagnosed on fine-needle aspiration (FNA).[456] It is more prevalent in Asians.[7] It commonly occurs in females with male to female ratio of 1:5-1:7 and peak incidence is in the middle age (30-50 years). It has a prevalence rate of 1-4% and incidence of 30-60/1,00,000 population per year.[8]
Incidence of HT seems to be increasing in the recent times.[9] It has become 10 times more common than it was until the early 1990s. This increase in the incidence has been linked to excess iodine intake, particularly in coastal areas.[10] FNA is highly sensitive in diagnosing HT, with a diagnostic accuracy rate of 92%, however diagnosis is likely to be missed when cytology smears show evidence of hyperplasia as in Grave's disease or abundant colloid.[11] Patients usually present with diffuse enlargement of the thyroid gland or less frequently with one or two prominent nodules.[8] It is the most common cause of hypothyroidism in those areas where iodine levels are sufficient.
Autoantibodies against thyroglobulin (Tg) and thyroid peroxidase (TPO) antigens are clinically most important for diagnosis.[12]
It is important to diagnose HT because patients subsequently become hypothyroid and require lifelong thyroxin supplementation. Thyroid carcinomas and malignant lymphomas can occur in HT which emphasizes the need for long-term follow-up.[13] This study was carried out to review the cytomorphologic spectrum of HT and correlate with clinical findings including thyroid function and antibody profile.
MATERIALS AND METHODS
Medical records of 52 HT patients between April 2006 and December 2012 were retrieved and reviewed. Diagnosis was based on the clinical and FNA features. All patients underwent FNA in cytology clinic. Aspiration/non-aspiration technique was used. After prior written consent, FNA was done with standard technique and aseptic precautions by using 10 cc disposable syringe and 23-25 gauge needle. Two to four rapid passes were given. More passes were given in non-aspiration technique. Material obtained was smeared on glass slides. Slides were stained with Leishman's and Hematoxylene and Eosin (H and E) stains. Cases in which, material obtained was not satisfactory, a repeat aspiration was done. In case of multiple nodules, aspiration was done from more than one nodule.
Detailed clinical history, radiological findings, status of thyroid function (T3, T4, thyroid-stimulating hormone [TSH]) and thyroid antibody were noted. Surgical specimens of thyroid, received for histological examination were 10% formalin fixed and paraffin processed. Three to four micron thick sections were stained with H and E stain.
Cellularity was adequate in all patients. Criteria used for diagnosis of HT on FNA smears was: mixture of Hurthle cells, lymphocytes/lymphocytes in various stages of maturation and follicular cells in variable proportion with scanty or no colloid. Based on the amount of the lymphocytic infiltrate, smears were categorized in Group 1, Group 2 and Group 3. Smears were also looked for other associated features such as anisonucleosis, histiocytes, plasma cells, eosinophils, giant cells, epithelioid cells and fire flares. Clinical findings were correlated with cytomorphologic features in each group.
RESULTS
Out of 52 patients, 94.23% (n = 49) were females and 5.75% (n = 3) were males. Age of the patients ranged from 17 to 64 years with 75% (n = 39) in 2nd, 3rd and 4th decades [Table 1]. All the patients had a history of goiter.
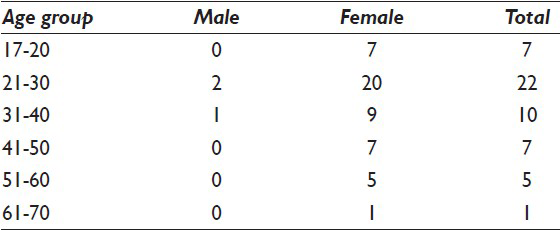
Table 2 shows the nature of thyroid enlargement, cytomorphologic features in three groups, thyroid function and TPO antibody titer. On local examination, 67.30% (n = 35) had diffuse goiter, 30.76% (n = 16) had uneven enlargement of thyroid and 1.92% (n = 1) had solitary nodule. Thyroid hormone evaluation revealed 46.15% (n = 24) hypothyroid, 23.07% (n = 12) hyperthyroid and 15.38% (n = 8) each subclinical hypothyroid and euthyroid. The serum TPO antibody titers were elevated in 32 patients. The 20 patients had normal titer. Ultrasonography (USG) showed diffusely altered parenchyma with hypoechogenic hypervascular goiter in 53.84% (n = 28) and micro nodules in 32.69% (n = 17) patients. Echogenic septations were seen in 25% (n = 13) and dominant nodules in 3.84% (n = 2) patients.
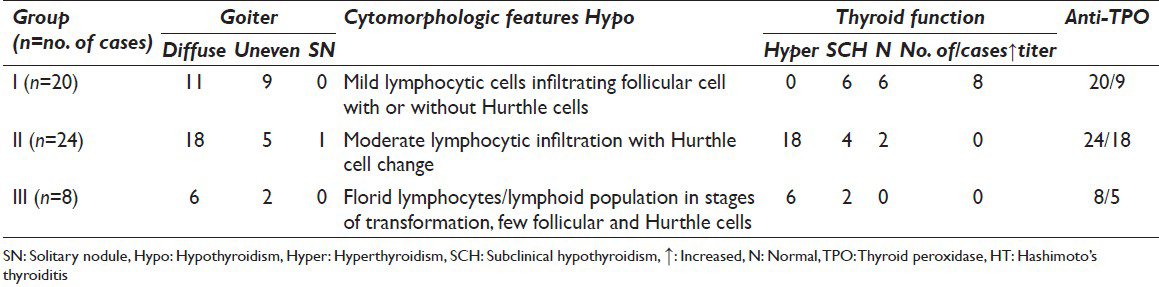
Table 3 shows the frequency of all cytomorphologic features of 52 cases in FNA smears. Based on the amount of lymphocytic infiltrate and other cell types, we defined the criteria for each group and categorized them into three groups. The smears were seen by two independent cytologists. Quantitative criteria's used for cytologic grouping were increased lymphocytes on the background, lymphocytes/lymphocytes in stages of maturation infiltrating thyroid follicular cell clusters and Hurthle cells [Table 2]. High concordance rate was noted between the two observers. In all three groups, increased lymphocytes were seen on the background.

Group I (n = 20) patients showed mild lymphocytic infiltrate in thyroid follicular cell clusters with or without Hurthle cells [Figure 1].
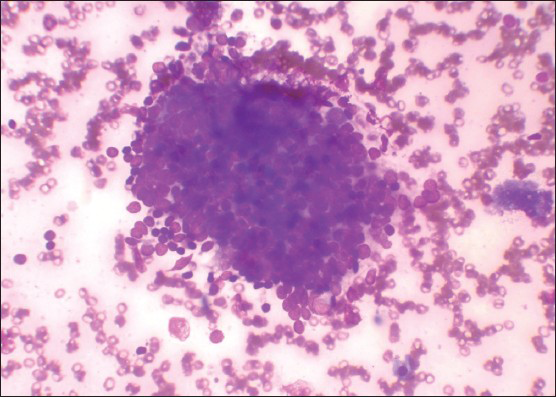
- Mild lymphocytic infiltrate in follicular cells cluster and increased background lymphocytes (Leishman's stain, ×400)
Group II (n = 24) patients showed moderate lymphocytic infiltrate with evidence of follicular cell destruction and Hurthle cells [Figure 2].

- Moderate lymphocytic infiltrate in follicular cells cluster with Hurthle cells (Leishma's stain, ×400)
Group III (n = 8) patients showed dense lymphocytic infiltrate/lymphoid cells in stages of transformation with very few follicular and Hurthle cells at places [Figures 3 and 4].
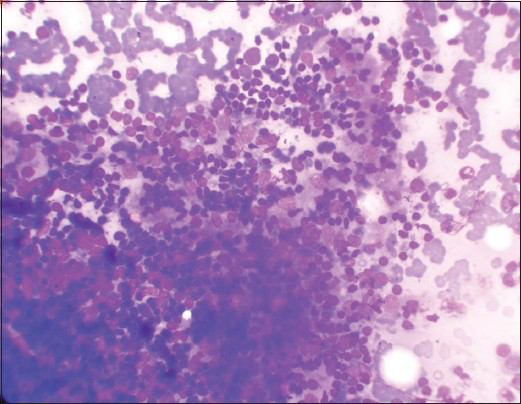
- Dense lymphocytic infiltrate in follicular cells cluster (Leishman's stain, ×400)

- Numerous lymphoid cells in stages of transformation (Leishman's stain, ×400)
In two patients, partial thyroidectomy was done due to pressure symptoms. Histopathology examination confirmed the diagnosis of HT.
DISCUSSION
HT is an autoimmune chronic inflammatory disease of the thyroid gland. It involves infiltration of thyroid gland by T and B lymphocytes which are reactive to thyroid antigens. Activated B cells secrete thyroid autoantibodies. Cytotoxic T lymphocytes are largely responsible for destruction of thyroid parenchyma. In the long run, follicular architecture is totally destroyed and replaced by fibrosis. The active phase of the disease is transient with clinical manifestation of thyrotoxicosis. Evolution and destructive phases manifest with subclinical and overt hypothyroidism.[2] Incidence of HT seems to be increasing in recent times.[1415]
Out of 52 patients, maximum patients (n = 39) were in 2nd, 3rd and 4th decades [Table 1]. This is in contrast to the previous study conducted by Vanderpump et al. from United Kingdom in which the patients were mainly older women with a mean age at diagnosis being 59 years.[16] Bhatia et al.[2] from India found majority patients in 3rd and 4th decade. The reason for this disparity may be due to the occurrence of HT in young patients in iodine deficient areas such as ours which is non-costal.[17] Despite the coverage of national iodine deficiency diseases control program in India, iodine deficiency is still prevalent in many parts of India. It occurs in older individuals in iodine sufficient areas.[6] Many authors have linked increased incidence of HT particularly in coastal areas due to excesses intake of iodine.[14151617] We therefore feel more work should be carried out in respect to etiological factors especially about dietary habits of people in non-coastal areas.
Maximum patients (n = 35) had diffuse goiter. Uneven enlargement of thyroid (n = 16) was seen in a substantial number of patients. Discrete nodular presentation is well-known in HT.[1819] Nodules represent an early stage of the disease. Higher incidence of nodularity in our study possibly can be due to younger age of the patients and early stage of the disease at the time of diagnosis.
Hypothyroidism (n = 24) in fairly large number of patients in our study is understandable due to an advanced stage of the disease at the time of diagnosis and represent destructive phase of the disease. Normal T3 and T4 levels in the presence of elevated TSH indicate subclinical hypothyroidism and represent evolutional phase of the disease. Incidence of subclinical hypothyroidism in our study was 15.38% (n = 8). Bagchi et al.[20] found incidence of 8.17% in their study. Hashitoxicosis is a transient hyperthyroid phase of HT.[21] It is due to acute aggravation of thyroid autoimmunity induced destruction of thyroid follicles. Incidence in our study was 23.07% (n = 12). Most patients recover from hyperthyroidism. Similar observations were made in our study. None of these patients had classical clinical features of graves disease and FNA smears of these patients showed classical features of HT. Higher incidence in our study is attributed to particular presentation at the time of FNA. Small proportion of patients may continue to have persistent thyroid dysfunction.
Serum TPO antibody titers were elevated in 61.52% (n = 32) patients. Autoantibodies against Tg and TPO antigens are clinically most important for diagnosis. Previous reports reported elevation of titers in up to 95% of the patients.[22] There is a controversy, whether anti TPO alone is sufficiently reliable to diagnose HT. Up to 20% adult females with no clinical disease have detectable Tg/TPO antibodies, raising the question about their pathogenic significance.
USG of thyroid showed hypoechogenic hypervascular goiter in 53.84% (n = 32). Micro nodules were seen in 38.40% (n = 20) patients, which are highly diagnostic of HT.[23] Other features such as echogenic septations and dominant nodules were also seen in variable numbers. The low prevalence of hypoechogenic goiter in our study is possibly due to more number of cases in the younger age group. Similarly thyroid volume varies significantly with factors such as age, sex, height and place of living and hence, western data cannot be compared with Indian population.[2425]
It is not rare for patients with HT to have neither symptoms nor physical signs. Clinical features and serum findings when used alone to diagnose HT, diagnosis will be missed in many cases.[26]
Two basic patterns of HT can be recognized on cytology which are believed to correspond to different phases of the disease. (I) Classic HT: It occurs in older individuals who are more often hypothyroid. Cytology smears show increased lymphocytes on the background and infiltrating the follicular cell clusters. (II) Florid lymphocytic pattern: It occurs in younger individuals and show dominant lymphoid population in stages of maturation. Epithelial cells may be inconspicuous.[27] In our study, classic HT was seen in 44 patients. Florid lymphocytic pattern was seen in 8 patients, out of which 2 patients were in the age group of 17-20 years, 5 were in 21-30 and 1 was in 41-50. It is now widely accepted that lymphocytic thyroiditis and HT represent different manifestations of autoimmune thyroiditis, however many authors use the term synonymously.
Aspiration from more than one site increased cellular yield and was useful for achieving correct diagnosis in our study. Similar observations were made by Hamburger.[26]
Cytomorphologic features of Group I, II and III were correlated with clinical nature of thyroid enlargement, thyroid function and TPO antibodies values. Out of 20 Group I patients, substantial number of patients had uneven enlargement of thyroid (n = 9), euthyroid function (n = 8) and subclinical hypothyroidism (n = 6). TPO antibodies titers were elevated in less than half of the cases (n = 9). These findings in Group I cases suggest milder form and early stage of the disease. Nature of thyroid enlargement, thyroid function and TPO antibodies values in Group II and III patients suggested more severe form and later stage of the disease in a fair number of cases [Table 2]. Other cytomorphologic features listed in Table 3 were seen in cytologic smears in variable numbers.
Despite its superiority, FNA has some diagnostic pitfalls in diagnosing HT. Cytomorphologic features of sub-acute lymphocytic thyroiditis sometimes overlap with HT. Inflammatory infiltrate in sub-acute thyroiditis is not uniformly lymphocytic but is mixed and with evidence of more severe tissue destruction.
Florid lymphocytic thyroiditis at times can be difficult to distinguish from lymphoma on cytology smears especially in adults. Adequate sampling and careful search for other cytologic features of HT reduced the diagnostic pitfalls.
Diagnosis of HT is likely to be missed in cytology smears which show marked follicular hyperplasia as in Grave's disease or abundant colloid.[11] None of our patients showed fire flares in cytology smears.
Variable amount of colloid was seen in three patients. Out of which one patient showed abundant colloid in cytology smears. Cytology smears of this patients showed numerous lymphoid cells in stages of transformation which helped in establishing the correct diagnosis [Figure 5].
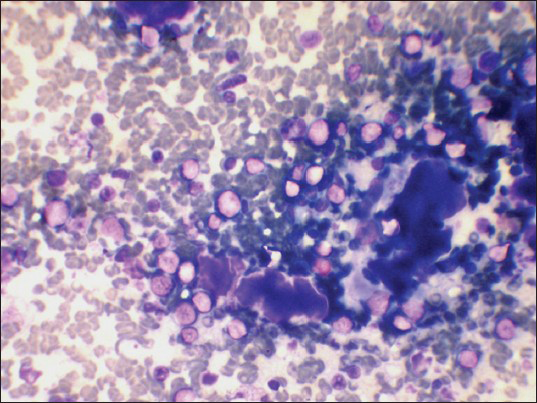
- Abundant colloid and lymphoid cells in stages of transformation (Leishman's stain, ×400)
A study by Ekambaram et al.[8] observed higher association of HT with eosinophilic inflammatory infiltrate in cytology smears. This was in contrast with our study. Eosinophils were seen in only one patient.
Follicular cells showing some features of papillary carcinoma and numerous Hurthle cells in cytology smears of HT can be a diagnostic pitfall.[8] Two patients in our study showed marked Hurthle change in cytology smears [Figure 6] and needed to be differentiated from Hurthle cell neoplasm. Repeat aspiration from other site showed lymphocytes in Hurthle cell clusters [Figure 7, arrows].

- Hurthle cells (Leishman's stain, ×400)

- Occasional lymphocytes in Hurthle cells cluster (arrows H and E, ×400)
Mild anisonucleosis is not uncommon in benign lesions of thyroid and they may be the histiocytes or regenerating epithelial cells.
In various surgical series, the prevalence of malignancy in HT ranges from 0.4% to 28%.[2829] It is well-known that almost all thyroid lymphomas arise in the setting of HT. They induce reactive lymphoid proliferation leading to the development of mucosa associated lymphoid tissue lymphoma, which can lead to an aggressive lymphoma.[30] We did not come across any associated malignancy in HT on FNA. Possible reasons can be due to the fact that many of our patients were newly diagnosed, younger age of the patients at the time of diagnosis and no significant long-term follow-up data is available.
Other autoimmune disorders may co-exist with HT.[3132] No significant evidence of other autoimmune disorders was present in our patients.
CONCLUSION(S) AND SUMMARY
HT is a disease of young and middle age and mostly occur in women. In the early stage and mild form of the disease, results of thyroid function and TPO antibodies are quite variable. Increased lymphocytes on the background and/or lymphocytic infiltration of thyroid follicular cell clusters are pathognomic of HT. FNA is the gold standard for diagnosis. In the presence of abundant colloid, follicular hyperplasia and predominant Hurthle cells in cytology smears, careful interpretation of cytomorphologic features should be done to minimize the diagnostic errors. Aspiration from more than one site minimizes the diagnostic pitfalls.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
We all authors declare that we have no competing interests or any financial competing interest in the manuscript.
AUTHORSHIP STATEMENT BY ALL AUTHORS
We all authors declare that we have sufficiently worked in the work and we have fulfilled authorship criteria. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article.
SSC has contributed in conception, design, interpretation of data and revision final approval. CRG contributed in design, interpretation of data, drafting final approval. SAB contributed in design, interpretation of data, drafting, final approval. NG contributed acquisition of data, analysis, drafting, final approval. KG contributed acquisition of data, analysis, drafting, final approval.
Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study is a retrospective analysis and correlation of clinical findings and FNA findings.
Before performing FNA, written consent of the patients was taken which is mentioned in material and methods.
We take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Lymphocytic thyroiditis: Is cytological grading significant? A correlation of grades with clinical, biochemical, ultrasonographic and radionuclide parameters. Cytojournal. 2007;4:10.
- [Google Scholar]
- Chronic (Hashimoto's) thyroiditis. In: DeGroot LJ, Jameson JL, eds. Endocrinology Vol 2. (4th ed). Philadelphia: Saunders Publication; 2001. p. :1471-80.
- [Google Scholar]
- Aspiration cytology of Hashimoto's thyroiditis in an endemic area. Cytopathology. 2002;13:31-9.
- [Google Scholar]
- Racial and age-related differences in incidence and severity of focal autoimmune thyroiditis. Am J Clin Pathol. 1994;101:698-702.
- [Google Scholar]
- Significance of eosinophils in diagnosing Hashimoto's thyroiditis on fine-needle aspiration cytology. Indian J Pathol Microbiol. 2010;53:476-9.
- [Google Scholar]
- Hashimoto's thyroiditis: Countrywide screening of goitrous healthy young girls in postiodization phase in India. J Clin Endocrinol Metab. 2000;85:3798-802.
- [Google Scholar]
- Changed presentation of Hashimoto's thyroiditis in North-Eastern Sicily and Calabria (Southern Italy) based on a 31-year experience. Thyroid. 2008;18:429-41.
- [Google Scholar]
- Lymphoid infiltrate. In: Schroder G, ed. Fine Needle Aspiration Cytology-Diagnostic Principles and Dilemmas (1st ed). Germany: Springer; 2006. p. :99-101.
- [Google Scholar]
- Organ specific autoimmune diseases. In: McPherson RA, Pincus MR, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods (21st ed). USA: Elsevier; 2007. p. :953.
- [Google Scholar]
- High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid. 2003;13:485-9.
- [Google Scholar]
- Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab. 2008;93:1751-7.
- [Google Scholar]
- The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43:55-68.
- [Google Scholar]
- Cytomorphologic aspects of thyroiditis. A study of 51 cases with functional, immunologic and ultrasonographic data. Acta Cytol. 1987;31:687-93.
- [Google Scholar]
- Hashimoto's thyroiditis: Cytodiagnostic accuracy and pitfalls. Diagn Cytopathol. 1997;16:531-6.
- [Google Scholar]
- Diagnosis of chronic lymphocytic thyroiditis (nodular presentation) by needle aspiration. Acta Cytol. 1981;25:513-22.
- [Google Scholar]
- Thyroid dysfunction in adults over age 55 years. A study in an urban US community. Arch Intern Med. 1990;150:785-7.
- [Google Scholar]
- Measurement of circulating thyroid microsomal antibodies by the tanned red cell haemagglutination technique: Its usefulness in the diagnosis of autoimmune thyroid diseases. Clin Endocrinol (Oxf). 1976;5:115-25.
- [Google Scholar]
- Micronodulation: Ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med. 1996;15:813-9.
- [Google Scholar]
- Thyroid volumes in schoolchildren of the Emirates. J Endocrinol Invest. 2003;26:56-60.
- [Google Scholar]
- Thyroid sonography in autoimmune thyroiditis. A prospective study on 123 patients. Acta Endocrinol (Copenh). 1990;122:391-5.
- [Google Scholar]
- Fine needle biopsy of thyroid nodules: Avoiding the pitfalls. N Y State J Med. 1986;86:241-9.
- [Google Scholar]
- Hashimoto's thyroiditis: Fine-needle aspirations of 50 asymptomatic cases. Diagn Cytopathol. 1994;11:141-5.
- [Google Scholar]
- Coexistence of papillary carcinoma and Hashimoto's thyroiditis. Acta Clin Croat. 2009;48:9-12.
- [Google Scholar]
- Thyroidectomy for Hashimoto's thyroiditis: Complications and associated cancers. Thyroid. 2008;18:729-34.
- [Google Scholar]
- Histopathologic and immunohistochemical features of Hashimoto thyroiditis. Indian J Pathol Microbiol. 2011;54:464-71.
- [Google Scholar]
- Chronic urticaria in patients with autoimmune thyroiditis: Significance of severity of thyroid gland inflammation. Indian J Dermatol Venereol Leprol. 2011;77:477-82.
- [Google Scholar]








