Translate this page into:
Metastatic mesothelioma to the thyroid
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 69 year-old male patient with a history of malignant mesothelioma treated with chemotherapy and surgical resection with removal of the right lung and right pleural pneumonectomy was clinically in remission for 1 ½ years. A positron emission tomography (PET) scan revealed limited uptake in the right pleural space, thought to represent post-surgical changes, and uptake in the left thyroid lobe. Thyroid ultrasound revealed a solid left lobe nodule with peripheral vascularity and absent microcalcifications. Fine needle aspiration cytology showed a microfollicular arrangement of cytologically bland cells with variable Hürthle cell changes initially interpreted as suspicious for Hürthle cell neoplasm. Review at multidisciplinary conference raised the possibility of metastatic mesothelioma, supported by immunohistochemical studies in the cell block. The patient opted for left hemithyroidectomy with isthmusectomy which confirmed malignant mesothelioma. Repeat PET scan 6 months later revealed no further uptake in the thyroid bed, with limited uptake in the right pleural space. Metastatic tumors to the thyroid are uncommon with only one previous description of metastasis to the thyroid by mesothelioma. Metastasis of cytologically low grade tumors such as mesothelioma present problems for cytology due to the potential for overlap with the variable appearances of thyroid neoplasms. The value (if any) of ancillary tests, including mutation testing, expression profiling and immunohistochemistry is discussed.
Keywords
Nuclear medicine-imaging
pathology-thyroid cytology
thyroid cancer-clinical
thyroid cancer-genetics
INTRODUCTION
On an average, 4-7% of the adult populations in the United States have palpable thyroid nodules. The incidence is more common in females than males and thought to increase with age and decrease with iodine intake.[1] The prevalence is even higher (19-67%) when detected by ultrasound, computed tomography (CT) scans, radioactive iodine studies and positron emission tomography (PET) scanning. The clinical importance of these studies is to rule out thyroid cancer, which can occur in 5-15% of cases.[1] Metastases to the thyroid are rare.
CASE REPORT
The present case report is about a 69 year-old Caucasian male with 40 pack year smoking history who was initially hospitalized for shortness of breath. His other medical conditions included hypertension, hyperlipidemia, gout, depression. CT of the chest showed a right upper lobe consolidation with a loculated right pleural effusion. PET scan revealed right pleural uptake (SUV 5.03) with no uptake in the right lung or mediastinum. Pleural biopsy confirmed malignant mesothelioma of epitheloid type and he received four cycles of chemotherapy with carboplatin and pemetrexed prior to thoracotomy with pleural pneumonectomy. Gross specimen revealed 12.5 cm × 12 cm × 5 cm mass extending into the right main stem bronchus. 13 hilar and 41 mediastinal lymph nodes were removed and all were negative on pathology.
Follow-up PET scan 1-½ years later revealed uptake in the right thorax felt to represent post- surgical changes and a fluorodeoxyglucose avid nodule in the left thyroid lobe (SUV 6). Thyroid ultrasound revealed a homogenous thyroid gland with right lobe measuring 4.3 cm × 1.6 cm × 1.1 cm, left lobe measuring 4.3 cm × 2.0 cm × 1.8 cm and isthmus was 0.4 cm. There was a 2.9 cm × 1.55 cm × 1.98 cm solid, isoechoic nodule, without calcifications, and with increased peripheral vascularity in the inferior pole of the left lobe [Figure 1] corresponding to the area of PET uptake.
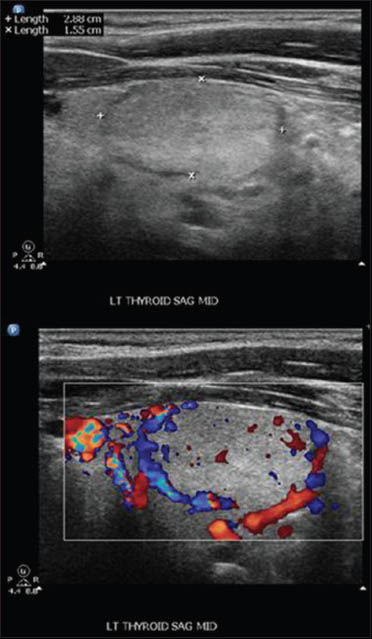
- Thyroid ultrasound: Left lobe nodule well circumscribed measuring 2.9 × 1.55 × 1.98 cm without calcifications but with peripheral vascularity
Fine-needle aspiration (FNA) of this nodule was prepared by liquid-based cytology with a ThinPrep with the residual material processed into a Cellient cell block (Hologic, Corporation, Marlborough Massachusetts). The FNA was diagnosed as suspicious for Hürthle cell neoplasm based on the presence of predominantly microfollicular groups of cells with abundant Hürthle-like cytoplasm [Figure 2]. The possibility of metastatic mesothelioma was raised at multidisciplinary thyroid FNA conference upon review, given the patient's history and review of findings on FNA. The cytology showed two distinctively different cell populations [Figure 3, panel A]. The Hürthle cells were arranged in broad two dimensional sheets with abundant granular cytoplasm and central nucleoli. The possible mesothelial cells were arranged in poorly formed glands or solid nests, and they showed waxy cytoplasm. There was no lymphocytic thyroiditis. Material in the cell block was stained for cytokeratin (CK) 5/6 and calretinin and found to be positive in the potential mesothelial population but negative in the Hürthle cell population [Figure 3, panels B and C]. The reverse was found for thyroid transcription factor-1 (TTF-1) and thyroglobulin staining.
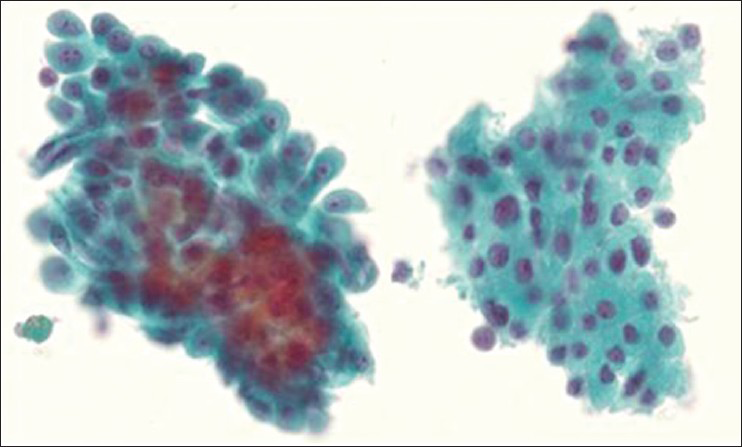
- Thyroid FNA ThinPrep: On the left are the mesothelioma cells, which are in three-dimensional groupings compared to the Hürthle cell population on the right, that forms two dimensional sheets. The mesothelioma cells have waxy cytoplasm with multiple nucleoli and more nuclear variation. The Hürthle cell population has abundant granular cytoplasm and generally one nucleolus. Papanicolaou stain ×600
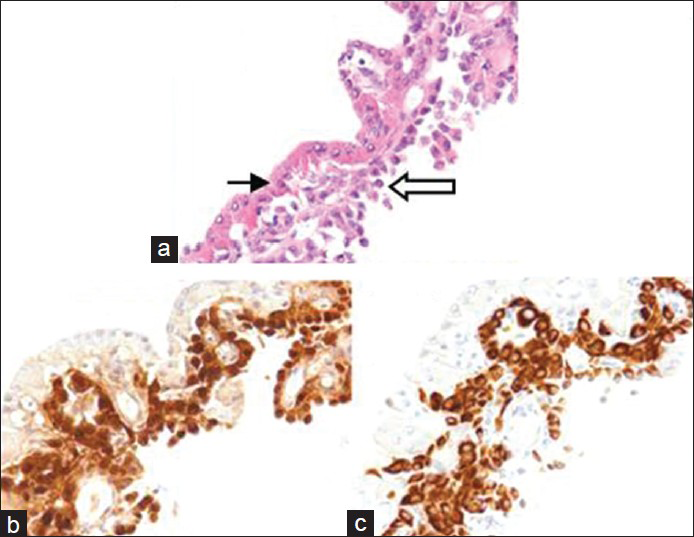
- Thyroid FNA cell block: Panel A shows two distinct populations, with Hürthle cells (thin arrow) and mesothelioma cells (open arrow). Panels B and C show calretinin and keratin 5/6 staining, respectively, in the mesothelioma cell population, but not in the Hürthle cell population. (H and E ×400)
The patient underwent left hemithyroidectomy with isthmusectomy due to the rarity of a diagnosis of metastatic mesothelioma to the thyroid and the uncertainty of how best to treat this. Final pathology confirmed the presence of multiple microscopic foci of metastatic mesothelioma that were again positive for immunostains CK 5/6 and calretinin, negative for TTF-1 and thyroglobulin [Figure 4]. Follow-up PET scan post-operatively revealed no further uptake in the thyroid bed and limited uptake in the right pleural space.
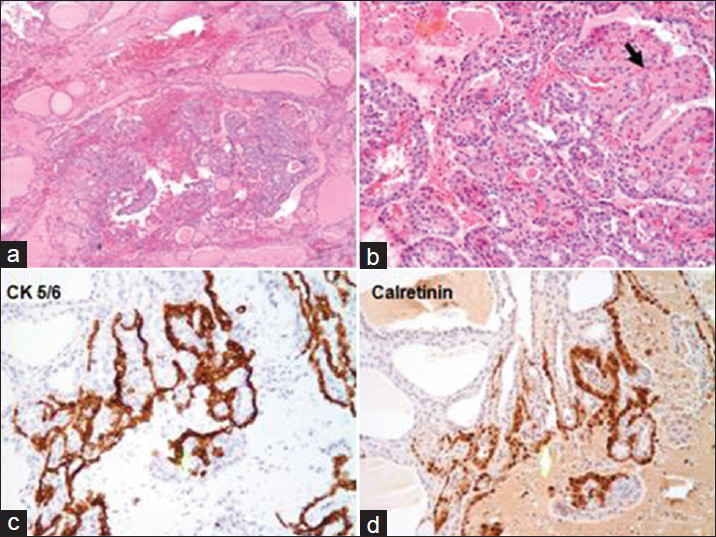
- Surgical Resection: Panel A (×40) shows nodular thyroid tissue. Panel B (×40): Two populations of cells seen within the nodule. Hürthle cells (black arrow) shows abundant pink cytoplasm. However, the tissue contains mostly mesothelial cells (white arrow) which are smaller polygonal cells with less cytoplasm. Panel C (×100): Mesothelial cells are positive for keratin 5/6. Panel D (×100): Mesothelial cells are positive for calretinin. Other immunostains (not shown) that were positive in the mesothelial cells include CK7 and HBME1, while TTF-1 and Thyroglobulin were both negative
DISCUSSION
Focal PET positive thyroid nodules have a higher risk of malignancy. More than 1/3 of these nodules are malignant, of which about 4% represent metastasis to the thyroid.[2] Metastasis can occur with equal frequency to normal and goiterous thyroid tissue.[3] However, metastasis to the thyroid may be difficult to recognize, especially if it occurs before the primary cancer has been diagnosed.[4] In a series of 25,000 FNA's reviewed to ascertain the incidence of metastatic cancer to the thyroid, 25 cases (0.1%) were identified as non-thyroid in origin.[3] The tumors that spread to the thyroid gland included kidney (8 cases), lung (7 cases), breast (5 cases) and one case each of cervical cancer/colon cancer/malignant melanoma/malignant mesothelioma/rhabdomyosarcoma. However, in only 5 of these cases was there cytological suspicion of metastasis without a clinical suspicion. In 9 of these cases, a positive FNA on cytopathology was originally indistinguishable from primary thyroid cancer prior to surgical pathology, as the primary tumor (predominately breast and renal carcinoma) was not known at the time of FNA.
Therefore, it can be difficult to distinguish primary thyroid tumors from metastasis on FNA, especially if there is no clinical suspicion of metastasis. Most thyroid tumors are relatively uniform and most metastases tend to have more pleomorphism. However, medullary thyroid carcinoma and anaplastic thyroid carcinoma are typically more pleomorphic and some metastases, such as this mesothelioma, can be relatively uniform. Specific features such as mucin or keratinization can point toward a metastasis, but not invariably. Immunohistochemical studies could therefore be the most helpful ancillary study. The absence of thyroglobulin and calcitonin staining point toward metastasis if anaplastic thyroid carcinoma is excluded.[4] Two realms of ancillary molecular testing, including mutational analysis using polymerase chain reaction (PCR) and single step reverse transcriptase PCR, have begun to be widely applied in the US in order to resolve atypical follicular cells of undetermined significance.[5] These tests may also be able to help resolve whether lobectomy or total thyroidectomy is needed in cases that are suspicious for follicular or Hürthle cell neoplasm. There is no data on how the Veracyte Affirma® gene expression profiling test would work in setting of metastasis to the thyroid. It is designed to detect normal/benign thyroid nodules by comparing the FNA washings to known gene characteristics in the Veracyte identification pool of benign or malignant nodules.[6] One could argue that the test could be positive due to the presence of a non-thyroid population. On the other hand, the majority of the cells in this patient's FNA were normal Hürthle cells, so it is possible that the assay would predict that the nodule is benign. Mutations in BRAF, RET, TRK, RAS and PAX8/PPARγ are found in 70% of follicular thyroid cancers.[7] With the exception of BRAF and N-RAS mutations, the other mutations are fairly specific for tumors of the thyroid. BRAF is commonly mutated in melanomas, Langerhans cell histiocytosis, a subset of colonic tumors and rare lung adenocarcinomas. Of importance, many metastases including mesothelioma would test negative for these mutations.
To the best of our knowledge, this is the second reported case where in an ante-mortem diagnosis of metastasis mesothelioma to the thyroid was made, as the first was identified as 1 of a series of 25,000 cases.[3] However, in a post-mortem study of 318 patients with malignant mesothelioma metastasis to the thyroid was found in 22 (7%) of patients.[8]
CONCLUSION
Metastatic tumors to the thyroid are rare, representing less than 1% of all thyroid malignancies[9] or 4% of thyroid malignancies identified by PET scanning.[2] They may closely mimic the appearance of primary thyroid neoplasm. Metastases should always be considered in patients with a previous history of cancer. Immunohistochemistry may be required to distinguish metastases from primary tumors.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
There are no conflicts of interest
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors have participated sufficiently in the study and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
As this is case report without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent). Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: A systematic review. Thyroid. 2012;22:918-25.
- [Google Scholar]
- Metastatic tumors in fine needle aspiration biopsy of the thyroid. Acta Cytol. 1991;35:722-4.
- [Google Scholar]
- Metastases to the thyroid gland: Diagnosis by aspiration cytology. Diagn Cytopathol. 1995;13:209-13.
- [Google Scholar]
- Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance" Cancer Cytopathol. 2010;118:17-23.
- [Google Scholar]
- Information for clinicians: Commercially available molecular diagnosis testing in the evaluation of thyroid nodule fine-needle aspiration specimens. Thyroid. 2013;23:131-4.
- [Google Scholar]
- Postmortem findings of malignant pleural mesothelioma: A two-center study of 318 patients. Chest. 2012;142:1267-73.
- [Google Scholar]
- Secondary malignancy of the thyroid gland: A case report and review of the literature. Thyroid. 1994;4:297-300.
- [Google Scholar]








