Translate this page into:
Determination of HER-2 status on FNAC material from breast carcinomas using in situ hybridization with dual chromogen visualization with silver enhancement (dual SISH)
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
During the last years, HER-2 status kits and protocols for chromogen visualization of hybridization signals have come on the market. The first generation using chromogen visualization used single color probes. The second generation, now emerging on the market, uses dual chromogen visualization. The aim of this study has been to test a new dual color chromogen kit (Ventana INFORM HER2 Dual Colour ISH Roche®) and compare the results with our in-house method(s). The material consisted primarily of cytological material from invasive breast carcinomas in 49 women. Dual SISH was done on all 49 cytological and histological specimens. The histological specimens were treated according to the manufacturer’s recommendations. The procedure was modified in several steps in order to adapt it to the cytological material. Hybridization failed in two cytological specimens. Dual SISH showed concordant results on cytological and histological material as to amplified/not amplified. The included cases had the same HER-2 expression in the invasive and the in situ components on histology. Four IDC showed HER-2 amplification (8.5%). Polysomy was found in two cases. All dual SISH results except for one concurred with the results of the in-house method(s) (1/47=2.1%). The dual SISH is suitable for cytological examination of HER-2 status. The protocol must be optimized for cytological material.
Keywords
Breast carcinoma
dual SISH
fine needle aspiration cytology
HER-2
in situ hybridization
liquid based material
INTRODUCTION
Breast carcinoma is a heterogeneous disease. The aggressiveness and prognosis of the tumors vary from low-grade carcinomas with an excellent prognosis to high-grade carcinomas with a poor prognosis. Prognostic and predictive markers such as estrogen and progesterone receptor status, HER-2 status and Ki-67 index are routinely evaluated in all carcinomas according to the recommendations of the Norwegian Breast Cancer Group (www.nbcg.no)[1] and national recommendations (www.helsedirektoratet.no/vp/multimedia/archive/00021/Nasjonalt_handlingsp_21559).[2] This is usually done on a preoperative biopsy or the surgical specimen. If necessary, all of the markers can also be evaluated on cytological material, but is most commonly used in metastatic settings where surgery or a biopsy is not feasible.[3] Cytological material is suitable for examining HER-2 using in situ hybridization (ISH)[3–6] and capping of tumor cell nuclei (meaning that in a histological section only part of, and not the whole nucleus, is represented) is not an issue. FISH (fluorescent in situ hybridization) protocols for HER-2 determination can normally be used on cytological material without or with only minor changes in the procedure for histological specimens.
During the last years, HER-2 status kits and protocols for chromogen visualization of hybridization signals have come on the market. These have been preferred in many pathology laboratories. The morphology is the same as in immunohistochemistry (IHC) and immunocytochemistry (ICC). The slides can be read using bright field microscopes and can be stored in the same way as the other cytological and histological specimens. The first generation using chromogen visualization used single color probes.[7–11] Kim et al,[5] demonstrated its usefulness in cytological specimens. The second generation, now emerging on the market, uses dual chromogen visualization.[12] The aim of this study has been to test a new dual color chromogen kit (Ventana INFORM HER2 Dual Colour ISH Roche®) and compare the results with our in-house method(s).
MATERIALS AND METHODS
The material consisted primarily of cytological material from breast lesions in 50 women. All cases had been preoperatively investigated with FNAC. The morphological preoperative diagnoses were based on direct, air - dried and Giemsa stained smears. Additional liquid based material (primarily suspended in ThinPrep Cytolyt and stored in ThinPrep Preservcyt (Hologic™, Crawley, West Sussex, UK) was available for estrogen and progesterone receptor status as well as HER-2 status. One lesion was cytologically benign and was included as benign “control”. Histologically, it was a benign phyllodes tumor. In situ hybridization failed in two cytological specimens. This left us with concordant cytological and histological material from 47 invasive carcinomas.
The surgical specimens had been routinely processed and diagnosed, including HER-2 status. In addition to IHC (Ventana INFORM®), FISH (previous in-house method, Ventana INFORM FISH®) and (single probe) SISH (current in-house method, Ventana INFORM SISH®) were used. IHC and FISH were in-house methods in the department from 2003 to 2008. Single probe SISH then replaced FISH as in-house method. The material for this study was collected during 2008 - early 2009. Until late 2008, both IHC and ISH were done routinely on all new primary breast carcinomas for QC purposes. These comprised 29 cases (14 cases IHC + single probe SISH; 19 cases IHC + single probe FISH). A shorter period using single probe SISH as the primary test followed (14 cases) before the department started to use IHC as the primary test and with additional SISH according to national guidelines (that is when IHC is 2+).
Dual SISH (Ventana INFORM HER2 Dual Colour ISH, Roche®) was done on all 47 cytological and histological specimens. The histological specimens were treated according to the manufacturer’s recommendations. The procedure was modified in several steps in order to adapt it to the cytological material [Table 1]. This was done prior to the study investigation. Strong and clearly identifiable signals for both HER-2 gene and CEP17 were demanded for optimization.
| Day 1 |
| Fix slides in 4% neutral buffered formalin (NBF) for 2 h at the bench |
| Wash slides in Tris buffered saline (TBS) 2×5 min |
| Load slides in the staining machine and start the protocol for cytological material |
| Cell conditioning: Incubate slides in mild followed by standard cell conditioning buffer (CC2) for 8 min each. |
| After enzyme (protease 3) incubation for 8 min, warm up slides to 47°C prior to hybridization |
| Incubate slides with HER-2 DNA probe and hybridize for 6 h at 47°C |
| Warm up slides up to 67°C and incubate in SISH stringent wash buffer 3×8 min. |
| Incubate slides with Red ISH-probe (Chr17 Probe) at 44oC for 4 min and further for 2 h. |
| Wash slides in Red ISH stringent wash at 59°C for 3×8 min. |
| Counterstain slides with hematoxylin II for 8 min followed by post counterstain with Bluing reagent for 4 min. |
| Day 2 |
| Wash slides in warm tap water for 10 min. |
| Air dry slides completely (e.g. 15 min at 56°C) |
| Coverslip with non-alcoholic/non-xylen based mounting medium |
HER-2 scoring was identical for cytological and histological specimens and was according to the manufacturer’s instructions. A ratio HER-2/CEP17 of > 2.2 was regarded as amplification. A ratio of less than 1.8 was considered not amplified and 1.8 – 2.2 as equivocal. Signals in at least 20 non-overlapping nuclei in two different fields were counted. Alternatively, in overlapping groups, the total number of nuclei and signals were counted and a mean calculated. A mean signal number and a ratio between the HER-2 and CEP17 were calculated. Polysomy was defined as a mean signal number of > 3 and a ratio of < 1.8.
Tumor subtype, grading as well as ER/PgR and routinely performed HER-2 status were retrieved from the pathology files. At the time of the study, cut-off for a positive ER/PgR was 10%. This has since been changed to 1%.
RESULTS
There were 39 ductal (IDC), 5 lobular (ILC), 2 mucinous and 1 invasive papillary carcinoma. Of these, 11 were grade 1, 27 were grade 2 and 9 were grade 3. Estrogen receptor (ER) was strongly positive (that is in > 50% of the tumor cells) in 40 cases and negative in 7 cases. Progesterone receptor (PgR) was negative in 15 cases, positive in < 50% of the tumor cells in 12 cases and positive in > 50% of the tumor cells in 20 cases. All ILC and mucinous carcinomas as well as the papillary carcinoma were ER/PgR positive. Tumor sizes were as follows: pT1b =8, pT1c =16, pT2 =16 and pT3 =7. Axillary lymph node metastases were found in 25 cases, whereas 22 cases were N0.
The red (CEP17) and silver (HER-2 gene) chromogen signals could be read at ×40 magnification, but were easier to evaluate under oil immersion (×100) [Figure 1a and b]. The optimal signal size and “sharpness” depended on the post hybridization washing conditions and were different for the two chromogens. The final procedure represented a compromise allowing both signals to be read easily [Table 1]. The differences in the cytological and histological procedures are highlighted in Table 2. As can be seen, a number of steps were modified, including type of buffer, hybridization temperature and stringent wash temperature. CC2 buffer gave better results as RB and the pretreatment could be somewhat shorter than for histological specimens. Likewise, the optimal hybridization and stringent wash temperatures were somewhat lower when using cytological preparations.
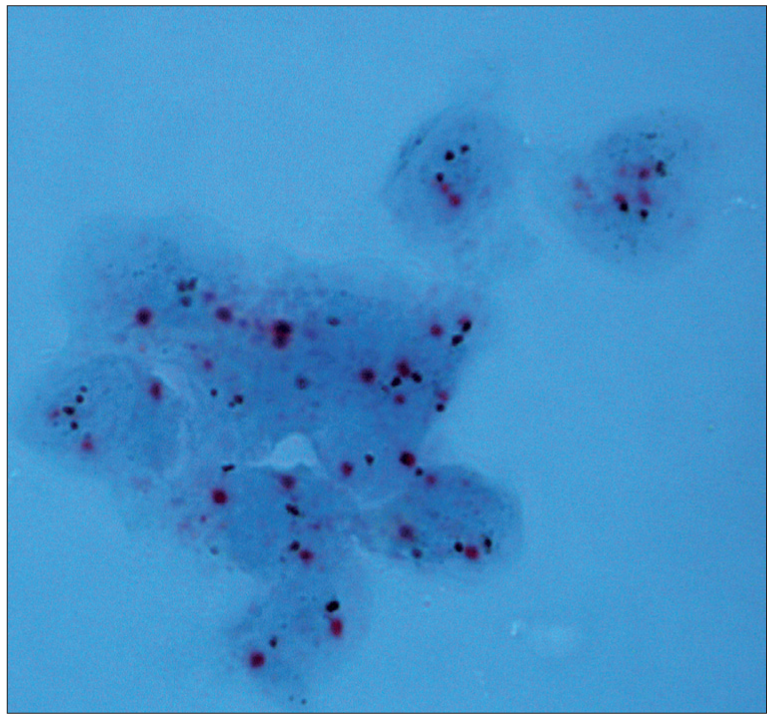
- Dual color SISH. Magnification × 1000. Cytological specimen, direct smear. Non-amplified with two signals of CEP17 (red) and HER-2 gene (black) per nucleus
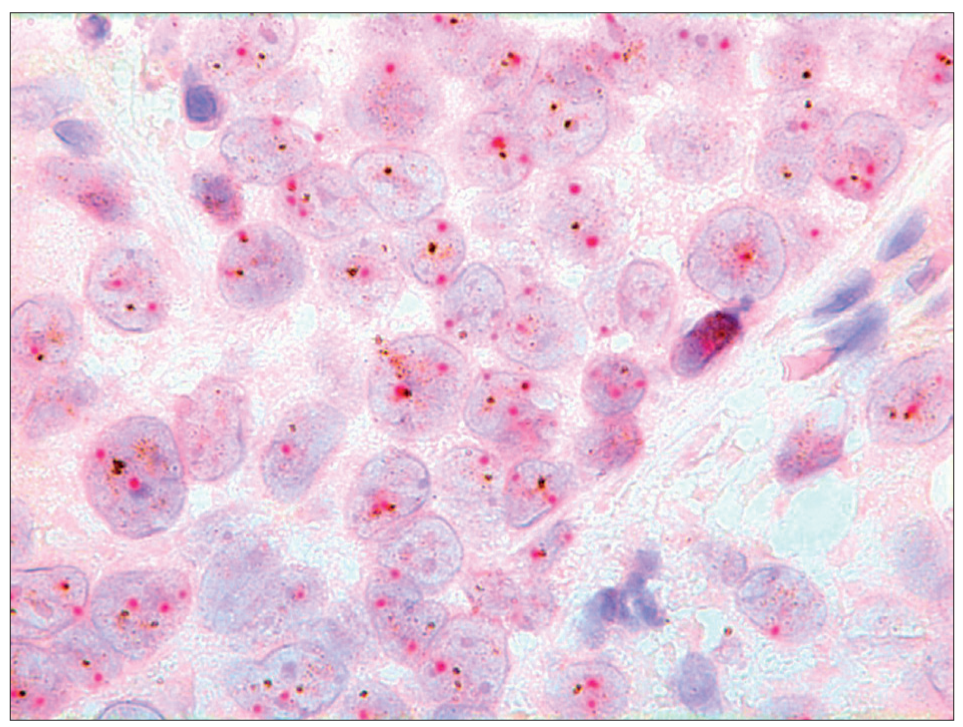
- Dual color SISH. Magnification × 1000. Histological specimen. Non-amplified with two signals of CEP17 (red) and HER-2 gene (black) per nucleus
| Cytological protocol | Histological protocol |
|---|---|
| Cell conditioning:- Cell conditioning buffer (CC2) | Cell conditioning:- Reaction buffer (RB) |
| Citrate-buffer with pH 6.0 and containing | Tris-buffer with pH 7.6 -7.8 and containing |
| Ethylene glycol | Tris (hydroxy methyl) aminomethane |
| Dodecyl sodium sulfate | Acetic acid |
| Hydrous citric acid | Proclin |
| Sodium metabisulfate | |
| Mild CC2 8 min | Mild RB 8 min |
| Standard CC2 8 min | Standard RB 12 min |
| Extended RB 8 min | |
| SISH hybridization temperature 47°C | SISH hybridization temperature 52°C |
| SISH stringent wash temperature 67°C | SISH stringent wash temperature 72° C |
Dual SISH showed 96% concordant results on cytological and histological study material. An equivocal ratio was found in two cytological specimens (1.94 and 1.97). These two cytological cases were listed as non-amplified. The corresponding histological ratios were 1.51 and 1.52, respectively. No case was found to be amplified on the cytological specimen and non-amplified on histological specimen or vice versa. The included cases had the same HER-2 expression in the invasive and the in situ components on histology. Four IDC showed HER-2 amplification (8.5%) on dual SISH [Figure 2a and b]. Corresponding in – house HER-2 status was based on IHC + FISH in one case, on IHC + SISH in one case, and on IHC 3+ alone in two cases. Polysomy [Figure 3] was found in 2 cases. All dual SISH study results (both cytological and histological) except for one concurred with the routine HER-2 status results of the in-house method(s) (1/47=2.1%). The discordant case was amplified on both cytological and histological specimens using dual SISH with ratios of 4.18 and 3.64, respectively. Routine IHC had been 2+, but the current in-house SISH showed only 1-2 signals per nucleus of both HER-2 gene and CEP17. An overview of signal counts and ratios is shown in Table 3.
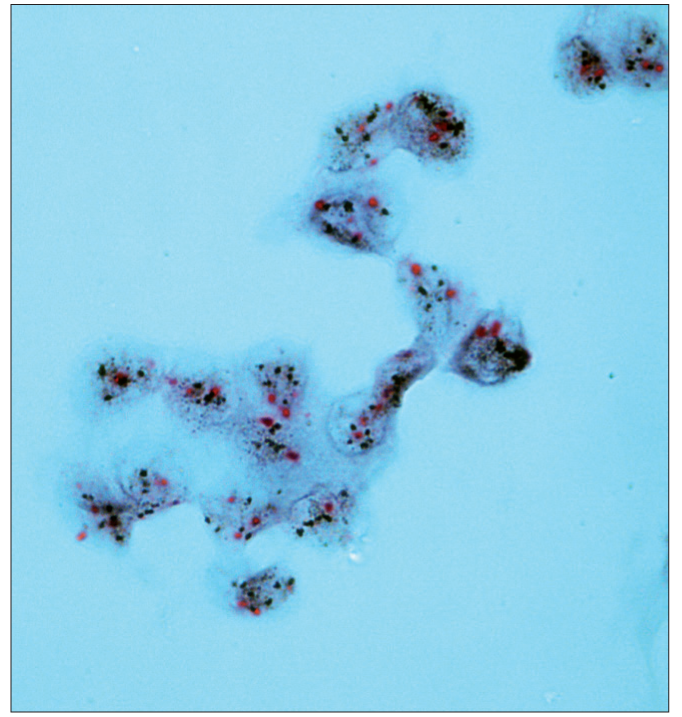
- Dual color SISH. Magnification × 1000. Cytological specimen, liquid based preparation. Two CEP17 (red) signals and from 5 to > 10 HER-2 gene signals (black) per nucleus. Amplification.
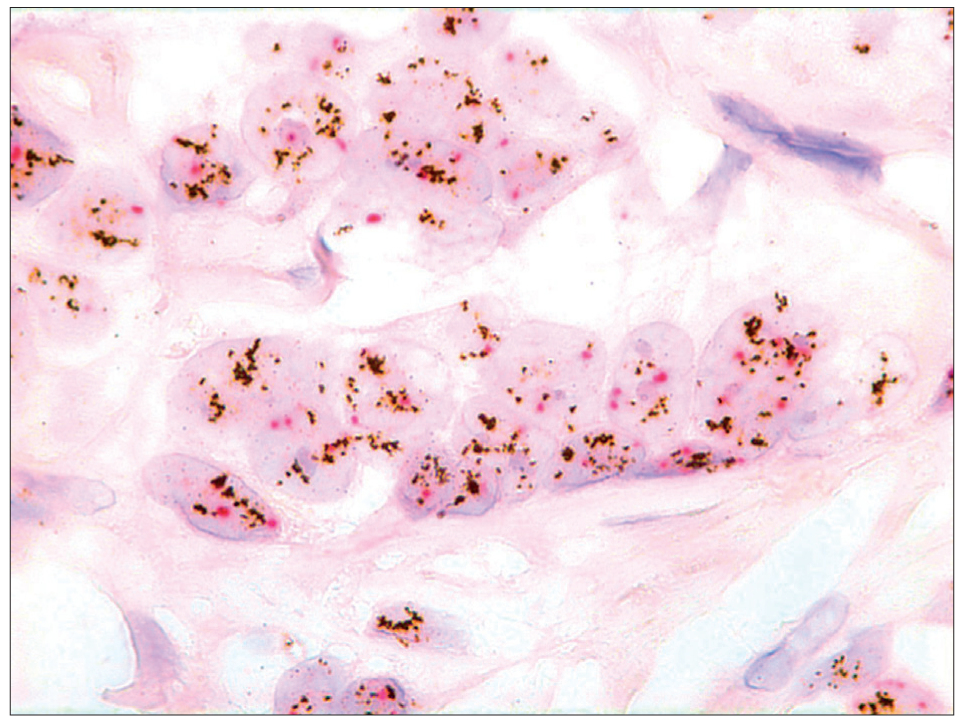
- Dual color SISH. Magnification × 1000. Histological specimen. Two CEP17 (red) signals and a highly increased number of HER-2 gene signals (black) per nucleus. Amplification.
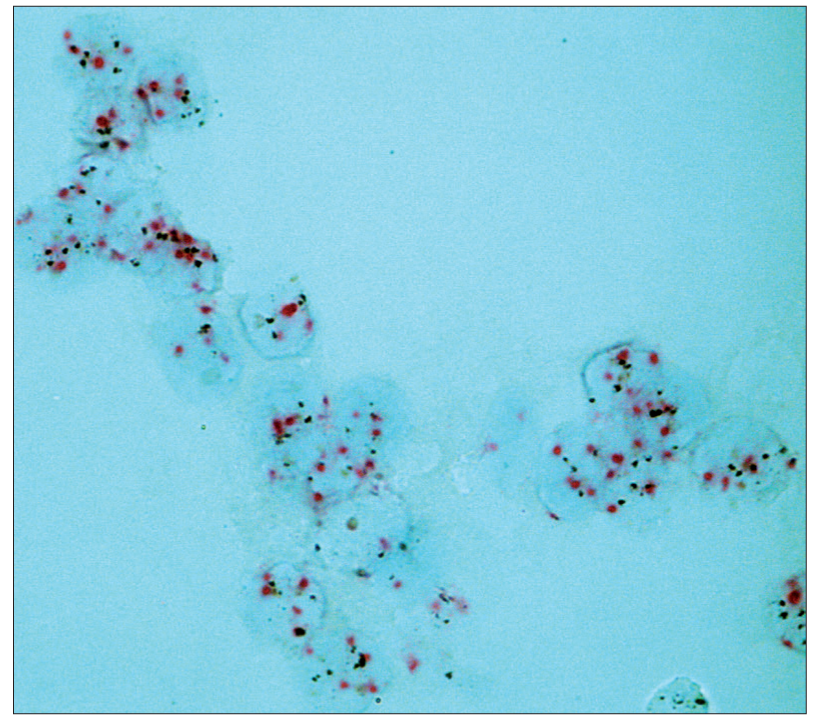
- Dual color SISH. Magnification × 1000. Cytological specimen. Liquid based preparation. Polysomy with increased number of both CEP17 (red) and HER-2 gene (black) per nucleus
| Mean | Median | Minimum | Maximum | |
|---|---|---|---|---|
| Number of HER-2 gene signals CYT/HIST non-amplified (=43) | 2.09/1.76 | 1.95/1.6 | 1.26/1.08 | 4.1/3.25 |
| Number of CEP17 signals CYT/HIST non-amplified | 1.84/1.54 | 1.75/1.45 | 1.13/1 | 3.05/2.95 |
| Ratio HER-2/CEP17 CYT/HIST non-amplified | 1.14/1.15 | 1.11/1.14 | 0.74/0.68 | 1.97/1.54 |
| Number of HER-2 gene signals CYT/HIST amplified (=4) | 10.25/8.69 | 10.5/7.83 | 8/5.8 | 12/13.29 |
| Number of CEP17 signals CYT/HIST amplified | 2.21/1.98 | 2.27/2 | 2/1.8 | 2.3/2.1 |
| Ratio of HER-2/CEP17 CYT /HIST amplified | 4.61/4.37 | 4.61/3.82 | 4/3.2 | 5.22/6.65 |
DISCUSSION
The ASCO/CAP guidelines for HER-2 testing[13] state that “If laboratories choose to use alternative fixatives other than buffered formalin, the laboratory is obligated to validate that fixative’s performance against the results of testing of the same samples fixed also in buffered formalin and tested with the identical HER2 assay, and concordance in this situation must also be 95%.” A FAQ web site[14] to these guidelines answer the question “Do the guidelines exclude HER2 testing of cytology specimens (fluids and aspirates) that have been fixed in 95% ethanol rather than formalin?” as follows: “Since cytology specimens are not ordinarily fixed in formalin, that sentence is not directly applicable, but labs performing HER2 testing on such specimens must document that they validated their methods and achieved acceptable concordance, in this case by comparing staining of alcohol fixed cytology specimens with routinely processed (formalin-fixed) tissue sections”. The complete concordance between dual color SISH results on cytological and histological specimens in this study as to amplification/no amplification as well as a concordance of 97.9% with the in-house method(s) indicates that this method and protocol is well suited to investigate HER-2 status on cytological material.
In contrast to FISH, where the histological protocol can be used practically unchanged for cytological specimens, the dual SISH method required adjustment of several steps in the protocol. The first crucial step is fixation. The primary fixation of cytological specimens is usually alcohol-based (ethanol or methanol) or acetone/acetic acid. Cross-linking is thus not occurring and demasking as when using formalin fixation is not necessary. However, formalin may also be used for fixation of cytological material. Several articles have reported different fixation methods for optimal sensitivity of ICC[15–19] and some have concluded that formalin is a good choice of fixative for ICC.[17] The same applies to ISH on cytological material. Here also, formalin fixation is a good option.[20]
Primary or post fixation of cytological material requires demasking, but usually much less than histological material. In this study, the specimens were post fixed in 10% formalin for 2h. Shorter or longer fixation periods resulted in weak or no gene and/or CEP17 signals.
Type of buffer is known to influence the results in immunocyto- and histochemistry and give anything from a false negative or weak positive reaction to a strong positive staining. In this study, optimal type of buffer and time for preconditioning of cytological material was different compared to the standard histological procedure given by the manufacturer. CC2 is a citrate buffer with PH 6 whereas RB is a Tris buffer with a higher of PH 7.6-7.8. As these modifications are not necessary using a FISH procedure, the differences may relate mainly to the reaction of the chromogen substances and not to the hybridization step.
Mean signal counts were higher in the cytological specimens as expected, because there is no capping of the nuclei. The ratios, however, were virtually the same in the concurrent cytological and the histological specimens with the exception of two cases with an equivocal ratio on cytological specimen and a clearly non-amplified ratio on the histological specimen. The number of amplified and polysomic cases was somewhat low. This is probably due to inclusion bias.
Chromogen ISH has a number of advantages. The morphology is familiar to what we see and evaluate in our everyday diagnostics, both in cytological and histological specimens. The cytomorphology is recognizable and the risk of including benign cells in our evaluation is minimal. The signals can be read in bright field microscope and the specimens may be stored alongside with the diagnostics slides in the department.
One of the objections to evaluating the results of ancillary studies on cytological material from primary breast carcinomas is that there may be a mixture of cells from the invasive component and from a component of ductal carcinoma in situ. This does not preclude testing for HER-2 (or ER/PgR) in metastatic settings, provided the institutions have adequate methods. In this study, the included cases had the same HER-2 expression in the invasive and the in situ components on histology.
In conclusion, cytological material is suitable for HER-2 examination using dual chromogen visualization with silver enhancement (Dual SISH) in situ hybridization (Ventana INFORM HER2 Dual Colour ISH Roche®). The protocol must be modified and optimized for cytological material.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
No competing interest to declare by any of the authors.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version was read and approved. All authors qualify for authorship as defined by ICMJE http://www.icmje.org/#author Each author participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from institutional Review Board (IRB) (or its equivalent) of all the institutions associated with this study.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model(authors are blinded for reviewers and reviewers are blinded for authors)through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2010/7/1/21/70968
REFERENCES
- Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av pasienter med brystkreft. In “Nasjonale faglige retningslinjer 15-1524” 2007 (National action program and recommendations for diagnostics, treatment and follow-up of patients with breast cancer)
- [Google Scholar]
- The role of breast FNAC in diagnosis and clinical management: A survey of current practice. Cytopathology. 2008;19:271-8.
- [Google Scholar]
- Fluorescence in situ hybridization in diagnostic cytology. Hum Pathol. 2007;38:1137-44.
- [Google Scholar]
- Chromogenic in situ hybridization analysis of HER-2/neu status in cytological samples of breast carcinoma. Cytopathology. 2004;15:315-20.
- [Google Scholar]
- Immunocytochemical evaluation of HER-2/neu on fine-needle aspirates from primary breast carcinomas. Diagn Cytopathol. 2003;28:142-6.
- [Google Scholar]
- Chromogenic in situ hybridization: A practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000;157:1467-72.
- [Google Scholar]
- Chromogenic in situ hybridization in tumor pathology. Methods Mol Med. 2004;97:133-44.
- [Google Scholar]
- Chromogenic in situ hybridization is a reliable method for detecting HER2 gene status in breast cancer. Am J Clin Pathol. 2009;131:490-7.
- [Google Scholar]
- Performance of chromogenic in situ hybridization on testing HER2 status in breast carcinomas with chromosome 17 polysomy and equivocal (2+) herceptest results. Am J Clin Pathol. 2009;132:228-36.
- [Google Scholar]
- Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007;451:19-25.
- [Google Scholar]
- Dual-colour chromogenic in situ hybridization for testing of HER-2 oncogene amplification in archival breast tumours. J Pathol. 2006;210:3-9.
- [Google Scholar]
- American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18-43.
- [Google Scholar]
- An evaluation of potentially suitable fixatives for immunoperixidase staining of estrogen receptors in imprints and frozen sections of breast carcinoma. Pathology. 1988;20:320-5.
- [Google Scholar]
- Optimal fixation conditions for immunocytochemical analysis of estrogen receptor in cytologic specimens of breast carcinoma. Cancer. 2003;102:34-40.
- [Google Scholar]
- Immunostaining of estrogen receptor, progesterone receptor, MIB1 antigen and c-erbB-2 oncoprotein in cytologic specimens: A simplified method with formalin fixation. Diagn Cytopathol. 1997;17:127-33.
- [Google Scholar]
- Immunocytochemical detection of prognostic markers in breast cancer: Technical considerations. Cytopathology. 1999;10:308-16.
- [Google Scholar]
- Expression of estrogen and progesterone receptors in cytologic specimens using various fixatives. Diagn Cytopathol. 1996;15:78-83.
- [Google Scholar]
- Method development for DuoCISH HER-2 on FNAC material from breast carcinomas. Cytopathology. 2009;20:110.
- [Google Scholar]








